Abstract
This review article is an update of what should be known for practicing basic lung ultrasound in the critically ill (LUCI) and is also of interest for less critical disciplines (e.g. pulmonology). It pinpoints on the necessity of a professional machine (not necessarily a sophisticated one) and probe. It lists the 10 main signs of LUCI and some of the main protocols made possible using LUCI: the BLUE protocol for a respiratory failure, the FALLS protocol for a circulatory failure, the SESAME protocol for a cardiac arrest and the investigation of a ventilated acute respiratory distress syndrome patient, etc. It shows how the field has been fully standardised to avoid confusion.
Key points
A simple ultrasonography unit is fully adequate, with minimal filters, and provides a unique probe for integrating the lung into a holistic, whole-body approach to the critically ill.
Interstitial syndrome is strictly defined. Its clinical relevance in the critically ill is standardised for defining haemodynamic pulmonary oedema, pneumonia and pulmonary embolism.
Pneumothorax is strictly and sequentially defined by the A′-profile (at the anterior wall in a supine or semirecumbent patient, abolished lung siding plus the A-line sign) and then the lung point.
The BLUE protocol integrates lung and venous ultrasound findings for expediting the diagnosis of acute respiratory failure, following pathophysiology, allowing prompt diagnosis of pneumonia, haemodynamic pulmonary oedema, exacerbated chronic obstructive pulmonary disease or asthma, pulmonary embolism or pneumothorax, even in clinically challenging presentations.
Educational aims
To understand that the use of lung ultrasound, although long standardised, still needs educational efforts for its best use, a suitable machine, a suitable universal probe and an appropriate culture.
To be able to use a terminology that has been fully standardised to avoid any confusion of useless wording.
To understand the logic of the BLUE points, three points of interest enabling expedition of a lung ultrasound examination in acute respiratory failure.
To be able to cite, in the correct hierarchy, the seven criteria of the B-line, then those of interstitial syndrome.
To understand the sequential thinking when making ultrasound diagnosis of pneumothorax.
To be able to use the BLUE protocol for building profiles of pneumonia (or acute respiratory distress syndrome) and understand their limitations.
To understand that lung ultrasound can be used for the direct analysis of an acute respiratory failure (the BLUE protocol), an acute circulatory failure (the FALLS protocol) and even a cardiac arrest (SESAME protocol), following a pathophysiological approach.
To understand that the first sequential target in the SESAME protocol (search first for pneumothorax in cardiac arrest) can also be used in countless more quiet settings of countless disciplines, making lung ultrasound in the critically ill cost-, time- and radiation-saving.
To be able to perform a BLUE protocol in challenging patients, understanding how the best lung ultrasound can be obtained from bariatric or agitated, dyspnoeic patients.
Short abstract
What you need to know to practice basic lung ultrasound in the critically ill: the BLUE, FALLS and SESAME protocols http://ow.ly/uDx630aNUN1
Ultrasound was a quiet field. When ultrasonography of the heart became the domain of the cardiologists and that of the uterus, the purview of gynaeco-obstetricians, the rest of the body was quietly given to the radiologists. The advent of lung ultrasound, a field ignored (and perhaps rejected [1]) by experts from the beginning, has changed the landscape. We are glad to see that pioneering work, such as that of CEURF (Cercle des Echographistes d’Urgence et de Réanimation Francophones), has contributed to taking ultrasound out of its traditional place as a “minor technique”, especially at the era of computed tomography (CT) and magnetic resonance imaging, to become a first-line clinical tool.
Currently, lung ultrasound is used by increasing numbers of physicians and, more interestingly, by increasing numbers of specialties. We are not keen on strong words and prefer to use soft ones: lung ultrasound has originated a change in clinical practice. Where are we now?
This review article is an opportunity to show that lung ultrasound has created a unique situation in medicine, from the biomedical engineering point of view: engineers are beginning to come back to older technologies, favouring more efficient use of lung ultrasound in the critically ill (LUCI).
The philosophy of LUCI
Doctors have used various tools for assessing lung function, from the stethoscope to CT [2–4]. After a long and cautious phase of observation, they increasingly began to adopt lung ultrasound, opening up a new “philosophy”. In fact, the inclusion of LUCI changes many aspects of the essence of ultrasound, and not only critical ultrasound (all doctors with a stethoscope in their pocket should be affected). In the philosophy of LUCI, there is no space for confusion; the machine, the probe, the signatures and the applications are considered with a holistic perspective.
The tools for practicing LUCI
The development of the tools for LUCI is the main change in the field in recent years. Previously, we had been using a 1982 technology (the ADR-4000) to develop, since 1989 (after underground years from 1984), critical care ultrasound, including of the lung. We still use everyday a 1992 Japanese technology (the latest update, being purely cosmetic, in 2008).
CEURF has defined seven criteria that an ultrasound machine must meet for you to be able to face the worst (i.e. cardiac arrest). Fortunately, the same equipment and approach are also perfect for other applications, from inserting a central venous line to checking bladder emptying, etc.). Although a given ultrasound machine may not necessarily meet all of our criteria, the more criteria your equipment does meet, the better prepared you will be for the greatest range of applications.
1) The machine must be small (for rapidly accessing the patient at the bedside). In hospitals, the obstacles are lateral, which means that a laptop is of use only if its lateral dimensions (cart included) are small (smaller than our 1992 reference of 32 cm wide). We ask the user to consider that they have never seen their laptops outside the cart. The cart, in addition, allows one to keep the machine clean (see later), to work with two hands (we are unable to perform critical ultrasound with only one hand), to have all of the equipment on site (interventional equipment, etc.) and to work at an ergonomic height. The cart is really a mandatory adjunct. Only few professions will take advantage of handheld machines (e.g. those working, like us, in medical aeroplanes).
2) The image must meet a minimal quality standard. Ultrasound is a nice example of vicious circle: when digital technologies came, this was a spectacular step backward in image quality. Now, they are little by little reaching the quality of the older analogue technologies. Hence, the need for compound and harmonics, which (see later) have sacrificed the lung to the advantage of less critical organs.
3) The machine must have a fast start-up time. Long delays must be avoided in cases of cardiac arrest. It helps if the machine is used many times a day. Ours has a 7-s start-up time, which is the fastest machine in the market.
4) A universal probe is needed. Fortunately, the Japanese microconvex probe we has use since 1992 has quite perfect resolution (see figures), range (from 0.6 to 17 cm, i.e. a whole-body approach) and length (8 cm, giving the possibility to scan posterior areas in supine, ventilated patients).
