Abstract
Objective
To assess the immunosuppressive effect of R-CHOP in patients with B-cell lymphoma at 2 years.
Methods
Parameters of humoral and cell-mediated immunity were assessed in 89 patients with diffuse large B-cell lymphoma or follicular lymphoma before and after 6–8 cycles of R-CHOP-14 or R-CHOP-21 regimen.
Results
Data on pre- and posttreatment serum IgG (sIgG) levels were available for all 89 patients, while the corresponding data on serum CD20+, CD3+, CD4+, and CD8+ lymphocyte counts were available in only 43. Median sIgG levels significantly decreased from 1,221 mg/dl (baseline) to 733 mg/dl (after chemotherapy) (p < 0.001). Although CD20+ and CD4+ cell counts decreased (p < 0.001), no significant effect of chemotherapy on CD3+ and CD8+ cell counts was observed. CD20+ cell counts were restored to baseline levels at the 12-month follow-up. sIgG levels and CD4+ cell counts were not completely restored at 24 months, indicating a sustained immunosuppressive effect of R-CHOP in these patients. The incidence of infections over the 2-year period was 16.3–23.6%.
Conclusion
The immunosuppressive effect of R-CHOP in newly diagnosed cases of B-cell lymphoma tends to persist for >2 years, although sIgG levels were restored more quickly than CD4+ cell counts.
Key Words: B-cell lymphoma, R-CHOP therapy, Immune system, Serum IgG, CD4+ lymphocyte
Introduction
R-CHOP therapy is the standard first-line treatment for B-cell lymphoma [1,2,3]. In Japan, R-CHOP therapy is confined to the standard first-line treatment for diffuse large B-cell lymphoma (DLBCL). However, the standard first-line treatment for follicular lymphoma (FL) has not been determined, although R-CHOP or R-COP therapy is often conventionally used. CHOP therapy comprises cytotoxic drugs, namely cyclophosphamide, doxorubicin, vincristine, and prednisolone (steroid); R-CHOP therapy includes rituximab with the abovementioned drugs. Rituximab is a chimeric monoclonal antibody against CD20 and exerts antineoplastic effects by producing antibody-dependent cellular cytotoxicity, particularly by inducing complement-dependent cytotoxicity [4,5]. In a previous study, R-CHOP therapy significantly prolonged time to treatment failure as well as overall survival among patients with symptomatic, advanced-stage FL compared with CHOP therapy [6]. In a subsequent study, R-CHOP therapy significantly increased the rates of complete responses, decreased the rates of treatment failure and relapse, and improved event-free and overall survival of elderly patients with newly diagnosed DLBCL compared with CHOP therapy [7]. Moreover, R-CHOP therapy was reportedly effective in patients with non-Hodgkin's lymphoma, and it is recognized as the standard treatment for B-cell lymphoma [8,9].
In previous studies [10,11], B cells were reconstituted after rituximab monotherapy, whereas B cell count was rapidly depleted and slowly recovered over 3–6 months and required approximately 1 year for complete restoration. In contrast, rituximab monotherapy did not have a significant effect on CD3+, CD4+, and CD8+ T-cell counts. In agreement, an immunological study showed that circulating B cells disappeared early after rituximab monotherapy, whereas T-helper cells (CD3+/CD4+), T-suppressor cells (CD3+/CD8+), and NK cell count remained stable [12,13,14,15]. Although the influence of rituximab monotherapy on immune system restoration have been examined in several studies, only few studies have described the influence of R-CHOP therapy on immune system restoration.
Nonetheless, Kurokawa et al. [16] reported the recovery of B-cells over 1 year and CD4+ T-cells and immunoglobulin over 2 years after the chemotherapy in patients with B-cell lymphoma who received R-CHOP-like regimen (cyclophosphamide, 750 mg/m2 on day 1; pirarubicin, 50 mg/m2 on day 1; vincristine, 1.4–2 mg on day 1; prednisolone, 100 mg/body on days 1–5; and rituximab 375 mg/m2 administered before each of the cycles). Moreover, no previous studies reported the immune function restoration for 2 years after R-CHOP therapy. Thus, we conducted a retrospective study where the influence of R-CHOP therapy was investigated after 2 years on immune system restoration and infection rate in patients with B-cell lymphoma.
