Abstract
BACKGROUND
Natural killer (NK) cells have shown promise in the treatment of malignancy. However, the widespread use of these cells may be limited by both the lack of resources and the expertise needed to manufacture them and the apparent need to use only fresh cells. The NHLBI-sponsored Production Assistance for Cellular Therapies group was established to provide the resources and expertise to carry out cell therapy research, including support of clinical trials. Here we describe the qualification of in transit activation of an NK-cell therapy product in preparation for a Phase I clinical trial at a distant medical center.
STUDY DESIGN AND METHODS
Nonmobilized apheresis mononuclear cell collections were CD3+ cell depleted, placed into culture bags with interleukin (IL)-2, and shipped from Minneapolis/Saint Paul, Minnesota, to Columbus, Ohio, and back to Minneapolis/Saint Paul, under warm, monitored temperatures. Products underwent quality control (QC) testing including cell count, immunophenotyping, viability, endotoxin, sterility culture, and cytotoxicity assays. One product tested the relative importance of IL-2 and controlled incubation.
RESULTS
The length of shipment ranged from 14 to 16 hours, and temperatures were well controlled. QC testing was acceptable based upon previous in-house experience. Controlled incubation was not necessary for successful activation of NK cells, but IL-2 appeared essential.
CONCLUSION
The need for novel cell therapies to be infused as fresh products may be a limitation for various cell types. However, we have shown that NK cells can be successfully shipped in the fresh state (allowing 48 hr from apheresis to product infusion) for use at clinical centers. Although IL-2 is critical for NK-cell activation, a 37°C, 5% CO2 incubator is not.
Allogeneic natural killer (NK) cells have potential to treat cancer and improve outcomes after hematopoietic transplantation in part because of their enhanced activity when they are not inhibited by “self” major histocompatibility Class I antigens which engage inhibitory killer immunoglobulin-like receptors.1 In hematopoietic stem cell transplantation, choosing donors with killer immunoglobulin-like receptor ligands lacking in the recipient may prevent relapse and promote long-term disease-free survival in hematologic malignancies.2 Infusion of allogeneic NK cells performed outside of transplantation in the adoptive transfer setting to treat refractory acute myeloid leukemia has been reported.3 Successful adoptive transfer and in vivo expansion is dependent on lymphodepleting chemotherapy and administration of exogenous interleukin-2 (IL-2). Given this promise, plans for multicenter clinical trials establishing a definitive role for NK cells in cancer and transplantation therapy are needed.
One method by which NK-cell products are prepared has been reported by our group previously.4 Briefly, this involves large-scale immunomagnetic bead selection of a mononuclear cell (MNC) apheresis product under current good manufacturing practices (cGMP), enriching for NK cells. The NK-cell–enriched product is incubated overnight with IL-2 in gas-permeable bags placed in a temperature- and CO2-controlled incubator. All current clinical trials obtain donor products and proceed with processing using the premise that maximal NK-cell potency is best derived from fresh cells. This is based on the primary mechanism of NK cells to mediate direct target cell cytotoxicity. Although cytokine production and CD107a degranulation can be demonstrated from frozen cells, direct cytotoxicity is clearly diminished after cryo-preservation and thawing.5 This remains as a potential barrier for banking and “off the shelf” NK-cell products as it results in diminished function in vivo. It also underlies a limitation of broad use of NK cells, which has been limited to centers capable of clinical-scale selection and cell activation under cGMP.
The National Heart, Lung, and Blood Institute (NHLBI) of the National Institutes of Health has developed a mechanism to test regionalization of the manufacture of cellular therapy products through the Production Assistance for Cellular Therapies, or PACT, group. Using these resources, we tested the hypothesis that fresh MNCs can be collected at a distant regional transplant center, shipped for processing, and returned with NK cells enriched and successfully activated for infusion into the patient at the originating transplant center within 48 hours of initial collection.
MATERIALS AND METHODS
NK-cell product preparation
NK cells were prepared from nonmobilized peripheral blood MNC apheresis collections on an apheresis system (COBE Spectra, CaridianBCT, Inc., Lakewood, CO) from seven normal research donors under an institutional review board (IRB)-approved protocol (IRB Code 0407M61943; PI: DM). Cells were CD3+ cell depleted using a cell selection system (Miltenyi CliniMACS, Miltenyi Biotec GmbH, Bergisch Gladbach, Germany) and split into two equal fractions. One of the fractions was combined with X-VIVO 15 without gentamicin and phenol red (Lonza, Inc., Walkersville, MD), supplemented with 10% human AB serum (Valley Biomedical, Winchester, VA) and 1000 IU/mL IL-2 (Proleukin, Novartis Corp., East Hanover, NJ) in Teflon bags (VueLife, American Fluoroseal Corp., Gaithersburg, MD), and shipped. The other half of the T-cell-depleted product was similarly handled except that IL-2 was omitted, and the product was not shipped. This served as the control in the cytotoxicity assays.
