Abstract
During the last decade, probiotics have been established to be important mediators of host immunity. Their effects on both innate and adaptive immunity have been documented in the literature. Although several reports have correlated different strains of bacteria as probiotics, their effects on immunity vary. Clearly, there is a complex interplay between various constituents of probiotics and the immune response in humans. The role of probiotics on natural killer (NK) cells in the gut has been the subject of a few reports. In this review, we summarize the reported findings on the role of probiotics in the activation of gut-associated NK cells and the response of NK cells to stimuli elicited by probiotics and their microenvironment. The effects of probiotics on the activation of NK cells and their secretion of immune factors (e.g., interferon–γ, tumor necrosis factor–α, interleukin-2, etc.) are discussed in regard to their clinical significance in various diseases. Current investigations are being pursued, in particular, on the role of probiotics-activated NK cells in promoting the adaptive immune response against pathogens.
Keywords: GALT, immunity, infection, natural killer, probiotics
I. INTRODUCTION
The human intestinal microbiome is composed of nearly 1014 microorganisms, nearly 100 times greater than the overall genomic composition of an individual. Critical to their functions is to synthesize amino acids and vitamins and extract energy from polysaccharides that are not absorbable.1 Furthermore, these microorganisms act against pathogens and support the activity of the immune system, thereby contributing to overall intestinal wall integrity.2 The composition of microorganisms present in the gut is highly variable to external influences such as diet, age, medications, illness, stress, and lifestyle.1 The majority of bacteria present in this environment consist of the Gram-positive species lactobacilli and bifidobacteria, comprising 85% of the microbiome.3 Additionally, the microbiota is also composed of viruses, fungi, and protozoans.2 The human microbiota is continuously shaped and changed starting at birth, staying constant throughout adulthood, and decreasing during old age. The microbiota is so closely associated with the human race, that without this symbiosis, our species would have not existed.4
II. PROBIOTICS
The microbiota is highly influenced by a variety of factors that can shape and change the overall composition of the gut environment, and this may benefit the host. Probiotics are thought to be significantly involved in affecting the gut microbiome. The Food and Agriculture Organization (FAO) of the United Nations alongside the World Health Organization (WHO) have defined a probiotic to be “live microorganisms, which when administered in adequate amounts, confers a health benefit on the host.”5 Lactic acid bacteria (LAB) such as Lactobacillus species and Bifidobacterium are most commonly used for probiotic supplementation in foods, mainly yogurts.6,7 It has been proposed that lactobacilli reside in both the stomach and upper portion of the small intestines, whereas bifidobacteria reside in the colon, where the environment is anaerobic.8 The FAO/WHO also state, “there is good evidence that specific probiotics are safe for human use and are able to confer some health benefits on the host, but these benefits cannot be extrapolated to other strains without additional experimentation.”5
Primarily, probiotics have been found in yogurts and fermented milks throughout the history of humanity, and these food items contain excellent nutritional properties.9 In examining the milk of both Caucasian and Bulgarian shepherds to compare the quality of life between these shepherds and American men, Metchnikoff mentioned that there are microorganisms that are not harmful to human health, thus suggesting that “the dependence of the intestinal microbes on the food makes it possible to adopt measures to modify the flora in our bodies and to replace the harmful microbes by useful microbes.”10 However, Metchnikoff’s intuition behind these microorganisms was faced with criticism, because the microorganisms were not apparent in these fermented foods. Yet, the health benefits of the yogurts were widespread, leading the microbiologist Shirota to discover certain bacterial strains that were able to contribute to bacterial pathogenic defense in the gut. Shirota isolated and characterized a Lactobacillus strain called Lactobacillus casei and successfully implemented this lactobacillus strain into a probiotic beverage called Yakult that produced exceptional nutritional benefits.9 The contributions that have been made to understand the biology of probiotics yielded sufficient insights to unravel the dynamics of microflora in the gut and the variability in bacterial composition when presented with these beneficial strains.
The native composition of intestinal bacterial species was determined by analysis via the 16s ribosomal RNA subunit through polymerase chain reactions and was sequenced bioinformatically.2 Approximately 60% to 80% of the native bacterial species were isolated, and the distribution of microbiota did not appear to be uniform among individuals. The bacterial populations were relatively low in the stomach, increased in concentration further down the intestinal tract, reached high concentration levels in the colon, and mainly consisted of Gram-negative anaerobic bacteria. The diversity of the microbiota is sensitive to both internal disturbances such as shifts in pH, motility, and the immune system and external disturbances such as diet or medications.4 The microbiota changes at different stages of life with the bacterial colonization of the gut during either vaginal or caesarean birth with breastfeeding but remains relatively stable during adulthood. Yet, the inclusion of probiotics into an infant and a malnourished person’s diet suggests it to be beneficial, producing immune-enhancing effects to relieve or prevent diseases or disorders.11,12
Probiotics are commensal bacteria that help to reinforce and maintain the permeability of the host intestinal cell wall.12 LAB, the most common probiotic supplement, has been associated with binding to microfold cells, which are follicle-associated epithelium cells found in Peyer’s patches in the intestine; this binding leads to the stimulation of surrounding lymphoid tissue via an antigen transport. 13 The lymphoid tissue in the gut is the largest tissue in the body, making the mucosal lining a major interaction of lymphocytes and antigens.14 Probiotics and other commensal bacteria present in the lumen of the gut are important in the regulatory role of dendritic cells (DCs), which are responsible for sampling the environment for any pathogenic invaders and releasing cytokines or chemokines to stimulate an immune response.15,16 Certain strains of probiotics have been seen to enhance innate immunity (specifically, phagocytosis and NK cell activity), but probiotics do not appear to have much of a desired effect on acquired immunity.17 NK cells are part of the innate immune system and are known for their cytokine secretion and killing of virally infected and tumor cells; hence, NK cells protect the host from pathogens and transformed cells by their targeted elimination. These activities underlie the importance of maintaining the cytotoxic function of NK cells at a normal range.18 It has been reported that NK cell activity could be enhanced by certain strains of probiotics in humans.19 In this review, we examine the effects of probiotics on gut immunity, specifically describing how NK cell activity and subsequently innate immunity are affected by the addition of certain probiotic strains into a human daily diet.