Subjects and Methods
Patients who received 6–8 cycles of R-CHOP or R-COP regimens as initial treatments for DLBCL or FL were recruited between April 2004 and April 2011 from the Fujita Health University Hospital. Patients received 375 mg/m2 rituximab on day 1 of CHOP therapy comprising 750 mg/m2 cyclophosphamide, 50 mg/m2 doxorubicin, and 1.4 mg/m2 vincristine on day 1, and 50 mg/m2 or 100 mg prednisolone on days 1–5 of 14- or 21-day cycles. Patients who were treated with rituximab monotherapy were not included, and doses of cyclophosphamide, doxorubicin, vincristine, and prednisolone were reduced by 20% in patients aged >70 years. Regular clinical observations were continued for over 2 years, and immune function was assessed according to CD4+, CD8+, and CD20+ lymphocyte counts using a Cytomics FC500 (Beckman Coulter Inc., Calif., USA), and serum IgG (sIgG) levels were determined using a JCA-BM6010 (JEOL Ltd., Tokyo, Japan). For measurement of lymphocyte counts, we used an FITC-labeled CD4 murine monoclonal IgG antibody fraction, a PE-labeled CD8 murine monoclonal IgG antibody fraction, and a PE-labeled CD20 murine monoclonal IgG antibody fraction (Beckman Coulter). Polyclonal rabbit anti-human IgG/FITC rabbit F(ab′)2 Code F0185 (Dako Denmark A/S, Denmark) were used for measurement of sIgG levels.
Kinetic data were collected before and after the projected cycles of R-CHOP therapy and at 3, 6, 9, 12, 15, 18, 21, and 24 months after the completion of treatment. Only follow-up samples that were collected within 1 month of each defined time point were used in analyses, and the number of patients included is presented for each time point. Immunological parameters were determined before and after the cycles of R-CHOP therapy and at 6, 12, 18, and 24 months after the completion of the treatment. Only follow-up samples that were collected within 2 months of each time point were included in the analyses. The present retrospective investigation was approved by the Institutional Review Board of the Fujita Health University School of Medicine and conformed to the provisions of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards. There was a limitation in gathering information because this study involved a retrospective investigation. We confirmed the tolerance level and gathered information on the condition, which was constant.
Statistical Analysis
Data are expressed as medians (first quartile-third quartile), and differences in lymphocyte subset counts and sIgG levels before and after chemotherapy were identified using the Wilcoxon signed-rank test. Pairwise differences in laboratory parameters were identified using the Mann-Whitney U test, and multiple comparisons were performed using the Kruskal-Wallis test. Effective ratios at each time point were compared using the χ2 test. Data expressed as percentages were assessed by the χ2 test. Univariate analysis was performed to identify risk factors. Variables with a significance level of <20% were included in the multivariate logistic regression model. The Hosmer-Lemeshow statistical test was used to verify goodness of fit of the developed model. Statistical analyses were conducted using SPSS v22.0 (IBM Corporation, Armonk, N.Y., USA), and differences were considered significant at a p value of <0.05.
Results
Baseline Characteristics
We investigated the kinetic data of sIgG levels in 89 patients and of lymphocyte count in 43 of those patients; no pairwise differences between the groups in terms of the baseline data for the abovementioned investigations were found (table 1).
Table 1.
Patient characteristics at baseline
| sIgG (n = 89) | Lymphocyte subsets (n = 43) | p value | |
|---|---|---|---|
| Age, years | 65 (57– 74) | 65 (57 – 74) | 0.82 |
| Male gender, % | 53.9 | 53.4 | 0.96 |
| sIgG, mg/dl | 1,214 (1,021– 1,450) | 1,187 (1,031 – 1,419) | 0.84 |
| Lymphocyte subsets | |||
| CD20+ cell counts, /µl | – | 144 (67 – 289) | |
| CD3+ cell counts, /µl | – | 941 (637 – 1,196) | |
| CD4+ cell counts, /µl | – | 590 (303 – 786) | |
| CD8+ cell counts, /µl | – | 302 (186 – 464) | |
| CD4+/8+ ratio | – | 1.78 (1.28 – 2.55) | |
| Pathology | 0.69 | ||
| DLBCL | 61 | 28 | |
| FL | 28 | 15 | |
| Grade 1, 2, 3 | 10, 12, 6 | 7, 6, 2 | |
| Ann Arbor stage I, II, III, IV | 26, 22, 20, 21 | 15, 13, 6, 9 | 0.62 |
| Chemotherapy | 0.63 | ||
| R-CHOP-14 × 6 times | 55 | 23 | |
| R-CHOP-21 × 6 | 12 | 4 | |
| R-CHOP-21 × 8 | 3 | 3 | |
| R-CHOP-21 × 4 after R-COP-21 × 4 | 10 | 8 | |
| Others | 9 | 5 | |
| Doses of rituximab, n | 6 (6– 8) | 7 (6 – 8) | 0.054 |
Variables that do not follow a normal distribution are expressed as median (interquartile range). IFRT = Involved-field radiation therapy.
