Abstract
Neuropsychiatric adverse events have been reported in influenza patients with and without exposure to oseltamivir (Tamiflu®), triggering speculation as to whether oseltamivir may be interacting with any human receptors and contributing to such neuropsychiatric events. In this study, the in vitro selectivity profile of oseltamivir prodrug and active metabolite was investigated. Both compounds lacked clinically relevant pharmacological activities on human, rodent and primate neuraminidases and on a panel of 155 other molecular targets, including those relevant for mood, cognition and behavior. Neuropsychiatric adverse events observed in influenza patients are likely a phenomenon caused by the infection rather than by oseltamivir.
Keywords: Influenza, Neuraminidase, Oseltamivir, Tamiflu, Selectivity, Neuropsychiatric adverse event
1. Introduction
Oseltamivir (Tamiflu®) is a widely used antiviral medication for the treatment and prophylaxis of infections with influenza A and B subtypes, including the A/H5N1 virus. Following oral administration, oseltamivir phosphate (oseltamivir phosphate = oseltamivir prodrug; F. Hoffmann-La Roche Ltd.) is rapidly converted by high-capacity esterases to the active metabolite, oseltamivir carboxylate (Supplementary Fig. S1) (He et al., 1999). In influenza patients, oseltamivir carboylate (oseltamivir carboxylate D-tartrate= oseltamivir active metabolite; F. Hoffmann-La Roche Ltd.) potently inhibits the viral neuraminidases (NA) that are essential for the release of progeny viruses from infected host cells (Moscona, 2005), which substantially reduces the severity and duration of the symptoms of influenza, or prevents their onset (Oxford, 2005). In adults, the standard oseltamivir treatment regimen of 75 mg twice daily for 5 days achieves steady-state plasma Cmax values of 185.7 nM and 1.3 μM for oseltamivir phosphate and oseltamivir carboxylate, respectively. In contrast, central nervous system (CNS) exposure is much lower, with extrapolated brain Cmax levels of ~ 6.7 nM and ~ 77 nM for oseltamivir phosphate and oseltamivir carboxylate, respectively. Plasma pharmacokinetics are similar in Caucasian and Japanese subjects (Schentag et al., 2007).
During the 2005/06 and 2006/07 influenza seasons, an increased number of neuropsychiatric adverse events was reported in influenza patients with and without exposure to oseltamivir. Although rare, events were most commonly reported in Japan and were most frequently observed in children and young adolescents. While no causality could be demonstrated, these findings generated discussions around the CNS tolerability of oseltamivir (Okumura et al., 2006; Goshima et al., 2006). Roche, the manufacturer of oseltamivir, explored possible reasons for the increased reporting (Toovey et al., 2008). These investigations involved: a review of the company’s preclinical and clinical datasets; an assessment of all post-marketing spontaneously reported neuropsychiatric adverse events in the Roche Global Safety Database; and an assessment of the available epidemiological data on the incidence of neuropsychiatric adverse events in patients with and without influenza and/or exposure to oseltamivir. From this review, it could be concluded that the incidence of neuropsychiatric adverse events in influenza patients receiving oseltamivir was no higher than in those who did not receive oseltamivir (Wilcox and Zhu, 2007; Blumentals and Song, 2007), and that there was no plausible mechanism by which oseltamivir phosphate or oseltamivir carboxylate could induce or exacerbate these events (Toovey et al., 2008).
As part of the above review, existing data on the pharmacological activity of oseltamivir phosphate and oseltamivir carboxylate were reexamined. As dictated by its rational design based on the X-ray crystal structure of the influenza virus NA (Lew et al., 2000), oseltamivir carboxylate was shown to be a potent and selective inhibitor of influenza virus NAs (He et al., 1999; Mendel et al., 1998). In a series of new experiments, the existing selectivity profile of oseltamivir was further extended to include previously unexplored targets. Here, we describe the conduct and outcomes of these experiments.
2. Materials and methods
2.1. Drugs
Oseltamivir phosphate and oseltamivir carboxylate D-tartrate were from F. Hoffmann-La Roche Ltd. (Basel, Switzerland). All other drugs were obtained at the highest available purity from Sigma (Buchs, Switzerland), Tocris Bioscience (Bristol, United Kingdom) and Invitrogen (Basel, Switzerland) unless otherwise stated.