III. THE IMMUNE SYSTEM: INNATE VERSUS ACQUIRED IMMUNITY
The immune system is a critical component of the host organism because it protects its host from a variety of infectious agents including bacteria, viruses, fungi, and parasites. The cells that of the immune system, white blood cells, are spread across the body and travel via the lymphatic system and bloodstream. These cells develop and mature either in primary lymphoid organs such as the bone marrow (BM) or the thymus or in secondary lymphoid organs such as the lymph nodes (LNs), spleen, or the gut-associated lymphoid tissue (GALT). The immune system is divided into two components: the innate immune system and the acquired immune system. The innate or natural immune system is that part of the immune system that arises when an individual is born, whereas the acquired or specific immune system is the component of the immune system that develops during multiple exposures to infectious agents. A well-functional immune system is able to distinguish pathogenic, threatening organisms from those that are nonthreatening, beneficial, or nonharmful.17
The immune system is ever changing and consistently shaped by the antigens and microorganisms to which it is exposed. Breast milk is the first exposure to the immature immune system after birth, because it contains a variety of antigens from the mother.20 However, near the end of life, the immune system becomes highly dysregulated, leading to the progressive loss of acquired immunity and, ultimately, a greater chance of infection. The process of decreased acquired immunity is called immunosenescence.21 Interestingly, innate immunity seems not to be affected by aging compared to acquired immunity.17
The gut immune system, the GALT, is important to understand because the dynamic of probiotics and NK cells depends on its complex nature. The GALT includes the physical barrier of the intestines and the mucous lining that accompanies it, in addition to both the innate and adaptive components of the immune system. The immune cells in the intestinal wall are organized into specific structures called Peyer’s patches, located under the epithelium near an area called the lamina propria.22
Gut innate immunity communicates with endogenous microbiota to improve growth, survival, and inflammatory control of the intestinal environment.3 Innate immunity is able to distinguish pathogenic from nonpathogenic agents via pattern recognition receptors (PRRs) and Toll-like receptors (TLRs). This helps the immune cells to determine similar recognition sites that are present on microorganisms. These sites or patterns are called pathogen-associated molecule patterns and help to target pathogens by specific surface markers such as lipopolysaccharides (LPSs), peptidoglycan, flagellin, and many others.23,24 Furthermore, TLRs are found on many cells of the innate immune system including macrophages, neutrophils, and dendritic cells (DCs).3 Microbe-associated molecule patterns from the microbiota can regulate the gut innate immune system, leading to an increase in the activation of nuclear factor–kappa beta (NF-κβ) signaling pathway. This response leads to a production of various cytokines, thereby stimulating antigen-presenting cells for the induction of an immune response. This results in the activation of naïve T cells, indicating that innate immunity interacts significantly with adaptive immunity.23,24
The lamina propria, as mentioned previously, houses specialized structures called Peyer’s patches that contain a large number of immune cells such as macrophages, DCs, T cells, and immunoglobulin A (IgA)-secreting β cells. Adaptive immunity is dependent on GALT, primarily in these Peyer’s patches and mesenteric LNs (MLNs).25 The lamina propria also contains cluster of differentiation 4 (CD4T), CD8T, and β cells. These β cells become IgA-secreting plasma cells when activated in the lamina propria. Antigens sampled in this region are taken up by DCs and then travel through the lymphatic draining vessels to the either the MLNs or other gut lymphoid tissues.26 The innate and adaptive components of immunity are critical for survival. Cross talk between these two components provides a more substantial response to protect the host from various pathogens.
IV. NK CELLS AND IMMUNITY
NK cells are a component of the innate immune system, constitute nearly 10% of all peripheral lymphocytes, and are characterized by surface CD56 expression but no CD3 expression.27 NK cells are composed of heterogeneous subsets of lymphocytes, whose roles are important in both innate and adaptive components of the immune system.28 Large granular cells that are primarily involved in targeting and controlling pathogenic infections and tumor malignancies.29 NK cells are found in blood, BM, and the spleen and have a role in protecting the host from infectious agents and preventing harmful transformations. Furthermore, NK cells do not express receptors for specific antigens that require the lysis of tumor cells.30 Farag et al.30 determined that NK cells have their own repertoire of receptors that can associate with either major histocompatibility complex (MHC)-I–like molecules or other ligands. NK cells are either activated by immunoreceptor tyrosine-based activating motifs (ITAMs) or inhibited by immunoreceptor tyrosine-based inhibitory motifs in their cytoplasmic tails.31
The development of NK cells in requires interaction between both MHC-I and inhibiting receptors.32 This interaction is called NK cell education and helps to determine the activation levels of mature NK cells. The activation threshold of each NK cell depends on the strength of the inhibitory signals, adapting to a particular MHC type for their host.33 Mature NK cells are defined to have surface CD56 expression but lack surface CD3 expression.34 NK cells can be divided into two major subsets: CD56dim (low density) and CD56bright (high density).28 CD56dim NK cells are the more cytotoxic of the two subsets and comprise nearly 90% of all NK cells, whereas the other 10% of NK cells are CD56bright. The CD56dim NK cells have also high expression of CD16 Fc receptor γ (FcRγ) III, which is important for binding to targets that are coated with antibodies via their FcRs and initiate antibody-dependent cellular cytotoxicity.35,36
NK cells are involved in cytotoxicity and cytokine production, resulting in cell-mediated lysis through cytotoxic molecules, perforin, granzymes, and the tumor necrosis factor–α (TNF-α) family of ligands.18 Perforin causes pore formation on the target cell membrane.37 Granzymes, which are serine esterases, stimulate apoptosis in the target cell.38 TNF-α, the Fas ligand, and TNF-related apoptosis-inducing ligand are involved in activating apoptotic pathways in target cells for induction of apoptosis.39 Additionally, NK cytotoxicity can be divided into its ability to target tumor and virally infected cells or antibody-driven lysis by IgG antibodies targeting pathogens.28
The main receptors present on NK cells are killer immunoglobulin receptors for MHC-I.40 These receptors, along with many others present on the NK cell surface, help to regulate stimulation or inhibition of cell-specific lysis.18 The activating receptors help NK cells to have a strong stimulus with the recruitment of adaptor molecules, such as diaminopimelic acid (DAP)10, DAP12, and FcRγ, which contain ITAMs.28 Inhibitory receptors oppose these activating receptors with inhibiting motifs at their cytoplasmic tails. These receptors can then activate downstream sites, such as Src homology region 2 domain-containing phosphatase-1 (SHP-1) and SHP-2, that can oppose the signals of activating receptors and their ITAMs.41,42 Lysis of a tumor or virally infected cell will occur when the NK cell binds to surface ligands on the target cell; however, lysis will be inhibited when the target cell contains an MHC-I–like molecule such as the human leukocyte antigen (HLA). When inhibitory receptors on NK cells interact with HLA without the activating receptor/ligand complex, the target cell will not be lysed because of a net negative signal. But when the activating receptor binds to the ligand, an overall activation signal will occur, resulting in lysis.18 Various cytokines can increase the number of NK cells and stimulate the activation of these cells to target a broader spectrum of cells, especially those that are affected by nonactivated NK cells; cytokines that are included in this process typically involve interferon-γ (IFN-γ), interleukin-2 (IL-2), IL-12, IL-15, or IL-18.43 Thus, NK cells’ lysing ability depends on the activating and inhibiting receptors that have a role in forming complexes of NK target cells and are stimulated by the levels of cytokines present in the environment.
To combat tumor cells and viruses, NK cells release their own cytokines that help to destroy these pathogens. NK cells enhance the production of IFN-γ and IL-12 via dendritic cell (DC)–cytokine activity.44 DCs are important for initiating immune responses against bacteria and continuously sample the surrounding peripheral tissues to detect pathogens. DC/NK cross talk is regulated by LAB by increasing NK cytolytic potential. Various strains of LAB may be useful for balancing cytokines and can result in more active type-1 immune responses in the body. DC cells help NK cells to acquire complete functions (and vice versa) that are critical for generating adaptive immunity against pathogens. NK cells require stimulation by DC cells to kill their targets as part of the cross-talk mechanism between DC and NK cells.7 Thus, NK cells are largely critical in the innate immune system as well as the adaptive immune system for promoting cytokine production and cytotoxicity for cell-mediated lysis of tumor cells and for virally infected cells to maintain homeostasis in the body.
V. NK ACTIVITY IN THE GUT
The GALT encompasses MLNs, Peyer’s patches, cryptopatches, and isolated lymphoid follicles; the latter three compose the lamina propria of the intestine.45,46 As stated above, NK cells develop in blood, BM, and LNs and exit these locations after their development to migrate via the bloodstream to secondary lymphoid structures. NK cells in the gut have varying phenotypes based on their maturation and function. The current understanding of NK development and migration models suggests that NK cells emerge from BM and are a mixture of both mature and immature NK (iNK) cells.47
NK cells that are present in the region of the gut are classified under the innate lymphoid cells–1 subset.48 Intestinal NK cells are different from other NK cells seen in the blood and more likely resemble “helper” NK cells.49 NK cells found in the gut appear to be predominantly CD56bright, but few express the CD16 phenotype. This subset may indicate that these NK cells are similar to iNK cells found in secondary lymphoid tissues.50,51 Furthermore, iNK cells may indicate cells that originate from NK precursors that developed from either BM or LNs, whereby CD56bright NK cells in humans produce large quantities of IFN-γ, although these cells exhibit low cytolytic activity during in vitro testing.51 After an infection, there are significant changes in the frequency of NK cells in both the small intestine and lamina propria.