Immune Function before and after Chemotherapy
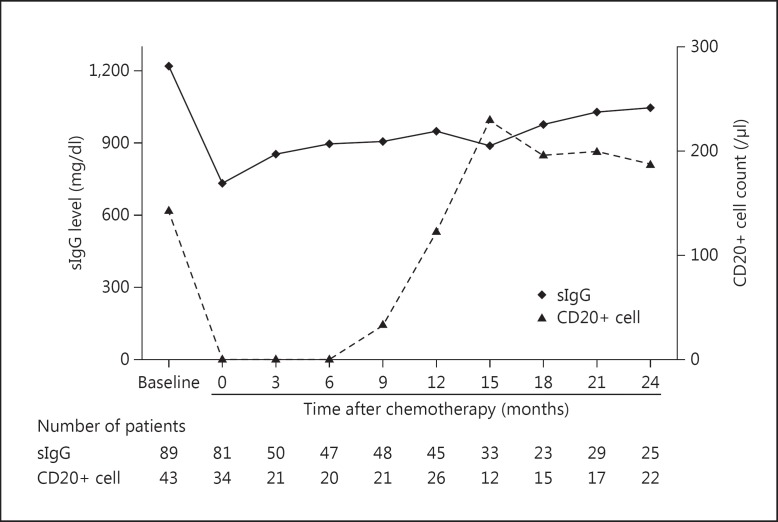
Humoral (sIgG level) and cellular immune parameters (CD20+, CD3+, CD4+, and CD8+ cell counts as well as CD4+/8+ ratios) were determined before and after chemotherapy and at 3, 6, 9, 12, 15, 18, 21, and 24 months (±1 month) after the completion of chemotherapy. In these analyses (fig. 1), median sIgG levels significantly decreased (40%) from 1,214 mg/dl before to 733 mg/dl after chemotherapy (p < 0.001). However, sIgG levels were significantly recovered after 3 months (fig. 1).
Fig. 1.
Kinetics of sIgG level and CD20+ cell count in peripheral blood. The data were obtained before and after chemotherapy, and at 3, 6, 9, 12, 15, 18, 21, and 24 months (±1 month) after chemotherapy completion. Data are expressed as medians.
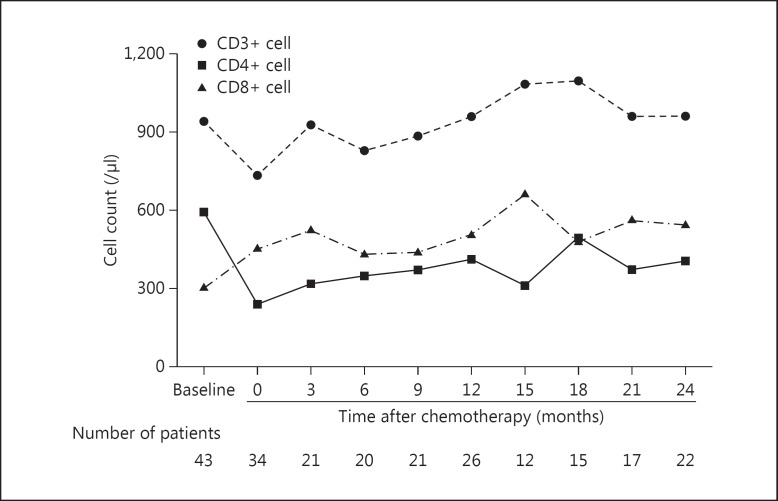
The median baseline CD20+ cell count was 144/µl and decreased rapidly to 0/µl after chemotherapy completion (p < 0.001; fig. 1). Subsequently, CD20+ cell counts remained low for 6 months and then recovered to approximately 85.7% of baseline levels at 1 year and to 100% at 2 years after chemotherapy. In contrast, CD3+ lymphocyte counts did not decrease following chemotherapy (p = 0.126, fig. 2), although CD4+ cell counts decreased from 590/µl at baseline to 239/µl after chemotherapy (p < 0.001), representing a 40.5% decrease in median CD4+ cell counts. CD8+ cell counts after chemotherapy (fig. 2) did not differ from those at baseline (p = 0.207). The median baseline CD4+/8+ ratio was 1.78 and was significantly decreased to 0.80 (p < 0.001) after chemotherapy and remained low for the subsequent 2 years.
Fig. 2.
Kinetics of each lymphocyte subset (CD3+, CD4+, and CD8+) cell count in peripheral blood. The data were obtained before and after chemotherapy, and at 3, 6, 9, 12, 15, 18, 21, and 24 months (±1 month) after chemotherapy completion. Data are expressed as medians.
Restoration of sIgG Levels and CD4+ Cell Counts after Chemotherapy
Both sIgG levels and CD4+ cell counts were significantly decreased after chemotherapy and did not return to baseline values at 6, 12, 18, and 24 months (±2 months) after chemotherapy. However, baseline slgG levels (table 2) did not differ between the four time points, and the percentages of baseline slgG levels slightly increased over the study period. CD4+ cell counts at follow-up were expressed as percentages of baseline counts in table 3 and did not differ between follow-up time points. The restoration of sIgG levels and CD4+ cell counts did not differ between the patient groups aged >70 years and ≤70 years at 6 and 24 months (data not shown).