Shipment and receipt of NK-cell products
Cells were packaged for transport in an insulated cardboard shipping container (Insulated Shipper-PUR, Tegrant Corp., Hayward, CA) similar to those used for platelet transport. Temperature-stabilizing packs (Cold Ice gel pack, Cold Ice, Inc., Oakland, CA) brought to 33°C were placed around the product to maintain approximate body temperature during shipment. A continuous temperature-monitoring device (SP425 temperature data logger, Dickson, Addison, IL) was placed adjacent to the culture bags containing the cell product. The containers were sealed and shipped overnight from Minneapolis/Saint Paul, Minnesota, to Columbus, Ohio, and back to Minneapolis/Saint Paul via commercial courier (AirNet Systems, Inc., Columbus, OH). Upon return to the laboratory, the cells were washed, resuspended in 5% human serum albumin (Baxter Healthcare, Deerfield, IL), and then analyzed.
Quality control analysis
Quality control (QC) testing included total nucleated cell (TNC) count (Coulter MD II, Beckman-Coulter, Brea, CA), percent NK (CD3−/56+) cells, percent T (CD3+) cells, viability by 7-aminoactinomycin (7-AAD; FACSCalibur, BD, Franklin Lakes, NJ), culture (Bactec, BD), endotoxin quantification (LAL Kinetic-QCL, Lonza, Walkersville, MD), and Gram stain. Cytotoxicity assays were performed as previously described6 and, briefly, consisted of assaying the NK-cell products and controls (CD3-depleted products without IL-2 incubation) for their ability to induce tumor lysis in three cell lines: K562 (American Tissue Type Collection [ATCC], Rockville, MD), Raji (ATCC), and MCF7 (ATCC). Aside from the NK-cell immunophenotyping, which is measured on sample before incubation with IL-2 due to workflow constraints on actual patient samples, all other lot release testing is performed on the final, washed product as this is infused into the patient.
Determination of the importance of IL-2 versus controlled incubation on NK-cell activation
A seventh NK-cell product was collected and processed to determine how critical controlled incubation (37°C, 5% CO2) or IL-2 were for NK-cell activation of the final product. The apheresis MNC product was CD3+ cell depleted and divided into four equal fractions. Two of the fractions (one with IL-2 and the other without IL-2) were packaged and shipped to Columbus and back to Minneapolis/Saint Paul and processed. The other two fractions (one with IL-2 and the other without IL-2) were placed into a 37°C, 5% CO2 incubator in the laboratory rather than being shipped.
RESULTS
Table 1 summarizes the results of the product QC testing, which includes analyzing by flow cytometry the percentage of the product that has an NK-cell phenotype (CD3−CD56+) and the absolute number of T cells (CD3+), as well as the TNC count, the percentage of viable cells in the product, and testing for microbiologic contamination. Previously established lot release testing specifications4 were met for all runs with the exception of one NK-cell product, which contained 18% NK cells after CD3 depletion. The lot release specification established with the FDA is for an NK-cell product to contain at least 20% CD56+/CD3− NK cells.4 We explored this product deviation further and found the starting apheresis-derived cell MNC product contained only 6% NK cells, a content lower than what is usually obtained from normal volunteers (11 ± 4%).4
TABLE 1.