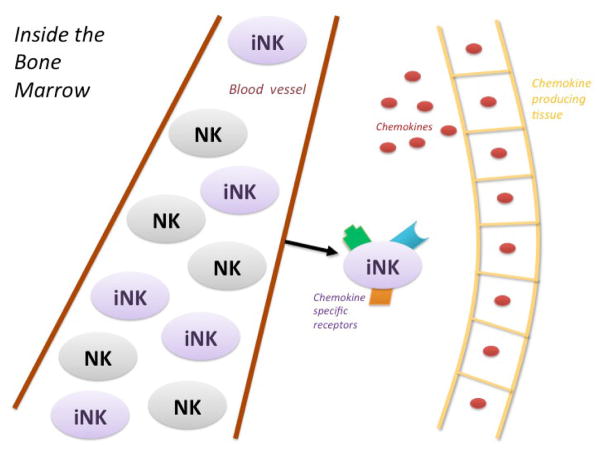
NK cells in the intestinal tract exhibit the distinct NK22 receptor, which is identified via NKp46 and is involved in the tissue remodeling process.52,53 As intestinal NK cells localize to the intestinal wall, they express chemokine receptor 6 (CCR6) and transcription factor RAR-related orphan receptors-γt (RORγt), which are mucosa-homing cytokines.52,54 NK cells express a variety of chemokine-associated receptors such as CXCR1, CCR2, CCR7, CCR5, and CX3CR1.55 The iNK cells are influenced by chemokines secreted by various peripheral organs such as the gut and liver, resulting in their migration to these same tissues (Fig. 1).56 Additionally, these RORγt+ NKp46+ NK cells produce IL-22. IL-22 in mice was seen to protect the integrity of the intestinal wall and increase epithelial repair against bacterial infection via the signal transducer and transcription 3 signaling pathway, resulting in the production of antimicrobial proteins.57 The diversity of the expression of receptors on NK cells may indicate that the tissues are able to regulate the migration of the cells to specific organs such as the gut epithelium.47 Furthermore, it has been suggested that NK cells in the gut require priming for activation by varying cytokines that include IFN-γ, IL-15, and IL-18.49 In the gut, iNK cells are primed to the gut mucosa by interacting with CCR7+ CD103+ DCs, resulting in the expression of CCR9.58,59
FIG. 1.
Activation and maturation of iNK cells in the BM by probiotics. NK cells are released into the peripheral blood from the BM as a mixture of both immature and mature cells. iNK cells are modified and gain specific receptors from chemokines released by certain mucosal tissues such as the gut or liver. These chemokines induce the migration of iNK cells to peripheral tissues.
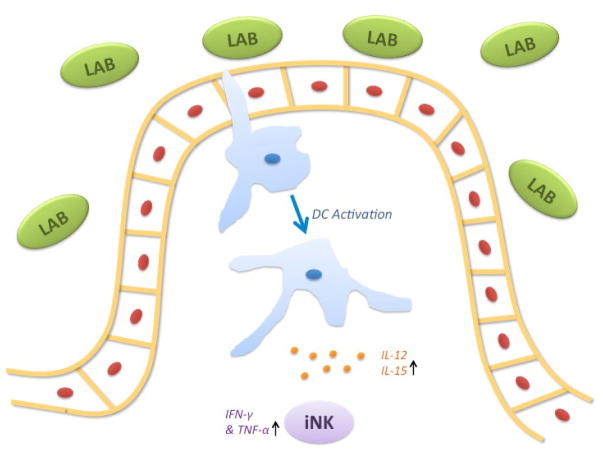
NK cells are exposed to various antigens in the gut including commensal bacteria. The NK cells display a variety of proinflammatory cytokines such as IFN-γ, TNF, IL-2, IL-17, and IL-22 when exposed to these commensal bacteria. These cytokines activate the innate immune system and cytolytic activity of NK cells. This indicates that NK cells are critical in innate immunity and tolerance against nonpathogenic bacteria that may also include probiotic strains found in commercial products.49,50,60 iNK cells cooperate with resident antigen presenting cells (APCs), primarily DCs, interacting with the intestinal microbiota. Inactivated dendritic cells sample the intestinal epithelium and are activated and matured by commensal bacteria, such as probiotics, that encompass many LAB (Fig. 2).7 The probiotics are recognized by DCs via PRRs, resulting in the release of various cytokines.61 The cytokines released, mainly IL-12, regulate both T-cell and NK cell responses to the gut, inducing the production of IFN-γ, favoring T-helper 1 (Th1) differentiation, that forms a connection between both the innate and adaptive components of the immune system.62 Additionally, IL-12–producing DCs increase the expression of CD69 in these gut NK cells.63
FIG. 2.
Interaction of iNK cells with APCs in the gut. iNK cells are stimulated by APCs, mainly DCs. DCs are activated by interacting with commensal or pathogenic bacteria; in this case, LAB. APCs increase IL-12 production in response to these bacteria, thereby stimulating the increase in NK cell production of INF-γ and TNF-α. This stimulation overall increases NK cell activation, cytotoxic activity, and expression of CD69.
It is hypothesized that NK cells may directly interact with nonmethylated CpG motifs of bacterial DNA via their own TLRs, specifically TLR-9, thereby enhancing their cytotoxic activity.7 The process by which NK cells interact with the gut microbiota is unclear, but it has been hypothesized that NK cells may infiltrate the intestinal wall and become intraepithelial lymphocytes, allowing them to respond to the gut microbiota (Fig. 3).47 Therefore, NK cells are stimulated and require several different cytokines to carry out and activate other nearby cells. The addition of probiotics helps to stimulate specific cytokines that regulate NK cell responses and secretions, leading to a form of a cross talk between innate immunity and adaptive immunity.
FIG. 3.
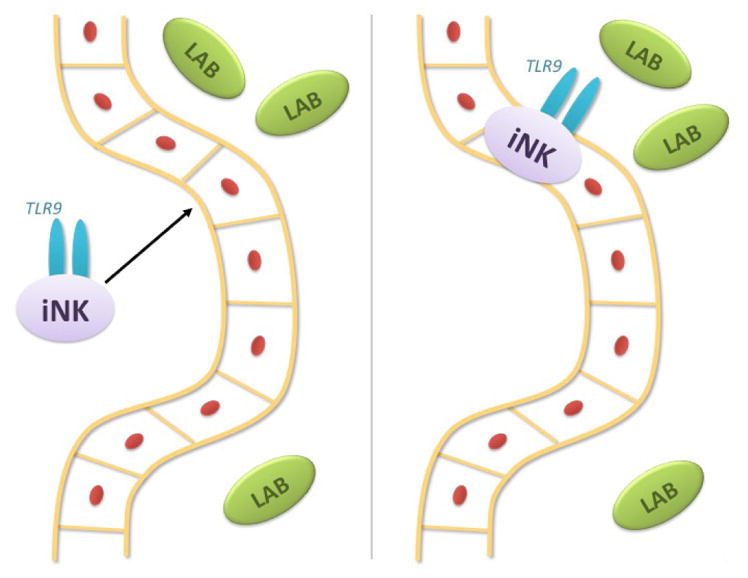
Interaction of NK cells with gut bacteria and probiotics. It is hypothesized that iNK cells may infiltrate the intestinal epithelium and interact with the nonmethylated CpG motif in bacterial DNA via its own TLR-9 and increasing its own cytolytic activity during this interaction.