Table 2.
sIgG levels
| Period after chemotherapy | Patients | sIgG at baseline/after chemotherapy, mg/dl | % of baseline | p valuea |
|---|---|---|---|---|
| 6 months | 55 | 1,183 (1,031 – 1,397)/913 (748 – 1,048) | 77.0 (62.8 – 91.6) | <0.001 |
| 12 months | 58 | 1,226 (1,021 – 1,407)/990 (798 – 1,226) | 82.7 (68.9 – 95.0) | <0.001 |
| 18 months | 38 | 1,185 (1,031 – 1,420)/1,026 (824 – 1,259) | 85.6 (73.4 – 95.2) | <0.001 |
| 24 months | 24 | 1,205 (1,094 – 1,341)/1,049 (907 – 1,400) | 94.7 (74.3 – 104.2) | 0.027 |
| p valueb | 0.993/0.081 | 0.085 | ||
The data were obtained before chemotherapy and at 6, 12, 18, and 24 months (±2 months) after chemotherapy completion. All data are expressed as median (first quartile-third quartile). % of baseline = Baseline divided by After chemotherapy × 100.
Wilcoxon signed-rank test.
Kruskal-Wallis test.
Table 3.
CD4+ cell counts
| Period after chemotherapy | Patients | CD4+ cell counts at baseline/after chemotherapy, /µl | % of baseline | p valuea |
|---|---|---|---|---|
| 6 months | 26 | 603 (329 – 777)/333 (255 – 495) | 60.1 (49.5 – 83.7) | 0.001 |
| 12 months | 29 | 544 (281 – 777)/410 (256 – 595) | 66.2 (47.8 – 129.9) | 0.037 |
| 18 months | 25 | 544 (230 – 777)/465 (193 – 535) | 73.0 (51.2 – 123.2) | 0.045 |
| 24 months | 22 | 669 (298 – 923)/403 (284 – 585) | 72.9 (52.8 – 115.3) | 0.038 |
| p valueb | 0.811/0.741 | 0.575 | ||
The data were obtained before chemotherapy and at 6, 12, 18, and 24 months (±2 months) after chemotherapy completion. All data were expressed in a form of median (first quartile-third quartile). % of baseline = Baseline divided by After chemotherapy x 100.
Wilcoxon signed-rank test.
Kruskal-Wallis test.
Restoration Rates
Percentages of patients with 70% or 90% restoration of baseline sIgG levels and CD4+ cell counts were calculated (table 4). The percentages of patients with 70% and 90% CD4+ cell count restoration rates did not differ between the 6- and 24-month time points, whereas the percentages of patients with restored sIgG levels were significantly greater at 24 months than at 6 months. Specifically, 70% recovery rates of sIgG levels from baseline increased over time, and at 24 months, 85.2 and 51.9% of the present patients achieved 70 and 90% restoration of sIgG, respectively. No patients developed hypogammaglobulinemia at 24 months. To explore the risk factors which cannot achieve 70% restoration of baseline sIgG levels, univariate analysis was performed to identify risk factors, and variables with a significance level of <20% were included in the multivariate logistic regression model. Multivariate logistic regression analysis did not demonstrate an association with the aforementioned parameters (data not shown). Additionally, 90% restoration of baseline sIgG levels and 70 or 90% restoration of baseline CD4+ cell counts did not demonstrate an association either (data not shown).
Table 4.
Percentage of patients with 70 or 90% restoration of baseline sIgG level or CD4+ cell count
| sIgG level |
CD4+ cell count |
|||||
|---|---|---|---|---|---|---|
| 6 M (n = 55) | 24 M (n = 24) | p value | 6 M (n = 26) | 24 M (n = 22) | p value | |
| 70% restoration | 60.0% | 85.2% | 0.021 | 42.3% | 63.6% | 0.141 |
| 90% restoration | 27.3% | 51.9% | 0.029 | 23.1% | 45.5% | 0.101 |
The data were obtained at 6 and 24 months (±2 months) after chemotherapy completion. Data are expressed as the percentage of population.
Infectious Complications
The number of patients who suffered infections between starting chemotherapy and the last follow-up was counted (table 5). The rate of infection during chemotherapy was 30.3–32.6%, and the rate of infection during the observation period was 16.3–23.6%.
Table 5.
Infectious complications between the start of chemotherapy and the last follow-up
| sIgG (n = 89) |
Lymphocyte subsets (n = 43) |
|||
|---|---|---|---|---|
| during chemotherapy | during observation | during chemotherapy | during observation | |
| Cytomegalovirus | 0 | 0 | 0 | 0 |
| Pneumocystis pneumonia | 0 | 2 (2.2) | 0 | 0 |
| Herpes simplex virus | 5 (5.6) | 3 (3.4) | 4 (9.3) | 3 (7.0) |
| Herpes zoster virus | 2 (2.2) | 3 (3.4) | 3 (7.0) | 1 (2.3) |
| Hepatitis B virus reactivation | 0 | 0 | 0 | 0 |
| Candida | 4 (4.5) | 2 (2.2) | 4 (9.3) | 2 (4.7) |
| Febrile neutropenia | 6 (6.7) | 1 (1.1) | 3 (7.0) | 0 |
| Other infections | 10 (11.2) | 10 (11.2) | 0 | 1 (2.3) |
| Total | 27 (30.3) | 21 (23.6) | 14 (32.6) | 7 (16.3) |
Values indicate n (%) of patients affected.
