Abstract
Immunotherapeutic strategies for malignant glioma have to overcome the immunomodulatory activities of M2 monocytes that appear in the circulation and as tumor associated macrophages (TAM). M2 cell products contribute to the growth-promoting attributes of the tumor microenvironment (TME) and bias immunity towards type 2, away from the type 1 mechanisms with anti-tumor properties. To drive type 1 immunity in CNS tissues we infected GL261 tumor-bearing mice with attenuated rabies virus (RABV). These neurotropic viruses spread to CNS tissues trans-axonally where they induce a strong type 1 immune response that involves Th1, CD8 and B cell entry across the blood brain barrier and virus clearance in the absence of overt sequelae. Intranasal infection with attenuated RABV prolonged the survival of mice bearing established GL261 brain tumors. Despite the failure of virus spread to the tumor, infection resulted in significantly enhanced tumor necrosis, extensive CD4 T cell accumulation and high levels of the proinflammatory factors IFNy, TNFa, and iNOS in the TME merely 4 days after infection, before significant virus spread or the appearance of RABV-specific immune mechanisms in CNS tissues. While the majority of g CD4 cells appeared functionally inactive, the proinflammatory changes in the TME later resulted in the loss of accumulating M2 and increased M1 TAM. Mice deficient in the Th1 transcription factor Tbet did not gain any survival advantage from RABV infection, exhibiting only limited tumor necrosis and no change in TME cytokines or TAM phenotype, highlighting the importance of type 1 mechanisms in this process.
Keywords: rodent, monocytes/macrophages, Th1/Th2 cells, neuroimmunology, tumor immunity
Introduction
Standard of care treatments for malignant glioma offer poor prognosis contributing to an interest in immunotherapeutic strategies. While certain early phase trials of various cell based vaccines and checkpoint inhibitors have shown some promise, most have failed to improve long term survival of patients with highly malignant glioblastoma multiforme (GBM), and none have been approved as standard treatment (1). These studies have reaffirmed that the immunomodulatory nature of the glioma tumor microenvironment (TME) is a key hurdle that must be overcome for successful immunotherapy.
Infiltrating tumor associated macrophages (TAM) are undoubtedly a major contributor to the immunomodulatory nature of the malignant glioma TME (2). These TAM closely resemble M2 macrophages in phenotype, factor expression and function, and are likely to arise from monocytes polarized in the periphery in response to M-CSF and other factors prior to their infiltration into tumor tissue (3–5). TAM elaborate numerous products that can contribute to tumor promotion including growth factors, angiogenic factors, such as VEGF, as well as anti-inflammatory cytokines including IL-10 and TGF-β (6–8). In addition, TAM express elevated levels of PD-L1 and decreased levels of costimulatory markers and MHC class II, which together result in inhibited anti-tumor T cell function (9, 10).
Evidence that glioma malignancy is driven by the infiltration and intratumoral activity of cells either resembling M2 monocytes or with similar functional properties comes from studies in animal models and glioma patients. In the murine orthotopic GL261 glioma model, increasing the ratio of CD11b+ spleen cells implanted with tumor cells accelerates tumor growth (11). In humans, astrocytoma malignancy is closely associated with the levels of M2 TAM; high levels of intratumoral and systemic M2 cells correlate with poor prognosis and resistance to therapy (12–15), and inhibition of M2 polarization inhibits glioma progression (16,17).