VI. ROLE OF PROBIOTICS IN ACTIVATION OF HUMAN NK CELLS
Several studies have shown that PRRs, TLRs, and Nod-like receptors are important for keeping a stable relationship between both the gut and microbiota.64,65 The activation of TLRs leads to an up-regulation of proinflammatory mediators that facilitate the immune defense of the host. Probiotics have benefited the treatment of several diseases such as viral gastroenteritis, postantibiotic-associated diarrhea, necrotizing enterocolitis, and many others.66 With this understanding of probiotic benefits, several studies have been conducted to examine the effects of probiotics on NK cells and their interactions with innate immunity.
Several strains of probiotics have been studied to observe the beneficial effects on NK cell activity. Bacillus polyfermenticus, commonly referred to as Bispan strain, is used to treat certain long-term intestinal disorders because this strain’s endospores successfully reach the target intestinal wall.67 Kim et al.68 examined the effects of the Bispan strain to determine whether it increased the levels of IgG and NK cell counts. Twenty-five males between the ages of 20 and 35 years old were assigned to either a control (n = 12) or experimental (n = 13) group supplemented with a placebo or B. polyfermenticus for 8 wk. During strain supplementation, the overall concentration of IgG was 12% higher in the experimental group than in the control group. Furthermore, it was seen that CD4+ helper T cells, CD8+ cytotoxic T cells, and CD56+ NK cells in the experimental group were 32%, 28%, and 35% higher, respectively, when compared to the control group. B. polyfermenticus oral administration was shown to have immunoenhancing effects in these test subjects, resulting in modulation of innate, humoral, and cell-mediated immune responses.68 The immunoenhancing effects of B. polyfermenticus on these immune systems provide more evidence that several nutritional and therapeutic effects ascribed to probiotics, specifically LAB, include metabolic stimulation of vitamin synthesis, gut flora stabilization, and carcinogen detoxification, among many others.69
Probiotics also have varying effects on the immune system during different stages of life and have different benefits for certain age groups, which is similar to how the microbiota in our gut is constantly changing over the course of life. In young adults, the effects of probiotic supplementation in diet have been shown to increase concentrations of IgA, the number of NK cells, and NK cell activity.70 In elderly adults, two 3-wk trials of Bifidobacterium lactus HN019 supplementation increased phagocytosis of monocytes, polymorphonuclear cells, and NK cell tumoricidal activity.71,72 Additionally, similar results of phagocytic and NK cell activities occurred in middle age and elderly populations when these two groups were given Lactobacillus rhamnosus HN001 supplements during a 3-wk period.73 Thus, age is a contributing factor in the activity of NK cells and other components of the immune system during probiotic supplementation.
Certain strains of microbiota appear to have a modulatory effect on GALT, increasing innate immunity functionality, activating DCs, and stimulating NK cells via a direct cytochemical process by pathogens invading the mucosal wall of the host intestine.74 DC and NK cells are important in early response against cancer and viral infections that can result in NK activation, DC maturation, or DC death, indicating that DC and NK cells have an influence on the innate and consequently the adaptive immune system.75–77 LAB have been shown to have significant influence on DCs, thereby activating NK cells. This leads to the hypothesis that DCs that interact with LAB and go through maturation can also stimulate NK cells.7 This interaction is seen in several different subsets of DCs such as blood DCs and LN DCs that are matured by IL-12–inducing LAB and activate NK cells to produce IFN-γ63,78 confirming the belief that IL-12 is critical for NK cell production of IFN-γ.79 Certain LAB might be involved in Th1 skewing via the intermediate of IFN-γ–producing NK cells, whereas a larger variety of LAB strains may increase NK cell number and NK cytotoxicity as a result of NK/DC interaction.7
One of the most studied strains of LAB, Lactobacillus casei Shirota (LcS), is currently manufactured in Japan as a commercial beverage, used for its benefits as a probiotic for improving human health. LcS is known to increase the number of beneficial bacteria in the intestines, improve the overall composition of beneficial bacteria, and potentially replace pathogenic bacteria that invade the gut.80 Many studies have been conducted to observe the immunomodulatory effects of LcS on the gut immune system, in particular, NK cell activity within the innate immune system. One in vitro study conducted by Dong et al.,6 examined the effects of LcS and characterized its immunomodulatory properties on immune function when using human peripheral blood mononuclear cells (PBMCs). The researchers observed that during LcS supplementation, LcS promoted NK cell activity and induced the surface expression of CD69 and CD25 on both CD8+ and CD56+ subsets without any other stimulus present. In the absence of any LPSs, LcS induced production of IL-1β, IL-6, TNF-α, IL-12, and IL-10, whereas during exposure to LPSs, LcS enhanced IL-1β production. The study also examined the effects of monocytes on LcS-induced immunity and determined that monocytes reduced the impact of LcS on NK cells and cytokine production, indicating that LcS activated cytotoxic lymphocytes in both the innate and adaptive immune systems, allowing LcS to stimulate the destruction of infected or malignant cells.6 This interaction between monocytes and NK cells was similar to results of a study that examined the effects of monocytes on LcS-induced NK cell activity.81 However, in a similar study conducted by Seifert et al.,82 LcS was observed alongside NK activity in a group of healthy men (n = 68) with reduced activity who were supplemented with LcS for 4 wk. Proportions of NK cells and other leukocytes were observed and the authors determined that LcS supplementation had no significant effect on the number of NK cells and function or phagocytosis, respiratory burst, or cytokine production of PBMCs.82 Another study also examined Shirota’s effects on NK activity in smokers. These researchers observed that taking probiotic supplements of Shirota increased cytotoxicity activity and CD16+ cell numbers, contradicting the previous study’s conclusion.83
Lactobacillus strains have been extensively studied to understand their probiotics effects on NK cell activity. Three strains of lactobacilli, Lactobacillus plantarum, L. paracasei, and L. rhamnosus GG (LGG), and the pathogen Salmonella typhimurium were examined by Mileti et al.84 to observe immunomodulatory properties in the immune system. L. plantarum exhibited the most effective induction of inflammatory cytokines, whereas L. paracasei was least effective. L. paracasei did not greatly induce cytokine release, inhibited DCs’ potential to release inflammatory cytokines, and drove a response for Th1 cells against salmonella. This finding describes a new property to characterize probiotics to be either direct or indirect in inhibiting DC activation via inflammatory bacteria. Probiotics would then need to be divided into either immunostimulatory or immunomodulatory activities, based on how each strain interacted with specific components of the immune system.84 Knowledge of probiotic strains and their interactions with the gut immune system is necessary to determine whether the use of probiotics as a therapeutic agent in inflammatory bowel disease is beneficial, because current research into clinical efficacy is limited.85
One aspect of probiotics that must be acknowledged is whether certain strains better enhance specific components of the immune system than other probiotic strains. For instance, heat-killed LcS, Lactobacillus acidopholus ATCC 4356, and Bifidobacterium breve ATCC 15700NK have been reported to increase NK cell activity and enhance NK activation.86
Dong et al.87 conducted another study on probiotics and immune function in in vitro samples that further expanded the number of tested probiotics to six (four Lactobacillus strains and two Bifidobacterium strains). The expression levels of both CD69 and CD25 were analyzed as two activation markers that are expressed by lymphocytes, T cells, and NK cells on specific lymphocytes. The study suggested that some preferential activation of cytotoxic CD8+ T cells occurred with all strains, indicating that probiotics help to partially activate cytotoxic T cells. The authors also observed that the strains significantly increased the production levels of IL-1β, IL-6, IL-10, TNF-α, granulocyte macrophage–colony-stimulating factor (GM-CSF), and macrophage inflammatory protein–1α (MIP-1α).87 Thus, the referred literature indicates the potentiation of NK cell activity and increased production of cytokines with certain strains of probiotics.
VII. CONCLUDING REMARKS
The microbiota in the gut is a highly variable and easily influenced environment that can be affected by a number of various factors such as age, diet, medication, and lifestyle.3 Probiotic supplementation in our diet can be substantial and beneficial in improving the immune response in the gut. NK cell activity is critical for mediating an appropriate immune response against pathogens. The interaction of NK cells and DCs suggests an important role for NK cells in properly initiating and regulating immune responses.7 NK/DC cross-talk cooperation is an important complex that would benefit from interaction with probiotics to stimulate NK cell activity and increase cytolytic potential to help prime the immune system against harmful pathogens.
