Abstract
Groundwater samples (111) from six different boreholes located in two geographical areas were examined for the presence of legionellae over a 7-year period. The number of Legionella isolates detected was generally low. The colonization of the aquifers was not uniform, and the persistence of Legionella was independent of the hydraulic pumps and the plumbing system present in the borehole. A total of 374 isolates identified by fatty acid methyl ester analysis belonged to Legionella pneumophila, L. oakridgensis, L. sainthelensi, and L. londiniensis. In area 1, L. oakridgensis constituted the major population detected, exhibiting only one random amplified polymorphic DNA (RAPD)-PCR profile. L. sainthelensi strains were less frequently isolated and also displayed a single RAPD profile, while L. pneumophila was only sporadically detected. In contrast, L. pneumophila comprised the vast majority of the isolates in area 2 and exhibited six distinct RAPD patterns, indicating the presence of different genetic groups; three L. londiniensis RAPD types were also detected. Two of the L. pneumophila and one of the L. londiniensis RAPD types were persistent in this environment for at least 12 years. The genetic structure of L. pneumophila groundwater populations, inferred from rpoB and dotA gene sequences, was peculiar, since the majority of the isolates were allied in a discrete group different from the lineages containing most of the type and reference strains of the three subspecies of L. pneumophila. Furthermore, gene exchange events related to the dotA allele could be envisioned.
Legionellae are facultative intracellular gram-negative bacteria that may cause Legionnaires’ disease (legionellosis), an occasionally fatal pneumonia, as well as much more common mild flulike lung infections. Both forms of disease are caused by inhalation of aerosolized water contaminated with virulent strains.
Legionella spp. are ubiquitous in aqueous environments, where they parasitize and multiply in protozoa. Several amoebae and some ciliated protozoa are known to be potential environmental hosts for legionellae (1). This host-parasite interaction has been extensively studied and is considered to be the fundamental link to the ecology and the pathogenesis of these bacteria. It is now clear that the association of legionellae with protozoa is a major factor regulating the presence of the bacteria in the environment. Indeed, protozoa provide a necessary environment for the growth of legionellae, and they enhance the resistance of these organisms to adverse environmental conditions (2).
Forty-nine species of Legionella with >70 serogroups have been classified to date (26), including five species designated Legionella-like amoebic pathogens that have been isolated only from protozoa (3, 26). Despite the large species diversity, ∼90% of disease cases are caused by Legionella pneumophila, especially strains belonging to serogroup 1, while the remainder are generally caused by strains of other serogroups of L. pneumophila, L. micdadei, and L. longbeachae (52). However, L. longbeachae constitutes 31% of the isolates involved in community-acquired disease in Australia and New Zealand (52).
The infection caused by the inhalation of legionellae depends on the ability of these organisms to enter and to multiply within alveolar macrophages, causing the destruction of these phagocytes and damage to the pulmonary tissues (21). Some virulence traits, such as high cytopathogenic activity, have been recognized in several L. pneumophila strains that are more virulent than others, but a set of distinctive characteristics correlated with increased pathogenicity could not be clearly defined (4). The Dot/Icm complex, however, is critical for the virulence capability of Legionella. The dot/icm genes, initially identified in L. pneumophila, are also present in all other Legionella spp. studied (4). This complex is thought to constitute a type IV-like secretion system that is capable of injecting effector molecules into the host cell, which allows Legionella to evade the endocytic pathways, probably by delaying the phagosome-lysosome fusion in macrophages, among other roles in the pathogenicity to macrophages (11).
It is not completely clear why specific legionellae are the major causes of disease; some of these organisms could have greater inherent virulence, or they could simply be more abundant in environments that promote their dispersal to humans. Understandably, the vast majority of the studies of the distribution of legionellae have been performed in man-made aquatic environments, since they are generally implicated in the dispersal of legionellae to humans. Legionella species, primarily L. pneumophila, have been frequently sought in air-conditioning cooling towers, potable-water distribution systems, and associated fixtures, where they are found in high numbers and can be dispersed to humans (5, 12, 33, 45).
In these environments, water temperature and other physical and chemical parameters influence colonization (18, 50). Moreover, the association among legionellae, amoebae, and other protozoa generally found in biofilms is known to be very important in promoting their growth (6, 13, 47, 48). The colonization of surface water and other natural environments, such as hydrothermal areas and thermally altered lakes and streams, by Legionella spp. has been less investigated (8, 17, 32, 44). The presence and persistence of Legionella spp. in groundwater is poorly documented, and the role of this environment as a potential natural reservoir of legionellae has not yet been investigated (16). However, the increased use of this kind of water by large human populations concerns public health authorities, since a large number of pathogens are known to contaminate groundwater (31).
We determined the presence of Legionella spp. and identified them for several years in water samples collected from different boreholes in two distinct areas venting hydrothermal water. We also evaluated the persistence of Legionella strains in these groundwater environments by typing the isolates using random amplified polymorphic DNA (RAPD)-PCR. Finally, sequence analysis of a housekeeping gene (rpoB) and a virulence-related gene (dotA) was used to define the genetic organization of the L. pneumophila populations isolated and their relationship with type and reference strains of L. pneumophila.
MATERIALS AND METHODS
Sampling sites.
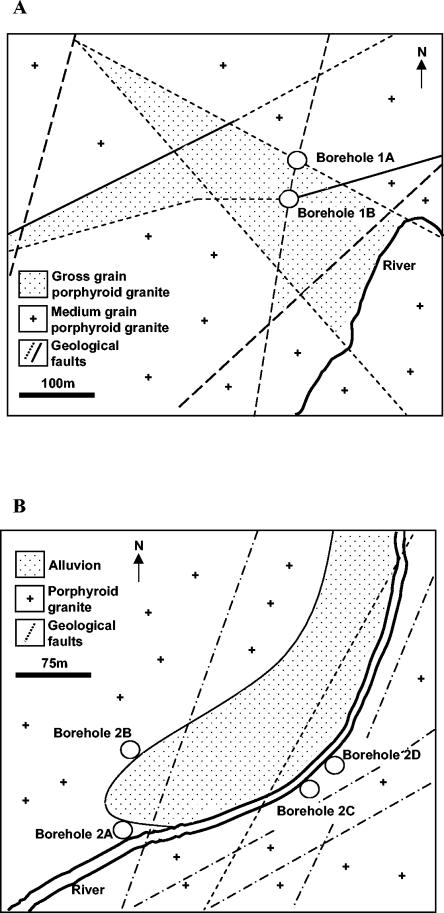
Groundwater samples were collected from two hydrothermal areas in central Portugal designated 1 and 2. The areas are geologically similar and largely dominated by granite formations (Fig. 1). However, different degrees of fragmentation and weathering can be clearly observed. Groundwater in area 1 reaches the surface through two boreholes, designated 1A and 1B, located 62 m from each other and along the same geological fracture (Fig. 1A). Borehole 1A has a diameter of 10 in. and was drilled vertically to a depth of 63.5 m, while 1B has a diameter of 8 in. and was drilled with a tilt of 85° from horizontal to a depth of 307 m. Borehole 1A is completely encased in a stainless steel jacket, while well 1B is encased in a stainless steel jacket for the upper 38 m. Both wells are artesian, but they are equipped with a plumbing system and hydraulic pumps to supply a spa. The water vents at 35.8 and 34.5°C from 1A and 1B, respectively, with a pH of 8.4.