5) A simple device is needed. While most clinicians do not have the skill of traditional experts (radiologists, cardiologists, etc.), in the event of a cardiac arrest, the comfort afforded by this skill is of no value. In our day-to-day practise, we use only three buttons (B mode/M mode, depth and gain). For cardiac arrest, we use no buttons at all (see later). We have never used Doppler ultrasonography in the past 28 years, including in other areas (venous assessment, etc.). The filters and facilities (dynamic noise, average, compound and harmonics), which are not bad for obtaining beautiful images of plain organs, can hinder LUCI: precious artefacts are erased and real-time imaging is affected by the informatic systems that rebuild the image, resulting in a confusing delay.
6) The unit must be compact, for efficient cleaning. Our 1982 and 1992 technologies have a flat keyboard. We developed a simple, fast and efficient cleaning protocol, detailed in all our textbooks [5].
7) The machine must be affordable. Criteria 4 and 5 make this possible. Our machine cost €15 000 in 1992.
We find many modern machines often make performing lung ultrasound a bit difficult, despite being accustomed to using them in many workshops. If we (with 28 years of experience in our intensive care unit (ICU) and 32 years in total) struggle, we would guess that doctors who have less experience will struggle more. In workshops, everything is quiet and calm. In extreme emergencies, every second is of the essence. We give here some tips for those who have laptops, since even the worst machine can be of help. We advise carefully deleting useless and deleterious filters, and bypassing all of them. I advise using the abdominal probe first, acknowledging also that it can rapidly becoming limiting (in posterior areas, in areas of difficult access, due to the need for a superficial resolution, etc.). However, we are glad to see that, increasingly since very recently, some manufacturers have begun to build machines with real simplicity. “Intelligent” machines are little by little elegantly competing with these laptops full of buttons and dust (and microbes), with endless start-up times, large widths, opaque algorithms, high cost, etc.
The seven principles of LUCI
The seven principles of LUCI have not changed since 2001 [6], apart from a slight update to principle 7.
1) A simple technique is still suitable.
2) The thorax is an area where air and water (gas and fluids for purists) can mingle. They follow the rules of gravity. One can define a macro-gas/fluid ratio (e.g. pleural effusion and aerated lung) and a micro-gas/fluid ratio, for defining the situation where in a minute place, the two elements coexist (e.g. an oedematous subpleural interlobular septum surrounded by air).
3) The lung is the widest organ; areas of interest must be defined (the BLUE points).
4) All signatures arise from the pleural line.
5) Artefacts, although usually considered a hindrance, have critical relevance in lung ultrasound.
6) The lung is the most vital organ. Like all vital organs, it is dynamic. The main dynamic is referred to as lung sliding.
7) All life-threatening disorders abut the chest wall. Update: and almost all, even small ones, have an extensive location (e.g. a pneumothorax can be small but visible in a rather large projection).
The technical approach to a critically ill patient
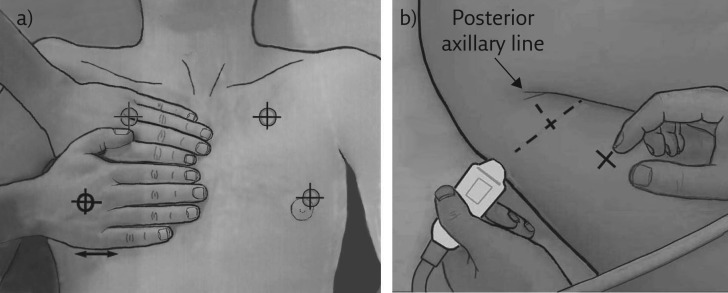
We must first define the areas of investigation. Those we use in an emergency comprise three strictly defined points, although LUCI principle 7 allows a certain flexibility. These BLUE points are defined by applying one’s hands to the patient’s thorax, in order to make finding the ultrasonographic field easier (figure 1).
Figure 1.
The BLUE points. The BLUE points respect LUCI principles 3 and 7. They have been made simple for expediting protocols without loss of information. a) The upper BLUE hand (here the hand of the operator, who has checked that the patient’s hand is approximately the same size; if not, rough adaptations are performed) is applied just below the clavicle and parallel to it, the tips of fingers touching the midline. The upper BLUE point is defined at the middle of the upper BLUE hand. The lower BLUE hand is applied just below. The lower BLUE point is defined at the middle of the palm of the lower BLUE hand. The heart is usually avoided using this way. The lung usually stops at the lower finger. b) The PLAPS point is defined by drawing a transverse line from the lower BLUE point until the posterior axillar line is reached (or better, as posterior as possible). Note that the insertion of the probe between the (supine, ventilated) patient and bed sometimes makes perfect acquisition difficult but this makes the posterior lung of such patients accessible to ultrasound.
In critical settings, movement comes from the lung, the chest wall (severe dyspnoea) and the whole body of critically ill, anxious patients. The physician should not add their own movement and should hold the probe gently but firmly in order to be able to understand the dynamics of lung ultrasound. Severe dyspnoea generates contractions of the accessory muscles, in which case, some expertise is required, although the procedure is made easier by using a standardised analysis, mostly using the M-mode (describing Keye’s sign; the Avicenne sign would be too long in the given volume).
The main signatures seen in the critically ill
Considering the way LUCI is sometimes described and taught, we will take the opportunity here to remind you of the definitions of the disorders. Our training centre aims to explain our choice (of equipment, probe and signatures) with the result of demonstrating that all confusion, which is still frequent in this field, can be avoided because LUCI as taught by CEURF is completely standardised. There is no room for confusion in LUCI. Often, lectures on lung ultrasound deal with pleural effusion, lung consolidation, and more rarely, interstitial syndrome and pneumothorax, but the normal pattern is rarely described. A brief description of the normal lung surface is critical, since it is seen in various diseases.
Normal lung surface
Merlin’s space is defined in a longitudinal scan as the surface delimited by the pleural line, the shadow of the ribs and the bottom of the screen. Lung sliding is strictly defined as a homogeneous twinkling (shimmering, sparkling or glittering) of Merlin’s space. This definition indicates that lung sliding must begin at the pleural line. The A-line is a repetition of the pleural line in the Merlin’s space. At the anterior chest wall in a supine or semirecumbent patient, lung sliding with A-lines strictly defines the A-profile. The A-profile is a practical term for sharing maximal information in minimal time. See figure 2 for more details.
