Abstract
As imaging techniques are ever-evolving, this article aims to provide a brief overview of the various modalities including their limitations. The ability of imaging for evaluation of implant osseo-integration will be addressed and also the role of imaging in assessing septic and aseptic loosening, with a particular focus on adverse tissue reactions, will be discussed. Specific features when imaging the big joints such as shoulder, hip, knee and ankle joint will also be outlined.
Overall, a lack of standardisation and validity was noted and despite the gross variety of imaging modalities, there is no technique covering all aspects required for evaluation of implant fixation and septic and aseptic loosening. Each imaging modality has a role, depending on the information required and anticipated. The choice of imaging technique should not be primarily based on medical considerations but also on availability, accessibility, expertise and costs. Plain radiographs alone have been recommended in cases of suspected peri-prosthetic joint infections, given the lack of evidence for additional imaging techniques in this context. For aseptic loosening, ultrasound and plain radiographs may serve as initial screening tools. Metal artefact reducing sequences (MARS) MRI are advancing cross-sectional imaging and are likely to promote their role in patient evaluation.
We conclude that imaging is one essential part in the work-up of patients with total joint replacements, within a specific clinical context. Close teamwork between experienced radiologists and orthopaedic surgeons is required for optimal patient care.
Cite this article: EFORT Open Rev 2017;2. DOI: 10.1302/2058-5241.2.160058. Originally published online at www.efortopenreviews.org
Keywords: imaging techniques, total joint replacement, adverse tissue reaction, peri-prosthetic loosening, osseo-integration
Why it is important for an orthopaedic surgeon to know about imaging modalities in peri-prosthetic assessment
Imaging is one essential part in the work-up of patients with total joint replacements. Although the actual scans are not done by the orthopaedic surgeon, understanding the respective advantages and disadvantages of each imaging modality is required for optimal patient care.
With regard to failed joint replacements, pre-operative assessment is critical for determining the underlying failure mechanisms in order to prevent it from happening again. Implant type, position and stability need to be determined and any defects in the bone and soft tissue should be noted. It goes without saying that detected abnormalities based on imaging should be assessed for consistency with the patients’ history and findings from the physical examination before coming to a final impression.
The evidence shows that implant failure is most commonly for two reasons, aseptic loosening or infection, where both of them may significantly affect the patient’s quality of life. As treatment depends on diagnosis, an early and accurate decision is crucial as delayed revision surgeries have been associated with poorer outcome.1,2 Ideally, decision making should be achieved without any invasive diagnostic method, but in a quick, cost-effective and reliable way. With a steady increase in demand for joint arthroplasty in young adults and an aging population the absolute number of (failed) implants is expected to rise, necessitating the appropriate imaging techniques.
As imaging modalities are ever-evolving, this article aims to provide a brief overview of the various techniques including their limitations. We will then assess the ability of imaging in evaluating implant osseo-integration and discuss the role of the various imaging modalities in the evaluation of septic loosening and aseptic loosening. A particular focus will be on adverse tissue reactions. Finally, specific features when imaging big joints, such as shoulder, hip, knee and ankle, will be determined. This paper will be completed with some suggestions for future directions. The authors would like to highlight that all information is evaluated from an orthopaedic perspective. Technical details will not be addressed.
Which considerations should be taken into account prior to imaging?
Various imaging modalities for peri-prosthetic assessment exist. An overview of the different techniques and their respective advantages and disadvantages is provided in Table 1. Generally speaking, there is no “one-fits-all” solution. Each imaging modality has a role, depending on the information required and anticipated. Apart from medical considerations, factors such as availability, accessibility, costs and the need for expertise need to be taken into account as discussed in more detail below .
Table 1.
A comparison of most commonly-used imaging modalities in peri-prosthetic assessment summarised and modified after a review by Nam et al.50
| Imaging modality | Advantages | Disadvantages | |
|---|---|---|---|
| Non-nuclear scanning test | Ultrasound | • Easily available and accessible • Quick to perform • Low costs • May be used to guide injections and biopsy • Can differentiate between cystic and solid lesions • Allows imaging in motion • Not affected by metal artifacts • No ionising radiation |
• Highly dependent on the operator • Poor in assessing bony lesions • Poor in defining exact extension of abnormalities • Poor in evaluating deep lesions • Patient factors affect transmission |
| Radiograph | • Easily available and accessible • Good for bony details • Low costs |
• Ionising radiation • Lack of soft tissue contrast |
|
| Nuclear scanning tests | Magnetic resonance imaging (MRI) | • Good soft-tissue contrast including neurovascular structures • 3D imaging • No ionising radiation |
• Metal artefacts • Varying sequencing protocols • Time-consuming |
| Computerised tomography (CT) | • Non-invasive • Good for bony details and implant positioning • 3D imaging • May be used to guide biopsy |
• Ionising radiation • Varying sequencing protocols • Relatively poor in soft tissue contrast • High costs |
|
| Positron emission tomography (PET) | • Good in evaluating lytic lesions | • Ionising radiation • Time-consuming • Limited accessibility |
|
| Bone scintigraphy | • Good in characterising bone metabolism | • Ionising radiation • Risk of allergic reaction to the tracer • Time-consuming |
|
| Single-photon emission computed tomography (SPECT) | • 3D imaging • Good in characterising bone metabolism |
• Time-consuming • Ionising radiation • Limited accessibility |