FIG. 1.
Geological formations in the vicinity of the sampling sites in central Portugal. (A) Sampling sites in area 1. (B) Sampling sites in area 2.
Area 2 had a natural spring that became obsolete when two boreholes, designated 2A and 2B, were drilled to supply a new spa. Both boreholes were drilled vertically at a distance of 14 m from each other. These wells were used between 1992 and 2000, when they were decommissioned and filled with cement. Two new boreholes (2C and 2D) were then drilled and used as sources of groundwater for the spa. Wells 2C and 2D (with 10-in. diameters) are located 74 m from each other and were drilled vertically to a depth of 91 and 85 m, respectively (Fig. 1B). All of the wells have stainless steel-jackets and are artesian but, to supply the spa, are equipped with hydraulic pumps and plumbing systems.
The water temperature in wells 2A and 2B was 48°C, while in boreholes 2C and 2D it was 45.5°C, with a pH of 7.9.
Sampling and isolation of Legionella spp.
A total of 66 groundwater samples from area 1 and 45 samples from area 2 were collected at irregular intervals over a 7-year period. To evaluate the influence of the plumbing system and the hydraulic pumps present in one borehole on colonization of the groundwater by Legionella, we removed the inner stainless steel pipe that brings water to the surface, the hydraulic pump, and the electrical cable of well 1A. On that occasion, the components were swabbed to collect biofilm samples. Afterwards, the water in the borehole, without the entire plumbing system, was chlorinated to 50 ppm, which was maintained for 48 h by continuously pumping chlorine through a sterile silicone tube that reached the bottom of the well. The pH of the water in the borehole was adjusted to 7.0 by pumping in an HCl solution to increase the disinfecting effect of the chlorine. Water samples were collected before, during, and after this procedure. We also collected water samples at different depths with a disinfected silicone tube connected to a peristaltic pump.
All water samples were recovered in sterile 2-liter containers, transported at ambient temperature, concentrated, and plated within 24 h. The water was filtered through 45-mm-diameter membrane filters (Gelman Supor 200; 0.2-mm pore size). The filters were placed in small sterile plastic bags containing 10 ml of the original water. The bags containing the filters were rubbed manually for a few minutes to remove organisms from the filters. One portion of the concentrated sample (0.1 ml) was spread directly on the surface of buffered charcoal yeast extract (BCYE) medium containing glycine, vancomycin, polymyxin B, and cycloheximide, designated GVPC; other portions of the concentrated sample were subjected to acid and heat treatments before being spread on GVPC (14). The plates were incubated in a normal atmosphere at 37°C for up to 9 days.
Presumptive Legionella sp. colonies were counted and subcultured on BCYE and BCYE without cysteine. All isolates that grew only on BCYE alone were considered putative Legionella strains, assigned separate designations, and maintained at −80°C in 5% (wt/vol) yeast extract with 15% (vol/vol) glycerol.
Identification of Legionella spp.
Identification of Legionella isolates was performed by analysis of the fatty acid methyl ester (FAME) profiles. Cells were harvested, and FAMEs were obtained by saponification, methylation, and extraction, as described previously by Kuykendall et al. (25); separated; identified; and quantified with the MIS library generation software (Microbial ID Inc., Newark, Del.). The identification was made by comparison of the FAME profiles with a previously constructed database that allows the identification of the vast majority of Legionella spp. (15).
Random amplified polymorphic DNA typing.
Several isolates were selected for RAPD-PCR analysis, taking into consideration the species, the site, and the date of sampling. The RAPD-PCR profiles were also compared with those obtained from isolates recovered 12 years before from a spring in area 2 by Marrão et al. (32). We also determined the RAPD profiles of type strains of each species found in the groundwater samples.
Crude cell lysates were used as DNA templates for RAPD typing as described elsewhere (51). Amplification reactions were performed with a total volume of 50 μl and standardized as follows: 1.5 U of Taq polymerase, 1.5 mM MgCl2 (Pharmacia Biotech), 0.2 mM (each) deoxynucleoside triphosphate, 0.6 μM primer OPA3 (5′-AGTCAGCCAC-3′), and 2.0 μl of crude cell lysates. Samples were subjected to 45 cycles of amplification (Perkin-Elmer model 240) as follows: 1 min at 94°C, 1 min at 34°C, and 2 min at 72°C, followed by a final extension step of 7 min at 72°C (40). The fragments were analyzed by electrophoresis in a 2% agarose gel in Tris-acetate-EDTA buffer.
Genetic structure of L. pneumophila groundwater populations.
L. pneumophila isolates from areas 1 and 2 with different RAPD profiles were selected for sequencing of variable regions of the rpoB and dotA genes to infer the genetic structures of these populations. The same regions of L. pneumophila subsp. pascullei strains U8W (ATCC 33737T), U7W (ATCC 33736), and MICU B (ATCC 33735) were also sequenced. The extraction of genomic DNA was carried out as described by Rainey et al. (36). The amplification of the genes and sequencing of the purified PCR products were carried out as described by Ko et al. (23). The purified reaction mixtures were electrophoresed on a model 310 Genetic Analyzer (Applied Biosystems, Foster City, Calif.).
The phylogenetic analysis was performed after manual checking of the quality of the sequences using the Bioedit editor (19) and alignment against sequences of 21 type and reference strains of L. pneumophila obtained from the public databases (Table 1), using the multiple-alignment CLUSTAL X software package (43). The method of Jukes and Cantor (22) was used to calculate evolutionary distances; phylogenetic dendrograms were constructed using the neighbor-joining method (39), and tree topologies were evaluated by performing bootstrap analysis of 1,000 data sets using the MEGA2 package (24). The amino acid sequences were deduced with the MEGA2 package from the 300- and 360-bp DNA sequences for the partial rpoB and dotA gene sequences, respectively.
TABLE 1.