Although the CNS is considered somewhat immunologically privileged, immunity can be readily generated to brain tumor antigens in animal models. Mice immunized in the flank with GL261 tumor cells are protected against a subsequent intracranial (i.c.) challenge though GL261-cell specific IgG1 isotype antibodies generated in response to the immunization, revealing a type 2 immune bias (18). Similarly, sera from GBM patients obtained prior to the onset of therapy generally contain tumor-reactive IgG2/IgG4 antibodies suggesting that there has been type 2 /Th2 immune recognition of tumor antigens (19,20). This is consistent with the polarization of monocytes to M2 and tumor progression rather than therapeutic anti-tumor immunity which is considered to require a type 1/Th1 response. Type 1 immunity is associated with the activation of Th1 CD4+ and CD8+ T cells, the production of cytokines such as IFN-γ, TNFα, and IL-12, enhanced M1-polarization of monocytes/macrophages, and a reduction in Treg activity, which in the case of tumor antigens can result in cell infiltration into tumor tissues and tumor cell destruction through a variety of mechanisms (21–25). The use of a dendritic cell (DC)-based vaccine to provoke Th1 anti-tumor immunity in glioma patients has shown therapeutic promise (26). In a mouse model of glioma, promoting a Th1 anti-tumor response via the adenovirus mediated intratumoral expression of IL-12 has been shown to enhance T cell infiltration and tumor cytotoxicity, survival, and long lasting protection (27). Therapies that shift both the myeloid and T cell response, toward M1 and Th1, respectively, can further enhance these anti-tumor effects (17). However, such therapies are dependent upon delivering the immune effectors into tumor tissues which may be problematic particularly at early, treatable stages in glioma formation where the blood brain barrier (BBB) may still be relatively intact.
The difficulty in delivering type 1 immune effectors into CNS tissues can be overcome by the use of virus infection. In addition to the IL-12 adenoviral construct described above, the utility for glioma therapy of several oncolytic viruses that typically induce a type 1 immune responses has been assessed. However the focus of these studies has been on the oncolytic properties of the virus, and the effects of the associated type 1 immune mechanisms have not been thoroughly examined (28, 29). To determine if type 1 immune mechanisms induced by virus infection may impact glioma growth in the absence of tumor cell lysis, we have used the attenuated, neurotropic rabies virus (RABV) SPBN-GAS. This RABV strain contains multiple attenuating mutations in its glycoprotein that prohibit its reversion to pathogenicity, ensuring its safety even in immunocompromised animals (30, 31). Such viruses spread to the brain via axons, bypassing the BBB, then replicate in neurons and astrocytes causing minimal cell death (32). Unlike pathogenic strains, attenuated RABV trigger changes in the neurovasculature that allow immune effector entry into CNS tissues (33). In normal mice this results in the production of high levels of type 1 cytokines and virus neutralizing antibody in the CNS tissues and, ultimately, clearance of the virus without histological or clinical evidence of pathology. Type 1 immune mechanisms are central to virus clearance from the CNS as mice lacking Tbet, the transcription factor associated with the development of Th1 cells, have a severe deficit in this process despite developing a strong RABV-specific Th2 response (31, 34–36). To determine if the induction of a type 1 response in CNS tissues would alter glioma growth, we infected congenic C57BL/6 and Tbet-/- mice bearing intracranial GL261 tumors with SPBN-GAS, monitoring the immune responses to tumor and viral antigens, cytokine production in the TME, and tumor growth.
Methods
Mice
Eight to ten week old male wild-type C57BL/6 mice and Tbet-/- mice on a C57BL/6 background were obtained from The Jackson Laboratory (Bar Harbor, ME), or raised at Thomas Jefferson University from The Jackson Laboratory founder animals. All procedures were conducted in accordance with Public Health Service Policy on Humane Care and Use of Laboratory Animals under protocols approved by the Institutional Animal Care and Use Committee of Thomas Jefferson University (Animal Welfare Assurance Number A3085-01).
GL261 maintenance and implantation
The GL261 cell line was acquired from the National Cancer Institute. Cells were grown in RPMI supplemented with 10% fetal bovine serum (ΔFBS, Corning Inc., Corning, NY), 4mM L-glutamine (Thermo Fisher, Waltham, MA), 50ug/ml gentamicin (Thermo Fisher, Waltham, MA), and 0.05 mM 2-mercaptoethanol (Sigma-Aldrich, Saint-Louis, MO) at 37°C in 5% CO2. Prior to implantation GL261 cells were harvested with 0.25% Trypsin (Corning Inc., Corning, NY), then washed and suspended in 4°C PBS. For i.c. implantations mice were anesthetized with isoflurane (Vedco, St. Joseph, MO) and 105 GL261 cells in 2μl PBS were stereotactically injected in the right cerebral cortex, 1mm anterior to the bregma and 1mm to the right of the midline at a depth of 3mm. Normal control and RABV-infected control mice received surgeries during which PBS alone was injected.