2.2. Neuraminidase assay with recombinant human neuraminidases and brain extracts
The cDNA of all four human NA genes were obtained from Origene Technologies Inc. (Rockville, USA) and sequence verified by double stranded DNA sequence analysis (Microsynth AG, Balgach, Switzerland). A cDNA encoding for the viral NA from the oseltamivir-sensitive influenza strain A/Beijing/39/1975 H3N2 was generated by gene synthesis (Sloning BioTechnology GmbH, Puchheim, Germany). The recombinant neuraminidases were expressed either by in vitro translation using the Wheat Germ Rapid Translation System (RTS; Roche Applied Biosciences, Rotkreuz, Switzerland; Neu3 and Neu4) or by transient transfection (Neu1, Neu2, viral NA) in Chinese Hamster Ovary (CHO) cells. In vitro translation was performed essentially as described by the manufacturer, and the enzymatic activities obtained by in vitro translation were used without further purification for selectivity testing in appropriate dilutions of assay buffer (see Supplemental methods). For transient transfection, CHO cells grown in serum-free suspension cultures were transfected by nucleofection (AMAXA AG, Cologne, Germany) with NA cDNA in the pcDNA3.1 Topo TA expression vector (Invitrogen, Basel, Switzerland) and cultivated for 1–2 days under standard conditions (Neu2, viral NA) or for 5 days in the presence of 0.5 μg/ml rapamaycin (Neul). Transient transfection controls included mock-transfected cells (no plasmid) and cells transfected with NA-unrelated cDNA (encoding the G protein-coupled receptor, vasopressin 1b). Lysates prepared from transfected cells were used directly for selectivity testing.
For native non-human primate (Macaca fascicularis) brain NA activity, brain tissue extracts were prepared from fresh brain tissue in buffer solution (320 mM sucrose, 5 mM HEPES, pH 7.4, protease inhibitors) and subsequently separated into microsomal, membranous and cytosolic fractions by differential centrifugations. Homogenates of microsomal, membranous and cytosolic fractions were used for selectivity testing.
For all NAs, the selectivity of oseltamivir phosphate and oseltamivir carboxylate (F. Hoffmann-La Roche Ltd.1, Basel, Switzerland) was assessed by a NA inhibition assay, as described by Potier et al. (1979) with modifications (see Supplemental methods).
2.3. Pharmacological assays for non-NA assays
Oseltamivir phosphate and oseltamivir carboxylate were tested for pharmacological activity on a panel of molecular drug targets either at two concentrations, 3 and 30 μM, respectively, or in a dose-response manner up to 30 μM (metabotropic glutamate receptors, mGlu 2 and 5). Pharmacological tests in an electrophysiological GABAA patch-clamp assay, as well as radioligand binding and functional tests on mGlu2 and mGlu5 were performed at F. Hoffmann-La Roche Ltd. (Basel, Switzerland; see Supplemental information) and all other pharmacological tests were performed at CEREP (Poitiers, France; see Supplementary Table S1 and Supplementary Table S2 of Appendix A for full account of targets and assay conditions). Results were expressed as the percent inhibition of specific binding (radioligand binding assays) or the percent inhibition or stimulation of specific functional activity (functional assays).
3. Results
3.1. Selectivity of oseltamivir phosphate and oseltamivir carboxylate for human and non-human primate neuraminidases
Four human NAs are known to be encoded in the human genome, each with a different subcellular location: Neu1 occurs in lysosomes as part of a multi-enzyme complex; Neu2 is a cytosolic protein; and Neu3 and Neu4 are membrane-associated enzymes (Monti et al., 2002). The selectivity of oseltamivir carboxylate and oseltamivir phosphate for NAs, previously established on the basis of extracts of influenza virus preparations and human liver tissue (Mendel et al., 1998), was established with recombinant enzymes in order to account for those NAs that might be insufficiently represented in liver. In addition, the activity of oseltamivir carboxylate and oseltamivir phosphate was tested on a representative viral NA with known sensitivity to oseltamivir (influenza strain A/Beijing/39/1975 H3N2).