Accession numbers of rpoB and dotA genes of L. pneumophila type and reference strains used
| Subspecies | Serogroup | Strain | Accession no.
|
|
|---|---|---|---|---|
| rpoB | dotA | |||
| L. pneumophila subsp. pneumophila | 1 | Philadelphia 1 (ATCC 33152T) | AF367748 | AY036018 |
| L. pneumophila subsp. pneumophila | 1 | Knoxville-1 (ATCC 33153) | AY036036 | AY036019 |
| L. pneumophila subsp. pneumophila | 1 | SF9 | AY036037 | AY036020 |
| L. pneumophila subsp. pneumophila | 1 | OLDA (ATCC 43109) | AY036038 | AY036021 |
| L. pneumophila subsp. pneumophila | 2 | Togus-1 (ATCC 33154) | AY036039 | AY036022 |
| L. pneumophila subsp. pneumophila | 3 | Bloomington-2 (ATCC 33155) | AY036040 | AY036023 |
| L. pneumophila subsp. fraseri | 4 | Los Angeles-1 (ATCC 33156T) | AY036041 | AY036024 |
| L. pneumophila subsp. fraseri | 5 | Dallas 1E (ATCC 33216) | AY036042 | AY036025 |
| L. pneumophila subsp. pneumophila | 6 | Chicago 2 (ATCC 33215) | AY036043 | AY036026 |
| L. pneumophila subsp. pneumophila | 7 | Chicago 8 (ATCC 33823) | AY036044 | AY036027 |
| L. pneumophila subsp. pneumophila | 8 | Concord 3 (ATCC 335096) | AY036045 | AY036028 |
| L. pneumophila subsp. pneumophila | 9 | IN-23-G1-C2 (ATCC 35289) | AY036046 | AY036029 |
| L. pneumophila subsp. pneumophila | 10 | Leiden 1 (ATCC 43283) | AY036047 | AY036030 |
| L. pneumophila subsp. pneumophila | 11 | 797-PA-H (ATCC 43130) | AY036048 | AY036031 |
| L. pneumophila subsp. pneumophila | 12 | 570-CO-H (ATCC 43290) | AY036049 | AY036032 |
| L. pneumophila subsp. pneumophila | 13 | 82A3105 (ATCC 43736) | AY036050 | AY036033 |
| L. pneumophila subsp. pneumophila | 14 | 1169-MN-H (ATCC 43703) | AY036051 | AY036034 |
| L. pneumophila subsp. fraseri | 15 | Lansing 3 (ATCC 35251) | AY036052 | AY036035 |
| L. pneumophila subsp. pascullei | 5 | U8W (ATCC 33737T) | AJ746049 | AJ746052 |
| L. pneumophila subsp. pascullei | 5 | U7W (ATCC 33736) | AJ746050 | AJ746053 |
| L. pneumophila subsp. pascullei | 5 | MICU B (ATCCC 33735) | AJ746051 | AJ746054 |
Nucleotide sequence accession numbers.
The partial rpoB and dotA gene sequences determined for strains of L. pneumophila subsp. pascullei were deposited in the EMBL data library under accession numbers ATCC 33737T (rpoB, AJ746049; dotA, AJ746052), ATCC 33736 (rpoB, AJ746050; dotA, AJ746053), and ATCC33735 (rpoB, AJ746051; dotA, AJ746054).
RESULTS
Legionella sp. isolation and identification.
Sixty-six groundwater samples were collected from area 1 (Table 2). Legionellae were isolated from all 33 groundwater samples from borehole 1A over a 7-year period. During the same period, legionellae were never recovered from borehole 1B (Table 2). The number of legionellae recovered from borehole 1A samples varied between 3 × 102 and 2.4 × 104 CFU liter−1. Isolates identified as L. oakridgensis were the most frequently recovered and were detected in all of the samples; strains identified as L. sainthelensi were isolated from 15 of the 33 groundwater samples collected from this borehole. Isolates of L. pneumophila were recovered only once (Table 2). While borehole 1A was being dismantled, legionellae were always detected, except when chlorine was added to the groundwater. These same three species were detected in 3 of 10 biofilm samples taken by swabbing the stainless steel plumbing system and the hydraulic pump; 7 samples were negative for legionellae (Table 3). The numbers of legionellae recovered from the biofilm and from groundwater during dismantling were slightly higher than in the groundwater (Table 3).
TABLE 2.
Enumeration, identification, and RAPD types of Legionella spp. isolated from groundwater samples collected from borehole 1A in area 1
| Sampling datea | CFU liter−1 | Legionella sp. | RAPD type |
|---|---|---|---|
| 06-1997 | 1.8 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 07-1997 | 2.5 × 103 | L. oakridgensis | LO1 |
| 08-1997 | 5.5 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 09-1997 | 3.6 × 103 | L. oakridgensis | LO1 |
| 10-1997 | 1.3 × 104 | L. oakridgensis | LO1 |
| 11-1997 | 5 × 102 | L. oakridgensis | LO1 |
| 12-1997 | 2.4 × 104 | L. oakridgensis | LO1 |
| 01-1998 | 2.4 × 104 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 02-1998 | 2.4 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 04-1998 | 2 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 07-1998 | 1.2 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| L. pneumophila | LP1 | ||
| 09-1998 | 1.1 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 10-1998 | 9.5 × 102 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 11-1998 | 4 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 12-1998 | 3 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 05-1999 | 7 × 102 | L. oakridgensis | LO1 |
| 12-1999 | 8 × 102 | L. oakridgensis | LO1 |
| 03-2000 | 6.5 × 102 | L. oakridgensis | LO1 |
| 06-2000 | 1.2 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 09-2000 | 1.5 × 103 | L. oakridgensis | LO1 |
| 10-2000 | 1 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 11-2000 | 3 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 03-2001 | 3 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 06-2001 | 1.6 × 103 | L. oakridgensis | LO1 |
| 07-2001 | 3.5 × 103 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| 09-2001 | 8 × 102 | L. oakridgensis | LO1 |
| 12-2001 | 3.5 × 102 | L. oakridgensis | LO1 |
| 03-2002 | 3 × 102 | L. oakridgensis | LO1 |
| 06-2002 | 1.3 × 103 | L. oakridgensis | LO1 |
| 08-2002 | 1.9 × 103 | L. oakridgensis | LO1 |
| 10-2002 | 9 × 102 | L. oakridgensis | LO1 |
| 04-2003 | 4.4 × 103 | L. oakridgensis | LO1 |
| 06-2003 | 2.2 × 103 | L. oakridgensis | LO1 |
Month-year.
TABLE 3.