Artefacts due to the presence of metal are a well-known problem in cross-sectional imaging, especially in magnetic resonance imaging (MRI). Metal artefact reduction sequence (MARS)-MRI is a method of minimising metal artefacts without grossly compromising image quality. Specifically, one MRI technique, slice encoding for metal artefact correction (SEMAC), has been reported as favourable in the presences of metal implants.3–6 Compared with conventional MRI however, MARS-MRI is more time-consuming and image quality is inferior due to reduced resolution and signal-to-noise ratio.7 Interestingly, fewer artefacts in MARS-MRI have been found for implants made of titanium or oxidised zirconium as compared with cobalt-chromium which may be explained by differences in susceptibility.8
Bone loss is a typical feature in implant loosening. Multiple classification systems have been designed to assess bone loss and guide treatment. Most of these grading systems are based on whether or not the defect is contained. It is important however to acknowledge that true bone loss is often underestimated on pre-operative radiographs as reported in a retrospective study of 31 patients with symptomatic TKAs and osteolytic lesions confirmed by computed tomography (CT). Plain radiographs however detected only 17% of the osteolytic lesions.9 From our own experience, we feel that this is especially true in certain situations – bone loss due to osteolysis around the acetabular cup of a total hip arthoplasty (THA) and the femoral component of the total knee arthroplasty (TKA). The underlying reason is the complex curved design of those implants components which cover the extent of bone loss. Therefore, size, symmetry and extent of bone loss are ultimately determined intra-operatively after implant removal. A standardised evaluation of any image obtained should be a given as small alterations may provide important information. This may also improve validity of radiological reports as poor inter-observer reproducibility has been reported for peri-prosthetic osteolysis.10
Is imaging useful in evaluating osse-ointegration of the implant?
Osseo-integration, the fusion between implant and bone, is crucial for implant survival and functional outcome, and therefore has predictive potential for the overall success of the total joint replacement. It is commonly defined as a state with no progressive movement between bone and implant.11 Bony ingrowth allowing final fixation is known as secondary fixation. Traditionally, evaluation of osseo-integration has been dominated by use of histology-based methods which may be impracticable in clinical practise. Improving imaging techniques however are increasingly contributing to the understanding of osseo-integration. In pre-clinical studies for example, µ-CT and its ability to generate 3D images has gained importance.12,13
Clinically, most orthopaedic surgeons use plain radiographs for evaluation of implant fixation. In X-rays, successful implant fixation is generally suggested by cortical thickening, bony sclerosis around the total joint replacement and periosteal reaction. A specific key feature for stability is the presence of “spot welds”, cancellous hypertrophy between the prosthesis and the endosteal surface.14
Recently, in vivo bone remodelling in response to implantation of a total joint replacement has been evaluated in a prospective study of 28 patients undergoing stemless shoulder prosthesis for primary osteoarthritis of the shoulder using single-photon emission computed tomography (SPECT)/CT.15 It has been demonstrated that primary osseo-integration is almost completed within the first three months, with the highest metabolic activity at the superior aspect of the stem. The latter has been attributed to different loading conditions of the bone.15 This study demonstrates the ability of nuclear imaging to evaluate the extent and timing of bone remodelling processes secondary to total joint implantation while providing 3D information.
What is the role of imaging in peri-prosthetic joint infections (PJI)?
According to the Philadelphia Consensus Statement on PJI,16 plain radiographic signs suggestive of PJI may include “signs of loosening of previously well-fixed components (particularly loosening seen within the first five years post-operatively)”. Osteolysis or bone resorption around the prosthetic components should not be considered to be related to wear of the bearing surface, particularly if seen at less than five years post-operatively, without subperiosteal elevation or transcortical sinus tracts. It is important to note that plain radiographs are generally normal in the setting of PJI.16 Despite the fact that plain radiograph were recommended to be performed in all cases of suspected PJI, it is not always seen as an accurate diagnostic marker.17 We recommend ruling out septic causes with detailed physical examination, serological test and plain radiograph (Fig. 1)
Fig. 1.
A practical imaging alogrithm when dealing with painful joint on follow up.17,18.
Other imaging modalities are currently not thought to have a direct role in the diagnosis of PJI but have been suggested for differential diagnosis. This recommendation was based on the lack of data for MRI and CT in diagnosing PJI18,19 and because, whilst nuclear imaging has be granted some value in that context, these imaging techniques are still not likely to be advised mainly due to its high costs. In agreement with the above, we would like to underscore the necessity of taking intra-operative samples, where possible, for histological and microbiological testing in the process of diagnosing PJI, apart from the clinical evaluation and blood testing for inflammatory markers.
What is the role of different imaging modalities in aseptic loosening?
According to the recommendations provided by the multidisciplinary consensus statement on the use and monitoring of metal-on-metal (MoM) bearings for THA and hip resurfacing, radiographs should be performed in all patients during follow-up, complemented by other imaging techniques such as ultrasound, CT scan and/or MARS-MRI in cases where any abnormalities are detected. Similarly, additional imaging has been recommended for high serum cobalt values (above the range of 2 µg/L to 7 µg/L).20
Serial radiographs offer an evaluation over time and therefore allow the detection of minimal changes. Thus they are important in demonstrating implant loosening. From a clinical perspective, a rapid time course is worrying – infection and adverse tissue reaction must be excluded. Besides being the image modality performed most often, plain radiographs are also useful with respect to identification of implant class, type, positioning and fixation/loosening, particularly with osteolysis. Change in implant position, also termed as “component migration”, indicates loosening. In the majority of cases, the obtained information indicates the necessity for revision surgery. Surprisingly however, there is no standardisation of frequency of imaging required during follow-up.