Summary of product testing*
| QC assay | Result | Lot release specification | Number of runs passed lot release |
|---|---|---|---|
| NK-cell phenotype (% CD3−/CD56+), before IL-2 incubation | Mean: 29.9 Range: 17.6–38.9 |
≥20.0 | 5/6 |
| NK-cell phenotype (% CD3−/CD56+), after shipment | Mean: 26.7 Range: 18.9–40.0 |
NA | NA |
| T (CD3+)-cell dose (×105/kg), after shipment | Mean: 0.31 Range: 0.03–0.75 |
<5.00 | 6/6 |
| TNCs (×109), before shipment | Mean: 2.79 Range: 1.16–5.26 |
NA | NA |
| TNCs (×109) of the split product, after shipment | Mean: 1.32 Range: 0.60–2.20 |
NA | NA |
| TNC dose (×107/kg), after shipment | Mean: 1.88 Range: 0.86–2.98 |
<3.00 | 6/6 |
| Viability (% 7-AAD negative cells), pre-shipment | Mean: 95 Range: 92–97 |
>70 | 6/6 |
| Viability (% 7-AAD–negative cells), after shipment | Mean: 95 Range: 92–97 |
>70 | 6/6 |
| Endotoxin, LAL method (EU/kg), after shipment | Mean: <3.15 | <5.00 | 5/5 |
| Bacterial/fungal culture (BacTec), after shipment | No growth | No growth | 6/6 |
| Gram stain, after shipment | No organisms | No organisms | 6/6 |
Immunophenotyping (CD3−/CD56+ and CD3+), TNC count, viability (7-AAD by flow cytometry), endotoxin level, Gram stain, and bacterial/fungal cultures were performed on the indicated samples. The QC variables required before the product can be released to a patient are listed where appropriate.
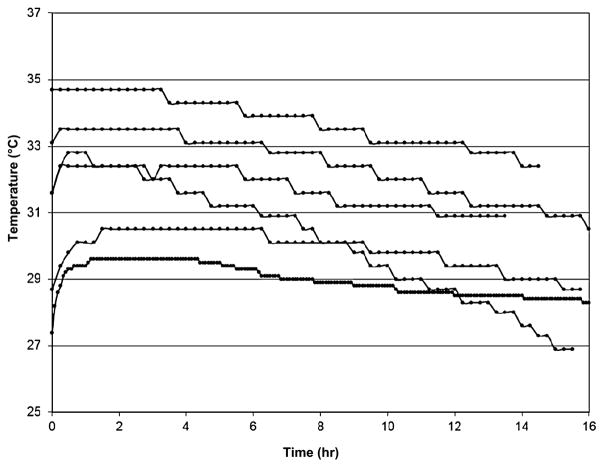
The length of shipment ranged from 14 to 16 hours, with an average of 4 hours in flight. The temperature consistently showed a gradual cooling over time during the product shipment (Fig. 1). The temperature during shipment ranged from 26.9 to 34.7°C.
Fig. 1.
Temperature profile for shipment of the NK-cell products. Automated electronic thermometers recorded the temperature inside the shipping containers that the NK-cell products experienced during roundtrip shipment from Minneapolis/Saint Paul, Minnesota, to Columbus, Ohio. Time of shipment ranged from 14 to 16 hours.
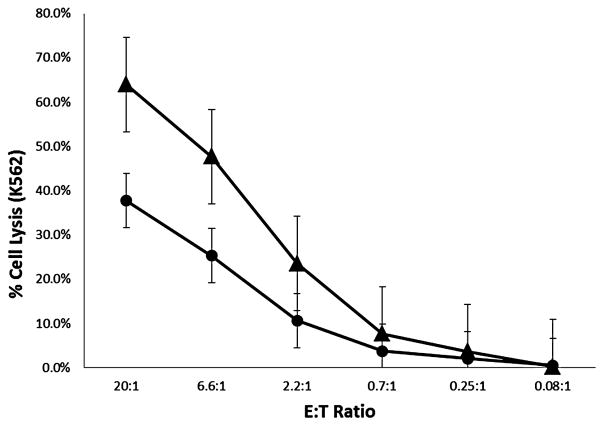
To determine whether shipment of the NK-cell products affected their in vitro antitumor activity, cytotoxicity assays using the NK-cell–sensitive cell line K562 were performed on five of the six NK-cell products. All five NK-cell products that were shipped with IL-2 exhibited potent lytic activity against K562 targets (Fig. 2). The antitumor activity of the shipped NK-cell product was significantly higher (p = 0.026 at 20:1 effector cell to tumor cell ratio) than that of the control cells from each product (i.e., not shipped or stimulated with IL-2).
Fig. 2.
Summary of cytotoxicity data. Cytotoxicity assays were performed on products shown in Table 1. The mean percentage of tumor cell lysis ± standard error is shown as a function of the effector cell to tumor (target) cell ratio (E : T). The results of the cytotoxicity assay for the shipped, IL-2–stimulated cells (▲) were compared to the activity of same cells that were not incubated with IL-2 and not shipped (●).