Quantitative real-time PCR
At 12 and 22 days after tumor implantation, CNS tissues from GL261-implanted animals were dissected, homogenized in TriReagent (MRC, Cincinnati, OH) by passaging through a 20-gauge needle and total RNA was extracted using the RNeasy minikit (Qiagen, Valencia, CA) according to manufacturer's protocol. cDNA was synthesized using oligo dT primers, dNTP, and Moloney Murine Leukemia Virus Reverse Transcriptase (Promega, Madison, WI). Quantitative real-time PCR (qPCR) was performed using iQ Supermix or iQ SYBRR Green Supermix (Bio-Rad Laboratories, Hercules, CA), for specific mRNAs with a Bio-Rad iCycler (BioRad, Hercules, CA). The Primer/probe sets (IDT, Coralville, IA) used for L13, SPBN-GAS, CD4, CD8, κ-L chain, IFN-β and IFN-γ were described previously (31) with additional sets listed in Table 1. All probes are dual labeled 5′-6-FAM and 3′-BHQ-1. Sample mRNA copy numbers were normalized to the housekeeping gene L13.
Table I. Primer and probe sequences for qrtPCR.
| Gene | Forward Primer | Reverse Primer | Probe |
|---|---|---|---|
| CXCL10 | 5′-TAC TGT AAG CTA TGT GGA GGT GCG-3′ | 5′-AAC TTA GAA CTG ACG AGC CTG AGC-3′ | 5′-TTC ACC ATG TGC CAT GCC CAG GCT-3′ |
| GZMB | 5′-TCG ACC CTA CAT GGC CTT AC-3′ | 5′-TTG CTG GGT CTT CTC CTG TT-3′ | 5′-TGT CAC TTT GGG GGC CCA CA-3′ |
| ICAM-1 | 5′-CTG CAG ACG GAA GGC AGA TGG T-3′ | 5′-GAG CTA AAG GCA TGG CAC ACG TA-3′ | 5′-CCT GCT GCC CAT CGG GGT GGT GAA-3′ |
| iNOS | 5′-TGG CTA CCA CAT TGA AGA AGC TG-3′ | 5′ TCT GGC TCT TGA GCT GGA AGA AA-3′ | 5′-TGG CCA CCA AGC TGA ACT TGA GCG A-3′ |
| TGF-β2 | 5′-GTG GCT TCA CAA CAA AGA CA-3′ | 5′-TCG CTT TTA TTC GGG ATG AT-3′ | |
| TNFα | 5′-AGG TTC TCT TCA AGG GAC AAG-3′ | 5′-GCA GAG AGG AGG TTG ACT TTC-3′ | 5′-CAC ACC GTC AGC CGA TTT GCT ATC-3′ |
Viral infection and in vitro viral inhibition curves
SPBN-GAS, a variant of the SAD B19 virus with two mutations in the glycoprotein that attenuate the virus and prevent reversion to a pathogenic strain, was propagated in NA cells as described elsewhere (30). Mice were anesthetized with isoflurane and infected with 105 focus forming units (f.f.u.) intranasally (i.n.) or 104 f.f.u. in the left or right cortex. Virus titration in both NA and GL261 cells was performed in 96-well plates when cells were approximately 80% confluent. Virus was added to media in ten-fold dilutions. To assess viral spread inhibition, GL261 cells were grown in supplemented RPMI which was removed at various time points. Cellular debris was removed by centrifugation and conditioned media was applied in two-fold serial dilutions to NA cells that were then infected with virus at 104 f.f.u./ml. Alternatively, conditioned media was applied directly to NA cells and virus was serially titrated. For both virus assays, plates were incubated for 48hr at 34°C, then fixed in 80% acetone at 4°C. FITC-conjugated anti-RABV RNP (Fujirebio Diagnostics, Malvern, PA) was applied with Evans Blue counterstain (J.T. Baker, Phillipsburg, NJ). Virus f.f.u. were counted using a fluorescent microscope.