The results of the recombinant human and influenza virus experiments are presented in Fig. 1. In summary, for all 4 human NAs no inhibition was observed up to a concentration of oseltamivir phosphate and oseltamivir carboxylate of 1 mM, with partial inhibitions (about 20–40%) at higher concentrations. For Neu1 (Fig. 1A), oseltamivir phosphate and oseltamivir carboxylate showed no inhibitory activity at concentrations up to 1 mM and a partial inhibition at higher concentrations. For Neu2 (Fig. 1B) and Neu3 (Fig. 1C), oseltamivir phosphate and oseltamivir carboxylate showed no inhibition at concentrations up to 5 mM and a partial inhibition at higher concentrations. Against Neu4 (Fig. 1D), neither oseltamivir phosphate nor oseltamivir carboxylate had an inhibitory activity at concentrations up to 2 mM and a trend for an inhibitory activity at higher concentrations. In contrast to the mammalian NAs, influenza virus NA (Fig. 1E) was inhibited by oseltamivir carboxylate with an 1C50 of 0.3 nM, in full agreement with 1C50 values recorded with the NA activity derived from influenza virus preparations (Mendel et al., 1998).
Fig. 1.
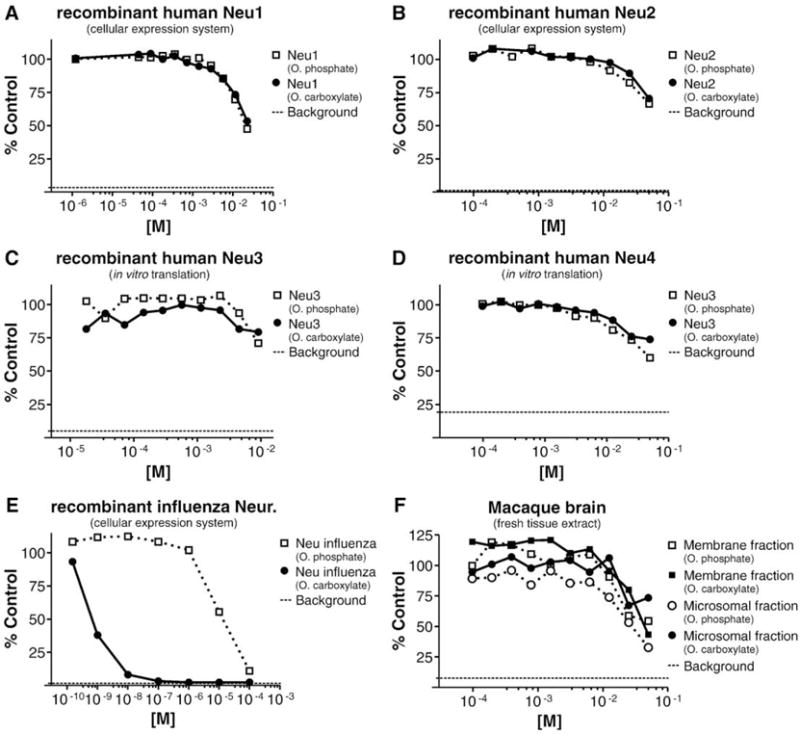
Assessment of oseltamivir phosphate and oseltamivir carboxylate for inhibitory activity against recombinant human NAs Neu1–4, recombinant influenza virus NA, and brain extract NA activity. Human (A–D) and influenza (E; strain A/Beijing/39/1975 H3N2) NAs were expressed by in vitro translation or by transient transfection in CHO cells. Brain extracts (F) were prepared from fresh Macaca fascicularis brain tissue. NA assays as described by Potier et al. (1979) with modifications (see Supplemental methods for assay details). For the transient transfection, the separation between the NA activity present in lysates of CHO cells either mock transfected or transfected with human or influenza NAs was ~ 3-fold for Neu1, ~ 10-fold for Neu2, and > 10-fold for influenza NA. Background: Signal obtained with mock in vitro translation (C and E) or with the reaction mixture lacking cell or tissue lysates (A, B, E, and F).‘% control’: Calculated as‘% control’ = 100 × RFU (test sample) /RFU (reference sample), with RFU = relative fluorescent unit; test sample = mixture of NA activity, substrate, and oseltamivir phosphate/oseltamivir carboxylate; reference sample = mixture of NA activity and substrate without oseltamivir phosphate or oseltamivir carboxylate.