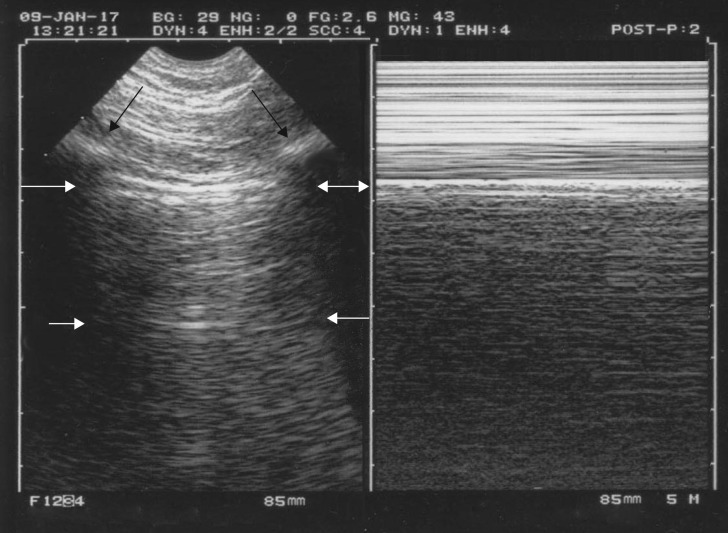
Figure 2.
The A-profile. This single figure shows multiple data. The bat sign appears in the left image. The upper and lower ribs (black arrows) indicate the location of the pleural line (upper white arrows), which must be 0.5 cm (in adults) below the rib line. This works in extreme conditions (major dyspnoea, agitation, etc.). The pleural line always indicates the parietal pleura but indicates the visceral pleura only when joined. Merlin’s space defines the area located between the pleural line, the shadow of the ribs and the bottom of the image. The A-line (lower white arrows) is the repetition of the pleural line at a standardised distance, the skin–pleural line distance. The A-line indicates gas below the pleural line. The seashore sign: on the right image, one can see an upper rectangle (called Keye’s space) with a stratified pattern and a lower rectangle with a sandy pattern (the M-mode of Merlin’s space) both separated exactly by the pleural line (continuation of the white arrows from the left image). Note that with intelligent technology, both images are not only aligned but, mostly, exactly aligned, unlike on many modern machines. The seashore sign demonstrates lung sliding. We can see that lung sliding arises strictly from the pleural line, not 1 mm above or below. The A-profile is the anterior association of both images, i.e. A-lines plus lung sliding. The A-profile indicates a pulmonary artery occlusion pressure <18 mmHg, a basic datum in a circulatory failure (FALLS protocol).
Pleural effusion
Pleural effusion is clearly defined using the concept of the lung line, a regular line roughly parallel to the pleural line. At CEURF, no tone (hypo- or hyperechoic) is needed, which is an advantage because then only anechoic effusions appear anechoic (if the pleural effusion was defined as an anechoic collection, the most life-threatening cases would not be diagnosed) (figure 3).
Figure 3.
Pleural effusion. Longitudinal scan at the PLAPS point. The pleural effusion is defined not because of (as shown here) a hypoechoic tone but by the appearance of the lung line (lower white arrows), which indicates the visceral pleura. The pleural line, which is clearly visible (upper white arrows) indicates here only the parietal pleura. All effusions, anechoic or echoic, can be diagnosed using the lung line. The volume of this effusion can be measured using a simple index. Note the underlying nontranslobar lung consolidation (subpleural, of course) with the shred sign (black arrows). The quad sign just describes the rough trapezium defined by the pleural line, the shadow of the ribs and the lung line. This is a typical example of (mixed) PLAPS.
Lung consolidation
The shred sign (or fractal sign) is of value for immediately diagnosing nontranslobar cases of lung consolidation, which are the most frequent (figure 4). The term “subpleural” is useless in the world of LUCI: each time a consolidation is seen using ultrasound, whether it is minute or huge, it is subpleural. We would guess that those who use this term mean “small” consolidations, so we would advise the term “small” be used instead of “subpleural”. In translobar cases, no shred sign can be seen and the image observed is a whole anatomical lung with a tissue-like pattern that one may call, temporarily, the lung sign (figure 5). Atelectasis is a notion that generates some confusion. To begin with, many authors oppose atelectasis to consolidation: atelectasis is a (retractile) consolidation. Authors describe passive atelectasis, due to pleural effusion (and we ask, so where does the effusion come from?). The only worthwhile atelectasis is the obstructive form. Complete obstructive atelectasis (e.g. foreign body aspiration) generates immediate functional signs (abolition of lung sliding with, usually, lung pulse) and delayed morphological signs: usually no air bronchogram but if any, never dynamic ones; and loss of lung volume with attraction of the surrounding organs.
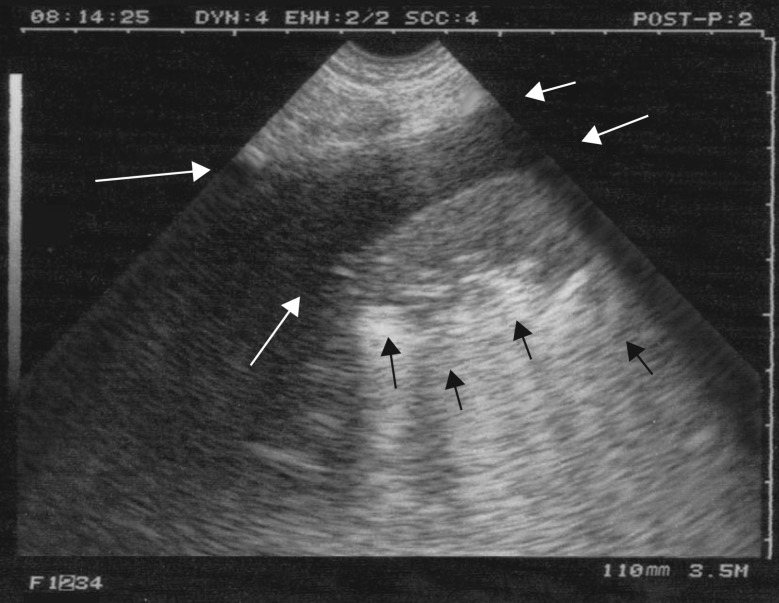
Figure 4.
Nontranslobar lung consolidation. Longitudinal scan at the PLAPS point. No lung line is present, i.e. there is no pleural effusion. A tissue-like image with a mostly fractal, deep boundary with the aerated underlying lung, the shred sign (or fractal sign), makes the diagnosis of a nontranslobar lung consolidation (which is, of course, subpleural). This is an example of PLAPS. If found anteriorly, this would be an example of the C-profile. Black arrows: fractal line. White arrows: ribs.