The rate of infection in the subgroup with ≥70% of baseline sIgG levels was 26.1% during chemotherapy and 47.8% during the observation period. The corresponding rate in the subgroup with <70% of baseline sIgG levels was 25.0 and 75.0%, respectively. In both groups, no significant differences were observed between the rates of infection during chemotherapy and that during the observation period (p = 0.56, 0.64, respectively). Similarly, no significant differences were observed between the group with ≥90% of baseline sIgG levels and that with <90% of baseline sIgG levels (data not shown).
The rate of infection in the subgroup with ≥70% of baseline CD4+ cell counts was 8.3% during chemotherapy and 25.0% during observation. The corresponding rates of infection in the group with <70% of baseline CD4+ cell counts were 30.0 and 50.0%, respectively. Infection rates in the group with <70% of baseline CD4+ cell counts were higher (both during chemotherapy and observation period) than those in the group with ≥70% of baseline CD4+ cell counts. However, the between-group difference was not statistically significant (p = 0.45, 0.44). Similarly, no significant differences were observed when we compared the patients with ≥90% of baseline CD4+ cell counts and those with <90% of baseline CD4+ cell counts (data not shown).
Discussion
In this study, we evaluated the effects of R-CHOP therapy on humoral and cell-mediated immunity in patients with newly diagnosed B-cell lymphoma of either DLBCL or FL. Our findings revealed marked decreases in sIgG levels and CD4+ counts immediately after treatment and subsequent restoration over 2 years. However, the restoration rates of CD4+ counts (indicative of cell-mediated immunity) were less than those of sIgG levels (indicative of humoral immunity).
Previously, Kurokawa et al. [16] reported the effects of CHOP-based chemotherapy containing rituximab on immune function in 66 patients with newly diagnosed malignant lymphoma. However, 21 patients (31.8%) were aged at least 70 years, and some patients received THP-CHOP therapy, in which doxorubicin was replaced with tetrahydropyranyl adriamycin. Hence, their study results were not solely reflective of R-CHOP therapy and may, thus, lack consistency with the results of the present study; in addition, the immunological effects of tetrahydropyranyl adriamycin remain uncharacterized. In contrast, to ensure the homogeneity of treatments in the present study, elderly patients aged ≥70 years received 20% reduced dosages of anticancer drugs. In addition, the reduced dosages of anticancer drugs did not influence the restoration of sIgG levels and CD4+ cell counts at 6 and 24 months. The generally lower immunity in older people is a plausible explanation, although further investigation is necessary to arrive at a definitive conclusion. Nonetheless, in agreement with Kurokawa et al. [16], gradual recovery of sIgG levels and CD4+ counts were observed over 2 years; the restoration rates of sIgG and CD4+ cells at 6, 12, and 18 months after treatment confirmed time-dependent recovery of these immune functions following R-CHOP therapy.
Moreover, although the 70% recovery rates of sIgG levels from baseline increased at 6 and 24 months, CD4+ counts were not improved. Kurokawa et al. [16] did not directly compare two variables in their study but reported a mean sIgG restoration rate of 93.9% at 2-year follow-up. Although the present data lack sufficient distribution normality for comparisons with previous studies, a subanalysis revealed considerably greater rates of 70% sIgG restoration than 90% restoration after 2 years. These analyses indicate that humoral and cell-mediated immunity have little probability of being fully recovered to baseline at 2 years following R-CHOP therapy in patients with newly diagnosed malignant lymphoma. On the other hand, the infectious complication rate was unexpectedly low at 16.3–23.6% in the observation period, whereas it was high for chemotherapy at 30.3–32.6%. The reason for this discrepancy was expected to be that a follow-up survey provides insufficient information because the observation period was just over 2 years. However, the infectious complication rate was not at all low. In patients at an increased risk of infectious diseases in particular, sIgG levels and CD4+ counts should be regularly monitored, or prophylactic antibiotics should be used.
