Abstract
Hearing and balance are rescued by a synthetic virus that delivers a transgene to the inner ear of neonatal mice.
Global health data reveal this sobering reality: either you have hearing loss, or chances are you care about someone who does. An estimated 32 million children and 328 million adults worldwide are affected by hearing loss that profoundly interferes with learning, interpersonal communication, and the ability to work productively1. Around 100 genes that cause non-syndromic hearing loss are known2, but efforts to develop gene-based therapies have struggled owing to a lack of suitable delivery vectors. In this issue, two companion studies report important progress in research on gene therapy for the inner ear. Landegger et al.3 first characterize a synthetic adeno-associated viral vector (AAV) that highly efficiently transduces auditory and vestibular sensory hair cells in the mouse inner ear in vitro and in vivo, and the human vestibular sensory epithelium in vitro. Pan et al.4 then apply this vector to rescue hearing and balance in a mouse model of a human deaf-blindness disease called Usher syndrome type 1c.
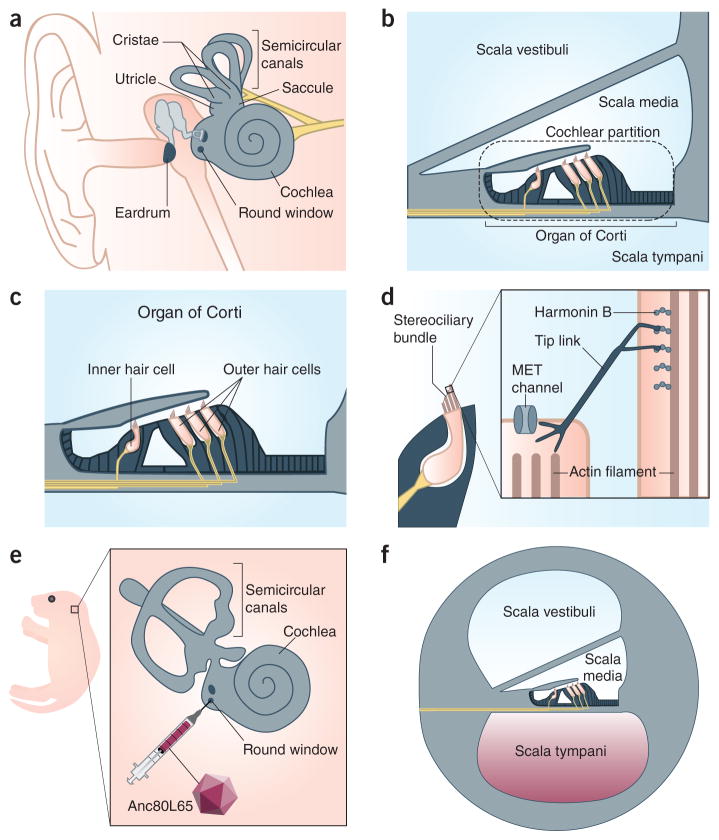
Hearing and balance are the two senses subserved by the inner ear (Fig. 1). The auditory sensory epithelium is located in the organ of Corti (Fig. 1b) and consists of one row of inner hair cells, three rows of outer hair cells, and supporting cells (Fig. 1c). The vestibular sensory epithelia, which control balance and spatial orientation, possess hair cells and supporting cells arrayed in the cristae of the semicircular canals and the maculae of the utricle and saccule (Fig. 1a). Energy transmitted by sound and movement deflects structures known as stereociliary bundles (Fig. 1d), actin-rich organelles on the apical surface of auditory and vestibular hair cells. This causes mechanically sensitive ion channels (Fig. 1d, inset) in the stereocilia to open, an initial step in the process of mechanotransduction that converts acoustic and proprioceptive stimuli into electrical impulses. The inner ear and brain masterfully collaborate as we listen to Mozart’s overture to The Magic Flute or experience the breathless thrills of the Kingda Ka roller coaster.
Figure 1.