In parallel with these recombinant protein studies, the selectivity of oseltamivir phosphate and oseltamivir carboxylate for the NAs present in non-human primate (M. fascicularis) and rat brain tissue was examined. Phylogenetic analysis (see Supplementary Fig. S2) suggests that human and primate NAs (Neu1–4) are very similar. Indeed, sequence alignments (see Supplementary Fig. S3) revealed that amino acid residues of human and macaque Neu2 are identical in a region within 5 Å of the Neu2 sialic acid binding site. In contrast, phylogenetic and sequence analyses demonstrated substantial differences between the human and rodent NAs. In non-human primate brain tissue, NA activity was detected in the microsomal fraction and the membrane fraction. No activity was present in the cytosolic fraction (data not shown). With both oseltamivir phosphate and oseltamivir carboxylate, no inhibition was detected in the microsomal and membrane fractions at concentrations up to 10 mM. A partial inhibition was observed at higher oseltamivir phosphate and oseltamivir carboxylate concentrations (Fig. 1F). In rat brain-derived tissue, no inhibitory activity was detected at concentrations up to 1 mM (data not shown). It cannot be excluded that the partial inhibition of recombinant human Neu1–4 by oseltamivir carboxylate and oseltamivir phosphate observed at higher concentrations represents a non-specific effect of both compounds, as suggested by the equal potency of oseltamivir carboxylate and oseltamivir phosphate for each of the human enzymes.
3.2. Selectivity of oseltamivir phosphate and oseltamivir carboxylate for non-neuraminidase targets
In a second series of experiments, the selectivity of oseltamivir phosphate and oseltamivir carboxylate against a comprehensive panel of 155 molecular targets unrelated to NAs was evaluated. The panel was composed of ion channels, receptors and enzymes, and included targets with high relevance for mood, cognition and behavior (e.g. neurotransmitter receptors and transporters for dopamine, serotonin, glutamate and GABA), cardiovascular function (e.g. adrenergic and purinergic receptors, hERG and other ion channels), endocrine and metabolic functions (e.g. androgen, estrogen and progesterone receptors, glucocorticoid and insulin receptors) and general cellular function (e.g. phosphodiesterases 1–6, MAP kinase, Na+/K+ ATPase, cathepsins D & L, caspases 3 and 8) (see Supplementary Table S1 and Supplementary Table S2 of Appendix A for a full list of targets).
In Table 1, the findings of the selectivity profiling on targets of high relevance for mood, cognition and behavior are presented. Against these targets and those in the wider profile (see Supplementary Table S1 and Supplementary Table S2 of Appendix A), no relevant pharmacological activity was detected for either oseltamivir phosphate or oseltamivir carboxylate. For eleven targets, low levels of activity were observed (20–41% inhibition/stimulation for oseltamivir phosphate and 20–27% inhibition for oseltamivir carboxylate at either 3 μM or 30 μM). In view of the intrinsic variability of single point measurements, 50% inhibition/stimulation was defined as a priori cut-off below which the observed activity was considered not relevant. For GABAA, oseltamivir phosphate and oseltamivir carboxylate revealed no significant activity in three different radioligand binding assays each employing a different radioligand (Table 1). Further examination in an electrophysiological patch-clamp assay revealed the lack of any significant, inhibitory, excitatory or modulatory activity of oseltamivir phosphate and oseltamivir carboxylate on the endogenous ligand GABA and pentobarbital (Fig. S4 of Appendix A).
Table 1.
Activity of OP and OC against molecular targets of high relevance for mood, cognition and behavior in binding or functional assays.