This retrospective analysis was limited to a small sample size (89 patients), reflecting a small number of patients who completed all measurements at several time points before and after treatment. Accordingly, the numbers of sIgG measurements differed from the estimates of lymphocyte cell numbers. However, no differences in age were identified between treatment groups, and all the included pharmacokinetic data were collected within ±1 month of designated time points. Nonetheless, percentage changes were calculated from data that were collected within ±2 months of baseline and at 24 months, allowing inclusion of a greater number of patients. Furthermore, baseline characteristics of patients showed much diversity, which may have impacted on the analysis. However, on multivariate analysis, we were able to account for baseline differences to some extent. On subgroup analyses of infection rates, no significant difference was observed in infection rates between patients with ≥70% of baseline sIgG levels (or ≥70% of baseline CD4+ cell counts) and those with <70% of baseline sIgG levels (or < 70% of baseline CD4+ cell counts, respectively). Out of 89 cases, complete data on IgG levels and CD4+ cell counts up to 24 months was available only for 24 and 22 cases, respectively. In addition, the relatively short follow-up period did not allow for meaningful estimates of the effect on overall survival and progression-free survival. The effect of R-CHOP therapy on survival requires an additional study with a longer duration of follow-up. Lastly, we did not investigate the effects of R-CHOP therapy on the immune system components other than IgG, B cells, and T cells. We believe that we need to investigate changes in other immune cells, such as NK cells, in future studies.
Several studies report the effects of rituximab monotherapy on immune parameters. In particular, Maloney et al. [10] examined the effect of rituximab monotherapy in 20 patients with relapsed B-cell lymphoma and reported no effects on mean CD3+, CD4+, and CD8+ cell counts or sIgG levels, although sIgG levels were reportedly decreased by ≥20% in 2 patients. Similarly, Anolik et al. [17] investigated the restoration of B cell numbers after rituximab monotherapy for non-Hodgkin lymphoma and reported sustained increases in percentages of transitional B cells and very slow increases in the number of memory B cells, which remained at very low levels even at 1 year after rituximab treatment. In three consecutive sponsor-initiated clinical trials of rituximab monotherapies in Japanese patients with relapsed or refractory B-cell lymphoma, weekly treatments with rituximab for 4 or 8 weeks led to no significant decreases in mean sIgG levels. Ghielmini et al. [14] found that prolonged treatment with rituximab monotherapy for FL induces the persistent depletion of B cells and progressive reduction of serum IgM levels. Hence, the effect of rituximab alone on the restoration of immunity after treatment remains unknown. On the other hand, multiple chemotherapy for initial or relapsed B-cell lymphoma was reportedly complicated by hypogammaglobulinemia, implying a clinical role for rituximab [18,19,20,21]. Among the cytotoxic agents of R-CHOP, cyclophosphamide and doxorubicin have well-documented detrimental effects on immunoglobulins and lymphocytes. However, the effects of rituximab in R-CHOP therapy on immune function remain unknown. Moreover, hypogammaglobulinemia has been reported in patients with autoimmune diseases following rituximab monotherapy [22,23]. Ricardo et al. [24] also compared treatments for chronic lymphocytic leukemia and indolent non-Hodgkin lymphomas, and showed that R-B therapy decreased CD4+ and CD8+ cell counts to a greater extent than R-CHOP therapy. In a recent study involving only a small number of patients with refractory or relapsed FL and mantle cell lymphoma, both CD4+ counts and sIgG levels did not completely recover at 1 year after R-B therapy [25]. Gafter-Gvili and Polliack [26] described that myelosuppression including lymphopenia occurs relatively frequently after R-B therapy and results in secondary hypogammaglobulinemia. In Japan, R-B therapy is not accepted as the standard first-line treatment for B-cell lymphoma. Therefore, direct comparisons between the aforementioned and the present studies were precluded by differing methodological approaches, and more rigorous comparisons with other chemotherapies are warranted. Thus, further studies are required to quantify the immunological effects of rituximab in R-CHOP therapy using direct comparisons with CHOP therapy.
Suppression of humoral and cell-mediated immunity tends to persist for >2 years after R-CHOP therapy in patients with newly diagnosed B-cell lymphoma, although sIgG levels were restored more quickly than CD4+ counts. These findings suggest that in patients at increased risk of infectious disease in particular, sIgG levels and CD4+ counts should be regularly monitored for >2 years after R-CHOP therapy, or prophylactic antibiotics should be used.
Disclosure Statement
The authors have nothing to disclose in association with the publication of this study.
Acknowledgments
We thank the participating patients for their contribution to this study and the staff of pharmacy, hematology, and nursing, Fujita Health University Hospital, that cooperated with our study.