Structure, function, and viral transduction of the inner ear. (a) The human inner ear. The vestibular sensory epithelium is located in the cristae of the three semicircular canals and the maculae of the utricle and saccule. The auditory sensory epithelium is contained within the coiled cochlea. The round window is covered by an uncalcified membrane. (b) The three fluid-filled compartments of the inner ear are called the scalae. Inner and outer sensory hair cells are in the organ of Corti. The cochlear partition oscillates in response to vibrations produced in the scala media by movement of the ear drum. (c) The organ of Corti within the cochlea presents one row of inner hair cells and three rows of outer hair cells which are surrounded by supporting cells (black). (d) The stereociliary bundle on the apical surface of hair cells consists of parallel stereocilia whose structural rigidity is established in part by filamentous actin. A proteinaceous tip link connects adjacent stereocilia and has an important role in the activation of the mechanoelectrical transduction (MET) channel. Harmonin-b is localized to the upper insertion point of the tip link, where it interacts with numerous proteins. (e) A postnatal day 1 mouse pup whose left inner ear (inset) is targeted for round window membrane injection with Anc80L65. (f) The solution containing Anc80L65 fills the scala tympani and then spreads to the other two scalae, gaining access to structurally and functionally immature neonatal hair cells.
Safe, efficient delivery of genes to target cells is often the rate-limiting step in the development of any gene therapy. The mammalian inner ear could not be more inhospitable to physical access. The intricate cytoarchitecture of the organ of Corti evolved to permit high-fidelity detection of sub-nanometer vibrations of the cochlear partition5 (Fig. 1b). The inner ear and its sensory epithelia are encased in the petrous portion of the temporal bone—one of the densest bones in the human body—which dampens oscillations that might otherwise interfere with hair cell mechanotransduction.
It is extremely difficult to safely breach this so-called bony labyrinth without causing mechanical compromise of the fluid-filled compartments of the cochlea and irreversible degradation of sensory function. In addition to a safe injection route, gene therapy requires a delivery modality that permits correct temporal and spatial expression of transgenes. Currently there are 27 active gene therapy clinical trials for ten diseases of the retina, but only one for severe-to-profound hearing loss and vestibular dysfunction. This disparity is largely due to the greater difficulty in establishing safe and efficient gene delivery to the inner ear.
AAV is a single-stranded DNA virus that infects dividing and quiescent mammalian cells but does not cause human disease or strong immune responses. Various AAV sero-types have been interrogated as gene therapy vectors to target multiple tissues. In a 2012 study, AAV2/1 encoding vesicular glutamate transporter 3 (VGLUT3) was shown to restore hearing after injection at postnatal days 1–3 through the round window membrane (Fig. 1e,f) of deaf VGLUT3 knockout mice (ref. 6). Fortuitously, VGLUT3 expression was restricted almost exclusively to inner hair cells, where VGLUT3 is natively expressed. However, for a different form of genetic deafness caused by mutations in the transmembrane channel-like genes, the inability of AAV2/1 to transduce outer hair cells limited hearing recovery7. Thus, a central goal in the field has been to identify a vector that can efficiently transduce both inner and outer hair cells.
In a search for more effective AAV variants, Vandenberghe and colleagues8 previously used ancestral sequence reconstruction to infer the viral sequence that may have been the ancestor of four AAV serotypes commonly used in gene therapy. The idea was that capsid variants upstream in the viral lineage might have different tropisms that would be advantageous for transducing cell types of interest. The authors synthesized a parental candidate called Anc80L65 and found that it transduced mouse muscle, liver, and retina, and non-human primate liver with high efficiencies8. Could this reverse-engineered, ancestral AAV outperform its extended modern family in the mammalian inner ear while escaping immune detection?
In a remarkable series of experiments from a team led by Stankovic, Holt, and Vandenberghe, Landegger et al.3 now show that Anc80L65 encoding GFP (Anc80L65-GFP) transduces 90–100% of inner and outer hair cells in both explanted mouse cochlear cultures and in mouse cochlea in vivo after neonatal administration through the round window membrane (Fig. 1e,f). The previous standard-bearer for hair-cell transduction efficiency was AAV2/1, which transduced nearly 100% of inner hair cells but few outer hair cells6,7. Anc80L65’s prowess is crucial because the vast majority of mutations that cause hearing loss disproportionately affect sensory hair cell structure and function2. In addition, Landegger et al.3 find that Anc80L65 efficiently transduces vestibular hair cells in the mouse utricular macula and the cristae.