Ultrasound has been successfully used for detection of pseudotumours in patients with large-diameter MoM THAs and hip resurfacing, regardless of the extent of symptoms.21–24 Ultrasound compared with MARS-MRI showed comparable sensitivity and specificity in detection of pseudotumours in a prospective cohort of 40 patients with large-diameter MoM heads.7
MARS-MRI has been reported as accurate in a series of 28 hips detecting wear-induced adverse tissue reaction, using conventional pre-operative radiographs and intra-operative information, when available, as a control.25 MARS-MRI is currently thought as most sensitive for quantification of peri-prosthetic osteolysis.26,27 Low T1 and high T2 signals around the implant components may be suggestive of implant loosening.28 Despite its reliability in describing abnormalities in MoM THAs, MARS-MRI fails to consistently differentiate the severity of those soft tissue changes.29 And it has also been shown that MRI images more than one-year-old should not been relied upon for decision-making or planning of revision surgery in failed MoM hips given the low sensitivity reported when using old images.30
CT has been reported to be superior to MARS-MRI in detecting and evaluating the extent of osteolysis but was less useful in detecting and classifying pseudotumours. Also little validity with reference to the extent of muscle atrophy was reported in a study evaluating 50 patients with MoM THA and unexplained pain, assessed by two observers who were blind for clinical data.31 Hybrid SPECT/CT of THA has recently been shown to be reliable in excluding aseptic loosening as well as being beneficial with reference to the extent and maturity of heterotopic ossification.32 CT may also be used for evaluation of acetabular cup position and version.33
Which specific features should be evaluated with reference to the joint being assessed?
Shoulder
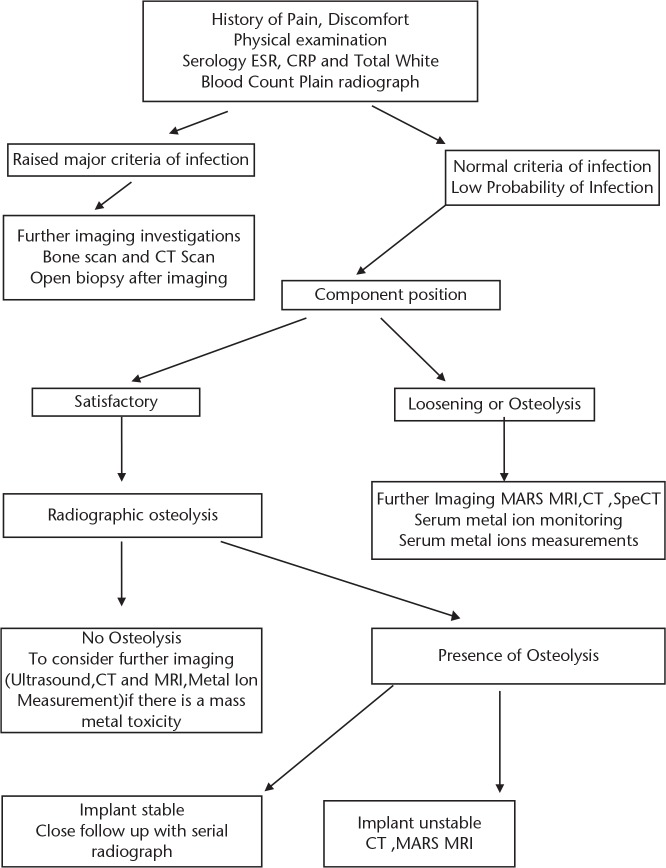
For the shoulder joint, the type of arthroplasty usually depends on the integrity of the rotator cuff, underlining the outstanding role of soft tissue assessment when imaging the shoulder. Ultrasound or MRI may be helpful in differentiating an intact cuff from (partial) tears with or without tendon retraction.34 Particular attention during pre-operative assessment should also be given to the morphology of the glenoid fossa as wear is typically more pronounced in its posterior aspect in primary osteoarthritis, which may even be functionally equivalent to glenoid retroversion. Clinically, this is of relevance as significantly increased stress within the cement mantle and the glenoid bone as well as increased micromotion at the bone-cement interface has been associated with glenoid retroversion.35 SPECT-CT scans have been found to be helpful in evaluating the extent of osteolysis especially over the glenoid bone (Fig. 2).
Fig. 2.
Spect-CT image of a 50-year-old male patient with pain in his left shoulder five years post-total shoulder replacement. Axial Spect-CT image outlining the pathology and osteolysis of glenoid bone.
Hip
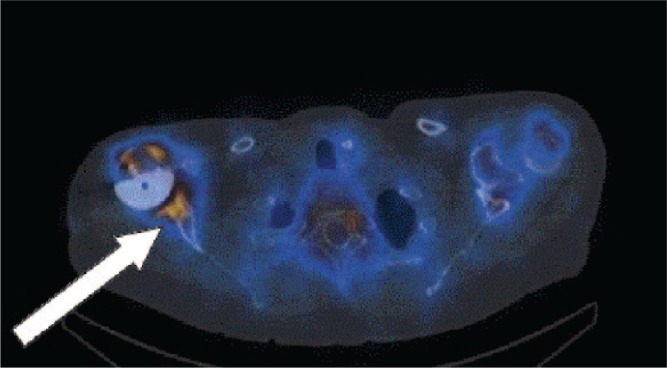
Alteration in stress distribution after implantation of a total joint replacement is a typical phenomenon; especially in the older generation with uncemented THA designs. Proximally, stress-shielding, transmitted by the relatively stiff femoral stem, may lead to decreased bone mineral density, appearing as increased porosity and a reduced thickness of the cortical bone. Distally, stress loading may lead to cortical thickening and sclerosis below the tip of the stem, also referred to as “a bone pedestal”, bridging the medullary canal (Fig. 3). This radiological sign however is not conclusive with regard to implant stability.36
Fig. 3.