NK cell activity and functionality are enhanced during the incorporation of probiotic strains in diet. Experimentation of NK activity among different strains of probiotics, specifically, commercial lactobacilli and bifidobacteria strains, indicates that NK activity is increased overall (with no differences between strains) and is not specific to any certain strain.87 Further research into expanding the number of commensal bacteria strains to test NK activity would be the next step in understanding how probiotics affect the gut immunity. More importantly, it will be important to examine which strains interact with NK cells, greatly facilitate their stimulation, and enhance their activity as well as frequency of NK cells present in the intestinal wall. The roles of probiotics in immunity in the literature are summarized in Table 1.
TABLE 1.
Summary of probiotics and their effects on NK cells and immunity
| Probiotic strain | Activity in the immune system | Cytokines produced | Other comments |
|---|---|---|---|
| LcS | Monocytes potentiate increased NK cell activity via LcS induction6 | IFN-γ, IL-1β, IL-6, IL-8 IL-10, IL-12, TNF-α, GM-CSF, and MIP-1α6,87 | Induced expression of CD69 and CD25 on CD8+ and CD56+ subsets of NK cells6 |
| Lactobacillus plantarum NCIMB8826 | Increased expression of CD69 and CD25 in NK cells, enhancing activation of NK cells87 | IL12p70, IFN-γ, TNF-α, MIP-1α, IL-1β, GM-CSF, and IL-887 | Up-regulation of MHC-II (HLA-DR) and CD80; induced phenotypic maturation of DCs84 |
| Lactobacillus rhamnosus GG (LGG) | Did not increase expression of CD69 or CD25 in lymphocytes87 | IL-1β, IL-8, and GM-CSF87 | Up-regulation of MHC-II (HLA-DR) and CD80; induced phenotypic maturation of DCs84 |
| Lactobacillus paracasei B21060 | Anti-inflammatory effect on DCs84 | TGF-β, TNF-α, and IL-12p7084 | Up-regulation of MHC-II (HLA-DR) and CD80; induced phenotypic maturation of DCs84 |
| Lactobacillus reuteri | NCIMB11951 Increased expression of CD69 and CD25 in NK cells, enhancing activation of NK cells87 | Increased expression of CD69 and CD25 in NK cells, enhancing activation of NK cells87 | |
| Bifidobacterium longum SP 07/3 | Increased expression of CD69 and CD25 in NK cells, enhancing activation of NK cells87 | Increased expression of CD69 and CD25 in NK cells, enhancing activation of NK cells87 | Good inducer of IFN-γ; Bifidobacterium strains were good inducers of IL-6, IL-10, and MCP-187 |
| Bifidobacterium bifidium MF 20/5 | Increased expression of CD69 and CD25 in NK cells, enhancing activation of NK cells87 | IL-1β, TNF-α, GM-CSF, IL-6, IL-8, IL-10, MCP-1, and MIP-1α87 | Good inducer of GM-CSF; Bifidobacterium strains were good inducers of IL-6, IL-10, and MCP-187 |
| Bacillus polyfermenticus (Bispan strain) | Increased levels of CD56+subset of NK cells68 | No cytokine expression levels reported | Enhanced levels of IgG production and modulation of CD4+ and CD8+ T cells68 |
CD, Cluster of differentiation (cell); DC, dendritic cell; GM-CSF, granulocyte macrophage–colony-stimulating factor; HLA-DR, human leukocyte antigen D related; IFN-γ, interferon-γ; IgA, immunoglobulin a; IL, interleukin; LcS, Lactobacillus casei Shirota; MHC-II, major histocompatibility complex class II; MIP-1α macrophage inflammatory protein–1α; NK, natural killer; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor–α
Another approach to stimulating NK cell activity would to examine the interaction among the ligands with probiotic bacteria and NK cells themselves. Understanding their complex interaction would be useful to help genetically modify the surface of probiotics to contain ligands that are known to actively stimulate and enhance gut NK cells. Genetic modification of probiotics would allow for greater interactions and more beneficial health benefits in improving innate immune and adaptive systems.
The observations derived from the referred studies should provide a strong rationale to emphasize targeting the ligand–receptor complexes between NK cells and probiotics, which can be used as novel immunotherapy treatment to help relieve the myriad of intestinal inflammatory diseases. These interactions could also be exploited to help those with conditions that require other forms of immunotherapy. A greater understanding into the processes that occur in the gut immune system would help to substantiate the health claims of probiotics.
Acknowledgments
We sincerely thank all of the graduate students and postdoctoral fellows associated with our project, who were responsible for some of the studies cited in this review. Some of these studies cited herein were supported, in part, by the National Institutes of Health grant numbers 5P50AT00151 and 5T@CA09120. We also acknowledge the support of the Jonsson Comprehensive Cancer Center at the University of California at Los Angeles. The assistance of Ailina Lao is greatly appreciated for the preparation of the figures.
ABBREVIATIONS
- BM
bone marrow
- DC
dendritic cell
- FcR-γ
Fc receptor gamma
- GALT
gut-associated lymphoid issue
- GM-CSF
granulocyte macrophage–colony-stimulating factor
- HLA
human leukocyte antigen
- IL
interleukin
- INF-γ
interferon-γ
- iNK
immature natural killer
- ITAM
immunoreceptor tyrosine-based activating motif
- LAB
lactic acid bacteria
- LcS
Lactobacillus casei Shirota
- LN
lymph node
- LPSs
lipopolysaccharides
- MIP-1α
macrophage inflammatory protein–1α
- MLNs
mesenteric lymph nodes
- NK
natural killer
- PRR
pattern recognition receptor
- TLR
Toll-like receptor
- TNF-α
tumor necrosis factor–α
References
- 1.Sommer F, Bäckhed F. The gut microbiota—masters of host development and physiology. Nat Rev Microbiol. 2013;11:227–38. doi: 10.1038/nrmicro2974. [DOI] [PubMed] [Google Scholar]
- 2.Gill H, Pop M, Deboy R, Eckburg P, Turnbaugh P, Samuel B, Gordon J, Relman D, Fraser-Liggett C, Nelson K. Metagenomic analysis of the human distal gut microbiome. Science. 2006;312:1355–9. doi: 10.1126/science.1124234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Purchiaroni F, Tortora A, Gabrielli M, Bertucci F, Gigante G, Ianiro G, Ojetti V, Scarpellini E, Gasbarrini A. The role of the intestinal microbiota and the immune system. Eur Rev Med Pharmacol Sci. 2013;17:323–3. [PubMed] [Google Scholar]
- 4.Giorgetti G, Brandimente G, Fabiocchi F, Ricci S, Fiamini P, Sandri G, Trolta M, Elisei W, Penna A, Lecca P, Picchio M, Tursi A. Interactions between innate immunity, microbiota, and probiotics. J Immunol Res. 2015;2015:501361. doi: 10.1155/2015/501361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.FAO/WHO. Expert consultation on evaluation of health nutritional properties of probiotics. FAO Food Nutr Paper. 2001:1–21. [Google Scholar]