Importantly, the group also carried out initial studies of safety in mice and of human cell transduction in vitro that support the potential of Anc80L65 for clinical translation. Anc80L65-GFP transduction does not alter auditory hair cell mechanotransduction in vitro and has no significant effect on hearing sensitivity or equilibrium when administered neonatally. Furthermore, only low levels of neutralizing antibody against the viral capsid are detected in serum, suggesting a favorable humoral response profile. In an experiment that inspires hope, Anc80L65-GFP efficiently transduces hair cells and supporting cells of human utricular sensory epithelium in vitro, suggesting that this synthetic AAV is a powerful starting point for developing appropriate vectors for use in the human inner ear.
In the companion paper, Géléoc and her colleagues4 show that gene augmentation achieved by Anc80L65-mediated transduction of the sensory epithelia rescues hearing and balance in a mouse model of severe human inner ear disease. Usher syndrome is the leading cause of deaf-blindness in the world, with 1–4 babies affected per 25,000 births9. Children with Usher syndrome type 1c (USH1C) are born deaf with severe disequilibrium, and experience vision abnormalities by early adolescence. Clinical care of children with Usher syndrome is tailored to the severity of their hearing, balance, and vision impairment, and may involve cochlear implants or hearing aids paired with assistive listening technologies, sign language, Braille instruction, low vision services, and mobility training. There is currently no clinical intervention that corrects the genetic mutation to restore inner ear function.
The Acadian version of USH1C is caused by a mutation of a scaffolding protein called harmonin (Fig. 1d), which is expressed in sensory hair cells of the inner ear and in photoreceptor cells of the retina. A single base change from G to A at position 216 of harmonin introduces a cryptic splice site, producing a frameshift, premature stop codon, and a predicted truncated protein10. While the spliceosome uses the cryptic splice site preferentially, a small amount of wild-type harmonin mRNA is still made. A mouse model of the disease in which the endogenous harmonin gene was replaced with the mutated human gene presents the same sensory phenotypes seen in affected children11.
Using this mouse model, Pan et al.4 carried out an elegant series of experiments demonstrating that neonatal administration of Anc80L65 encoding wild-type harmonin (Anc80L65-Harm) through the round window membrane (Fig. 1e,f) augments residual wild-type harmonin expression in mutant mice and restores hearing and balance. In vitro, Anc80L65-Harm corrects mechanotransduction currents in mutant inner hair cells. Cochlear administration of the therapy to neonatal mice substantially improves hearing sensitivity to low through intermediate frequency stimuli, as measured by auditory brainstem response (ABR) testing. Outer hair cell responses are intermediate between wild-type and mutant levels through the same frequency range, as measured by distortion product otoacoustic emissions (DPOAE) testing. ABR and DPOAE thresholds for lower frequency stimuli are robust through 6 months of age. Notably, startle response testing shows that treated mutant mice have a significant behavioral response to sound. They are also indistinguishable from control mice in quantitative assessments of balance function.
What makes this study so significant is the depth of auditory and vestibular rescue achieved by a single neonatal treatment. Taken together, the two studies3,4 rigorously validate an ancestral AAV capsid that enables robust transgene expression in sensory hair cells of the mouse inner ear. The work is certain to kick-start the development of new approaches to correct a diverse set of deafness genes.
Several challenges must still be overcome to make inner ear gene therapy a reality.
Pan et al.4 treated mutant mice just after birth, when mouse auditory hair cells are maturing, whereas treatment at postnatal day 10 was ineffective. This strict temporal requirement is not unique to Anc80L65 or harmonin. For example, AAV2/1-VGLUT3 mediated hearing recovery when delivered at postnatal days 1–3 but was less effective at postnatal days 10–12 (ref. 6). Similarly, intraperitoneal injection of an antisense oligonucleotide that corrects harmonin splicing in Ush1c mutant mice rescued low-to-intermediate-frequency hearing when administered at postnatal day 5 but was less effective at postnatal day 10 (ref. 10). These data suggest that there is a brief postnatal window prior to the onset of hearing when maturing mouse hair cells can be corrected by gene or antisense therapy. Whether a similar window exists for the human cochlea remains to be determined. As human hair cells are born at 12–14 weeks of gestation, with hearing in utero detectable at 19 weeks12, we have suggested that fetal gene therapy may be an appropriate approach to treat human hereditary deafness and balance dysfunction13.