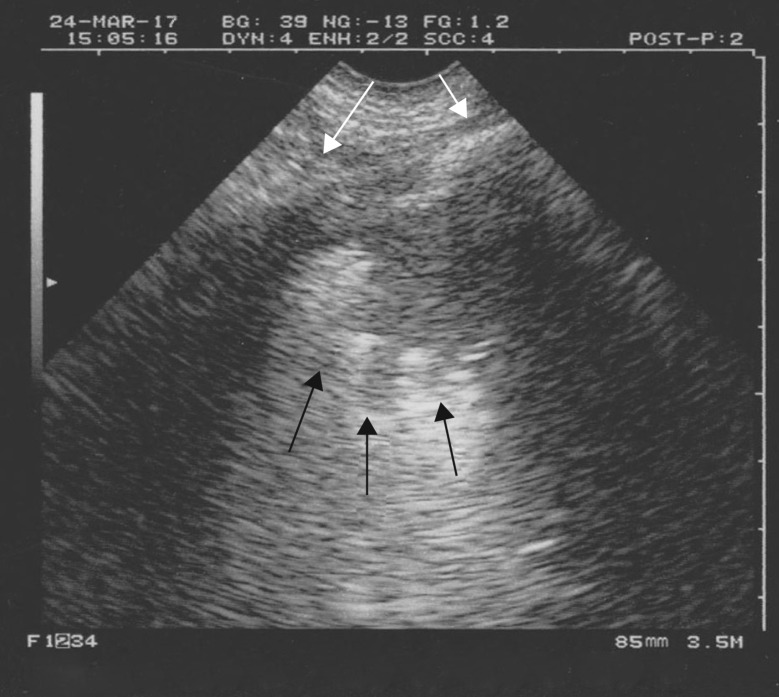
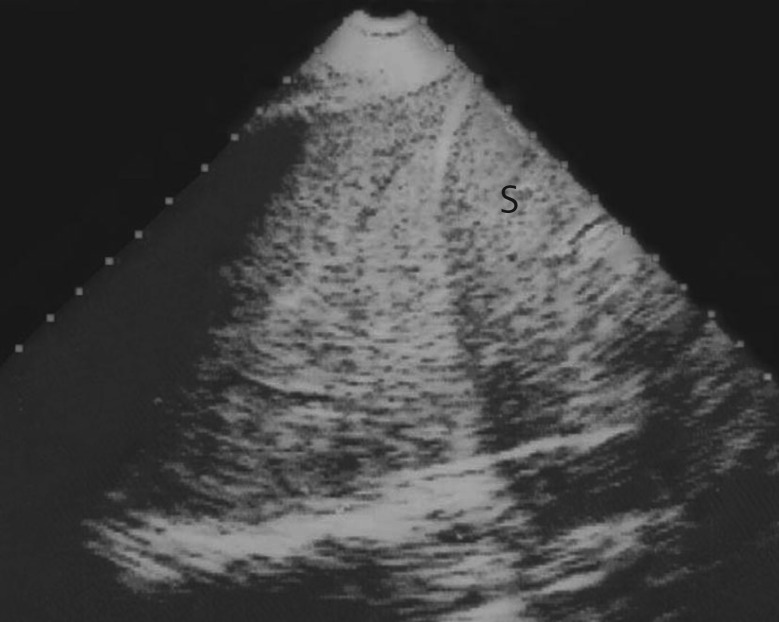
Figure 5.
Translobar lung consolidation. This is another example of PLAPS. Four points can be described here. First, in this longitudinal view at the PLAPS point, there is a huge, whole lower left lobe consolidation (subpleural, of course). Novice users often see here “a lung”, unaware that the normally aerated lung cannot be seen this anatomical way, so this sign may be called, simply, the “lung sign”. It indicates translobar consolidation. The deep border is rectilinear, not shredded: the mediastinal pleura. Second, no air bronchogram is visible: air bronchograms are not needed for the diagnosis (dynamic air bronchograms would allow consideration that this consolidation is not retractile, among several signs). Third, this consolidation is homogeneous (abscesses and necrosis can generate hypoechoic areas) and has no loss of volume: the spleen (S) is in the normal place, another sign that would demonstrate the nonretractile origin of this consolidation. Fourth, this image comes from an ADR-4000, a portable unit from 1982, which we used to describe all of the signatures of lung ultrasound (which shows that the modern machines, especially the laptop units, were not necessary for the birth of bedside lung ultrasound). The diaphragm can be seen between the lung and spleen.
Posterolateral alveolar and/or pleural syndrome
The notion of posterolateral alveolar and/or pleural syndrome (PLAPS) has great relevance in the BLUE protocol. At the PLAPS point, the detection of an alveolar, pleural, mixed or even ill-defined but otherwise structural image is called a PLAPS (figures 3–5). This can simplify the use of LUCI, especially in difficult (bariatric) patients, since the distinction does not impact the accuracy of the BLUE protocol (subtle distinctions should be made once the BLUE protocol is perfectly mastered, not before). Using the PLAPS point, very posterior disorders can be detected, which is better, of course, than with lateral views that are sometimes advised. Note that abdominal probes have a limited ergonomy, and the cardiac probes a limited resolution for the analysis of PLAPS in supine patients.
Interstitial syndrome
Interstitial syndrome is clearly defined when more than two B-lines are visible between two ribs. We propose a hierarchy of signs that allows universal definition (figure 6). Using this standardised definition, comet-tail artefacts such as the E-line (seen in the case of parietal emphysema) or Z-line (interference) will not be confused with B-lines. We would like to add that the expression “more than three B-lines” makes no sense for diagnosis.
Figure 6.
Interstitial syndrome and lung rockets. This figure presents most of the criteria of the B-line (the elementary sign). The B-line is defined using seven criteria, three of which are always present: a comet-tail artefact; arising from the pleural line; moving in concert with lung sliding (when lung sliding is present). The other four criteria are almost always present: long; well-defined; erasing A-lines; hyperechoic. This definition works in all situations and avoids confusion with other comet-tail artefacts that are not B-lines. The resulting sign, lung rockets, indicates that more than two B-lines are visible between two ribs. Three or four B-lines make the pattern called septal rockets and correlate with Kerley’s lines (subpleural interlobular septa). Five or more (the maximum seems to be 10) make a pattern called ground-glass rockets, as they correlate with ground-glass lesions. It is easy to count here six B-lines, a pattern correlating with ground-glass lesions on computed tomography. Lung rockets indicate interstitial syndrome.