Cementless total hip arthroplasty showing implant migration and tilt of the acetabular cup.
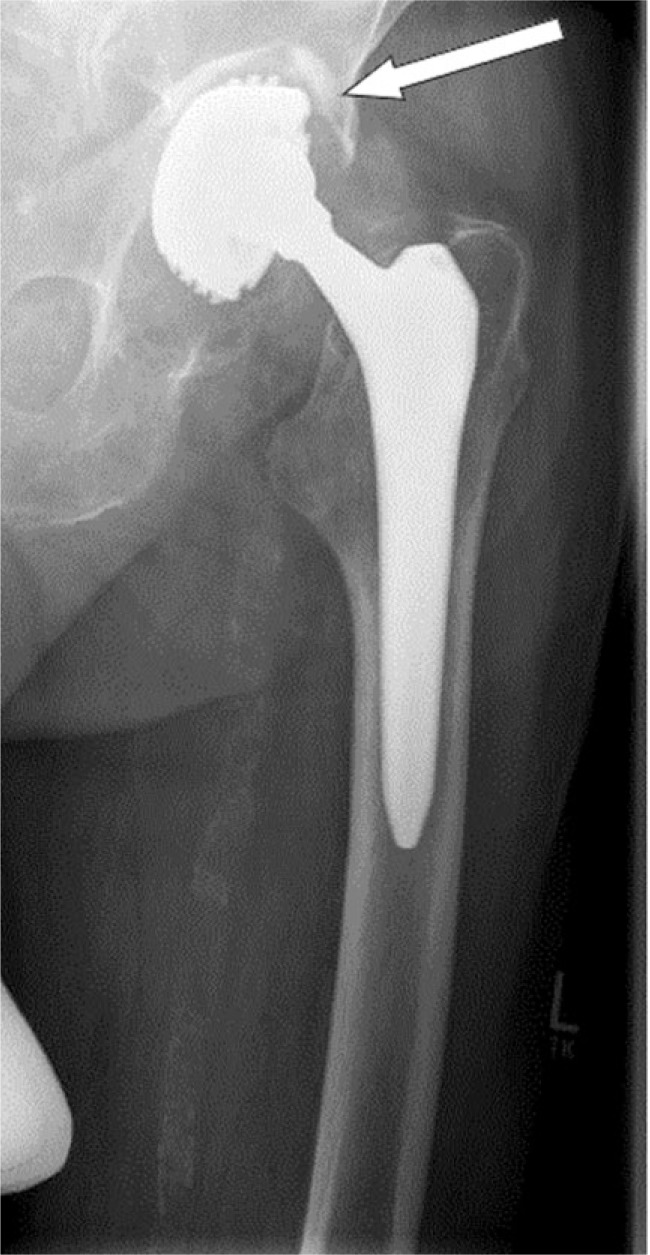
Radiolucencies along the cement-bone interface are commonly described using a classification system proposed by DeLee and Charnley 40 years ago.37 This allows radiologists and orthopaedic surgeons to speak a common language while avoiding equivocal descriptions. A thin radiolucent line separated by a dense sclerotic line parallel to the femoral stem along the bone-cement interface is a frequent finding and is not indicative of implant failure as long as there in no progression in bone loss. It has rather been thought of as the radiological appearance of fibrous membrane formation secondary to cement-bone interactions (Fig. 4).38
Fig. 4.
Cemented femoral stem in a total hip arthroplasty with evidence of gross subsidence, varus tilt and radiolucent lines along the cement mantle.
Loosening of the acetabular component typically appears as cranial migration of the cup or tilting. The “tear-drop” position may be used as a reference point. For the femoral stem, varus tilting and/or gross subsidence are characteristics of loosening which may even result in breakage of any locking screws. In the case of the uncemented stem, early subsidence of the hip stem indicates that the problem was an undersized stem to start with. Wearing-out of the polyethylene may appear as eccentric position of the femoral head in the acetabular cup. The use of the EBRA (Einzel-Bild-Roentgen-Analysis) method is a validated technique for quantifying the implant component migration and wear using plain anterioposterior radiographs of the pelvis.39
Apart from peri-prosthetic osteolysis, granulomatous reactions secondary to wear products may also lead to soft-tissue masses, commonly referred to as pseudotumours. MRI is able to delineate the soft-tissue extent, synovial thickness and volume of a pseudotumour (Fig. 5). Despite the relatively high prevalence of those adverse reactions especially in MoM THAs, the authors would like to emphasise that one should always exclude malignancies.40–43
Fig. 5.