| Inhibition (% control)
|
||||
|---|---|---|---|---|
| OP
|
OC
|
|||
| 3 μM | 30 μM | 3 μM | 30 μM | |
| Radioligand binding assays | ||||
| GABA (non-selective)a | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| GABAA (central, BZD)a | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| GABAA (TBPS site)a | 6 | n.i.d. | n.i.d. | 2 |
| Dopamine D1 receptor | n.i.d. | n.i.d. | n.i.d. | 8 |
| Dopamine D2S receptor | 11 | 11 | 10 | 1 |
| Dopamine D3 receptor | 1 | n.i.d. | n.i.d. | 1 |
| Dopamine D4 receptor (D4.4 variant) | n.i.d. | n.i.d. | n.i.d. | 3 |
| Dopamine D5 receptor | n.i.d. | 2 | 2 | n.i.d. |
| AMPA-type glutamate receptor | 4 | 17 | n.i.d. | n.i.d. |
| Kainate-type glutamate receptor | n.i.d. | 14 | 10 | 10 |
| NMDA-type glutamate receptor (PCP) | 14 | 23 | 21 | 12 |
| NMDA-type glutamate receptor | n.i.d. | 6 | 12 | 10 |
| Glycine-site on NMDAR (strychnine-insensitive) | n.i.d. | n.i.d. | 1 | 24 |
| Metabotropic glutamate receptor 2b,c | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| Metabotropic glutamate receptor 5b,c | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| Serotonin 5-HT1A receptor | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| Serotonin 5-HT1B receptor | 10 | 4 | n.i.d. | n.i.d. |
| Serotonin 5-HT1D receptor | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| Serotonin 5-HT2A receptor | 4 | 13 | 15 | n.i.d. |
| Serotonin 5-HT2B receptor | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| Serotonin 5-HT2C receptor | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| Serotonin 5-HT3 receptor | n.i.d. | n.i.d. | 1 | n.i.d. |
| Serotonin 5-HT4e receptor | n.i.d. | 13 | n.i.d. | n.i.d. |
| Serotonin 5-HT5A receptor | 6 | n.i.d. | n.i.d. | n.i.d. |
| Serotonin 5-HT6 receptor | 6 | 3 | 3 | 6 |
| Serotonin 5-HT7 receptor | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| Norepinephrine transporter | n.i.d. | n.i.d. | 5 | 11 |
| Dopamine transporter | 8 | 7 | 3 | 7 |
| GABA transporter | 15 | 15 | n.i.d. | 17 |
| Choline transporter (CHT1) | 9 | 5 | 14 | 14 |
| Serotonin transporter | n.i.d. | n.i.d. | 4 | n.i.d. |
| L-type Ca2+ channel (DHP site) | n.i.d. | n.i.d. | n.i.d. | 3 |
| L-type Ca2+ channel (diltiazem site) | 14 | 41 | 10 | 9 |
| L-type Ca2+ channel (verapamil site) | n.i.d. | 5 | n.i.d. | n.i.d. |
| N-type (voltage dep.) Ca2+ channel | 7 | n.i.d. | n.i.d. | n.i.d. |
| SK Ca2+ channel (non-selective) | n.i.d. | 2 | n.i.d. | n.i.d. |
| ATP sensitive K+ channel (Kir6.2) | 20 | 9 | 16 | 14 |
| Voltage gated K+ channel (a-DTX) | n.i.d. | n.i.d. | n.i.d. | −8 |
| Nicotinic acetylcholine receptor (α4β3, BGTX insensitive) | 5 | n.i.d. | n.i.d. | 11 |
| Na+ channel (site 2) | 11 | 38 | n.i.d. | 9 |
| Functional assays | ||||
| Acetylcholinesterase | n.i.d. | 1 | n.i.d. | n.i.d. |
| Monoaminoxidase A | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| Monoaminoxidase B | n.i.d. | n.i.d. | 3 | n.i.d. |
| Metabotropic glutamate rec. 2b,c,d | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
| Metabotropic glutamate rec. 5b,c,d | n.i.d. | n.i.d. | n.i.d. | n.i.d. |
n.i.d.: No inhibition detected.
Binding data complemented with electrophysiological data on human GABAA α1β2γ2, Fig. S4 of Appendix A.
Assay performed at F. Hoffmann-La Roche Ltd.; all other assays performed at CEREP, Poitiers, France Laboratories, Le bois l’Evêque, 86600 Celle l’Evescault, France.
Assay performed in dose-response up to 30 μM.
Functional assay run in agonist and antagonist mode.
