Abstract
Objective
Recent national initiatives from the White House and Institute of Medicine have focused on strategies to increase the accessibility and affordability of hearing loss treatment given the average cost of $4700 for bilateral hearing aids. More affordable direct-to-consumer hearing technologies are increasingly gaining recognition, but the performance of these devices has been poorly studied. We investigated the technical and electroacoustic capabilities of several direct-to-consumer hearing devices in order to inform otolaryngologists who may be asked by patients to comment on these devices.
Patients/Intervention
Nine direct-to-consumer hearing devices ranging in retail cost from $144.99 to $395.00 and one direct-to-consumer hearing device with a retail cost of $30.00.
Main Outcome Measure
Electroacoustic results and simulated real-ear measurements. Main electroacoustic measures are frequency response, equivalent input noise, total harmonic distortion, and maximum output sound pressure level at 90 dB.
Results
Five devices met all four electroacoustic tolerances presented in this study, 2 devices met three tolerances, 1 device met two tolerances, 1 device met one tolerance, and 1 device did not meet any tolerances. Nine devices were able to approximate 5 of 9 NAL targets within 10 dB while only three devices were able to approximate 5 of 9 NAL targets within a more stringent 5 dB.
Conclusion
While there is substantial heterogeneity among the selection of devices, certain direct-to-consumer hearing devices may be able to provide appropriate amplification to persons with mild-to-moderate hearing loss and serve as alternatives for hearing aids in specific cases.
Introduction
The prevalence of a clinically significant hearing loss doubles with each decade of life such that nearly two-thirds of adults over 70 years-old have an age-related hearing loss (ARHL)1. However, less than 20% of adults with significant hearing loss have hearing aids (HAs)2. The low rate of HA use is the result of myriad factors including cost, required time for multiple HA fitting visits with a hearing care professional, public awareness of the effects of ARHL, and limited awareness of hearing treatment options by medical providers3-5.
Given the prevalence and growing recognition of the impact of ARHL on public health6-9, there is a need for novel approaches to improve access to amplification for consumers. Direct-to-Consumer Devices (DTCDs), including both direct-to-consumer Has (available via internet sale) and Personal Sound Amplification Products (PSAPs), represent a potentially accessible and affordable ($100-400 per device) option. Many DTCDs can be self-fit by the user and mirror many capabilities of traditional HAs10. The direct-to-consumer (DTC) model utilized by these devices facilitates their ready availability to the public at lower price points. Current state-level regulations throughout the U.S. stipulate that HAs can only be sold through a licensed professional (e.g., audiology, HA dispenser). Hence, HAs cannot be directly purchased in a store where a hearing care professional is not present (e.g., at a drugstore) but can often be purchased over the internet because inter-state sales are not necessarily subject to state regulations and/or the company employs a licensed HA professional. In contrast, PSAPs by virtue of not being labelled explicitly as treating hearing loss (and hence not being considered as a HA from the perspective of the Food and Drug Administration) can be sold over the internet or in any store.
Direct-to-consumer devices have been available for several decades; however, recent advances in the level of technology available make this class of devices worth investigating as a potential low cost option for some adults with ARHL10. There is a paucity of research surrounding these devices in the healthcare literature.
Previous studies of DTCDs found that many products tended to over amplify the low frequencies with little to no amplification above 2000 Hz, which would render devices useless for much more common high-frequency hearing loss, and produced high levels of internal noise11-13. However, certain devices did meet some electroacoustic standards of traditional HAs, and were able to approximate some amplification targets.11
The purpose of this study was to conduct basic electroacoustic analyses on a current selection of DTCDs to understand the capabilities of these devices and provide evidence to make appropriate recommendations to patients.
Methods
Device Selection
Selected devices were a combination of products with the most user reviews on third party consumer and vendor websites as well as devices that were previously known to the investigators. Nine devices available for purchase on the internet were selected for inclusion in the study based on the following criteria: (a) devices had to be marketed for hearing loss or situational hearing, (b) devices had to be readily available to the public, and (c) devices had to be in the $150 to $400 price range. In addition, one readily available $30 device sold over the counter at a major drugstore chain was included. Table 1 summarizes the device characteristics.
Table 1.