Enumeration, identification, and RAPD types of Legionella spp. isolated during borehole 1A dismantling and disinfection
| Sample | CFU liter−1 | Legionella sp. | RAPD type |
|---|---|---|---|
| Groundwater at surface (before dismantling) | 3.8 × 105 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| Biofilm 1 | 2.4 × 105 | L. pneumophila | LP2 |
| L. sainthelensi | LS1 | ||
| Biofilm 2 | 2.4 × 105 | L. oakridgensis | LO1 |
| L. pneumophila | LP2 | ||
| L. oakridgensis | LO1 | ||
| Biofilm 3 | 2.4 × 104 | L. sainthelensi | LS1 |
| L. pneumophila | LP3 | ||
| Groundwater at surface (during chlorination) | —a | ||
| Groundwater at 55-m depth (after chlorination) | 3.8 × 104 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| Groundwater at 28-m depth (after chlorination) | 4.5 × 104 | L. oakridgensis | LO1 |
| L. sainthelensi | LS1 | ||
| Groundwater at surface (after chlorination) | 4 × 105 | L. oakridgensis | LO1 |
—, not detected.
Forty-five samples were collected from the four boreholes in area 2 over 7 years. The number of legionellae in this area varied between 5 × 101 and 1 × 104 CFU liter−1. The organisms were always detected in samples collected from borehole 2B, but on some occasions, none were recovered from other wells. Moreover, legionellae were not detected in groundwater samples recovered from the recently drilled boreholes 2C and 2D during the first year of operation (Table 4).
TABLE 4.
Enumeration, identification, and RAPD types of Legionella spp. isolated from groundwater samples collected in area 2
| Sampling datea | Borehole 2A
|
Borehole 2B
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| CFU liter−1 | Legionella sp. | RAPD type | CFU liter−1 | Legionella sp. | RAPD type | |||||
| 06-1997 | 3.5 × 102 | L. pneumophila | LP4,5 | NPb | ||||||
| L. oakridgensis | LO2 | |||||||||
| 07-1998 | 4.2 × 103 | L. oakridgensis | LO2 | NP | ||||||
| 03-2000 | 1.3 × 103 | L. pneumophila | LP4,5 | 5 × 101 | L. pneumophila | LP5,6 | ||||
| 04-2000 | —c | 3 × 102 | L. pneumophila | LP5 | ||||||
| 05-2000 | 2 × 102 | L. pneumophila | LP4,5 | 1.4 × 103 | L. pneumophila | LP4,6,7 | ||||
| 06-2000 | 1.3 × 103 | L. pneumophila | LP4,5 | 6.5 × 102 | L. pneumophila | LP4 | ||||
| 07-2000 | 5 × 10 | L. pneumophila | LP4,5 | 2 × 103 | L. pneumophila | LP4,5,6,7 | ||||
| 08-2000 | 1 × 102 | L. pneumophila | LP4 | 1.5 × 102 | L. pneumophila | LP4,5,6 | ||||
| 09-2000 | 2.3 × 103 | L. pneumophila | LP5 | 5 × 101 | L. pneumophila | LP4 | ||||
| 10-2000 | — | 3 × 102 | L. pneumophila | LP4 | ||||||
| 11-2000 | 1.5 × 102 | L. pneumophila | LP4,5 | 7 × 102 | L. pneumophila | LP4 | ||||
| 12-2000 | 6 × 102 | L. pneumophila | LP5 | 5.5 × 102 | L. pneumophila | LP4 | ||||
| 03-2001 | — | NP | ||||||||
| 06-2001 | 6.7 × 103 | L. pneumophila | LP5 | NP | ||||||
| 08-2001 | 6 × 103 | L. londiniensis | LL1 | NP | ||||||
| L. oakridgensis | LO2 | |||||||||
| Borehole 2C
|
Borehole 2D
|
|||||||||
| 09-2001 | — | — | ||||||||
| 10-2001 | — | — | ||||||||
| 09-2001 | — | — | ||||||||
| 12-2002 | — | — | ||||||||
| 05-2002 | — | — | ||||||||
| L. pneumophila | LP4 | L. pneumophila | LP5 | |||||||
| 09-2002 | 6 × 102 | L. londiniensis | LL1 | 3 × 103 | L. londiniensis | LL1 | ||||
| L. oakridgensis | LO2 | |||||||||
| 10-2002 | 6.5 × 102 | L. pneumophila | LP4 | 2.2 × 103 | L. pneumophila | LP5 | ||||
| L. oakridgensis | LO2 | L. londiniensis | LL1 | |||||||
| L. oakridgensis | LO2 | |||||||||
| 11-2002 | 1 × 102 | L. londiniensis | LL2 | 1 × 104 | L. pneumophila | LP5 | ||||
| L. londiniensis | LL1 | |||||||||
| 12-2002 | — | 4.5 × 103 | L. pneumophila | LP5 | ||||||
| 05-2003 | 1 × 103 | L. pneumophila | LP4 | 3.1 × 103 | L. pneumophila | LP5 | ||||
Month-year.
NP, not performed.
—, not detected.
The large majority of the isolates from area 2 were identified as L. pneumophila, although strains belonging to L. oakridgensis and L. londiniensis were also isolated. All isolates recovered from borehole 2B were identified as L. pneumophila, in contrast to the diversity found in the other wells (Table 4).
RAPD typing of Legionella isolates.
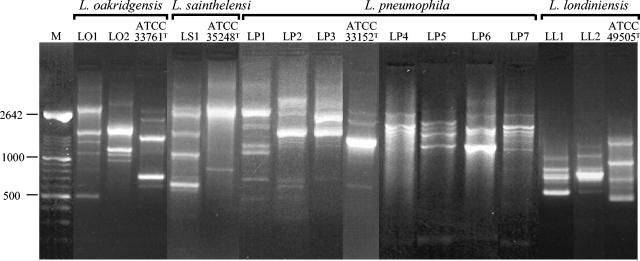
All 72 L. oakridgensis isolates typed from area 1 had only one RAPD type, designated LO1 (Fig. 2). Only one RAPD type, designated LS1, was obtained from 29 L. sainthelensi isolates typed from this area (Fig. 2). L. pneumophila isolates were less frequently detected in area 1 but revealed higher RAPD pattern diversity. Indeed, from the seven L. pneumophila isolates typed, we obtained three distinct RAPD profiles, namely, LP1, LP2, and LP3 (Fig. 2).
FIG. 2.
Agarose gel electrophoresis of RAPD types obtained from representative isolates of Legionella spp. detected in groundwater and biofilm samples from areas 1 and 2 and respective type strains. M, Marker XIV (Roche Molecular Biochemical). Values to the left are in base pairs.
Four different RAPD types, LP4 to LP7, were obtained from 103 L. pneumophila isolates in area 2 (Fig. 2). The four L. pneumophila RAPD types were obtained from isolates recovered from borehole 2B, while borehole 2A had isolates with RAPD types LP4 and LP5. In the more recent wells, we detected only strains with RAPD type LP4 from borehole 2C and RAPD type LP5 from 2D (Table 4). Two distinct RAPD types, LL1 and LL2, were obtained from 13 L. londiniensis isolates from area 2, while RAPD type LO2 was found in 11 L. oakridgensis isolates from this area (Fig. 2).