To determine the role of IL-2 and controlled incubation in activation of the NK-cell product, one MNC collection was divided into four fractions. Two fractions were shipped, one with IL-2 and one without IL-2. Similarly, the remaining two fractions were incubated in the laboratory at 37°C and 5% CO2, one with IL-2 and one without IL-2. At the end of the experiment, cell viability was similar between all four fractions and ranged from 92.9% to 95.7%. Cytotoxicity assays were performed to determine antitumor activity against the NK-sensitive “leukemia” cell line K562 (Fig. 3A) and the NK-resistant Raji “lymphoma” (Fig. 3B) and MCF7 “breast cancer” cell lines (Fig. 3C). Killing of K562 cells was preserved under all conditions and was higher with IL-2 than without. In marked contrast, killing of Raji and MCF-7 was only successful when IL-2 was present. The IL-2–activated NK-cell products showed similar levels of tumor lysis whether the cell product was shipped or incubated in the laboratory under standard culture conditions (Figs. 3A–3C).
Fig. 3.
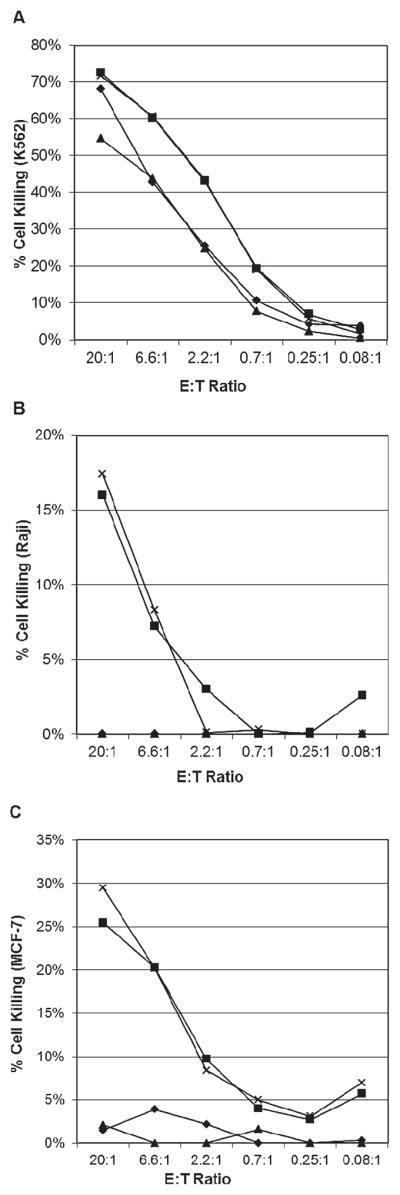
Relative importance of IL-2 and controlled incubation on NK-cell activation. One NK-cell product was split into four fractions: shipped and incubated with IL-2 (×), shipped without IL-2 incubation (▲), incubated in the laboratory (37°C, 5% CO2) with IL-2 (■), and incubated in the laboratory (37°C, 5% CO2) without IL-2 (◆). Each fraction was tested for cytotoxicity against three cell lines (A) K562, (B) Raji, and (C) MCF7 as a function of the effector cell to tumor (target) cell ratio (E : T). In (A) both fractions including IL-2 incubation (× and ■) overlap with × marks obscured. In (B) both fractions without IL-2 (▲ and ◆) overlap with no killing of target cells evident.
DISCUSSION
NK-cell–based immunotherapy has shown potential for treating a wide variety of malignancies.7 However, the level of expertise and the cGMP resources required to ensure a safe and potent cellular product could potentially preclude their widespread manufacture and use. In such cases, the shipment of an MNC donation to a central production facility and the expeditious return of the resultant cellular product to the patient may be a viable option that enables patients to have access to quality cellular therapies.
An obvious strategy to accommodate shipping and time constraints is to employ cryopreservation. A recent study validated the cryopreservation of MNCs for use in generation of cellular products without compromising quality.8 However, this group did not manufacture NK-cell products from the cryopreserved and thawed MNCs. It has been suggested that cryopreservation of NK cellular products may decrease their cytotoxic activity.5 In our experience, cryopreserved NK cells must be placed in culture for 1 to 2 days after thaw to return to prefreeze baseline cytotoxicity levels (J. Miller, unpublished). Until these issues are resolved, the use of cryopreserved cells remains in question for the widespread use of NK cells in clinical trials. Therefore, we set out to validate the shipment and in-transit activation of fresh NK cellular therapy products.