Pneumothorax
Little has changed in the ultrasonographic diagnosis of pneumothorax. Pneumothorax is still defined by a sequential approach, first recognising the A′-profile (which is anterior, by definition). This A′-profile is constant (figure 7). Second, finding a lung point, i.e. extending from the area with the A′-profile laterally until this point is found. The lung point is defined as follows: once, and only once, an A′-profile has been detected, the probe is moved until one finds the sudden appearance of lung signs (lung sliding and B-lines), synchronous with respiration (figure 8). The lung point indicates pneumothorax. It also indicates its volume: radio-occult and minor if anterior, moderate if lateral, substantial if seen at the PLAPS point, and major if para-Rachidian or not found. In this last case, the use of the extended BLUE protocol allows easy diagnosis, since major cases are recognised clinically and, here, ultrasound provides a critical, additional argument.
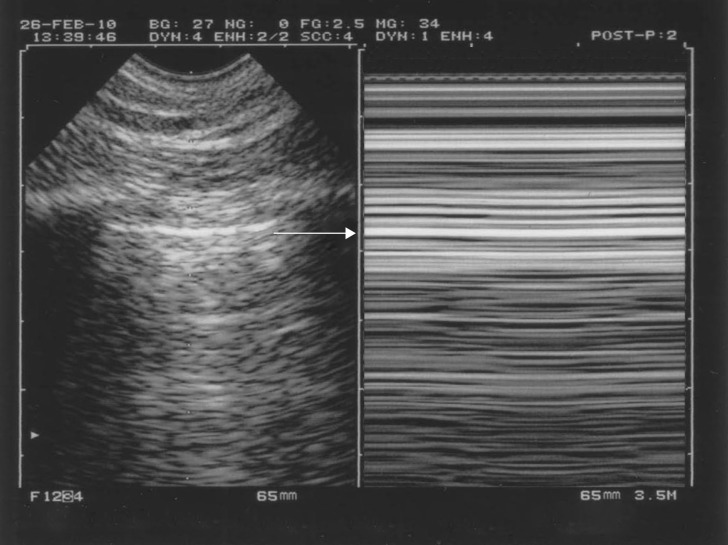
Figure 7.
Pneumothorax and the A′-profile. The A′-profile is defined anteriorly in supine patients. It comprises (anteriorly in supine patients) abolished lung sliding and the A-line sign (no B-line should be observed). Abolished lung sliding can be identified in M-mode (right image) by the stratospheric pattern, the “stratosphere sign”. Note the intelligent technology: both images are strictly aligned. The pleural line, which is impossible to detect by looking only at the right image, is clearly located using the left image (arrow). Note that there is no “barcode sign” at CEURF for several reasons, one of them being due to the recent introduction barcodes, which may add more confusion to the field.
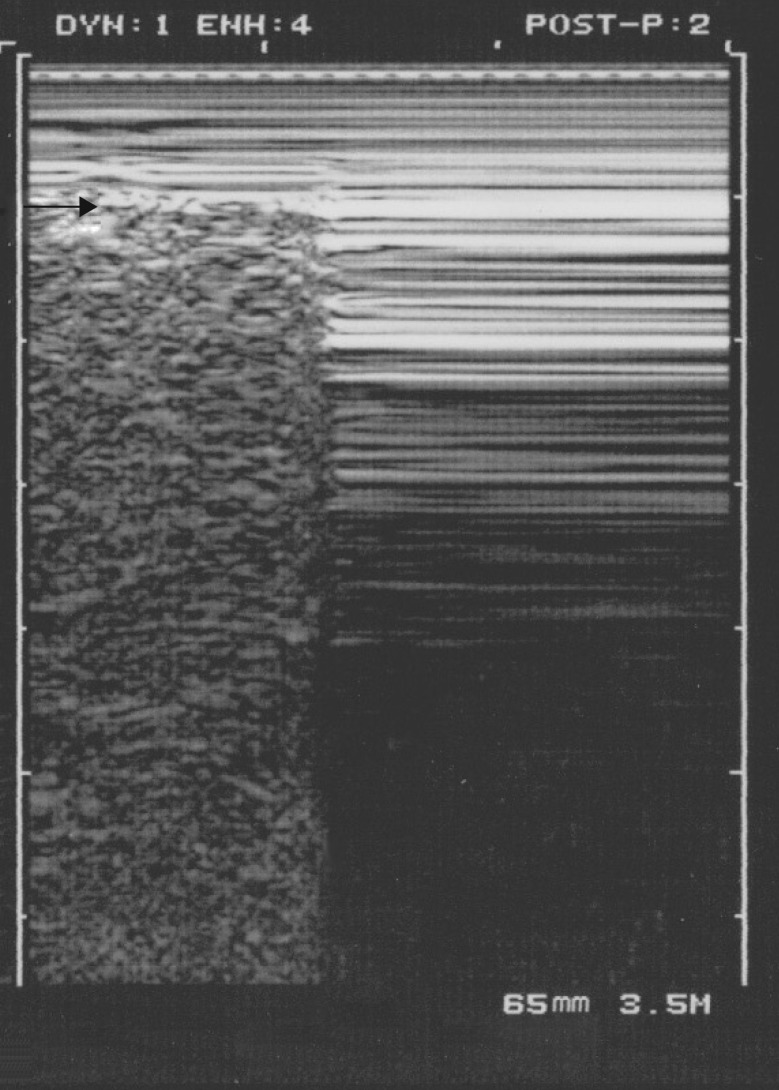
Figure 8.
Pneumothorax and the lung point. A characteristic lung point is shown. This M-mode image shows one main pattern of the lung point. At the point where the collapsed lung increases slightly its volume and touches more of the thoracic wall, one can see the lung pattern suddenly replacing the A′-profile, as shown using M-mode imaging. The lung point is a pathognomonic sign of pneumothorax. Never forget that a lung point must never be sought if no A′-profile has been identified in the first step (lest confusion occur, slowing the learning curve). Arrow: location of the pleural line.
The main clinical applications in the critically ill
Three main protocols are taught at CEURF that summarise the main needs of the critical care physician, i.e. prompt diagnosis of respiratory failure, circulatory failure and cardiac arrest. These are defined below.