ELISA and cell-based ELISA
RABV-specific antibody isotypes were assessed by ELISA. Serum samples were collected 0, 8 and 12 days after infection and incubated on UV-inactivated RABV ERA-coated plates. Antibody titers were determined using secondary antibodies specific for IgG as well as IgG1 and IgG2a as described previously (31). A cell-based ELISA was used to detect the level and isotype of GL261-specific antibodies as described previously (18). Antibody isotypes were detected with alkaline phosphatase-conjugated anti-mouse IgG (1:1000), IgG1, IgG2a or IgG2b (1:2000) (MP Biomedicals, Santa Ana, CA).
Histochemistry and immunofluorescence staining
Whole brains were snap frozen in Tissue-Tek O.C.T. compound at 12 and 22 days after implantation (Sakura Finetex, Torrence, CA) fixed in 95% ethanol, rinsed in water, stained with Mayer's hematoxylin solution and Eosin Y solution (Sigma Aldrich, St. Louis, MO), dehydrated and mounted with Richard-Allen Scientific™ Mounting Medium (ThermoFisher, Waltham, MA). Immunofluorescent staining was performed on sections fixed in cold methanol for 10 min at -20°C, rinsed in PBS and incubated with primary antibodies, diluted in PBS containing 2% BSA, 5% goat serum, and 0.25% Triton X-100, overnight at 4°C. Antibodies for RABV nucleoprotein, NeuN, and CD4 have been described previously (31) and additional reagents are listed in Table 2. Slides were then incubated with fluorescence-conjugated secondary antibodies and mounted with Vectashield® Hard Set™ mounting medium (Vector Laboratories, Inc., Burlingame, CA) containing DAPI. Brightfield and fluorescent images were acquired with a Leica DM6000 microscope with the Leica Application Suite v4 program (Leica Microsystems, Switzerland). Image brightness and contrast were adjusted using Photoshop CS5 software.
Table II. Primary and secondary Abs for immunohistochemistry.
| Target | Tag | Clone | Host | Isotype | Dilution | Supplier and reference |
|---|---|---|---|---|---|---|
| Primary Abs | ||||||
| CD11b | PE | M1/70 | Rat | IgG2b | 1/200 | BD Biosciences #553311 |
| CD11c | APC | HL3 | Hamster | IgG1 | 1/200 | BD Biosciences #550261 |
| CD31 | ER-MP12 | Rat | IgG2a | 1/1000 | AbD Serotec #MCA2388GA | |
| CD206 | Alexa Fluor 647 | MR5D3 | Rat | IgG2a | 1/100 | AbD Serotec #MCA2235A647 |
| F4/80 | Cl-A3-1 | Rat | IgG2b | 1/100 | AbD Serotec #MCA497 | |
| iNOS | N20 | Rabbit | IgG | 1/100 | Santa Cruz #sc-651 | |
| Ki67 | APC | 16A8 | Rat | IgG2a | 1/100 | Biolegend #652405 |
| Secondary Abs | ||||||
| Anti-rabbit | Alexa Fluor 488 | Goat | 1/200 | Life Technologies #A11008 | ||
| Anti-rat | Alexa Fluor 555 | Goat | 1/1000 | Life Technologies #A21434 |
In vitro iNOS induction
GL261 cells were cultured in 4- well Nunc™ Lab-Tek™ chamber slides (ThermoFisher, Rochester, NY) until confluent and treated with 0, 10, 100, or 1000 Units of recombinant mouse interferon-gamma (BD Bioscience, san Jose, CA) for 24 hours. Supernatant was removed from chamber wells and cells were washed once with PBS before fixation with ice-cold methanol for 10 minutes and staining as described above.
Luminex
Serum and tumors were collected from mice 12 days after GL261 implantation. Tumors were homogenized with a 20 gauge syringe and passed through a 70μm strainer, then cultured overnight in GL261 media and culture conditions. The level of relevant factors in serum and tumor supernatant was measured using MILLIPLEX MAP Mouse Cytokine/Chemokine Magnetic Bead Panels (Millipore, Darmstadt, Germany) according to manufacturer protocol. Duplicate samples were analyzed by a FlexMAP 3D (Luminex, Austin, TX).