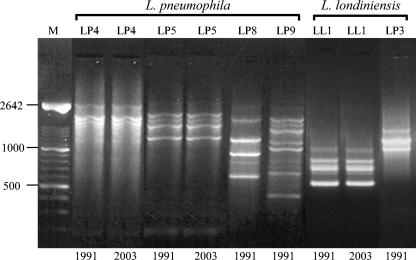
Representative L. pneumophila isolates (32 isolates) recovered 12 years before from area 2 possessed RAPD types LP4 and LP5, like the more recent isolates, but RAPD types LP8 and LP9 were obtained only from L. pneumophila strains isolated during the earlier study. RAPD types LP6 and LP7 were present in the population only during the previous 7 years (Fig. 3). One of the L. londiniensis profiles (RAPD LL1) also had a long persistence, while RAPD LL3 was only encountered 12 years before, after which it was no longer detected (Fig. 3). As expected, the RAPD profiles exhibited by the type strains of these species were distinct from those obtained from our isolates (Fig. 2).
FIG. 3.
Comparison of RAPD types obtained from strains isolated in 1991 and the more persistent RAPD types from 2003 in area 2. M, Marker XIV (Roche Molecular Biochemical). Values to the left are in base pairs.
Genetic structure of L. pneumophila groundwater isolates.
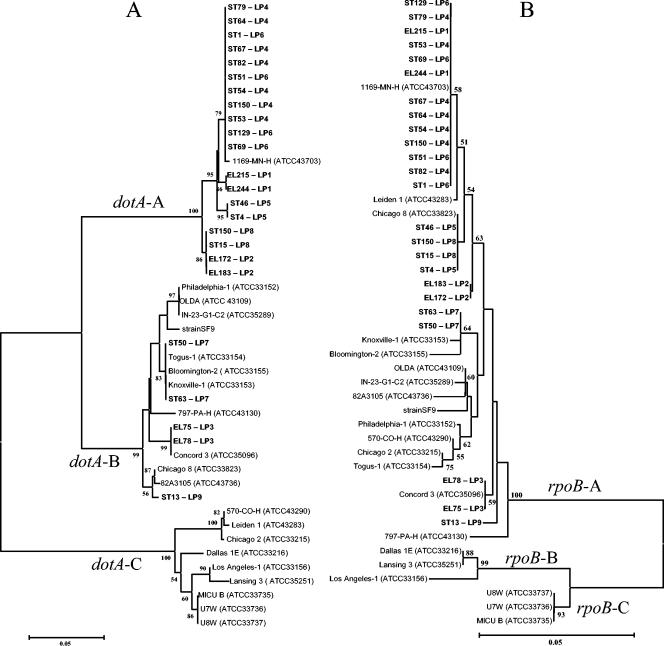
The population structure of the L. pneumophila isolates from groundwater of the two areas was analyzed using partial rpoB and dotA sequences and compared with those of L. pneumophila reference strains. The phylogenetic trees inferred from these genes (Fig. 4) demonstrate that strains with the same RAPD profile had identical rpoB and dotA gene sequences. Moreover, the same rpoB and dotA gene sequences were found in strains with different RAPD profiles.
FIG. 4.
Phylogenetic dendrograms based on analyses of partial sequences of dotA (A) and rpoB (B) of the groundwater isolates and of type and references strains of L. pneumophila. The trees were created using the neighbor-joining method. Isolates and the respective RAPD types characterized in this study are indicated in boldface. Branches supported by values of >50% in the bootstrap analysis (1,000 replications) are indicated. Scale bar, 5 inferred nucleotide substitutions per 100 nucleotides.
The topologies of the two trees were not congruent; some isolates had different relationships with each other and with the L. pneumophila reference strains, depending on the gene considered. Furthermore, the major clusters obtained from rpoB sequence analysis, which included the reference strains corresponding to the three different L. pneumophila subspecies, namely, L. pneumophila subsp. pneumophila, L. pneumophila subsp. fraseri, and L. pneumophila subsp. pascullei, were very discrete and could be clearly distinguished from each other (Fig. 4B). The dendrogram based on dotA sequence analysis was also composed of three principal clusters; one cluster (cluster dotA-A) included representative isolates of six different RAPD types that constituted the large majority of the strains recovered from the groundwater samples and one reference strain (1169-MN-H) of L. pneumophila subsp. pneumophila. Cluster dotA-B comprised the majority of the reference strains belonging to L. pneumophila subsp. pneumophila and representative strains of three RAPD types from groundwater. Finally, the third major cluster (cluster dotA-C) was composed exclusively of the type and reference strains of L. pneumophila subsp. fraseri, the type and reference strains of L. pneumophila subsp. pascullei, and three reference strains (570-CO-H, Leiden 1, and Chicago 2) that belong to L. pneumophila subsp. pneumophila (Fig. 4A). Moreover, some of the sequences of the dotA gene from the groundwater isolates with RAPD LP-1, LP-2, LP-5, LP-8, and LP-9 profiles did not have a close relationship with sequences from any of the reference strains. The same was true for types LP-2 and LP-9 in the tree derived from rpoB gene sequence analysis (Fig. 4).
The deduced amino acid sequences from the partial rpoB gene sequences of all isolates and reference strains were the same, despite the nucleotide differences detected. On the other hand, a remarkable difference in the amino acid sequences was deduced from the dotA nucleotide sequences (results not shown). The degree of amino acid diversity was different within each of the three principal dotA gene clusters. The mean diversity index calculated using the Mega2 equation was: 0.026 ± 0.010 for strains included in cluster dotA-A, 0.025 ± 0.009 for strains included in cluster dotA-B, and 0.045 ± 0.013 for strains included in cluster dotA-C.
DISCUSSION
Legionellae have been sporadically detected in groundwater (29, 30, 37). The increasing use of groundwater as a resource for potable water implies the need for more comprehensive data on the occurrence and distribution of legionellae in this type of environment. Furthermore, groundwater has been used, mainly in Europe, without being disinfected to supply therapeutic spas or bottled mineral water. Some cases of disease associated with exposure to contaminated water in spas have already been reported (8, 38).
The ability of Legionella spp. to persistently colonize aquifers was fully demonstrated by the consistent recovery of these organisms, over several years, in both areas studied. In area 1, nevertheless, we never detected legionellae in the samples collected from borehole 1B. The inability to detect legionellae in this borehole, which is only 62 m from 1A, where legionellae were always detected, could indicate the existence of localized environments within the aquifer, generating different conditions that would promote the development of Legionella or at least that could sustain these bacteria in a cultivable state. It should be noted that boreholes 1A and 1B have been drilled to different depths (63.5 m versus 307 m, respectively) and that one is only stainless steel jacketed to a depth of 38 m. These differences could influence colonization by and persistence of the organisms or their host protozoans. Our results could also indicate that the circulation of water through the aquifer is limited, leading to localized colonization of the aquifer by the microorganisms. Moreover, legionellae could not be recovered during the first year of sampling from the most recently drilled wells in area 2 (2C and 2D), indicating that colonization by the organisms is slow.