- 6.Dong H, Rowland I, Tuhoy K, Thomas L, Yaqoob P. Selective effects of Lactobacillus casei Shirota on T cell activation, natural killer cell activity and cytokine production. Clin Exper Immunol. 2010;161:378–88. doi: 10.1111/j.1365-2249.2010.04173.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizello V, Bonaccorsi I, Dongarra ML, Fink LN, Ferlazzo G. Role of natural killer and dendritic cell crosstalk in immunomodulation by commensal bacteria probiotics. J Biomed Biotech. 2011;2011:473097. doi: 10.1155/2011/473097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Harmsen H, Wildeboer-Veloo A, Raangs G, Wagendorp A, Klijn N, Bindels J, Welling G. Analysis of intestinal flora development in breast-fed and formula-fed infants by molecular identification and detection methods. J Pediatr Gastroenterol Nutr. 2000;30:61–7. doi: 10.1097/00005176-200001000-00019. [DOI] [PubMed] [Google Scholar]
- 9.Caramia G, Silvi S. Probiotics: From the ancient wisdom to the actual therapeutical and nutraceutical perspective. In: Malago J, Koninkx J, Marinsek-Logar R, editors. Probiotic bacteria and enteric infections. New York: Springer; 2011. pp. 3–38. [Google Scholar]
- 10.Metchnikoff E. The prolongation of life; optimistic studies. London: GP Putnam’s Sons; 1907. Lactic acid as inhibiting intestinal putrefaction; pp. 161–83. [Google Scholar]
- 11.Cucchiara S, Iebba V, Conte M, Schippa S. The microbiota in inflammatory bowel disease in different age groups. Digest Diseases. 2009;27:252–8. doi: 10.1159/000228558. [DOI] [PubMed] [Google Scholar]
- 12.Nova E, Warnberg J, Gomez-Martinez S, Diaz L, Romeo J, Marcos A. Immunomodulatory effects of probiotics in different stages of life. Br J Nutr. 2007;98:S90–5. doi: 10.1017/S0007114507832983. [DOI] [PubMed] [Google Scholar]
- 13.Corthesy B, Gaskins H, Mercenier A. Cross-talk between probiotic bacteria and the host immune system. J Nutr. 2007;137:781S–90S. doi: 10.1093/jn/137.3.781S. [DOI] [PubMed] [Google Scholar]
- 14.Hooper L, Macpherson A. Immune adaptations that maintain homeostasis with the intestinal microbiota. Nat Rev Immunol. 2010;10:159–69. doi: 10.1038/nri2710. [DOI] [PubMed] [Google Scholar]
- 15.Bancheraeu J, Steinman R. Dendritic cells and the control of immunity. Nature. 1998;392:245–52. doi: 10.1038/32588. [DOI] [PubMed] [Google Scholar]
- 16.Stagg A, Hart A, Knight S, Kamm M. Microbial-gut interaction in health and disease. Interactions between dendritic cells and bacteria in the regulation of intestinal immunity. Best Prac Res Clin Gastroenterol. 2004;18:255–70. doi: 10.1016/j.bpg.2003.10.004. [DOI] [PubMed] [Google Scholar]
- 17.Calder P. Feeding the immune system. Proc Nutr Soc. 2013;72:299–309. doi: 10.1017/S0029665113001286. [DOI] [PubMed] [Google Scholar]
- 18.Shereck E, Satwani P, Morris E, Cairo M. Human natural killer cells in health and disease. Pediatr Blood Cancer. 2007;49:615–23. doi: 10.1002/pbc.21158. [DOI] [PubMed] [Google Scholar]
- 19.Ho Y, Lu Y, Chang H, Lee S, Tsai M, Huang Y, Hsu T. Daily intake of probiotics with high IFN-γ/IL-10 ratio increases the cytotoxicity of human natural killer cells: A personalized probiotic approach. J Immunol Res. 2014;2014:721505. doi: 10.1155/2014/721505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bernt K, Walker W. Human milk as a carrier of biochemical messages. Acta Paed Suppl. 1999;88:27–41. doi: 10.1111/j.1651-2227.1999.tb01298.x. [DOI] [PubMed] [Google Scholar]
- 21.Burns E, Goodwin J. Immunodeficiency of aging. Drugs Aging. 1997;11(5):374–97. doi: 10.2165/00002512-199711050-00005. [DOI] [PubMed] [Google Scholar]
- 22.Mowat A. Anatomical basis of tolerance and immunity to intestinal antigens. Nat Rev Immunol. 2003;3:331–41. doi: 10.1038/nri1057. [DOI] [PubMed] [Google Scholar]
- 23.Ulevitch R. Endotoxin opens the Tollgates to innate immunity. Nat Med. 1999;5:144–5. doi: 10.1038/5504. [DOI] [PubMed] [Google Scholar]
- 24.Akira S, Hemmi H. Recognition of pathogen-associated molecular patterns by TLR family. Immunol Lett. 2003;85:85–95. doi: 10.1016/s0165-2478(02)00228-6. [DOI] [PubMed] [Google Scholar]
- 25.Garside P, Millington O, Smith K. The anatomy of mucosal immune response. Ann NY Acad Sci. 2004;1029:9–15. doi: 10.1196/annals.1309.002. [DOI] [PubMed] [Google Scholar]
- 26.Neutra M, Mantis N, Kraehenbuhl J. Collaboration of epithelial cells with organized mucosal lymphoid tissues. Nat Immunol. 2001;2:1004–9. doi: 10.1038/ni1101-1004. [DOI] [PubMed] [Google Scholar]
- 27.Timonen T, Ortaldo JP, Herberman R. Characteristics of human large granular lymphocytes and relationship to natural killer and K cells. J Exp Med. 1981;153:569–82. doi: 10.1084/jem.153.3.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Deniz G, van der Veen W, Akdis M. Natural killer cells in patients with allergic diseases. J Allergy Clin Immunol. 2013:527–35. doi: 10.1016/j.jaci.2013.07.030. [DOI] [PubMed] [Google Scholar]
- 29.Vivier E, Tomasello E, Baratin M, Walzer T, Ugolini S. Functions of natural killer cells. Nat Immunol. 2008;9:503–10. doi: 10.1038/ni1582. [DOI] [PubMed] [Google Scholar]
- 30.Farag S, Fehniger T, Ruggeri L, Velardi A, Caligiuri M. Natural killer cell receptors: new biology and insights into graft-versus-leukemia effect. Blood J. 2002;100:1935–47. doi: 10.1182/blood-2002-02-0350. [DOI] [PubMed] [Google Scholar]
- 31.Vivier E, Nunès J, Vèly F. Natural killer cell signaling pathways. Science. 2004;306:1517–9. doi: 10.1126/science.1103478. [DOI] [PubMed] [Google Scholar]
- 32.Raulet D, Held W, Correa I, Dorfman J, Wu M, Corral L. Specificity, tolerance and developmental regulation of natural killer cells defined by expression of class I-specific Ly49 receptors. Immunol Rev. 1997;155:41–52. doi: 10.1111/j.1600-065x.1997.tb00938.x. [DOI] [PubMed] [Google Scholar]
- 33.Brodin P, Karre K, Hoglund P. NK cell education: not an on-off switch but a tunable rheostat. Trends Immunol. 2009;30:143–9. doi: 10.1016/j.it.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 34.Cooper M, Fehniger T, Caligiuri M. The biology of human natural killer-cell subsets. Trends Immunol. 2001;22:633–40. doi: 10.1016/s1471-4906(01)02060-9. [DOI] [PubMed] [Google Scholar]
- 35.Lanier L, Le A, Civin C, Loken M, Phillips J. The relationship of CD16 (Leu-11) and Leu-19 (NKH-1) antigen expression on human peripheral blood NK cells and cytotoxic T lymphocytes. J Immunol. 1986;136:4480–6. [PubMed] [Google Scholar]
- 36.Leibson P. Signal transduction during natural killer cell activation: inside the mind of a killer. Immunity. 1993;81:1819–26. doi: 10.1016/s1074-7613(00)80441-0. [DOI] [PubMed] [Google Scholar]
- 37.Pao L, Sumaria N, Kelly J, van Dommelen S, Cretney E, Wallace M, Anthony D, Uldrich A, Godfrey D, Papadimitriou J, Mullbacher A, Degli-Esposti MA, Smyth MJ. Functional analysis of granzyme M and its role in immunity to infection. J Immunol. 2005;175:3235–43. doi: 10.4049/jimmunol.175.5.3235. [DOI] [PubMed] [Google Scholar]