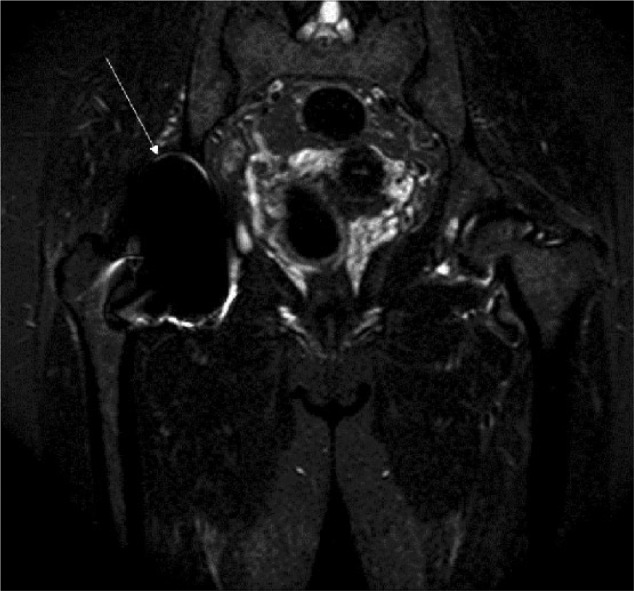
Adverse local tissue reaction with osteolysis in a 70-year-old man. Coronal Image of MR of metal-metal resurfacing hip arthroplasty system showing expansion of pseudocapsule and moderate amount of synovitis.
Knee
Importantly, plain radiographs of the knee should be obtained whenever possible in a weight-bearing mode. Although rather obvious, we sometimes still see patients which have been referred to us, bringing non-weight-bearing x-rays. In cases with severe deformities, we strongly recommend the three-foot standing anteroposterior view of the lower limb for evaluation of the anatomical and mechanical axes.
Component alignment is a surgically-modifiable factor, distributing mechanical forces to the adjacent bone and is considered as essential for successful implantation. Restoration of the anatomical tibial slope and limb axis for example has been found to increase post-operative flexion in posterior-stabilised TKA, provided that coronal alignment has been restored.44 Improved range of motion is a parameter which is expected to gain importance given the high expectations of more and more active patients seeking joint replacement.
Peri-prosthetic osteolysis in TKA is commonly found in the proximal tibia, below the tibial component. In our own institution however, we observed a high number of patients with uncemented NK II implants and screw fixation requiring revision surgery due to osteolysis around the screw holes at the medial side of the tibial component. The radiological appearance is comparable with osteolysis adjacent to screw tracks in the context of THA. We believe that the concept of the effective joint space proposed by Schmalzried et al may explain this pattern of bone loss (Fig. 6).45
Fig. 6.
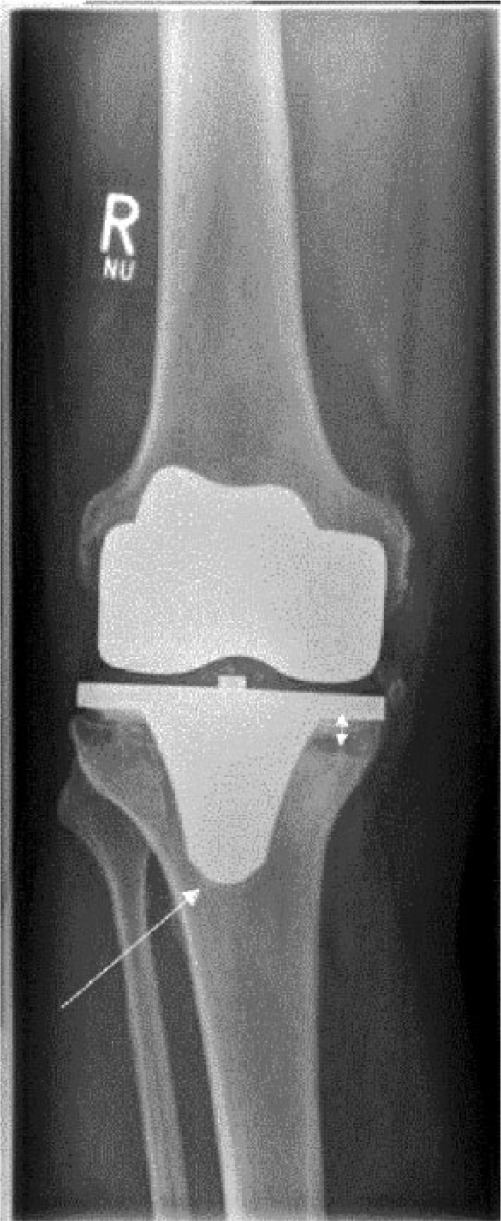
Anteroposterior knee radiograph of 65-year-old patient ten years post-total knee arthroplasty with increased radiolucent lines especially under the tibial component and the appearance of osteolysis and varus tilt.
Ankle
In the context of total ankle replacement, radiographic abnormalities are disproportionately observed. And large peri-prosthetic cysts, also known as “ballooning osteolysis”, are a typical feature in patients with failed total ankle replacements. Most radiographic analyses follow a classification system proposed by Besse et al using a ten-zone protocol.46 Although CT scans are known to describe the extent of ballooning osteolysis more accurately,47,48 no generally-accepted classification system exists so far for that image modality. From our own experience, we feel that CT scans are particularly helpful in assessing remaining bone stock at the time of pre-operative planning.49 (Fig. 7).
Fig. 7.