We enumerated legionellae from the water and from the plumbing structures by dismantling borehole 1A, followed by disinfecting the water column, in an attempt to determine the origin of the colonization. The results clearly indicate that legionellae were present in the groundwater, since the organisms were recovered from the water samples collected at different depths without the pumping system only when the chlorine became undetectable, indicating that groundwater had entered the borehole and reached the surface by natural pressure from the aquifer.
The numbers of Legionella spp. detected in groundwater were lower than those generally found in water samples collected from the man-made environments (5, 33, 49), perhaps because of smaller amounts of biofilms found in groundwater compared with the sometimes massive development of biofilms in surface waters and man-made aquatic environments (13, 32).
L. pneumophila represented the major legionella population persistently recovered from area 2, while L. oakridgensis was the most abundant species isolated from area 1. Remarkably, there are very few environments where L. pneumophila is not the predominant Legionella isolate. Indeed, potting soils in Australia are primarily colonized by L. longbeachae, and it has been suggested that this would be its natural habitat (42). The absence of L. pneumophila was also observed in another Portuguese hydrothermal area, where we isolated only L. sainthelensi and another unidentified Legionella sp. (15, 44).
RAPD analyses demonstrate the long persistence of some clones in groundwater. Distinct clones of L. pneumophila are known to be persistent, and it has been hypothesized that some of these clones are better suited to persist in man-made environments (10, 27, 35). We recognized, for example, two L. pneumophila clones and one L. londiniensis clone in the groundwater for 12 years in area 2, as well as the persistence of one clone each of L. oakridgensis and L. sainthelensi over 7 years in area 1. These results support the idea that the interaction between legionellae, the presence of particular protozoa, and physicochemical parameters may lead to the colonization and persistence of specific clones or species in aquatic environments.
The diversity of RAPD profiles was higher in area 2 than in area 1, which may be related to undetermined environmental parameters and/or to characteristics of the L. pneumophila clones that constitute the major population. Indeed, the genetic structure of these L. pneumophila populations, based on the dotA and rpoB sequence analyses, indicates that the majority of the isolates are closely related to a strain designated 1169-MN-H (7). On the other hand, Ko et al. (23), who used the same genes to analyze the structures of populations from clinical cases and man-made environments, found that the vast majority of the organisms clustered with other reference strains of the three subspecies of L. pneumophila.
Recently, the dotA gene, together with icmX, icmW, and icmV, were found to be the most variable genes in the entire dot/icm complex (34). Interestingly, the deduced amino acid sequences of the dotA gene from the isolates included in the dotA-A cluster (Fig. 4A) have a degree of diversity slightly higher than that calculated for the cluster (dotA-B) that included the majority of the reference strains belonging to L. pneumophila subsp. pneumophila. This result led us to speculate that the diversity of at least the dotA allele is higher in strains isolated from groundwater environments than in those recovered from man-made environments. These results appear to confirm the hypothesis that L. pneumophila isolates from clinical cases and man-made environments belong to a restricted subset of all clones in the species as a whole, as anticipated by Selander et al. (41).
The topologies of inferred rpoB and dotA trees are not similar, as previously observed (23), since the relationship between the L. pneumophila subspecies differs in the two trees. While the inferred rpoB tree agrees with phylogenetic and taxonomic analyses based on other methods (9, 20, 46), the inferred dotA tree does not, since the three subspecies cannot be separated from each other by the sequence analysis of this gene. The main discrepancies between the two trees may result from dotA gene exchange involving L. pneumophila (sensu lacto) strains. Gene exchange can occur by two distinct routes: homologous recombination between closely related individuals or lateral gene transfer between organisms belonging to more distant lineages (28). We may envision lateral gene transfer of dotA within all known reference strains of L. pneumophila subsp. fraseri and L. pneumophila subsp. pascullei and some strains of L. pneumophila subsp. pneumophila. Moreover, the fact that different clones (determined by RAPD analysis) have the same dotA or rpoB sequence genes may indicate the occurrence of homologous recombination events. These events may mediate the dispersal of advantageous alleles that may rise to high frequencies among genetically related individuals by means of sporadic selection events and may be an explanation for differences in the persistences of certain strains in a given environment.
The groundwaters investigated in this study are used, without any disinfection procedure, as water supplies in spas for therapeutic purposes, so that persistence of Legionella spp. is certainly a cause for concern for the spa officials and public health authorities. During the period of our investigations, no case of Legionella-related disease was detected in either area. The absence of disease may be related to the low number of legionellae detected in these groundwaters and the very stringent cleaning procedures adopted within both facilities that prevent proliferation in the water distribution systems. Lastly, the uniqueness of the majority of the dotA alleles detected in these L. pneumophila isolates could also lead us to hypothesize that the strains more frequently present in these particular environments are less pathogenic than others more common in man-made environments. This appealing hypothesis requires further investigation.
Acknowledgments
This research was funded by FCT/FEDER project POCTI/ESP/35661/2000.
We thank Isabel Pinhal for helping to isolate legionellae and Fernanda Nobre for FAME analysis.