Another key issue concerns cell-type specificity. Anc80L65 transduces not only sensory hair cells in the inner ear but also spiral ganglion neurons, supporting cells, and cells in the spiral limbus. In treated neonatal Ush1c mutant mice, the presumptive off-target expression of harmonin did not affect sensory function, although this is not likely to be the case for every therapeutic gene of interest. Efforts to limit transduction to specific cell types might involve further modification of the AAV capsid8 or use of cell-specific promoters. In addition, the packaging capacity of an AAV vector is <5 kb, which excludes larger deafness genes from efficient encapsidation, although new AAV technologies may partly circumvent this size limitation14.
The ability to model human disease in higher vertebrates using genome editing technology offers the field an unprecedented opportunity to advance human gene therapy for inner ear disease. The rhesus macaque has sensitive hearing in the low-frequency range that is also used by humans for oral communication. Validation of a gene therapy or pharmacotherapy that safely and effectively restores the speech frequency range in a macaque model of human genetic deafness would provide a compelling rationale for human clinical trials.
The hope is that recent progress in gene therapy for inner ear disease can unite philanthropists, biomedical industry leaders, private foundation executives, academic administrators, venture capitalists, and researchers in vigorous pursuit of the National Institute on Deafness and Other Communication Disorders’ primary mission to treat hearing loss. The work of Landegger et al.3 and Pan et al.4 keenly demonstrates that the field possesses the talent and creativity necessary for inner ear gene therapy to go viral.
Acknowledgments
Karen Thiebes, Ph.D., created the artwork for Figure 1. Work in the author’s laboratory is funded by the Hearing Health Foundation’s Hearing Restoration Project and by the National Institute on Deafness and Other Communication Disorders (NIDCD).
Footnotes
COMPETING FINANCIAL INTERESTS
The author declares no competing financial interests.
References
- 1.Olusanya BO, Neumann KJ, Saunders JE. Bull World Health Organ. 2014;92:367–373. doi: 10.2471/BLT.13.128728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Camp G, Smith RJH. [Accessed: January 28, 2017]; http://hereditaryhearin-gloss.org.
- 3.Landegger LD, et al. Nat Biotechnol. 2017;35:280–284. doi: 10.1038/nbt.3781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pan B, et al. Nat Biotechnol. 2017;35:264–272. doi: 10.1038/nbt.3801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ren T, He W, Kemp D. Proc Natl Acad Sci USA. 2016;113:9910–9915. doi: 10.1073/pnas.1607428113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Akil O, et al. Neuron. 2012;75:283–293. doi: 10.1016/j.neuron.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Askew C, et al. Sci Transl Med. 2015;7:295ra108. doi: 10.1126/scitranslmed.aab1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zinn E, et al. Cell Rep. 2015;12:1056–1068. doi: 10.1016/j.celrep.2015.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mathur P, Yang J. Biochim Biophys Acta. 2015;1852:406–420. doi: 10.1016/j.bbadis.2014.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lentz JJ, et al. Nat Med. 2013;19:345–350. doi: 10.1038/nm.3106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lentz JJ, et al. Dev Neurobiol. 2010;70:253–267. doi: 10.1002/dneu.20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hepper PG, Shahidullah BS. Arch Dis Child. 1994;71:F81–F87. doi: 10.1136/fn.71.2.f81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Depreux FF, et al. Nucleic Acids Res. 2016;44:9519–9529. doi: 10.1093/nar/gkw867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Trapani I, et al. Hum Mol Genet. 2015;24:6811–6825. doi: 10.1093/hmg/ddv386. [DOI] [PMC free article] [PubMed] [Google Scholar]