Statistical Analysis
Experimental data were plotted and statistical analyses were performed using Prism 5.01 (Graph Pad, Inc., San Diego, CA). Survival curves were assessed for significance with the Mantel-Cox test. ELISA, qPCR, and in vitro experiments were analyzed using the Mann-Whitney test. Statistically significant differences between groups are generally denoted as follows: * (p≤0.05), ** (p≤0.01) and *** (p≤0.001). Principle component analysis (PCA) and heat maps were generated to determine patterns in cytokine expression across samples in MeV (37). Luminex mean fluorescent intensity (MFI) values were median centered across each cytokine. Three component 3D PCA plots were calculated for tumor samples and sera samples separately. Statistically significant differences in individual cytokines were determined by Student's t test with p-values based on permutation (critical p value = .05).
Results
Infection with attenuated RABV extends the survival of normal but not Th1 deficient mice bearing i.c. GL261 tumors
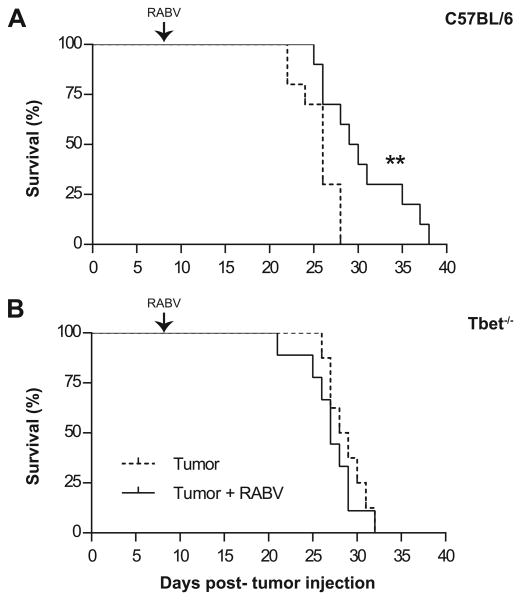
To determine if attenuated RABV infection, known to generate a strong type 1 immune response in CNS tissue, would prolong survival of mice with glioma, mice were implanted in the cerebral cortex with 105 GL261 cells, a dose that causes close to 100% tumor growth, and then intranasally (i.n.) infected with 105 f.f.u. of SPBN-GAS eight days later. Tumor-bearing C57BL/6 mice lived significantly longer when infected with RABV (Fig. 1A). This improved longevity evidently required the Th1 arm of the immune response, as there was no benefit from infection in glioma-bearing Tbet-/- mice (Fig. 1B). Neither wild type (WT) nor Tbet-/- mice exhibited clinical symptoms of RABV infection, and all mice had substantial tumor burdens at sacrifice.
Figure 1. RABV infection prolongs survival of tumor-bearing mice via a Th1-dependent mechanism.
Mice were stereotactically injected i.c. with 105 GL261 cells and then given i.n. PBS (dashed line) or 105 FFU SPBN-GAS (solid line) on day 8 and euthanized when moribund. (A) C57BL/6 mouse survival presented over time, (n=10) per group. Data is representative of two experiments. Statistically significant differences between tumor bearing mice and infected tumor bearing mice measured by Mantel-Cox test and is denoted as: **p< 0.01. (B) Tbet-/- mouse survival data are presented as percent survival over time. Tumor alone (n=8), tumor + RABV (n=9), difference NS.