REFERENCES
- 1.Abu Kwaik, Y., L. Y. Gao, B. J. Stone, C. Venkataraman, and O. S. Harb. 1998. Invasion of protozoa by Legionella pneumophila and its role in bacterial ecology and pathogenesis. Appl. Environ. Microbiol. 64:3127-3133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abu Kwaik, Y., L. Y. Gao, O. S. Harb, and B. J. Stone. 1997. Transcriptional regulation of the macrophage-induced gene (gspA) of Legionella pneumophila and phenotypic characterization of a null mutant. Mol. Microbiol. 24:629-642. [DOI] [PubMed] [Google Scholar]
- 3.Adeleke, A. A., B. S. Fields, R. F. Benson, M. I. Daneshvar, J. M. Pruckler, R. M. Ratcliff, T. G. Harrison, R. S. Weyant, R. J. Birtles, D. Raoult, and M. A. Halablab. 2001. Legionella drozanskii sp. nov., Legionella rowbothamii sp. nov. and Legionella fallonii sp. nov.: three unusual new Legionella species. Int. J. Syst. Evol. Microbiol. 51:1151-1160. [DOI] [PubMed] [Google Scholar]
- 4.Alli, O. A., S. Zink, N. K. von Lackum, and Y. Abu-Kwaik. 2003. Comparative assessment of virulence traits in Legionella spp. Microbiology 149:631-641. [DOI] [PubMed] [Google Scholar]
- 5.Atlas, R. M. 1999. Legionella: from environmental habitats to disease pathology, detection and control. Environ. Microbiol. 1:283-293. [DOI] [PubMed] [Google Scholar]
- 6.Barker, J. M., and R. W. Brown. 1994. Trojan horses of the microbial world: protozoa and the survival of bacterial pathogens in the environment. Microbiology 140:1253-1259. [DOI] [PubMed] [Google Scholar]
- 7.Benson, R. F., W. L. Thacker, H. W. Wilkinson, R. J. Fallon, and D. J. Brenner. 1988. Legionella pneumophila serogroup 14 isolated from patients with fatal pneumonia. J. Clin. Microbiol. 26:382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bornstein, N., D. Marmet, M. Surgot, M. Nowicki, A. Arslan, J. Esteve, and J. Fleurette. 1989. Exposure to Legionellaceae at a hot spring spa: a prospective clinical and serological study. Epidemiol. Infect. 102:31-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brenner, D. J., A. G. Staigerwalt, P. Epple, W. F. Bibb, R. M. McKinney, R. W. Starnes, J. M. Colville, R. K. Selander, P. H. Edelstein, and C. W. Moss. 1988. Legionella pneumophila serogroup Lansing 3 isolated from a patient with fatal pneumonia, and descriptions of L. pneumophila subsp. pneumophila subsp. nov., L. pneumophila subsp. fraseri subsp. nov., and L. pneumophila subsp. pascullei subsp. nov. J. Clin. Microbiol. 26:1695-1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chang, F., S. L. Jacobs, S. M. Colodny, J. E. Stout, and V. L. Yu. 1996. Nosocomial Legionnaires's disease caused by Legionella pneumophila serogroup 5: laboratory and epidemiologic implications. J. Infect. Dis. 174:1116-1119. [DOI] [PubMed] [Google Scholar]
- 11.Christie, P. J. 2001. Type IV secretion: intercellular transfer of macromolecules by systems ancestrally related to conjugation machines. Mol. Microbiol. 40:294-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colbourne, J. S., and P. J. Dennis. 1985. Distribution and persistence of Legionella in water systems. Microbiol. Sci. 2:40-43. [PubMed] [Google Scholar]
- 13.Colbourne, J. S., and P. J. Dennis. 1988. Legionella: a biofilm organism in engineered water systems?, p. 36-42. In D. R. Houghton, R. N. Smith, and H. O. W. Eggins (ed.), Biodeterioration 7: Proceedings of the Seventh International Biodeterioration Symposium. Elsevier Applied Science, London, England.
- 14.Dennis, P. J. 1988. Isolation of Legionellae from environmental specimens, p. 31-44. In T. G. Harrison and A. G. Taylor (ed.), A laboratory manual for Legionella. John Wiley & Sons Ltd., London, United Kingdom.
- 15.Diogo, A., A. Veríssimo, M. F. Nobre, and M. S. da Costa. 1999. Usefulness of fatty acid composition for differentiation of Legionella species. J. Clin. Microbiol. 37:2248-2254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Environmental Protection Office of Water. March, 2001 posting date. Legionella: drinking water health advisory. United States Office of Science and Technology, Environmental Protection Agency, Washington, D.C. [Online.] http://www.epa.gov/waterscience/humanhealth/microbial/legionellaha.pdf.
- 17.Fliermans, C. B., W. B. Cherry, L. H. Orrison, and L. Thacker. 1979. Isolation of Legionella pneumophila from nonepidemic-related aquatic habitats. Appl. Environ. Microbiol. 37:1239-1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Groothuis, D. G., H. R. Veenendaal, and H. L. Dijkstra. 1985. Influence of temperature on the number of Legionella pneumophila in hot water systems. J. Appl. Bacteriol. 59:529-536. [DOI] [PubMed] [Google Scholar]
- 19.Hall, T. A. 1999. BioEdit: a user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 41:95-98. [Google Scholar]
- 20.Hookey, J. V., N. A. Saunders, N. K. Fry, R. J. Birtles, and T. G. Harrison. 1996. Phylogeny of Legionellaceae based on small-subunit ribosomal DNA sequences and proposal of Legionella lytica comb. nov. for Legionella-like amoebal pathogens. Int. J. Syst. Bacteriol. 46:526-531. [Google Scholar]
- 21.Horwitz, M. A. 1983. The Legionnaires' disease bacterium (Legionella pneumophila) inhibits phagosome-lysosome fusion in human monocytes. J. Exp. Med. 158:2108-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jukes, T. H., and C. R. Cantor. 1969. Evolution of protein molecules, p. 21-132. In H. N. Munro (ed.), Mammalian protein metabolism. Academic Press, New York, N.Y.
- 23.Ko, K. S., H. K. Lee, M. Y. Park, M. S. Park, K. H. Lee, S. Y. Woo, Y. J. Yun, and Y. H. Kook. 2002. Population genetic structure of Legionella pneumophila inferred from RNA polymerase gene (rpoB) and DotA gene (dotA) sequences. J. Bacteriol. 184:2123-2130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kumar, S., K. Tamura, I. B. Jakobsen, and M. Nei. 2001. MEGA2: molecular evolutionary genetics analysis software. Bioinformatics 17:1244-1245. [DOI] [PubMed] [Google Scholar]
- 25.Kuykendall, L. D., M. A. Roy, J. J. O'Neill, and T. E. Devine. 1988. Fatty acids, antibiotic resistance, and deoxyribonucleic acid homology groups of Bradyrhizobium japonicum. Int. J. Syst. Bacteriol. 38:358-361. [Google Scholar]
- 26.La Scola, B., R. J. Birtles, G. Greub, T. J. Harrison, R. M. Ratcliff, and D. Raoult. 2004. Legionella drancourtii sp. nov., a strictly intracellular amoebal pathogen. Int. J. Syst. Evol. Microbiol. 54:699-703. [DOI] [PubMed] [Google Scholar]
- 27.Lawrence, C., M. Reyrolle, S. Dubrou, F. Forey, B. Decludt, C. Goulvestre, P. Matsiota-Bernard, J. Etienne, and C. Nauciel. 1999. Single clonal origin of a high proportion of Legionella pneumophila serogroup 1 isolates from patients and the environment in the area of Paris, France, over a 10-year period. J. Clin. Microbiol. 37:2652-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawrence, J. G. 2002. Gene transfer in bacteria: speciation without species? Theor. Popul. Biol. 61:449-460. [DOI] [PubMed] [Google Scholar]
- 29.Lieberman, R. J., L. C. Shadix, B. S. Newport, S. R. Crout, S. E. Buescher, R. S. Safferman, R. E. Stetler, D. Lye, G. S. Fout, and D. R. Dahling. 1995. Source water microbial quality of some vulnerable public ground water supplies, p. 1425-1436. In Proceedings of the 1994 Water Quality Technology Conference, part II. American Water Works Association, Denver, Colo.