Device details and electroacoutic and simulated real ear measure output
| Devices Overview | Electroacoustic and Simulated Real Ear Measures | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||||||||
| Device Name | Manufacturer | Retail Cost | Processor | Directionality/Remote Mic | Streaming Capability | Noise/Speech Processing6 | Frequency Range (Hz) | Equivalent Input Noise (dB) | Max OSPL 90 (dB) | Max OSPL Frequency (Hz) | Total Harmonic Distortion (%) | Approximation of NAL Frequency Targets (Number of targets)1 | |||
| 500 Hz | 800 Hz | 1600 Hz | Within 10 dB | Within 5 dB | |||||||||||
|
|
|
||||||||||||||
| Bean | Etymotic | $299.99 | Linear | No | No | No | 200-8000 | 24 | 101 | 3500 | 1 | 1 | 0 | 5 | 3 |
| Bean T7 | Etymotic | $299.99 | Linear | No | T-coil4 | No | 200-8000 | 31 | 100 | 4100 | 0 | 1 | 2 | 7 | 4 |
| CS-50+ | Soundworld Solutions | $349.99 | Digital | Directional2 | Bluetooth5 | Yes | 200-7300 | 27 | 112 | 2380 | 0 | 1 | 0 | 8 | 5 |
| LifeEar | LifeEar | $349.99 | Digital | No | No | Yes | 350-6000 | 20 | 115 | 900 | 0 | 0 | 1 | 6 | 2 |
| MD Hearing Aid AIR | MD Hearing Aid | $299.99 | Digital | No | No | No | 280-6300 | 31 | 122 | 750 | 1 | 2 | 5 | 7 | 3 |
| Songbird Ultra | Songbird Hearing | $395.00 | Digital | No | No | Yes | 350-6000 | 29 | 115 | 2120 | 0 | 0 | 2 | 9 | 7 |
| SoundHawk | SoundHawk | $349.99 | Digital | Remote Mic3 | Bluetooth5 | No | 200-7000 | 20 | 101 | 2240 | 0 | 1 | 0 | 7 | 6 |
| Tweak Basic | Tweak Hearing | $144.99 | Digital | No | No | Yes | 200-7000 | 24 | 111 | 2120 | 1 | 0 | 0 | 6 | 4 |
| Tweak Focus | Tweak Hearing | $249.99 | Digital | Directional2 | No | Yes | 200-7160 | 28 | 113 | 2200 | 1 | 0 | 0 | 5 | 3 |
| MSA30× | RCA | $30.00 | Linear | No | No | No | 350-4000 | 50 | 129 | 700 | 4 | 5 | 4 | 4 | 2 |
Bold indicates measure was within tolerance limits defined in methods section
Number of frequencies at which devices were able to approximate frequency targets during speech mapping with an average sound input within 10 dB and within 5 dB
Directionality refers to the device's ability to focus on sound signals in front of the patient rather than those behind the patient, thereby allowing the patient to focus on a signal of interest
A remote mic offers a portable microphone that can be placed next to a signal of interest to achieve a better signal-to-noise ratio
T-coil allows for direct input via a telecoil (copper wire); this is commonly used with landline telephones and large loop systems in amphitheaters and museums
Bluetooth allows for the devices to be customized/programmed via a smartphone and for direct input from the phone signal to the device
Noise/Speech processing refers to the algorithmic signal processing that attempts to enhance speech signals while reducing noise
T-coil was not active during electroacoustic and simulated real ear testing
Data Collection Procedures
Data were collected independently in replicate on two calibrated Audioscan Verifit 1.0 test boxes by different audiologists. Data were aggregated and compared, and repeated as appropriate when substantive differences were noted between the replicate datasets.
Electroacoustic Analysis
Electroacoustic measurements on all devices were performed using a HA 1 2cc coupler regardless of hearing device style in order to obtain independent electroacoustic data11. Tolerances for four measurements in this study were established based on previous literature, including American National Standards Institute (ANSI) S3.22-1987 standards, and a scan of current specification sheets for HAs appropriate for mild to moderate ARHL (Phonak Audeo RIC B-312, Oticon Ria2 RITE 85, and Starkey Muse RIC 312t)11,14. Devices were judged on their ability to meet these four set tolerances for a specific ARHL audience rather than compared against a set of commonly available HAs because HAs are already subject to regulation by the Food and Drug Administration and direct comparisons are nebulous as current ANSI standards allow manufacturers to set their own specifications which creates a wide array of specifications given the different technology levels and output capabilities of HAs. All HAs used as references to create tolerances would meet all tolerances based on their specification sheets. Electroacoustic measurements included:
Frequency range over which the device amplifies. Devices should amplify speech over 250 to 6000 Hz to cover the complete range of speech.
Total harmonic distortion (TDH) at 500, 800, and 1600 Hz, TDH represents the introduction of undesired harmonics into the signal. Higher TDH will distort the signal, causing unintelligibility. TDH was not to exceed 3% at any given frequency.
Equivalent internal noise (EIN). EIN represents the noise produced by the device during amplification. A higher EIN will mask the signal of interest and make listening more difficult for the user. A tolerance of < 28 EIN was used based on ANSI S3.22-1987.