4. Discussion
The testing of oseltamivir phosphate and oseltamivir carboxylate for pharmacological activity on recombinant human NAs Neu1–4, native non-human primate brain NA activity and viral NA confirmed the high selectivity of oseltamivir carboxylate for influenza virus NA. Of note, the assays involving the recombinant human NAs revealed no inhibition of NA activity by oseltamivir phosphate and oseltamivir carboxylate at concentrations up to 1 mM for Neu1, 5 mM for Neu2 and Neu3, and 2 mM for Neu4. Likewise, no inhibition of the NA activity present in macaque or rat brain tissue was identified with oseltamivir phosphate and oseltamivir carboxylate at concentrations up to 10 mM and 1 mM, respectively. In contrast with these findings, an IC50 of 0.3 nM was identified for oseltamivir carboxylate on the recombinant influenza virus NA, demonstrating a >3,000,000-fold separation between the potencies of oseltamivir carboxylate on the influenza virus and human host NAs, respectively; the weak inhibitory activity of oseltamivir phosphate on viral NA with an IC50 of about 10 μM was likely due to traces of oseltamivir carboxylate in the oseltamivir phosphate preparation employed (which contains <0.05% oseltamivir carboxylate), as well as minor hydrolysis of oseltamivir phosphate, which might have occurred under the assay conditions. When considering the clinical relevance of these findings, it is noteworthy that, in comparison to the steady-state Cmax levels of oseltamivir phosphate and oseltamivir carboxylate achieved with the standard dosage regimen, the lowest inhibitory oseltamivir phosphate and oseltamivir carboxylate concentrations identified (1 mM) represents an excess of ~ 5000-fold (oseltamivir phosphate) and ~ 750-fold (oseltamivir carboxylate) for the plasma concentrations (oseltamivir phosphate: 185.7 nM; oseltamivir carboxylate: 1.3 μM) and an excess of ~ 150,000-fold (oseltamivir phosphate) and ~ 13,000-fold (oseltamivir carboxylate) for the extrapolated brain tissue concentrations (oseltamivir phosphate: ~6.7 nM; oseltamivir carboxylate: ~77 nM).
Recently, Hata et al. (2008) reported a thorough study on the inhibitory activity of NA inhibitors (including oseltamivir carboxylate) on recombinant human NAs, revealing IC50 values for oseltamivir carboxylate of > 10 mM (human Neu1, Neu3, and Neu4) and >6mM (human Neu2), respectively. At concentrations up to 1 mM no or negligible inhibitory activity of oseltamivir carboxylate on any of the human NAs was observed. On NA activity of different viral strains, oseltamivir carboxylate showed a potent inhibitory activity, with IC50 values in the range of 1.6–7.1 nM. Our data are in full agreement with the data reported by Hata et al. Li et al. (2007) reported an inhibitory activity of oseltamivir carboxylate on multi-histidine tagged recombinant human wild type Neu2 expressed in E. coli and subsequently purified by Ni-NTA and gel filtration chromatography. The potency of oseltamivir carboxylate on this Neu2 preparation was reported with Michaelis constant (Km) = 2.136 mM and inhibition constant (Ki) = 0.432 mM. However, as no IC50 values were provided, no direct comparison with the datasets in this study and in Hata et al. can be made.
Further evidence for selectivity is provided by the pharmacological profiling of oseltamivir phosphate and oseltamivir carboxylate on a panel of targets unrelated to NAs, in which neither had a relevant activity on any of the molecular targets tested. The test concentration of 30 μM in this selectivity screen corresponds to a ~ 160-fold (oseltamivir phosphate) and ~23-fold (oseltamivir carboxylate) excess of the therapeutic plasma levels, and to a ~4500-fold (oseltamivir phosphate) and ~ 400-fold (oseltamivir carboxylate) excess of the extrapolated therapeutic brain tissue levels. The weak pharmacological activities observed on a small subset of targets at 30 μM are not clinically relevant in view of the therapeutic levels of oseltamivir phosphate and oseltamivir carboxylate in humans.
The results of the selectivity screening are further supported by a recent report by Satoh et al. (2007). In this study, neither oseltamivir phosphate nor oseltamivir carboxylate at concentrations up to 100 μM had any effect on dopamine, serotonin or norepinephrine uptake or release from rat brain synaptosomes. In addition, Satoh et al. (2007) reported that oseltamivir phosphate and oseltamivir carboxylate had no effect on [35S]-GTP-γS binding on whole rat brain membranes, demonstrating that neither oseltamivir phosphate nor oseltamivir carboxylate activate any of the Gαi- and Gαs-linked G protein-coupled receptors present in membranes prepared from whole rat brains.