RABV infection causes the early onset of tumor necrosis
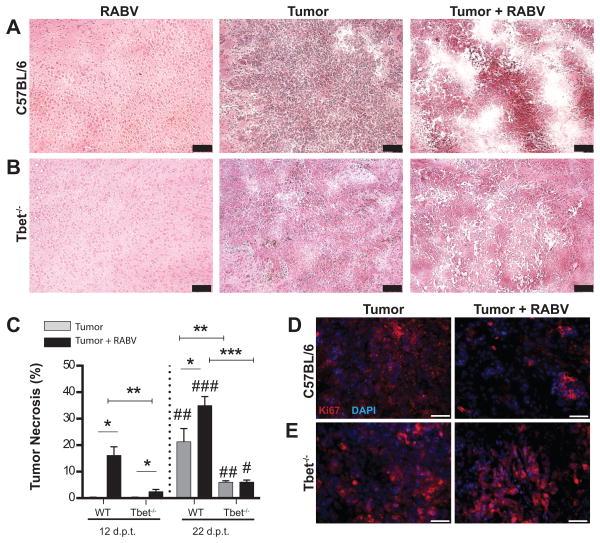
To gain insight into why RABV infection prolongs survival of C57BL/6 but not Tbet-/- mice implanted with GL261 cells, we assessed brain and tumor tissues by histopathology at 12 and 22 days after tumor cell implantation. Differences in tumor tissues between the groups of mice were evident by gross examination with only tumors from infected WT mice exhibiting extensive hemorrhage (not shown). Consistent with their increased survival, tumor tissues from C57BL/6 mice that received SPBN-GAS were considerably more necrotic by 4 days after infection (12 days after GL261 cell implantation) than those from uninfected C57BL/6 or infected and uninfected Tbet-/- mice (Fig. 2A). Despite increasing necrotic areas in tumors from uninfected wild-type mice, infection continued to be associated with greater tumor necrosis in these animals over the next 10 days (Fig. 2C). A slight elevation in tumor necrosis was detected in Tbet-/- mice as a consequence of infection at the early time point but no difference between infected and uninfected Tbet-/- mice was detected later (Fig. 2B-C). Notably, tumor necrosis remained significantly less in these animals than in C57BL/6 mice at 22 days after tumor implantation regardless of treatment. In support of the concept the that tumors in RABV-infected C57BL/6 mice are less viable, Ki67 staining revealed less tumor cell proliferation in these animals by comparison with uninfected counterparts and the Tbet-/- cohort (Fig. 2D-E).
Figure 2. GL261 tumors become necrotic early after RABV infection.
H&E staining was performed on C57BL/6 (A) and Tbet-/- (B) mouse brains at 12 d.p.t. for each cohort of infection controls, tumor alone, and tumor + RABV infection. Scale bar represents 100μm. (C) Percent necrosis was calculated in ImageJ as total necrotic area/ tumor area (pixels) from 2-6 sections/ brain, (n=2) for each condition in C56BL/6 mice and Tbet-/- mice at 12 and 22 d.p.t. Mann-Whitney test: *p<.05, **p<.01, ***p<.001 compared between infected and uninfected mice and WT and Tbet-/- mice. Mann-Whitney test comparing 22 d.p.t. time point to 12 d.p.t. time points: #p<.05, ##p<.01, ###p<.001. (D) Ki67 (red) staining of C57BL/6 and (E) Tbet-/- mouse brain tumors at 12 d.p.t. Fluorescent Images representative of 6 sections per mouse and 2 mice per condition. White scale bars represent 50μm.
RABV does not spread to tumor-bearing cortex or tumor, likely due to secretion of antiviral factors by the GL261 cells
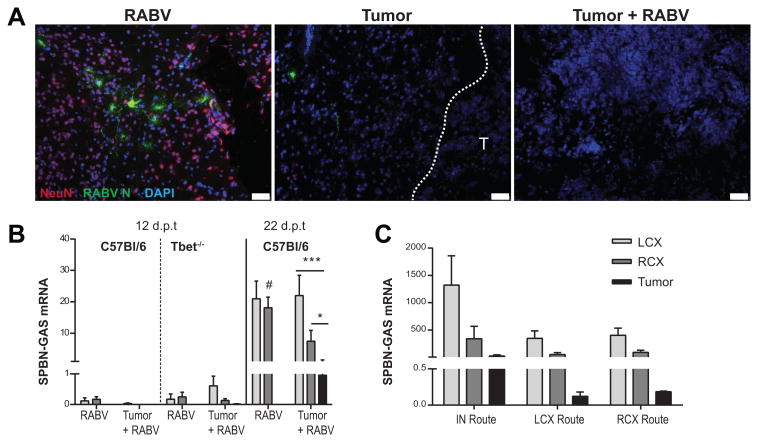
Tumor necrosis is evident in infected C57BL/6 mice as little as 4 days post-infection (12 days post-tumor cell implantation) which is considerably before SPBN-GAS given i.n. spreads through the cortex tissues of normal mice (31) suggesting the possibility that the presence of the glioma enhances virus spread. However, the pattern of staining for virus that emerged in tumor-bearing mice indicates otherwise. When virus becomes detectable in brain tissues by immunofluorescent staining for nucleoprotein at 7 days post-infection it is observed predominantly in the left cortex (LCX) as