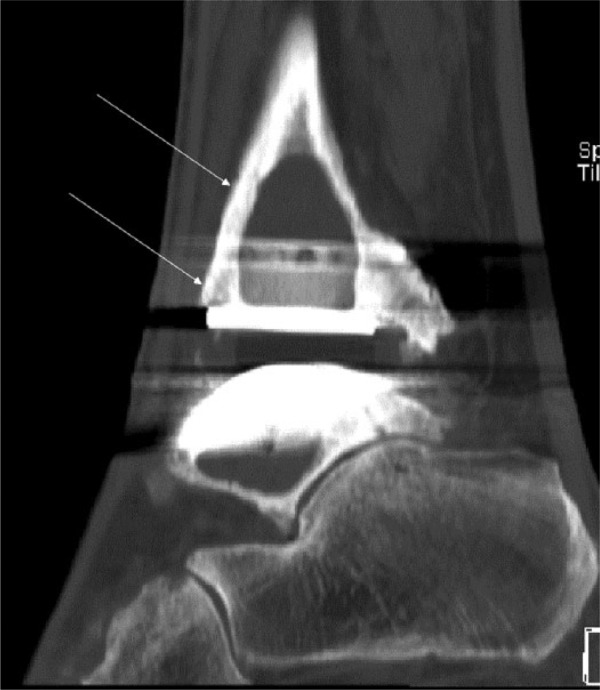
CT image of a 64-year-old female with peri-prosthetic loosening, periostal ballooning and osteolyses of the tibia and talus after ankle arthroplasty.
Limitations and future directions
On a large scale, a major drawback is the lack of a “gold standard” imaging technique in peri-prosthetic assessment. Consequently, various parameters have been referred to as “controls” which may not be representative and may also be subject to selection bias. Study outcome therefore needs to be carefully interpreted. Lack of validation also results in diverse diagnostic algorithms which sometimes even impair study comparison. Moreover, the literature also provides varying definitions for radiological findings such as “radiolucency”,”osteolysis”, “pseudotumour” and “adverse tissue reaction”. Reaching a consensus on a “gold standard”, standardisation of protocols and generating a common classification system in order to standardise the reporting of findings would however be of great benefit and in our opinion should be given priority. Prospective long-term studies are lacking, similarly a systematic qualitative and quantitative comparison of signal changes with a focus on sensitivity and specificity (e.g. meta-analysis for comparing different imaging modalities) is missing.
On a smaller scale, most individual study limitations are due to their retrospective nature. Future research may also seek reduction in radiation exposure and improvement of imaging quality, in particular for protocols which are intended to reduce metal artefacts. Non-invasive imaging modalities providing markers for a clear diagnosis or as a predictive marker for progression of any detected abnormalities would further improve patient care. All of the above however should be further supported by a better understanding of the normal way of how the body responds to an orthopaedic implant from initial primary fixation until final implant loosening.
While the role of imaging is well established in peri-prosthetic assessment, a systematic evaluation of the patient including taking history and a thorough clinical examination are essential for a conclusive diagnosis and necessary in making adequate treatment decisions. A close interdepartmental co-operation between radiology and orthopaedic surgery is fundamental and requires interdisciplinary expertise from both sides. Use of a single imaging modality may not always be sufficient and in doubtful cases should be supported by other imaging technique(s). Lack of validation and standardisation needs to be addressed with priority in order to develop study validity and comparison. Quality improvement of metal-artefact-reducing sequences is expected to allow more accurate image analysis and may also facilitate the generation of a common classification system.
Footnotes
Conflict of Interest: CHL reports that his institution received a grant from the European Commission. He is a board member of the German Society of Orthopaedics and Traumatology and consults for Waldemar Link GmbH & Co KG. All other authors declare no conflict of interest.
Funding
Although none of the authors has received or will receive benefits for personal or professional use from a commercial party related directly or indirectly to the subject of this article, benefits have been or will be received but will be directed solely to a research fund, foundation, educational institution, or other non- profit organization with which one or more of the authors are associated.