References
- 1.Persky DO, Unger JM, Spier CM, Stea B, LeBlanc M, McCarty MJ, Rimsza LM, Fisher RI, Miller TP, Southwest Oncology Group Phase II study of rituximab plus three cycles of CHOP and involved-field radiotherapy for patients with limited-stage aggressive B-cell lymphoma: Southwest Oncology Group study 0014. J Clin Oncol. 2008;26:2258–2263. doi: 10.1200/JCO.2007.13.6929. [DOI] [PubMed] [Google Scholar]
- 2.Pfreundschuh M, Trümper L, Osterborg A, Pettengell R, Trneny M, Imrie K, Ma D, Gill D, Walewski J, Zinzani PL, Stahel R, Kvaloy S, Shpilberg O, Jaeger U, Hansen M, Lehtinen T, López-Guillermo A, Corrado C, Scheliga A, Milpied N, Mendila M, Rashford M, Kuhnt E, Loeffler M, MabThera International Trial CHOP-like chemotherapy plus rituximab versus CHOP-like chemotherapy alone in young patients with good-prognosis diffuse large-B-cell lymphoma: a randomised controlled trial by the MabThera International Trial (MInT) Group. Lancet Oncol. 2006;7:379–391. doi: 10.1016/S1470-2045(06)70664-7. [DOI] [PubMed] [Google Scholar]
- 3.Fisher RI, LeBlanc M, Press OW, Maloney DG, Unger JM, Miller TP. New treatment options have changed the survival of patients with follicular lymphoma. J Clin Oncol. 2005;23:8447–8452. doi: 10.1200/JCO.2005.03.1674. [DOI] [PubMed] [Google Scholar]
- 4.van Meerten T, Hagenbeek A. CD20-targeted therapy: the next generation of antibodies. Semin Hematol. 2010;47:199–210. doi: 10.1053/j.seminhematol.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 5.Cragg MS, Glennie MJ. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood. 2004;103:2738–2743. doi: 10.1182/blood-2003-06-2031. [DOI] [PubMed] [Google Scholar]
- 6.Hiddemann W, Kneba M, Drevling M, Schmitz N, Lengfelder E, Schmits R, Reiser M, Metzner B, Harder H, Hegewisch-Becker S, Fischer T, Kropff M, Reis HE, Freund M, Wörmann B, Fuchs R, Planker M, Schimke J, Eimermacher H, Trümper L, Aldaoud A, Parwaresch R, Unterhalt M. Frontline therapy with rituximab added to the combination of cyclophosphamide, doxorubicin, vincristine, and prednisolone (CHOP) significantly improves the outcome for patients with advanced-stage follicular lymphoma compared with therapy with CHOP alone: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood. 2005;106:3725–3732. doi: 10.1182/blood-2005-01-0016. [DOI] [PubMed] [Google Scholar]
- 7.Coiffier B, Lepage E, Briere J, Herbrecht R, Tilly H, Bouabdallah R, Morel P, Van Den Neste E, Salles G, Gaulard P, Reyes F, Lederlin P, Gisselbrecht C. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346:235–242. doi: 10.1056/NEJMoa011795. [DOI] [PubMed] [Google Scholar]
- 8.Salles G, Seymour JF, Offner F, López-Guillermo A, et al. Rituximab maintenance for 2 years in patients with high tumour burden follicular lymphoma responding to rituximab plus chemotherapy (PRIMA): a phase 3, randomised controlled trial. Lancet. 2011;377:42–51. doi: 10.1016/S0140-6736(10)62175-7. [DOI] [PubMed] [Google Scholar]
- 9.Czuczman MS, Weaver R, Alkuzweny B, Berlfein J, Grillo-López AJ. Prolonged clinical and molecular remission in patients with low-grade or follicular non-Hodgkin's lymphoma treated with rituximab plus CHOP chemotherapy: 9-year follow-up. J Clin Oncol. 2004;22:4711–4716. doi: 10.1200/JCO.2004.04.020. [DOI] [PubMed] [Google Scholar]
- 10.Maloney DG, Grillo-López AJ, Bodkin DJ, White CA, Liles TM, Royston I, Varns C, Rosenberg J, Levy R. IDEC-C2B8: results of a phase I multiple-dose trial in patients with relapsed non-Hodgkin's lymphoma. J Clin Oncol. 1997;15:3266–3274. doi: 10.1200/JCO.1997.15.10.3266. [DOI] [PubMed] [Google Scholar]
- 11.Ghielmini M, Rufibach K, Salles G, Leoncini-Franscini L, Léger-Falandry C, Cogliatti S, Fey M, Martinelli G, Stahel R, Lohri A, Ketterer N, Wernli M, Cerny T, Schmitz SF. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK) Ann Oncol. 2005;16:1675–1682. doi: 10.1093/annonc/mdi320. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin P, Grillo-López AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. 1998;16:2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 13.Ghielmini M, Schmitz SF, Cogliatti S, Bertoni F, Waltzer U, Fey MF, Betticher DC, Schefer H, Pichert G, Stahel R, Ketterer N, Bargetzi M, Cerny T, Swiss Group for Clinical Cancer Research Effect of single agent rituximab given at the standard schedule or as prolonged treatment in patients with mantle cell lymphoma: a study of the Swiss Group for Clinical Cancer Research (SAKK) J Clin Oncol. 2005;23:705–711. doi: 10.1200/JCO.2005.04.164. [DOI] [PubMed] [Google Scholar]