Lastly, maximum output sound pressure level at 90 dB SPL (Max OSPL 90) and maximum output sound pressure level at 90 dB SPL frequency (Max OSPL frequency). Max OSPL 90 represents the maximum output of a device with an input of 90 dB while Max OSPL frequency represents the frequency of the peak output produced by the device. Based on a review of several HA manufacturer specifications for HAs regularly recommended for mild to moderate ARHL, Max OSPL 90 tolerance was set at no greater than 120 dB SPL.
Simulated Real Ear Measures
Speech mapping, a measurement of providing appropriate gain for a given hearing loss, was measured at soft (55 dB SPL), average (65 dB SPL), and loud (75 dB SPL) input levels. HAs are highly customizable and generally meet all targets appropriately. We determined whether each device could provide appropriate levels of amplification (within 5 dB or 10 dB SPL) at 9 frequencies (0.25 kHz to 6 kHz) based on National Acoustics Laboratories (NAL) NAL-NL2 and NAL-R prescriptive threshold-based targets15-17 for common mild-to-moderate ARHL configurations18.
Results
The mean electroacoustic results and consistency with defined tolerance limits for each device are presented in Table 1. Notably, five devices met all four of the electroacoustic criteria noted above: the Etymotic Bean, the SoundWorld Solutions CS-50+, the SoundHawk, the Tweak Basic and the Tweak Focus. The MSA30× did not meet any of the established electroacoustic criteria.
Table 1 indicates the number of frequencies at which each device was able to approximate target gain during speech mapping within 10 and 5 dB SPL. All but one device, the MSA30×, were able to approximate at least half (5 of 9) targets within 10 dB SPL. The SoundWorld Solutions CS-50+, the Songbird Ultra, and the SoundHawk were able to approximate half the targets within 5 dB SPL. These results, as well as those for other input levels, are also visualized in Figure 1.
Figure 1.
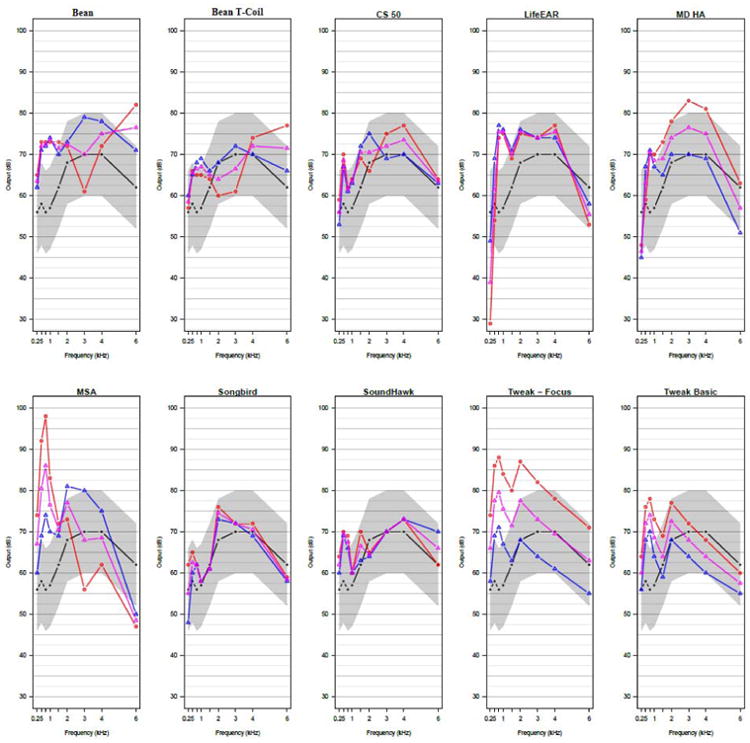
Visualization of each device's ability to approximate NAL targets at soft (55 dB SPL), average (65 dB SPL), and loud (75 dB SPL) input levels
Device speech mapping results are displayed whereas the black line represents the target with a shaded region of within 10 dB surrounding it. The blue line represents device output for soft input, pink represents device output for average input, and red represents device output for loud input.
Discussion
Our results demonstrate that certain DTCDs are comparable to traditional HAs in their electroacoustic output and ability to approximate NAL target gains in a well-controlled clinical environment. Specifically, the electroacoustic output of five devices from this sample were within defined tolerance limits on characteristics of frequency range, TDH, and EIN. In addition, most of the tested devices were able to match target within ±10 dB SPL across at least five of nine frequencies in a simulated real ear measure evaluation of the device output when using a typical ARHL configuration while three devices were within ±5 dB across at least five of nine targets. These results suggest certain DTCDs produce ample amplification across a wide enough frequency range with low enough distortion to meet the amplification needs of a person with mild-to-moderate ARHL. In contrast, one device readily available over-the-counter at retail drug stores (MSA30×) provided over-amplification of low frequencies while under amplifying the high frequencies and producing excessive internal noise consistent with prior studies investigating DTCDs11,12.