opposed to the right tumor-bearing hemisphere (RCX) or the tumor itself (Fig. 3A). Analysis of tissues for RABV nucleoprotein mRNA confirms this observation. RABV-infected mice that had undergone implantation surgery but received an i.c. injection of PBS without cells had equal levels of viral mRNA in the LCX and RCX at all time points. When necrosis is observed in tumor tissues from mice infected 4 days previously, the level of viral mRNA in the CNS tissue is very low and undetectable in the tumors of either C57BL/6 or Tbet-/- mice. By 14 days post-infection, when virus is replicating at higher levels in the CNS, viral message was higher in the LCX compared to RCX, and the levels in tumor tissues are significantly lower than in the right cortex, and in turn, significantly lower than those measured in the left cortex (Fig. 3B). These results suggested that the tumor may in fact be inhibiting virus spread to the area. To address this possibility, tumor-bearing and control mice were infected with 104 f.f.u. SPBN-GAS in either the left or right cortex, 12 days after tumor implantation in the right cortex (Fig. 3D). Infection in either cortex resulted in a similar trend to i.n. infection where virus preferably replicated in the non-tumor-bearing LCX and only minimally in tumor tissues.
Figure 3. RABV does not infect tumor or tumor-bearing cortex despite route of infection.
(A) Pattern of RABV spread assessed by immunofluorescence staining in infected tumor bearing mice 15 d.p.t. Labeling of NeuN (red), RABV N protein (green), and DAPI (blue). Images representative of 2 mice/condition and 6 sections/ mouse. Dashed white line and “T” indicates tumor border, scale bar represents 50μm. (B) Mice with and without tumor were infected i.n. 8 d.p.t. and CNS tissue was collected12 and 22 d.p.t. for virus qPCR detection, (n=5). (C) Tumor bearing C57BL/6 mice were infected 12 d.p.t. i.n with 105 FFU RABV (n=4), or i.c. in LCX (n=5) or RCX (n=5) with 104 FFU RABV and CNS tissue was collected 22 d.p.t. Viral mRNA expressed as mean ± SEM copies mRNA /1000 L13 in the LCX, RCX, or tumor. Statistical significance determined by Wilcoxon matched-pairs signed rank test where *p<.05, ***p<.001. Statistical difference between matching section in respective treatment groups measured by Mann-Whitney test and indicated by: #p<.05.
To test the hypothesis derived from the in vivo studies that products of GL261 cells may inhibit RABV replication and spread, we collected GL261 cell supernatants from cultures at various time points, measured secreted factors using a Luminex assay, and added the conditioned media to infected mouse neuroblastoma (NA) cells. Supernatants from NA cells, typically used to grow RABV, were used as controls. Compared to NA cells, GL261 cells secrete high levels of cytokines with anti-viral properties including RANTES, CXCL10, and Lif (Supplemental Fig. 1A). Moreover, GL261-conditioned media was found to inhibit virus infection and spread in NA cells (Supplemental Fig. 1B). Finally, we compared the infectivity of SPBN-GAS for NA and GL261 cells finding that the latter are highly resistant to infection with the virus (Supplemental Fig. 1C- D).