In line with the absence of sedative or proconvulsive properties of either prodrug or drug (Toovey et al. 2008), both oseltamivir phosphate and oseltamivir carboxylate at concentrations up to 30 μM lacked a significant activity on GABAA in radioligand binding assays employing rat cerebral cortex membranes, expressing most of the physiologically relevant receptor isoforms at different levels (Sieghart and Sperk, 2002), and three different radioligands (3H-GABA, a subtype unselective agonist ligand; 3H-flunitrazepam, a high affinity radioligand interacting with the benzodiazepine binding sites; 35S-TBPS, binding within the GABAA chloride channel pore). The lack of an agonistic, antagonistic or modulatory activity of oseltamivir phosphate and oseltamivir carboxylate on the endogenous ligand GABA or a representative benzodpazepine was demonstrated using patch-clamp recordings on recombinant α1β2γ2 GABAA receptor, representing the most abundantly expressed subunit composition in brain tissue (Fig. S4 of Appendix A; Whiting, 2003). Even though oseltamivir phosphate and oseltamivir carboxylate have not been tested individually on each possible GABAA subunit combination, our data collectively provide strong evidence for a lack of pharmacological activity of prodrug and drug on GABAA even at concentrations substantially above therapeutic levels.
Taken together, the above findings confirm that oseltamivir is a highly selective inhibitor of influenza virus NA and that both oseltamivir phosphate and oseltamivir carboxylate lack clinically relevant activities on human and rodent NAs and a broad range of NA-unrelated molecular targets. Moreover, in view of the reported neuropsychiatric adverse events in influenza patients, no relevant activity against molecular targets with high importance for mood, cognition and behavior was observed. The lack of off-target activities revealed by our pharmacological data support the notion that oseltamivir phosphate and oseltamivir carboxylate are unlikely to be involved in the genesis or exacerbation of neuropsychiatric adverse events in influenza patients, and that these neuropsychiatric adverse events represent a disease—rather than drug-related phenomenon (Toovey et al., 2008; Okumura et al., 2006; Goshima et al., 2006).
Acknowledgments
We would like to acknowledge the expert technical support provided by Christophe Fischer, Catherine Diener, Dieter Reinhardt, Christophe Schweitzer, Giuseppe Virgallita, Jennifer Beck, Monique Dellenbach, Elvira Da Silva, Muriel Brecheisen, and Aziz Cetinsu. We also acknowledge the medical writing support provided by Scott Malkin (Gardiner-Caldwell Communications, Macclesfield, UK), who assisted the development, revision and finalization of this manuscript. Funding for this support was provided by F. Hoffmann-La Roche Ltd., the manufacturers of oseltamivir.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at doi:10.1016/j.ejphar.2009.11.020.
Footnotes
Conflict of interest
Lothar Lindemann, Helmut Jacobsen, Diana Schuhbauer, Frederic Knoflach, Silvia Gatti, Joseph G. Wettstein, Hansruedi Loetscher, Tom Chu, Martin Ebeling, Eric Prinssen and Manfred Brockhaus currently are or until recently have been (Manfred Brockhaus) full-time employees of F. Hoffmann-La Roche Ltd. James C. Paulson is a scientific consultant for F. Hoffmann-La Roche Ltd.