Using our established QC indicators,4 we have shown that MNC-derived NK-cell products can be shipped fresh while maintaining the integrity of the product (Table 1). In one shipping study, we showed that IL-2 appeared to be important for activating the NK-cell product (Fig. 2). Further, we have shown that the in vitro cytotoxicity of the NK-cell products remains intact with shipment and tightly controlled incubation is not necessary (Fig. 3). Most importantly, we have demonstrated that centralized production and routine shipment of NK-cell therapies to distant clinical sites is feasible. In fact, this study enabled support of a recently completed Phase I clinical trial at Tufts Medical Center.9
Acknowledgments
Supported in part by the National Heart, Lung, and Blood Institute Contract N01-HB-47095 (Production Assistance for Cellular Therapies, or PACT), P01 CA111412 (JSM), and the BMT Research Fund and Children’s Cancer Research Fund (JEW, JSM).
The authors thank the staff of the Clinical Cell Therapy Laboratory, especially Sheryl Adams, MT (ASCP), Nancy Bostrom, MT (ASCP), and Darin Sumstad, MT (ASCP), and the QA and support staff at Molecular and Cellular Therapeutics.
ABBREVIATIONS
- cGMP
current good manufacturing practices
- NK
natural killer
- TNC(s)
total nucleated cell(s)
Footnotes
CONFLICT OF INTEREST
The authors have no conflicts of interest to disclose.
References
- 1.Moretta L, Locatelli F, Pende D, Marcenaro E, Mingari MC, Moretta A. Killer Ig-like receptor-mediated control of natural killer cell alloreactivity in haploidentical hematopoietic stem cell transplantation. Blood. 2011;117:764–71. doi: 10.1182/blood-2010-08-264085. [DOI] [PubMed] [Google Scholar]
- 2.Symons HJ, Leffell MS, Rossiter ND, Zahurak M, Jones RJ, Fuchs EJ. Improved survival with inhibitory killer immunoglobulin receptor (KIR) gene mismatches and KIR haplotype B donors after nonmyeloablative, HLA-haploidentical bone marrow transplantation. Biol Blood Marrow Transplant. 2010;16:533–42. doi: 10.1016/j.bbmt.2009.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Miller JS, Soignier Y, Panoskaltsis-Mortari A, McNearney SA, Yun GH, Fautsch SK, McKenna D, Le C, Defor TE, Burns LJ, Orchard PJ, Blazar BR, Wagner JE, Slungaard A, Weisdorf DJ, Okazaki IJ, McGlave PB. Successful adoptive transfer and in vivo expansion of human haploidentical NK cells in patients with cancer. Blood. 2005;105:3051–7. doi: 10.1182/blood-2004-07-2974. [DOI] [PubMed] [Google Scholar]
- 4.McKenna DH, Jr, Sumstad D, Bostrom N, Kadidlo DM, Fautsch S, McNearney S, Dewaard R, McGlave PB, Weisdorf DJ, Wagner JE, McCullough J, Miller JS. Good manufacturing practices production of natural killer cells for immunotherapy: a six-year single-institution experience. Transfusion. 2007;47:520–8. doi: 10.1111/j.1537-2995.2006.01145.x. [DOI] [PubMed] [Google Scholar]
- 5.Marti F, Miralles A, Peiro M, Amill B, de Dalmases C, Pinol G, Rueda F, Garcia J. Differential effect of cryopreservation on natural killer cell and lymphokine-activated killer cell activities. Transfusion. 1993;33:651–5. doi: 10.1046/j.1537-2995.1993.33893342746.x. [DOI] [PubMed] [Google Scholar]
- 6.Miller JS, Oelkers S, Verfaillie C, McGlave P. Role of monocytes in the expansion of human activated natural killer cells. Blood. 1992;80:2221–9. [PubMed] [Google Scholar]
- 7.Sutlu T, Alici E. Natural killer cell-based immunotherapy in cancer: current insights and future prospects. J Intern Med. 2009;266:154–81. doi: 10.1111/j.1365-2796.2009.02121.x. [DOI] [PubMed] [Google Scholar]
- 8.Whiteside TL, Griffin DL, Stanson J, Gooding W, McKenna D, Sumstad D, Kadidlo D, Gee A, Durett A, Lindblad R, Wood D, Styers D. Shipping of therapeutic somatic cell products. Cytotherapy. 2011;13:201–13. doi: 10.3109/14653249.2010.506507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Klingemann H, Grodman C, Cuttler E, Duque M, Kadidlo D, Klein A, Sprague KA, Miller KB, Comenzo RL, Kewalramani T, Yu N, Van Etten R, McKenna D. Autologous stem cell transplant recipients tolerate MHC haplotype—mismatched NK cell enriched donor lymphocyte infusions. doi: 10.1111/j.1537-2995.2012.03764.x. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]