- 38.Berthou C, Marolleau J, Lafaurie C, Soulie A, Dai Cortivo L, Bourge J, Benbunan M, Sasportes M. Granzyme B and perforin lytic proteins are expressed in CD34+ peripheral blood progenitor cells mobilized by chemotherapy and granulocyte colony-stimulating factor. Blood. 1995;86:3500–6. [PubMed] [Google Scholar]
- 39.Robertson M, Ritz J. Biology and clinical relevance of human natural killer cells. Blood. 1990;76:2421–38. [PubMed] [Google Scholar]
- 40.Carrillo-Buscamente P, Kesmir C, de Boer R. The evolution of natural killer cell receptors. Immunogenetics. 2015;68:3–16. doi: 10.1007/s00251-015-0869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yusa S, Catina T, Campbell K. SHP-1 and phosphotyrosine-independent inhibitory signaling by killer cell Ig-like receptor cytoplasmic domain in human NK cells. J Immunol. 2002;168:5047–57. doi: 10.4049/jimmunol.168.10.5047. [DOI] [PubMed] [Google Scholar]
- 42.Purdy A, Campbell K. SHP-2 expression negatively regulates NK cell function. J Immunol. 2009;183:7234–43. doi: 10.4049/jimmunol.0900088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maki G, Krystal G, Dougherty G, Takei F, Klingemann HG. Induction of sensitivity to NK-mediated cytotoxicity by TNF-α treatment: possible role of ICAM-3 and CD44. Leukemia. 1998;12:1565–72. doi: 10.1038/sj.leu.2401145. [DOI] [PubMed] [Google Scholar]
- 44.Agaugue S, Marcenaro E, Ferrantia B, Moretta L, Moretta A. Human natural killer cells exposed to IL-2, IL-12, IL-18, or IL-4 differentially modulate priming of naïve T cells by monocyte-derived dendritic cells. Blood. 2008;112:1776–83. doi: 10.1182/blood-2008-02-135871. [DOI] [PubMed] [Google Scholar]
- 45.Kanamori Y, Ishimaru K, Nanno M, Maki K, Ikuta K, Nariuchi H, Ishikawa H. Identification of novel lymphoid tissues in murine intestinal mucosa where cluster of c-kit+ IL-7R+ Thy-1+ lympho-hemopoietic progenitors develop. J Exp Med. 1996;184:1449–59. doi: 10.1084/jem.184.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Hamada H, Hiroi T, Nishiyama Y, Takahashi H, Masunaga Y, Hachimura S, Kaminogawa S, Takahashi-Iwanaga H, Iwanaga T, Kiyono H, Yamamoto H, Ishikawa Identification of multiple isolated lymphoid follicles on the anti-senteric wall of mouse small intestine. J Immunol. 2002;168:57–64. doi: 10.4049/jimmunol.168.1.57. [DOI] [PubMed] [Google Scholar]
- 47.Ivanova D, Krempels R, Ryfe J, Weitzmann K, Stephenson D, Gigley J. NK cells in mucosal defense against infection. Biomed Res Int. 2014;2014:413982. doi: 10.1155/2014/413982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Spits H, Artis D, Colonna M, Diefenbach A, Di Santo J, Ebert G, Koyasu S, Locksley R, McKenzie A, Mebius R, Powrie F, Vivier E. Innate lymphoid cells: a proposal for uniform nomenclature. Nat Rev Immunol. 2013;13:145–9. doi: 10.1038/nri3365. [DOI] [PubMed] [Google Scholar]
- 49.Sanos S, Diefenbach A. Isolation of NK cells and NK-like cells from the intestinal lamina propria. Methods Mol Biol. 2010;612:505–17. doi: 10.1007/978-1-60761-362-6_32. [DOI] [PubMed] [Google Scholar]
- 50.Leon F, Roldan E, Sanchez L, Camarero C, Bootello A, Roy G. Human small-intestinal epithelium contains functional natural killer lymphocytes. Gastroenterol. 2003;125:345–56. doi: 10.1016/s0016-5085(03)00886-2. [DOI] [PubMed] [Google Scholar]
- 51.Lindgren A, Yun C, Lundgren A, Sjoling A, Ohman L, Svennerholm A, Holmgren J, Lundrin S. CD8− natural killer cells are greatly enriched in the human gastrointestinal tract and have the capacity to respond to bacteria. J Innate Immun. 2010;2:294–302. doi: 10.1159/000286238. [DOI] [PubMed] [Google Scholar]
- 52.Satoh Takayama N, Vosshenrich C, Lesjean-Pottier S, Sawa S, Lochner M, Rattis F, Mention J, Thiam K, Cerf-Bensussan N, Mandelboim O, Eberl G, Di Santo JP. Microbial flora drives interleukin 22 production in intestinal NKp46+ cells that provide innate mucosal immune defense. Immunity. 2008;29:958–70. doi: 10.1016/j.immuni.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Colonna M. Interleukin-22-producing natural killer cells and lymphoid tissue inducer-like cells in mucosal immunity. Immunity. 2009;31:15–23. doi: 10.1016/j.immuni.2009.06.008. [DOI] [PubMed] [Google Scholar]
- 54.Satoh Takayama N, Lesjean-Pottier S, Vieira P, Sawa S, Eberl G, Vosshenrich C, Di Santo J. IL-7 and IL-15 independently program the differentiation of intestinal CD3-NKp46+ cell subsets from Id2-dependent precursors. J Exp Med. 2010;207:273–80. doi: 10.1084/jem.20092029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lopez M, Stanley M. Cytokine profile of mouse vaginal and uterus lymphocytes at estrus and diestrus. Clin Devel Immunol. 2005;12:159–64. doi: 10.1080/17402520500141010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gregoire C, Chasson L, Luci C, Tomasello E, Geissmann F, Vivier E, Walzer T. The trafficking of natural killer cells. Immunol Rev. 2007;220:169–82. doi: 10.1111/j.1600-065X.2007.00563.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pickert G, Neufert C, Leppkes M, Zheng Y, Wittkopf N, Warntjen M, Lehr H, Hirth S, Weigmann B, Wirtz S, Ouyang W, Neurath MF, Becker C. STAT3 links IL-22 signaling in intestinal epithelial cells to mucosal wound healing. J Exp Med. 2009;206:1465–72. doi: 10.1084/jem.20082683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Moretto M, Weiss L, Combe C, Kahn I. IFN-γ-producing dendritic cells are important for priming gut intraepithelial lymphocyte response against intracellular parasitic infection. J Immunol. 2007;179:2485–92. doi: 10.4049/jimmunol.179.4.2485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Guy-Grand D, Vasalli P, Ebert G, Pereira P, Burlen-Defranoux O, Lemaltre F, Di Santo J, Freltas A, Cumano A, Bandeira A. Origin, trafficking, and intraepithelial fate of gut-tropic T cells. J Exper Med. 2013;210:1839–54. doi: 10.1084/jem.20122588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Vivier E, Spits H, Cupedo T. Interleukin-22-producing innate immune cells: new players in mucosal immunity and tissue repair? Nat Rev Immunol. 2009;9:229–34. doi: 10.1038/nri2522. [DOI] [PubMed] [Google Scholar]
- 61.Hessle C, Andersson B, Wold A. Gram-positive bacteria are potent inducers of monocytic interleukin-12 (IL-12) while Gram-negative bacteria preferentially stimulate IL-10 production. Infection Immun. 2000;68:3581–6. doi: 10.1128/iai.68.6.3581-3586.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adoptive immunity. Nat Rev Immunol. 2003;3:133–46. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 63.Fink LN, Zeuthen LH, Ferlazzo G, Frokiaer H. Human antigen-presenting cells respond differently to gut-derived probiotic bacteria but mediate similar strain-dependent NK and T cell activation. FEMS Immunol Med Microbiol. 2007;51:535–46. doi: 10.1111/j.1574-695X.2007.00333.x. [DOI] [PubMed] [Google Scholar]