Respiratory failure: the BLUE protocol
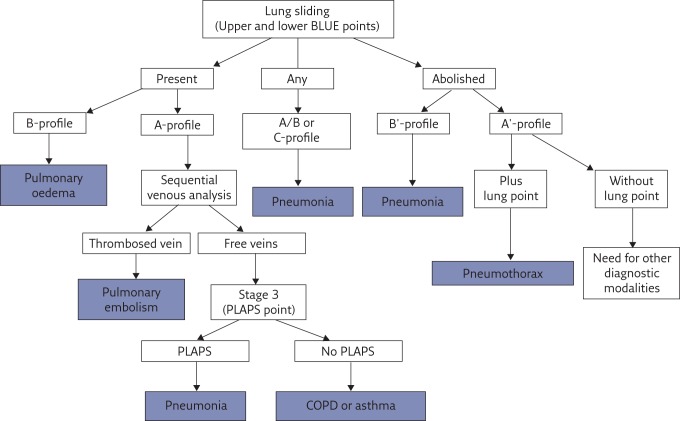
The BLUE protocol (figure 9) draws on the experience of 18 years of use of lung and venous ultrasound for standardising prompt diagnosis of the cause of a respiratory failure. The decision tree shows the six main diseases, which were seen in 97% of the adult patients seen in our emergency department and admitted to our ICU. Countless rare diseases make up the remaining 3%.
Figure 9.
Decision tree of the BLUE protocol. This decision tree, which has been made as simple as possible, may appear complex to some. In actual fact, it simplifies the huge body of knowledge that any physician dealing with respiratory failure should master (anatomy, physiology, pathophysiology, clinical signs, imaging and biological or other paraclinical signs). It is not designed to provide 100% of diagnoses of acute dyspnoea; it has been simplified with a target overall accuracy just over 90% (90.5%). Reproduced and modified from [7] with permission from the publisher.
Six profiles are defined at the anterior chest wall; one (the A-profile) giving three subprofiles, making eight profiles in all. The accuracy of each profile is presented in table 1.
The A-profile was described above (figure 2). It requires a venous analysis (the specific protocol is too long to describe here). The combination of the A-profile and a deep venous thrombosis (DVT), called the A-DVT profile, is associated with pulmonary embolism.
The B-profile combines lung sliding with lung rockets at the four anterior BLUE points. It is usually associated with haemodynamic pulmonary oedema.
The B′-profile combines abolished lung sliding with diffuse anterior lung rockets. It is associated with pneumonia.
The A/B-profile describes a half A-profile in one lung and a half B-profile in the other. It is associated with pneumonia.
The C-profile describes anterior lung consolidation (regardless of number or size), typically indicating pneumonia.
The A-profile with no DVT and with a PLAPS, called the A-V-PLAPS profile, is associated with pneumonia.
The A-profile with no DVT and no PLAPS, called the nude profile (everything normal) is linked to chronic obstructive pulmonary disease exacerbation or asthma.
The A′-profile combines anterior abolished lung sliding with exclusive A-lines. It is suggestive of pneumothorax, a definite diagnosis if a lung point is also present.
Many subtleties cannot be dealt with here, such as how to diagnose acute respiratory distress syndrome (ARDS) (briefly, the profiles are the same as for pneumonia [8]), how to manage rare diagnoses or multiple diagnoses, how to locate the heart when following the BLUE protocol (briefly, just after), and does the BLUE protocol work in children or neonates, in wealthy and poor areas of the world (briefly, yes) [5].
Table 1.
Accuracy of BLUE protocol
| Mechanism of dyspnoea | BLUE protocol profile | Sensitivity | Specificity | Positive predictive value | Negative predictive value |
| Acute haemodynamic pulmonary oedema | B-profile | 97% | 95% | 87% | 99% |
| Exacerbated COPD or severe acute asthma | Nude profile (A-profile with no DVT and no PLAPS) | 89% | 97% | 93% | 95% |
| Pulmonary embolism | A-profile with DVT | 81% | 99% | 94% | 98% |
| Pneumothorax | A′-profile (with lung point) | 88% | 100% | 100% | 99% |
| Pneumonia | All profiles# | 89% | 94% | 88% | 95% |
| B′-profile | 11% | 100% | 100% | 70% | |
| A/B-profile | 14.5% | 100% | 100% | 71.5% | |
| C-profile | 21.5% | 99% | 90% | 73% | |
| A-V-PLAPS profile | 42% | 96% | 83% | 78% |
COPD: chronic obstructive pulmonary disease; DVT: deep venous thrombosis. #: sensitivity calculated by adding the sensitivity of each of the four profiles. Reproduced and modified from [7] with permission from the publisher.
CEURF has attracted some attention regarding the terms seen in some of the literature and the use of the word “profile”, such as the B1, B2-profiles. These terms can cause confusion as they do not specify the quality of lung sliding, which is a critical datum. We use instead the simple and descriptive terms “septal rockets” and “ground-glass rockets” to avoid confusion (and aid memory). There is no confusion in the world of LUCI.
Acute circulatory failure: the FALLS protocol
In the absence of a firm gold standard for the clinical assessment of volaemia, we present here the approach that we propose for the community to work on. Briefly, in a case of circulatory failure of unknown cause, this protocol, which follows Weil’s classification of shock [9], harnesses the potential of lung ultrasound. Simple equipment, without Doppler, and the same probe as for the BLUE protocol are suitable. The FALLS (fluid administration limited by lung sonography) protocol (figure 10) begins with a pericardial analysis, followed by a simple evaluation of the right ventricle volume, then a search for a pneumothorax. If no disorder is found, an obstructive shock is thus rapidly ruled out. The absence of a B-profile makes the diagnosis of a cardiogenic shock with elevated pressures unlikely; in such cases, the patient usually has an A-profile or something equivalent. The only remaining causes of shock are hypovolaemic and distributive shock (i.e. usually septic shock in clinical practice). Fluid therapy is commenced at this step. Hypovolaemic shock resolves under fluid therapy. If the signs of shock remain, there is no clinical signal for discontinuing the fluid therapy, which is resumed with cautious analysis of the lung artefacts. If the fluid begins to saturate the interstitial compartment, an ultrasound interstitial syndrome appears, i.e. an early, infraclinical stage of pulmonary oedema [12–14]. Hypovolaemic shock resolves before saturating the lung interstitial compartment. When A-lines begin to turn into B-lines, the diagnosis of distributive (i.e. usually septic) shock is proposed as the likely cause of circulatory failure. Many common questions on this subject are answered in Chapter 30 of Lung Ultrasound of the Critically Ill: the BLUE Protocol [5]. The FALLS protocol is not proposed fully to solve the very expert question of haemodynamic assessment and is open to criticism.