As the variety of amplification options increases, otolaryngologists and other hearing care professionals have an opportunity to provide support and recommendations to consumers who wish to pursue DTCDs as a communication solution. While some adults will be able to explore products independently and find benefit, others may require more professional guidance from an audiologist for professional fitting of HAs and rehabilitative education.
This study is limited in that we only investigated a small sample of the many devices available in this emerging market. Moreover, these results are obtained in a clinical setting and do not represent real-world outcomes.
Conclusion
There is substantive heterogeneity in the quality and performance of DTCDs. Otolaryngologists and other hearing care professionals should be aware that some DTCDs meet objective electroacoustic criteria and have the ability to provide appropriate amplification for hearing loss. Our study shows some DTCDs meet the basic electroacoustic criteria necessary for aiding a person with mild to moderate ARHL and could be recommended to patients in need of alternative options.
References
- 1.Lin FR, Niparko JK, Ferrucci L. Hearing loss prevalence in the United States. Archives of internal medicine. 2011;171(20):1851–1852. doi: 10.1001/archinternmed.2011.506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chien W, Lin FR. Prevalence of hearing aid use among older adults in the United States. Archives of internal medicine. 2012;172(3):292–293. doi: 10.1001/archinternmed.2011.1408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Strom KE. Hearing Review 2013 dispenser survey: Dispensing in the age of internet and big box retailers. Hearing Review. 2014;21(4):22–28. [Google Scholar]
- 4.Knudsen LV, Oberg M, Nielsen C, Naylor G, Kramer SE. Factors influencing help seeking, hearing aid uptake, hearing aid use and satisfaction with hearing aids: a review of the literature. Trends in amplification. 2010;14(3):127–154. doi: 10.1177/1084713810385712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kochkin S. Marke-Trak VIII: 25-year trends in the hearing health market. Hearing Review. 2009;16(11):12–31. [Google Scholar]
- 6.IOM (Institute of Medicine) and NRC (National Research Council) Hearing loss and healthy aging: Workshop summary. Washington, D.C: The National Academies Press; 2014. [PubMed] [Google Scholar]
- 7.Lin FR, Hazzard WR, Blazer DG. Priorities for Improving Hearing Health Care for Adults: A Report From the National Academies of Sciences, Engineering, and Medicine. JAMA. 2016 doi: 10.1001/jama.2016.7916. [DOI] [PubMed] [Google Scholar]
- 8.President EOot, editor. Technology PsCoAoSa. Aging America & Hearing Loss: Imperative of Improved Hearing Technologies. Oct, 2015. [Google Scholar]
- 9.National Academies of Sciences E, and Medicine. Hearing Health Care for Adults: Priorities for Improving Access and Affordability. 2016 [PubMed] [Google Scholar]
- 10.Mamo SK, Reed NS, Nieman CL, Oh ES, Lin FR. Personal Sound Amplifiers for Adults with Hearing Loss. The American journal of medicine. 2015 doi: 10.1016/j.amjmed.2015.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Callaway SL, Punch JL. An electroacoustic analysis of over-the-counter hearing aids. American journal of audiology. 2008;17(1):14–24. doi: 10.1044/1059-0889(2008/003). [DOI] [PubMed] [Google Scholar]
- 12.Cheng CM, McPherson B. Over-the-counter hearing aids: electroacoustic characteristics and possible target client groups. Audiology : official organ of the International Society of Audiology. 2000;39(2):110–116. doi: 10.3109/00206090009073062. [DOI] [PubMed] [Google Scholar]
- 13.Chan Z, McPherson B. Over-the-Counter Hearing Aids: A Lost Decade for Change. BioMed Research International. 2015;2015:1–15. doi: 10.1155/2015/827463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Staab W, Lybarger S. Characteristics and use of hearing aids. Handbook of clinical audiology. 1994;4:664. [Google Scholar]
- 15.Dillon H. What'S new from NAL in hearing aid prescriptions? The Hearing Journal. 2006;59(10):10–16. [Google Scholar]
- 16.Keidser G, Dillon H, Flax M, Ching T, Brewer S. The NAL-NL2 prescription procedure. Audiology Research. 2011;1(1) doi: 10.4081/audiores.2011.e24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Byrne D, Dillon H. The National Acoustic Laboratories'(NAL) new procedure for selecting the gain and frequency response of a hearing aid. Ear and hearing. 1986;7(4):257–265. doi: 10.1097/00003446-198608000-00007. [DOI] [PubMed] [Google Scholar]
- 18.Dubno JR, Eckert MA, Lee FS, Matthews LJ, Schmiedt RA. Classifying human audiometric phenotypes of age-related hearing loss from animal models. J Assoc Res Otolaryngol. 2013;14(5):687–701. doi: 10.1007/s10162-013-0396-x. [DOI] [PMC free article] [PubMed] [Google Scholar]