- 64.Kamada N, Seo S, Chen G, Nunez G. Role of the gut microbiota in immunity and inflammatory disease. Nat Rev Immunol. 2013;13:321–35. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 65.Abreu M. Toll-like receptor signaling in the intestinal epithelium: how bacterial recognition shapes intestinal function. Nat Rev Immunol. 2010;10:131–44. doi: 10.1038/nri2707. [DOI] [PubMed] [Google Scholar]
- 66.Vieira A, Teixeira M, Martins F. The role of probiotics and prebiotics in inducing gut immunity. Front Immunol. 2013;4:445. doi: 10.3389/fimmu.2013.00445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jun K, Lee K, Kim W, Paik H. Microbiological identification of medical probiotic Bispan strain. Korean J Appl Microbiol Biotechnol. 2000;28:124–7. [Google Scholar]
- 68.Kim H, Park H, Cho I, Paik H, Park E. Dietary supplementation of probiotic Bacillus polyfermenticus, Bispan strain, modulates natural killer and T cell subset populations and immunoglobulin G levels in human subjects. J Med Food. 2006;9:321–7. doi: 10.1089/jmf.2006.9.321. [DOI] [PubMed] [Google Scholar]
- 69.Naidu AS, Bidlack WR, Clemens RA. Probiotics spectra of lactic acid bacteria (LAB) Crit Rev Food Sci Nutr. 1999;39:13–126. doi: 10.1080/10408699991279187. [DOI] [PubMed] [Google Scholar]
- 70.Olivares M, Diaz-Ropero M, Gomez N, Lara-Villoslada F, Sierra S, Maldonado J, Martin R, Rodriguez J, Xaus J. The consumption of two new probiotic strains, Lactobacillus gasseri CECT 5714 and Lactobacillus coryniformis CECT 5711, boosts the immune system of healthy humans. Int Microbiol. 2006;9:47–52. [PubMed] [Google Scholar]
- 71.Gill H, Rutherford K, Cross M, Gopal P. Enhancement of immunity in the elderly by dietary supplementation with the probiotic Bifidobacterium lactis HN019. Am J Clin Nutr. 2001;74:833–9. doi: 10.1093/ajcn/74.6.833. [DOI] [PubMed] [Google Scholar]
- 72.Chiang B, Sheih Y, Wang L, Liao C, Gill H. Enhancing immunity by dietary consumption of a probiotic lactic acid bacteria (Bifidobacterium lactis HN019): optimization and definition of cellular immune responses. Eur J Clin Nutr. 2000;54:849–55. doi: 10.1038/sj.ejcn.1601093. [DOI] [PubMed] [Google Scholar]
- 73.Sheih Y, Chiang B, Wang L, Liao C, Gill H. Systemic immunity-enhancing effects in healthy subjects following dietary consumption of the lactic acid bacterium Lactobacillus rhamnosus HN001. J Am Coll Nutr. 2001;20:149–56. doi: 10.1080/07315724.2001.10719027. [DOI] [PubMed] [Google Scholar]
- 74.Lee L, Mo J, Katakura K, Alkalay I, Rucker A, Liu Y, Lee H, Shen C, Cojocaru G, Shenouda S, Kagnoff M, Eckmann L, Ben-Neriah Y, Raz E. Maintenance of colonic homeostasis by distinctive apical TLR9 signaling in intestinal epithelial cells. Nature. 2006;8:1327–36. doi: 10.1038/ncb1500. [DOI] [PubMed] [Google Scholar]
- 75.Fernandez N, Lozier A, Flament C, Ricciardi-Castagnoli P, Bellet D, Suter M, Perricaudet M, Tursz T, Maraskovsky E, Zitvogel L. Dendritic cells directly trigger NK cell functions: cross-talk relevant in innate anti-tumor immune responses in vivo. Nature Med. 1999;5:405–11. doi: 10.1038/7403. [DOI] [PubMed] [Google Scholar]
- 76.Ferlazzo G, Tsang M, Moretta L, Melioli G, Steinman R, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–51. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Andoniou C, van Dommelen S, Voigt V, Andrews D, Brizard G, Asselin-Paturel C, Delale T, Stacey K, Trinchieri G, Degli-Esposti M. Interaction between conventional dendritic cells and natural killer cells is integral to the activation of effective antiviral immunity. Nat Immunol. 2005;6:1011–9. doi: 10.1038/ni1244. [DOI] [PubMed] [Google Scholar]
- 78.Fink L, Frokiaer H. Dendritic cells from Peyer’s patches and mesenteric lymph nodes differ from spleen dendritic cells in their response to commensal gut bacteria. Scan J Immunol. 2008;68:270–9. doi: 10.1111/j.1365-3083.2008.02136.x. [DOI] [PubMed] [Google Scholar]
- 79.Konstantinov SR, Smidt H, de Vos WM, Brujins SC, Singh SK, Valence F, Molle D, Lortal S, Altermann E, Klaenhammer T, van Kooyk Y. S layer protein A of Lactobacillus acidophilus NCFM regulates immature dendritic cells and T cell functions. Proc Natl Acad Sci USA. 2008;105:19474–9. doi: 10.1073/pnas.0810305105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Matsumoto K, Takada T, Shimizu K, Kado Y, Kawakami K, Makino I. The effects of a probiotic milk product containing Lactobacilli casei strain Shirota on the defecation frequency and the intestinal microflora of sub-optimal health state volunteers: a randomized placebo-controlled cross-over study. Biosci Microflora. 2006;25:39–48. [Google Scholar]
- 81.Shida K, Suzuki T, Kiyoshima-Shibata J, Shimada S, Nanno M. Essential roles of monocytes in stimulating human peripheral blood mononuclear cells with Lactobacillus casei to produce cytokines and augment natural killer cell activity. Clin Vaccine Immunol. 2006;13:997–1003. doi: 10.1128/CVI.00076-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Seifert S, Bub A, Franz C, Watzl B. Probiotic Lactobacillus casei Shirota supplementation does not modulate immunity in healthy men with reduced natural killer cell activity. J Immunol. 2011;141:978–84. doi: 10.3945/jn.110.136440. [DOI] [PubMed] [Google Scholar]
- 83.Reale M, Boscolo P, Bellante V, Tarantelli C, Di Nicola M, Forcella L, Li Q, Morimoto K, Muraro R. Daily of Lactobacillus casei Shirota increases natural killer cell activity in smokers. Br J Nutr. 2012;108:308–14. doi: 10.1017/S0007114511005630. [DOI] [PubMed] [Google Scholar]
- 84.Mileti E, Matteoli G, Iliev I, Rescigno M. Comparison of the immunomodulatory properties of three probiotic strains of lactobacilli using complex culture systems: predication for in vivo efficacy. PLoS One. 2009;4:e7056. doi: 10.1371/journal.pone.0007056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Issacs K, Herfarth H. Role of probiotic therapy in IBD. Inflamm Bowel Dis. 2008;14:1597–605. doi: 10.1002/ibd.20465. [DOI] [PubMed] [Google Scholar]
- 86.Medina M, Izquierdo E, Ennahar S, Sanz Y. Differential immunomodulatory properties of Bifidobacterium logum strains: relevance to probiotic selection and clinical applications. Clin Exp Immunol. 2007;150:531–8. doi: 10.1111/j.1365-2249.2007.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Dong H, Rowland I, Yaqoob P. Comparative effects of six probiotic strains on immune function in vitro. Br J Nutr. 2012;108:459–70. doi: 10.1017/S0007114511005824. [DOI] [PubMed] [Google Scholar]