Figure 10.
Decision tree of the FALLS protocol. The decision tree presented is here simplified in order to understand first the purpose of the FALLS protocol, to rapidly diagnose the cause of an unexplained circulatory failure. A full understanding of the FALLS protocol is needed to use this tool in clinical practice [5, 10]. Reproduced and modified from [11] with permission from the publisher.
Cardiac arrest: the SESAME protocol
The SESAME protocol requires an ultrasound machine with all of the advantages for optimal access to the patient, when each second matters (narrow machine, 7-s start-up time, only one probe and no buttons to set). Shockable causes are apart the SESAME protocol. The protocol begins by ruling out pneumothorax, a reversible cause of cardiac arrent, in a few seconds. It then assesses a particular vein that is involved in half of cases of massive pulmonary embolism, because we found it easier to see an on–off marker (DVT or no DVT) than a right ventricle enlargement, a finding that is less binary and is window-dependent; importantly, detecting a positive DVT by the BLUE protocol is 99% sensitive to pulmonary embolism. Then, the abdomen is explored to rule out a haemorrhage in a few seconds; then the pericardium, to rule out a pericardial tamponade, also easily and within a few seconds. If none of these four, highly reversible causes has been detected, the prognosis is worse. The heart is then imaged, window permitting. This usually occurs at the 40th second. We do not detail the cardiac data here; please read Chapter 31 of Lung Ultrasound of the Critically Ill: the BLUE Protocol [5] for details of this.
The SESAME protocol does not need any validation: each of the applications has been duly validated. We need only to highlight here the importance of an adapted ultrasound unit.
Only a few details will be explained here. Our microconvex probe is perfect for detecting both the pericardial effusion and the needle, which can therefore be promptly inserted and is far better than the cardiac probes. Inserting a venous line can be done very rapidly (better than with these vascular probes, which have unsuitable ergonomics).
The SESAME protocol can be used with no adaptation when a pneumothorax, a DVT, etc. are sought in less critical settings. The SESAME protocol is an opportunity to convince the community of the importance of a machine that is prepared for the worst but can perform the best with the same ease of use. Fortunately, some modern manufacturers begin to understand this.
The Extended BLUE protocol
The Extended BLUE (E-BLUE) protocol takes the best of the data extracted from history, physical examination, blood tests and other tests (if needed)to improve the accuracy of the BLUE protocol. Blood gases do not feature in the E-BLUE protocol (this test is painful and the information from it is not critical once a BLUE protocol has been performed). Thoracocentesis is one tool of the E-BLUE protocol; we must mention here that we do not recommend ultrasound during the puncture (this is of limited interest once the physician knows where to insert the needle). In rare instances, the E-BLUE protocol will ask for tools such as Doppler, expert echocardiography and transoesophageal echocardiography. In these cases, we simply use the DIAFORA (Doppler intermittently asked from outside in rare applications) approach: we call an expert who comes, during clinic hours, with the sophisticated machine and the three traditional probes.
Other clinical applications in critically ill and less critically ill patients
The Pink, Fever and CLOT protocols
The Pink protocol regards patients who are not “blue” (dyspnoeic) because they are under high oxygen and sedation, namely those with ARDS. It is more comprehensive than the BLUE protocol (large scanning including the lateral wall and apex). For those who wish to score lung injuries, please read again our words on the B-profile (above). The air/fluid ratios of the BLUE profiles are as follows.
the A′-profile contains 100% air;
the A-profile contains ∼99.5% air;
in our opinion, the B-profile with septal rockets has no less than ∼98% air and the B-profile with ground-glass rockets not less than roughly 75–95% air;
the B′-profiles will be a bit richer in fluids;
the C-profile indicates fluids; and
PLAPS indicate full loss of aeration.
The Fever protocol (not an acronym) details a simple way to search for the cause of a fever in a patient admitted to the ICU for a long time. The usual sites are: first, the lung; then the jugular or femoral veins, which appear often thrombosed; and the maxillary sinus.
Venous ultrasound can replace lung ultrasound in some cases. The CLOT (catheter-linked occult thromboses) protocol invites one to think differently when searching for a pulmonary embolism in ventilated patients (e.g. ARDS). If an internal jugular or femoral catheter is or has been present and if a DVT is present, we consider that the combination of a worsening of the clinical condition with the sudden disappearance of the DVT is a strong argument for evoking a pulmonary embolism.
The LUCIFLR project
Lung Ultrasound in the Critically Ill Favoring Limitation of Radiations (LUCIFLR) is a promising application, of major interest in paediatrics. It aims to decrease, in the three next decades, one-third of the bedside chest radiographs and two-thirds of urgent CTs, which is a reasonable target. Radiographs should of course not be eradicated (this would be suboptimal, as radiographs are sometimes of great interest; this would also result in a scary acronym).
LUCI can be applied in order to limit radiation dose in many instances, not only in the critically ill but also in less critical settings, including in family medicine. In many aspects, LUCI is superior to CT, such as for finding necrotising areas in pneumonia [15], assessing the dynamic of air bronchograms for distinguishing pneumonia from obstructive atelectasis [16], assessing lung compliance by studying lung sliding [17], and studying diaphragmatic disorders and anomalies in a pleural effusion. All these points of clear superiority, albeit with those of slight inferiority, can lead us to consider LUCI a reasonable bedside gold standard.
Which disciplines
In short, LUCI is of use in all those disciplines where the physician carries a stethoscope around the neck. This means, first, critical care, emergency medicine, pre-hospital medicine and all paediatric settings (the emergency department, neonatal ICU, etc.). Second, specialties such as cardiology, pulmonology, internal medicine, nephrology, gynaeco-obstetrics, geriatrics, etc. In fact, all disciplines that have to deal with the lung. Neonates and elderly patients benefit from the same approach, without real adaptation, since the signs are universal. In pulmonology, the puncture of superficial masses is an expert procedure that can be performed during clinic hours and we refer the reader to expert sources [18].
What of body habitus? Whether the patient is underweight or bariatric, the lung can be considered a superficial organ. In bariatric patients, the lung can be located confidently using the BLUE points. The anterior profiles of the BLUE protocol can usually be observed. The posterior disorders are more difficult to assess but the concept of the BLUE points allows precise definition of the boundary between the chest and abdomen (which is usually not well estimated clinically) and the concept of PLAPS allows detection of the presence of any structural image at this level.