References
- 1. Grammatopoulos G, Pandit H, Kwon YM, et al. Hip resurfacings revised for inflammatory pseudotumour have a poor outcome. J Bone Joint Surg [Br] 2009;91-B:1019-1024. [DOI] [PubMed] [Google Scholar]
- 2. Glyn-Jones S, Pandit H, Kwon YM, et al. Risk factors for inflammatory pseudotumour formation following hip resurfacing. J Bone Joint Surg [Br] 2009;91-B:1566-1574. [DOI] [PubMed] [Google Scholar]
- 3. Chen CA, Chen W, Goodman SB, et al. New MR imaging methods for metallic implants in the knee: artifact correction and clinical impact. J Magn Reson Imaging 2011;33:1121-1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Koch KM, Lorbiecki JE, Hinks RS, King KF. A multispectral three-dimensional acquisition technique for imaging near metal implants. Magn Reson Med 2009;61:381-390. [DOI] [PubMed] [Google Scholar]
- 5. Lu W, Pauly KB, Gold GE, Pauly JM, Hargreaves BA. SEMAC: Slice Encoding for Metal Artifact Correction in MRI. Magn Reson Med 2009;62:66-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sutter R, Ulbrich EJ, Jellus V, Nittka M, Pfirrmann CW. Reduction of metal artifacts in patients with total hip arthroplasty with slice-encoding metal artifact correction and view-angle tilting MR imaging. Radiology 2012;265:204-214. [DOI] [PubMed] [Google Scholar]
- 7. Garbuz DS, Hargreaves BA, Duncan CP, et al. The John Charnley Award: diagnostic accuracy of MRI versus ultrasound for detecting pseudotumors in asymptomatic metal-on-metal THA. Clin Orthop Relat Res 2014;472:417-423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ebraheim NA, Savolaine ER, Zeiss J, Jackson WT. Titanium hip implants for improved magnetic resonance and computed tomography examinations. Clin Orthop Relat Res 1992;275:194-198. [PubMed] [Google Scholar]
- 9. Reish TG, Clarke HD, Scuderi GR, Math KR, Scott WN. Use of multi-detector computed tomography for the detection of periprosthetic osteolysis in total knee arthroplasty. J Knee Surg 2006;19:259-264. [DOI] [PubMed] [Google Scholar]
- 10. Engh CA, Jr, Sychterz CJ, Young AM, et al. Interobserver and intraobserver variability in radiographic assessment of osteolysis. J Arthroplasty 2002;17:752-759. [DOI] [PubMed] [Google Scholar]
- 11. Brånemark PI. Osseointegration and its experimental background. J Prosthet Dent 1983;50:399-410. [DOI] [PubMed] [Google Scholar]
- 12. Ko CY, Lim D, Choi BH, Li J, Kim HS. Suggestion of new methodology for evaluation of osseointegration between implant and bone based on μ-CT images. Int J Precis Eng Manuf 2010;11:785-790. [Google Scholar]
- 13. Neldam CA, Lauridsen T, Rack A, et al. Application of high resolution synchrotron micro-CT radiation in dental implant osseointegration. J Craniomaxillofac Surg 2015;43:682-687. [DOI] [PubMed] [Google Scholar]
- 14. Engh CA, Bobyn JD, Glassman AH. Porous-coated hip replacement. The factors governing bone ingrowth, stress shielding, and clinical results. J Bone Joint Surg [Br] 1987;69-B:45-55. [DOI] [PubMed] [Google Scholar]
- 15. Berth A, März V, Wissel H, et al. SPECT/CT demonstrates the osseointegrative response of a stemless shoulder prosthesis. J Shoulder Elbow Surg 2016;25:e96-e103. [DOI] [PubMed] [Google Scholar]
- 16. Parvizi J, Gehrke T. International consensus on periprosthetic joint infection: let cumulative wisdom be a guide. J Bone Joint Surg [Am] 2014;96:441. [DOI] [PubMed] [Google Scholar]
- 17. Tigges S, Stiles RG, Roberson JR. Appearance of septic hip prostheses on plain radiographs. AJR Am J Roentgenol 1994;163:377-380. [DOI] [PubMed] [Google Scholar]
- 18. Love C, Tomas MB, Marwin SE, Pugliese PV, Palestro CJ. Role of nuclear medicine in diagnosis of the infected joint replacement. Radiographics 2001;21:1229-1238. [DOI] [PubMed] [Google Scholar]
- 19. Cyteval C, Hamm V, Sarrabère MP, et al. Painful infection at the site of hip prosthesis: CT imaging. Radiology 2002;224:477-483. [DOI] [PubMed] [Google Scholar]
- 20. Hannemann F, Hartmann A, Schmitt J, et al. European multidisciplinary consensus statement on the use and monitoring of metal-on-metal bearings for total hip replacement and hip resurfacing. Orthop Traumatol Surg Res 2013;99:263-271. [DOI] [PubMed] [Google Scholar]
- 21. Kwon YM, Ostlere SJ, McLardy-Smith P, et al. “Asymptomatic” pseudotumors after metal-on-metal hip resurfacing arthroplasty: prevalence and metal ion study. J Arthroplasty 2011;26:511-518. [DOI] [PubMed] [Google Scholar]
- 22. Pandit H, Glyn-Jones S, McLardy-Smith P, et al. Pseudotumours associated with metal-on-metal hip resurfacings. J Bone Joint Surg [Br] 2008;90-B:847-851. [DOI] [PubMed] [Google Scholar]
- 23. Williams DH, Greidanus NV, Masri BA, Duncan CP, Garbuz DS. Prevalence of pseudotumor in asymptomatic patients after metal-on-metal hip arthroplasty. J Bone Joint Surg [Am] 2011;93-A:2164-2171. [DOI] [PubMed] [Google Scholar]
- 24. Lainiala O, Elo P, Reito A, et al. Good sensitivity and specificity of ultrasound for detecting pseudotumors in 83 failed metal-on-metal hip replacements. Acta Orthop 2015;86:339-344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Potter HG, Nestor BJ, Sofka CM, et al. Magnetic resonance imaging after total hip arthroplasty: evaluation of periprosthetic soft tissue. J Bone Joint Surg [Am] 2004;86-A:1947-1954. [DOI] [PubMed] [Google Scholar]