- 30.Lye, D., G. S. Fout, S. R. Crout, R. Danielson, C. L. Thio, and C. M. Paszo-Kolva. 1997. Survey of ground, surface, and potable waters for the presence of Legionella species by enviroamp PCR Legionella kit, culture, and immunofluorescent staining. Water Res. 31:287-293. [Google Scholar]
- 31.Macler, B. A., and J. C. Merkle. 2000. Current knowledge on groundwater microbial pathogens and their control. Hydrogeol. J. 8:29-40. [Google Scholar]
- 32.Marrão, G. M., A. Veríssimo, R. G. Bowker, and M. S. da Costa. 1993. Biofilms as the source of Legionella spp. in hydrothermal areas and their dispersion into stream water. FEMS Microbiol. Ecol. 12:25-33. [Google Scholar]
- 33.Miller, R. D., and K. A. Kenepp. 1993. Risk assessments for Legionnaires' disease based on routine surveillance of cooling towers for Legionella, p. 211-216. In J. M. Barbaree, R. F. Breiman, and A. P. Dufour (ed.), Legionella current status and emerging perspectives. American Society for Microbiology, Washington, D.C.
- 34.Morozova, I., X. Qu, S. Shi, G. Asamani, J. E. Greenberg, H. A. Shuman, and J. J. Russo. 2004. Comparative sequence analysis of the icm/dot genes in Legionella. Plasmid 51:127-147. [DOI] [PubMed] [Google Scholar]
- 35.Plouffe, J. F., M. F. Para, W. E. Maher, B. Hackman, and L. Webster. 1983. Subtypes of Legionella pneumophila serogroup 1 associated with different attack rates. Lancet ii:649-650. [DOI] [PubMed] [Google Scholar]
- 36.Rainey, F. A., N. Ward-Rainey, R. M. Kroppenstedt, and E. Stackebrandt. 1996. The genus Nocardiopsis represents a phylogenetically coherent taxon and a distinct actinomycete lineage: proposal of Nocardiopsaceae fam. nov. Int. J. Syst. Bacteriol. 46:1088-1092. [DOI] [PubMed] [Google Scholar]
- 37.Riffard, S., S. Douglass, T. Brooks, S. Springthorpe, L. G. Filion, and S. A. Sattar. 2001. Occurrence of Legionella in groundwater: an ecological study. Water Sci. Technol. 43:99-102. [PubMed] [Google Scholar]
- 38.Rocha, G., A. Veríssimo, R. Bowker, N. Bornstein, and M. S. da Costa. 1995. Relationship between Legionella spp. and antibody titres at a therapeutic thermal spa in Portugal. Epidemiol. Infect. 115:79-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Saitou, N., and M. Nei. 1987. The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol. Biol. Evol. 4:406-425. [DOI] [PubMed] [Google Scholar]
- 40.Santos, P., I. Pinhal, F. Rainey, N. Empadinhas, J. Costa, B. Fields, R. Benson, A. Veríssimo, and M. da Costa. 2003. Gamma-Proteobacteria, Aquicella lusitana gen. nov., sp. nov., and Aquicella siphonis sp. nov. infect protozoa and require activated charcoal for growth in laboratory media. Appl. Environ. Microbiol. 69:6533-6540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selander, R. K., R. M. McKinney, T. S. Whittam, W. F. Bibb, D. J. Brenner, F. S. Nolte, and P. E. Pattison. 1985. Genetic structure of populations of Legionella pneumophila. J. Bacteriol. 163:1021-1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Steele, T. W., C. V. Moore, and N. Sangster. 1990. Distribution of Legionella longbeachae serogroup 1 and other legionellae in potting soils in Australia. Appl. Environ. Microbiol. 56:2984-2988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson, J. D., T. J. Gibson, F. Plewniak, F. Jeanmougin, and D. G. Higgins. 1997. The ClustalX Windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 24:4876-4882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veríssimo, A., G. Marrão, F. G. da Silva, and M. S. da Costa. 1991. Distribution of Legionella spp. in hydrothermal areas in continental Portugal and the island of São Miguel, Azores. Appl. Environ. Microbiol. 57:2921-2927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Veríssimo, A., G. Vesey, G. M. Rocha, G. Marrão, J. Colbourne, P. J. Dennis, and M. S. da Costa. 1990. A hot water supply as the source of Legionella pneumophila in incubators of a neonatology unit. J. Hosp. Infect. 15:255-263. [DOI] [PubMed] [Google Scholar]
- 46.Veríssimo, A., P. V. Morais, A. Diogo, C. Gomes, and M. S. da Costa. 1996. Characterization of Legionella species by numerical analysis of whole-cell protein electrophoresis. Int. J. Syst. Bacteriol. 46:41-49. [DOI] [PubMed] [Google Scholar]
- 47.Wadowsky, R. M., L. J. Butler, M. K. Look, S. M. Verma, M. A. Paul, B. S. Fields, G. Keleti, J. L. Sykora, and R. B. Yee. 1988. Growth-supporting activity for Legionella pneumophila in tap water cultures and implication of hartmannellid amoebae as growth factors. Appl. Environ. Microbiol. 54:2677-2682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wadowsky, R. M., and R. B. Yee. 1985. Effect of non-Legionellaceae bacteria on the multiplication of Legionella pneumophila in potable water. Appl. Environ. Microbiol. 49:1206-1210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wadowsky, R. M., R. B. Yee, L. Mezmar, E. J. Wing, and J. N. Dowling. 1982. Hot water systems as sources of Legionella pneumophila in hospital and nonhospital plumbing fixtures. Appl. Environ. Microbiol. 43:1104-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wadowsky, R. M., R. Wolford, A. M. McNamara, and R. B. Yee. 1985. Effect of temperature, pH, and oxygen level on the multiplication of naturally occurring Legionella pneumophila in potable water. Appl. Environ. Microbiol. 49:1197-1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wiedmann-al-Ahmad, M., H. V. Tichy, and G. Schon. 1994. Characterization of Acinetobacter type strains and isolates obtained from wastewater treatment plants by PCR fingerprinting. Appl. Environ. Microbiol. 60:4066-4071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yu, V. L., J. F. Plouffe, M. C. Pastoris, J. E. Stout, M. Schousboe, A. Widmer, J. Summersgill, T. File, C. M. Heath, D. L. Paterson, and A. Chereshsky. 2002. Distribution of Legionella species and serogroups isolated by culture in patients with sporadic community-acquired legionellosis: an international collaborative survey. J. Infect. Dis. 186:127-128. [DOI] [PubMed] [Google Scholar]