LUCI can be performed in both wealthy and resource-scarce countries: the applications developed by CEURF were defined using a simple, easy-to-purchase machine with a single, universal probe, without Doppler or other facilities. That which is of value in a sophisticated ICU is performed the same way in any other setting.
Some words before concluding
Have we defined holistic ultrasound? A discipline is holistic when each component must be considered together with the others in order to be able to understand the whole; each of these components impacts the others to result in a logical entirety.
To understand holistic ultrasound, one must see that critical ultrasound without the lung is nothing; this is beyond doubt. However, lung ultrasound is nothing without being integrated into a whole-body approach; this is why we use a system that naturally integrates the lung (same probe, same settings and the same philosophy of use, i.e. looking at the heart when the lung does not inform and looking at the lung when the heart does not inform).
This being said, let us focus on a peculiar point. It is increasingly seen in modern ultrasounds machines that there is a “lung” setting. We have mixed feelings about this. On the one hand, it indicates that some manufacturers are beginning to understand the spirit of critical ultrasound (which must include the lung to be a real discipline). On the other hand, we do not really understand the purpose of this setting because when making a whole-body ultrasound in critical situations, we use the same setting for everything (i.e. devoid of any filters) and this gives the best compromise for assessing all targets of the SESAME protocol. Instead of a “lung” setting, might we suggest a “SESAME”, “whole body” or “critical” setting?
Conclusions
In writing this article, the title of which turns around modernity, we were once more obliged to describe what we do daily with an “old” machine. Even the machine we used initially, the ADR-4000 of 1982, was able to make what clinicians of today call a revolution, even in the most modern settings. All applications of LUCI (and critical ultrasound, including simple heart ultrasound) were defined, assessed and developed using our “antique” technologies. There is no space for confusion in the world of LUCI. By considering the simplicity of the equipment, we have, in all, a tool that is the most suitable for dealing with the worst (SESAME protocol in cardiac arrest) but that also remains excellent in less critical settings (LUCIFLR programme), from sophisticated ICUs to more austere areas of the world. This is more than a point of view; this is a mandatory definition of critical ultrasound, a holistic discipline once the lung is included [19]. Fortunately, manufacturers are beginning to understand our requirements and our past (and present) will be the future of the next generation of physicians.
Acknowledgements and notes on videos
I thank François Jardin (Medical ICU, Boulogne, France), who made this project possible by allowing us to formally join his ICU.
Videos of the A-profile (normal anterior lung surface), B-profile (usually seen in haemodynamic pulmonary oedema), B′-profile (pneumonia/acute respiratory distress syndrome) and A′-profile (usually pneumothorax) mentioned in this article can be seen at will at http://www.ceurf.net/en/blueprotocol.htm
Footnotes
Conflict of interest None declared.
References
- 1.Weinberger SE, Drazen JM. Diagnostic procedures in respiratory diseases In: Harrison’s principles of internal medicine. 16th Edn. New York, McGraw-Hill, 2005; pp. 1505–1508. [Google Scholar]
- 2.Laënnec RTH. Traité de l’Auscultation Médiate, ou Traité du Diagnostic des Maladies des Poumons et du Cœur. Paris, J.A. Brosson & J.S. Chaudé, 1819. [Google Scholar]
- 3.Williams FH. A method for more fully determining the outline of the heart by means of the fluoroscope together with other uses of this instrument in medicine. Boston Med Surg J 1896; 135: 335–337. [Google Scholar]
- 4.Hounsfield GN. Computerized transverse axial scanning. Br J Radiol 1973; 46: 1016–1022. [DOI] [PubMed] [Google Scholar]
- 5.Lichtenstein D. Lung Ultrasound in the Critically Ill: the BLUE Protocol. Heidelberg, Springer-Verlag, 2016. [Google Scholar]
- 6.Lichtenstein D. Lung ultrasound in the intensive care unit. Res Signpost Recent Res Devel Respir Critical Care Med 2001; 1: 83–93. [Google Scholar]
- 7.Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134: 117–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pesenti A, Musch G, Lichtenstein D, et al. . Imaging in acute respiratory distress syndrome. Intensive Care Med 2016; 42: 686–698. [DOI] [PubMed] [Google Scholar]
- 9.Weil MH, Shubin H. Proposed reclassification of shock states with special reference to distributive defects. Adv Exp Med Biol 1971; 23: 13. [DOI] [PubMed] [Google Scholar]
- 10.Lichtenstein DA. BLUE-protocol and FALLS-protocol: two applications of lung ultrasound in the critically ill. Chest 2015; 147: 1659–1670. [DOI] [PubMed] [Google Scholar]
- 11.Lichtenstein D. Fluid administration limited by lung sonography: the place of lung ultrasound in assessment of acute circulatory failure (the FALLS-protocol). Expert Rev Respir Med 2012; 6: 155–162. [DOI] [PubMed] [Google Scholar]
- 12.Staub NC. Pulmonary edema. Physiol Rev 1974; 54: 678–811. [DOI] [PubMed] [Google Scholar]
- 13.Guyton CA, Hall JE. Textbook of medical physiology. 9th Edn. Philadelphia, W.B. Saunders Company, 1996. [Google Scholar]
- 14.Gargani L, Lionetti V, Di Cristofano C, et al. . Early detection of acute lung injury uncoupled to hypoxemia in pigs using ultrasound lung comets. Crit Care Med 2007; 35: 2769–2774. [DOI] [PubMed] [Google Scholar]
- 15.Lichtenstein D, Peyrouset O. Is lung ultrasound superior to CT? The example of a CT occult necrotizing pneumonia. Intensive Care Med 2006; 32: 334–335. [DOI] [PubMed] [Google Scholar]
- 16.Lichtenstein D, Mezière G, Seitz J. The dynamic air bronchogram. A lung ultrasound sign of alveolar consolidation ruling out atelectasis. Chest 2009; 135: 1421–1425. [DOI] [PubMed] [Google Scholar]
- 17.Lichtenstein D. Lung ultrasound (in the critically ill) superior to CT: the example of lung sliding. Korean J Crit Care Med 2017; 32: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mathis G. Chest Sonography. Heidelberg, Springer Verlag, 2008. [Google Scholar]
- 19.van der Werf TS, Zijlstra JG. Ultrasound of the lung: just imagine. Intensive Care Med 2004; 30: 183–184. [DOI] [PubMed] [Google Scholar]