- 26. Walde TA, Weiland DE, Leung SB, et al. Comparison of CT, MRI, and radiographs in assessing pelvic osteolysis: a cadaveric study. Clin Orthop Relat Res 2005;437:138-144. [DOI] [PubMed] [Google Scholar]
- 27. Weiland DE, Walde TA, Leung SB, et al. Magnetic resonance imaging in the evaluation of periprosthetic acetabular osteolysis: a cadaveric study. J Orthop Res 2005;23:713-719. [DOI] [PubMed] [Google Scholar]
- 28. Duggan PJ, Burke CJ, Saha S, et al. Current literature and imaging techniques of aseptic lymphocyte-dominated vasculitis-associated lesions (ALVAL). Clin Radiol 2013;68:1089-1096. [DOI] [PubMed] [Google Scholar]
- 29. Anderson H, Toms AP, Cahir JG, et al. Grading the severity of soft tissue changes associated with metal-on-metal hip replacements: reliability of an MR grading system. Skeletal Radiol 2011;40:303-307. [DOI] [PubMed] [Google Scholar]
- 30. Lainiala O, Elo P, Reito A, et al. Comparison of extracapsular pseudotumors seen in magnetic resonance imaging and in revision surgery of 167 failed metal-on-metal hip replacements. Acta Orthop 2014;85:474-479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Robinson E, Henckel J, Sabah S, et al. Cross-sectional imaging of metal-on-metal hip arthroplasties. Can we substitute MARS MRI with CT? Acta Orthop 2014;85:577-584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Dobrindt O, Amthauer H, Krueger A, et al. Hybrid SPECT/CT for the assessment of a painful hip after uncemented total hip arthroplasty. BMC Med Imaging 2015;15:18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Roth TD, Maertz NA, Parr JA, Buckwalter KA, Choplin RH. CT of the hip prosthesis: appearance of components, fixation, and complications. Radiographics 2012;32:1089-1107. [DOI] [PubMed] [Google Scholar]
- 34. Petscavage JM, Ha AS, Chew FS. Current concepts of shoulder arthroplasty for radiologists: Part 1–Epidemiology, history, preoperative imaging, and hemiarthroplasty. AJR Am J Roentgenol 2012;199:757-767. [DOI] [PubMed] [Google Scholar]
- 35. Farron A, Terrier A, Büchler P. Risks of loosening of a prosthetic glenoid implanted in retroversion. J Shoulder Elbow Surg 2006;15:521-526. [DOI] [PubMed] [Google Scholar]
- 36. Engh CA, Massin P, Suthers KE. Roentgenographic assessment of the biologic fixation of porous-surfaced femoral components. Clin Orthop Relat Res 1990;257:107-128. [PubMed] [Google Scholar]
- 37. DeLee JG, Charnley J. Radiological demarcation of cemented sockets in total hip replacement. Clin Orthop Relat Res 1976;121:20-32. [PubMed] [Google Scholar]
- 38. Pluot E, Davis ET, Revell M, Davies AM, James SL. Hip arthroplasty. Part 2: normal and abnormal radiographic findings. Clin Radiol 2009;64:961-971. [DOI] [PubMed] [Google Scholar]
- 39. Malak TT, Broomfield JA, Palmer AJ, et al. Surrogate markers of long-term outcome in primary total hip arthroplasty: a systematic review. Bone Joint Res 2016;5:206-214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Fabbri N, Rustemi E, Masetti C, et al. Severe osteolysis and soft tissue mass around total hip arthroplasty: description of four cases and review of the literature with respect to clinico-radiographic and pathologic differential diagnosis. Eur J Radiol 2011;77:43-50. [DOI] [PubMed] [Google Scholar]
- 41. Schmidt AH, Walker G, Kyle RF, Thompson RC., Jr Periprosthetic metastatic carcinoma. Pitfalls in the management of two cases initially diagnosed as osteolysis. J Arthroplasty 1996;11:613-619. [DOI] [PubMed] [Google Scholar]
- 42. O’Shea K, Kearns SR, Blaney A, et al. Periprosthetic malignancy as a mode of failure in total hip arthroplasty. J Arthroplasty 2006;21:926-930. [DOI] [PubMed] [Google Scholar]
- 43. Mallick A, Jain S, Proctor A, Pandey R. Angiosarcoma around a revision total hip arthroplasty and review of literature. J Arthroplasty 2009;24:323.e17-e20. [DOI] [PubMed] [Google Scholar]
- 44. Singh G, Tan JH, Sng BY, et al. Restoring the anatomical tibial slope and limb axis may maximise post-operative flexion in posterior-stabilised total knee replacements. Bone Joint J 2013;95-B:1354-1358. [DOI] [PubMed] [Google Scholar]
- 45. Schmalzried TP, Jasty M, Harris WH. Periprosthetic bone loss in total hip arthroplasty. Polyethylene wear debris and the concept of the effective joint space. J Bone Joint Surg [Am] 1992;74:849-863. [PubMed] [Google Scholar]
- 46. Besse JL, Brito N, Lienhart C. Clinical evaluation and radiographic assessment of bone lysis of the AES total ankle replacement. Foot Ankle Int 2009;30:964-975. [DOI] [PubMed] [Google Scholar]
- 47. Easley ME, Vertullo CJ, Urban WC, Nunley JA. Total ankle arthroplasty. J Am Acad Orthop Surg 2002;10:157-167. [DOI] [PubMed] [Google Scholar]
- 48. Hanna RS, Haddad SL, Lazarus ML. Evaluation of periprosthetic lucency after total ankle arthroplasty: helical CT versus conventional radiography. Foot Ankle Int 2007;28:921-926. [DOI] [PubMed] [Google Scholar]
- 49. Singh G, Reichard T, Hameister R, et al. Ballooning osteolysis in 71 failed total ankle arthroplasties. Acta Orthop 2016;87:401-405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Nam D, Barrack RL, Potter HG. What are the advantages and disadvantages of imaging modalities to diagnose wear-related corrosion problems? Clin Orthop Relat Res 2014;472:3665-3673. [DOI] [PMC free article] [PubMed] [Google Scholar]