- 14.Ghielmini M, Schmitz SF, Cogliatti SB, Pichert G, Hummerjohann J, Waltzer U, Fey MF, Betticher DC, Martinelli G, Peccatori F, Hess U, Zucca E, Stupp R, Kovacsovics T, Helg C, Lohri A, Bargetzi M, Vorobiof D, Cerny T. Prolonged treatment with rituximab in patients with follicular lymphoma significantly increases event-free survival and response duration compared with the standard weekly ×4 schedule. Blood. 2004;103:4416–4423. doi: 10.1182/blood-2003-10-3411. [DOI] [PubMed] [Google Scholar]
- 15.Rao A, Kelly M, Musselman M, Ramadas J, Wilson D, Grossman W, Shenoy S. Safety, efficacy, and immune reconstitution after rituximab therapy in pediatric patients with chronic or refractory hematologic autoimmune cytopenias. Pediatr Blood Cancer. 2008;50:822–825. doi: 10.1002/pbc.21264. [DOI] [PubMed] [Google Scholar]
- 16.Kurokawa T, Hase M, Tokuman N, Yoshida T. Immune reconstitution of B-cell lymphoma patients receiving CHOP-based chemotherapy containing rituximab. Hematol Oncol. 2011;29:5–9. doi: 10.1002/hon.947. [DOI] [PubMed] [Google Scholar]
- 17.Anolik JH, Friedberg JW, Zheng B, Barnard J, Owen T, Cushing E, Kelly J, Milner EC, Fisher RI, Sanz I. B-cell reconstitution after rituximab treatment of lymphoma recapitulates B cell ontogeny. Clin Immunol. 2007;122:139–145. doi: 10.1016/j.clim.2006.08.009. [DOI] [PubMed] [Google Scholar]
- 18.Cabanillas F, Liboy I, Pavia O, Rivera E. High incidence of non-neutropenic infections induced by rituximab plus fludarabine and associated with hypogammaglobulinemia: a frequently unrecognized and easily treatable complication. Ann Oncol. 2006;17:1424–1427. doi: 10.1093/annonc/mdl141. [DOI] [PubMed] [Google Scholar]
- 19.Casulo C, Maragulia J, Zelenetz AD. Incidence of hypogammaglobulinemia in patients receiving rituximab and the use of intravenous immunoglobulin for recurrent infections. Clin Lymphoma Myeloma Leuk. 2013;13:106–111. doi: 10.1016/j.clml.2012.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saikia TK, Menon H, Advani SH. Prolonged neutropenia following anti CD20 therapy in a patient with relapsed follicular non-Hodgkin's lymphoma and corrected with IVIG. Ann Oncol. 2001;12:1493–1494. doi: 10.1023/a:1012500524758. [DOI] [PubMed] [Google Scholar]
- 21.Yokohama A, Tsukamoto N, Uchiumi H, Handa H, Matsushima T, Karasawa M, Murakami H, Nojima Y. Durable remission induced by rituximab-containing chemotherapy in a patient with primary refractory Burkitt's lymphoma. Ann Hematol. 2004;83:120–123. doi: 10.1007/s00277-003-0758-2. [DOI] [PubMed] [Google Scholar]
- 22.De La Torre I, Leandro MJ, Valor L, Becerra E, Edwards JC, Cambridge G. Total serum immunoglobulin levels in patients with RA after multiple B-cell depletion cycles based on rituximab: relationship with B-cell kinetics. Rheumatology. 2012;51:833–840. doi: 10.1093/rheumatology/ker417. [DOI] [PubMed] [Google Scholar]
- 23.Marco H, Smith RM, Jones RB, Guerry MJ, Catapano F, Burns S, Chaudhry AN, Smith KG, Jayne DR. The effect of rituximab therapy on immunoglobulin levels in patients with multisystem autoimmune disease. BMC Musculoskelet Disord. 2014;15:178–186. doi: 10.1186/1471-2474-15-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.García Muñoz R, Izquierdo-Gil A, Muñoz A, Roldan-Galiacho V, Rabasa P, Panizo C. Lymphocyte recovery is impaired in patients with chronic lymphocytic leukemia and indolent non-Hodgkin lymphomas treated with bendamustine plus rituximab. Ann Hematol. 2014;93:1879–1887. doi: 10.1007/s00277-014-2135-8. [DOI] [PubMed] [Google Scholar]
- 25.Ito K, Okamoto M, Ando M, Kakumae Y, Okamoto A, Inaguma Y, Tokuda M, Yanada M, Yamada S, Emi N. Influence of rituximab plus bendamustine chemotherapy on the immune system in patients with refractory or relapsed follicular lymphoma and mantle cell lymphoma. Leuk Lymphoma. 2015;56:1123–1125. doi: 10.3109/10428194.2014.921298. [DOI] [PubMed] [Google Scholar]
- 26.Gafter-Gvili A, Polliack A. Bendamustine associated immune suppression and infections during therapy of hematological malignancies. Leuk Lymphoma. 2016;57:512–519. doi: 10.3109/10428194.2015.1110748. [DOI] [PubMed] [Google Scholar]