References
- Blumentals WA, Song X. The safety of oseltamivir in patients with influenza: analysis of healthcare claims data from six influenza seasons. Med Gen Med. 2007;9:23. [PMC free article] [PubMed] [Google Scholar]
- Goshima N, Nakano T, Nagao M, Ihara T. A clinical study of abnormal behaviors in patients with influenza. Infect Immun Child. 2006;18:371–376. [Google Scholar]
- Hata K, Koseki K, Yamaguchi K, Moriya S, Suzuki Y, Yingsakmongkon S, Hirai G, Sodeoka M, von Itzstein M, Miyagi T. Limited inhibitory effects of oseltamivir and zanamivir on human sialidases. Antimicrob Agents Chemother. 2008;52(10):3484–3491. doi: 10.1128/AAC.00344-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He G, Massarella J, Ward P. Clinical pharmacokinetics of the prodrug oseltamivir and its active metabolite Ro 64-0802. Clin Pharmacokinet. 1999;37:471–484. doi: 10.2165/00003088-199937060-00003. [DOI] [PubMed] [Google Scholar]
- Lew W, Chen X, Kim CU. Discovery and development ofGS 4104 (oseltamivir): an orally active influenza neuraminidase inhibitor. Curr Med Chem. 2000;7:663–672. doi: 10.2174/0929867003374886. [DOI] [PubMed] [Google Scholar]
- Li CY, Yu Q, Ye ZQ, Sun Y, He Q, Li XM, Zhang W, Luo J, Gu X, Zheng X, Wei L. A nonsynonymous SNP in human cytosolic sialidase in a small Asian population results in reduced enzyme activity: potential link with severe adverse reactions to oseltamivir. Cell Res. 2007;17:357–362. doi: 10.1038/cr.2007.27. [DOI] [PubMed] [Google Scholar]
- Mendel DB, Tai CY, Escarpe PA, Li W, Sidwell RW, Huffman JH, Sweet C, Jakeman KJ, Merson J, Lacy SA, Lew W, Williams MA, Zhang L, Chen MS, Bischofberger N, Kim CU. Oral administration of a prodrug of the influenza virus neuraminidase inhibitor GS 4071 protects mice and ferrets against influenza infection. Antimicrob Agents Chemother. 1998;42:640–646. doi: 10.1128/aac.42.3.640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monti E, Preti A, Venerando B, Borsani G. Recent development in mammalian sialidase molecular biology. Neurochem Res. 2002;27:649–663. doi: 10.1023/a:1020276000901. [DOI] [PubMed] [Google Scholar]
- Moscona A. Neuraminidase inhibitors for influenza. N Engl J Med. 2005;353:1363–1373. doi: 10.1056/NEJMra050740. [DOI] [PubMed] [Google Scholar]
- Okumura A, Kubota T, Kato T, Morishima T. Oseltamivir and delirious behavior in children with influenza. Pediatr Infect Dis J. 2006;25:572. doi: 10.1097/01.inf.0000219363.24938.62. [DOI] [PubMed] [Google Scholar]
- Oxford J. Oseltamivir in the management of influenza. Expert Opin Pharmac-other. 2005;6:2493–2500. doi: 10.1517/14656566.6.14.2493. [DOI] [PubMed] [Google Scholar]
- Potier M, Mameli L, Belisle M, Dallaire L, Melancon SB. Fluorometric assay of neuraminidase with a sodium (4-methylumbelliferyl-alpha-D-N-acetylneuraminate) substrate. Anal Biochem. 1979;94:287–296. doi: 10.1016/0003-2697(79)90362-2. [DOI] [PubMed] [Google Scholar]
- Satoh K, Nonaka R, Ogata A, Nakae D, Uehara S. Effects of oseltamivir phosphate (Tamiflu) and its metabolite (GS4071) on monoamine neurotransmission in the rat brain. Biol Pharm Bull. 2007;30:1816–1818. doi: 10.1248/bpb.30.1816. [DOI] [PubMed] [Google Scholar]
- Schentag JJ, Hill G, Chu T, Rayner CR. Similarity in pharmacokinetics of oseltamivir and oseltamivir carboxylate in Japanese and Caucasian subjects. J Clin Pharmacol. 2007;47:689–696. doi: 10.1177/0091270007299761. [DOI] [PubMed] [Google Scholar]
- Sieghart W, Sperk G. Subunit composition, distribution and function of GABA(A) receptor subtypes. Curr Top Med Chem. 2002;2:795–816. doi: 10.2174/1568026023393507. [DOI] [PubMed] [Google Scholar]
- Toovey S, Rayner CR, Prinssen E, Chu T, Donner B, Dutkowski R, Sacks S, Solsky J, Small I, Reddy D. Post-marketing safety assessment of neuropsychiatric adverse event risk in patients with influenza treated with oseltamivir. X International Symposium on Respiratory Viral Infections Singapore; 28th February–2nd March; 2008. Abstract. [Google Scholar]
- Whiting PJ. The GABAA receptor gene family: new opportunities for drug development. Curr Opin Drug Discov Dev. 2003;6:648–657. [PubMed] [Google Scholar]
- Wilcox M, Zhu S. Oseltamivir therapy appears not to affect the incidence of neuropsychiatric adverse events in influenza patients. 47th Interscience Conference on Antimicrobial Agents and Chemotherapy Chicago; 17–20 September 2007; 2007. (poster 114) [Google Scholar]