RABV infection promotes CD4 but not cytolytic CD8 T cell infiltration into glioma tissues
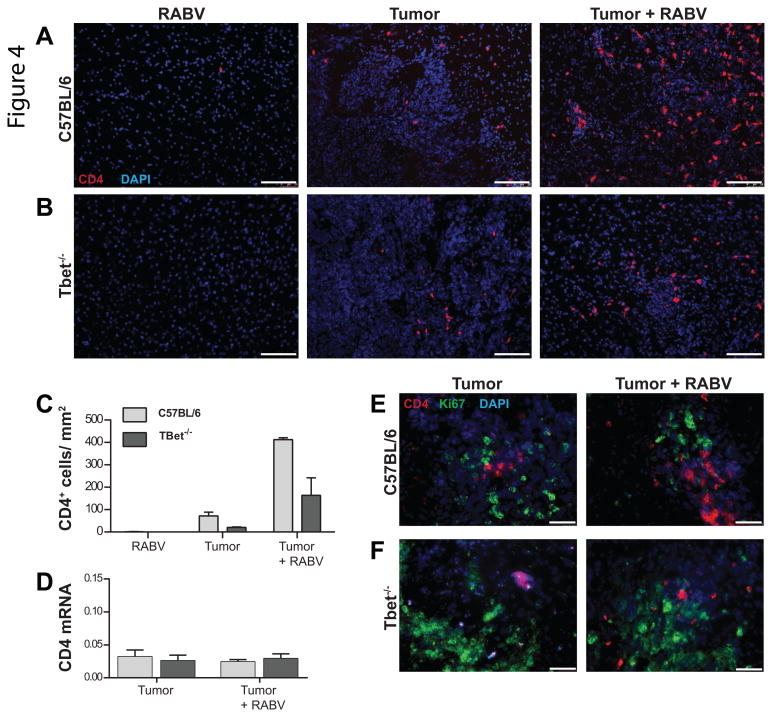
Attenuated RABV infection is known to promote the infiltration of immune cells specific for non-viral antigens into CNS tissues (34). Tumor tissues from infected C57BL/6 and, to a lesser extent, Tbet-/- mice show elevated levels of CD4+ T cell accumulation by immunofluorescence than non-infected controls at 4 days post-infection (Fig. 4A- C). CD4+ T cells were not seen in brain tissues from infected animals without tumors at this time point, and there were no CD4+ T cells present in the cortex surrounding the GL261 tumor in either infected WT or Tbet-/- mice. However, when tumor tissues were assessed for CD4 mRNA levels, no difference between infected and non-infected animals was seen (Fig. 4D). Consistent with this finding, Ki67 staining revealed that CD4+ cells in tumor tissue are not actively proliferating in either C57BL/6 or Tbet-/- tumor-bearing mice regardless of whether or not they had been given RABV (Fig. 4 E-F).
Figure 4. Enhanced CD4+ T cell accumulation occurs in glioma tissue of RABV infected mice.
Immunofluorescence staining performed 12 d.p.t in WT (A) and Tbet-/- (B) mice in infected controls and tumor bearing mice with and without infection. Immunolabeling of CD4+ cells (red) and DAPI (blue) at 10× where scale bar represents 100μm. (C) Quantification of CD4+ cells was performed with ImageJ with data presented as total CD4+ cells /mm2 tumor area. Immunofluorescence images and quantification data are representative of 2 mice/ condition and 6 sections/ mouse. (D) qPCR analysis of tumor tissue from C57BL/6 and Tbet-/- tumor-bearing mice with and without infection performed 12 d.p.t and expressed as CD4 mRNA copies/ 100 L13. n=5 for each condition in C57BL/6 and Tbet-/- mice, differences NS. (E) Immunofluorescence staining of Ki67 (green) and CD4 (red) staining of C57BL/6 and (E) Tbet-/- mouse brain tumors at 12 d.p.t. Fluorescent Images representative of 6 sections per mouse and 2 mice per condition. Images captured at 40×, white scale bars represent 50μm.
RABV infection results in the enhanced expression of ICAM on neurovascular endothelial cells which would be expected to support immune cell infiltration into CNS tissues (38). Infection increased ICAM mRNA expression in the tumors of C57BL/6 mice but not Tbet-/- mice (Supplemental Fig. 2A). However, no increase in CD8 or GZMB expression in tumors of either C57BL/6 or Tbet-/- mice 4 days following RABV infection was detected (Supplement Fig. 2B- C). Similarly, expression of the NK cell marker NKp46 was not significantly altered in tumors of infected WT or Tbet-/- animals (data not shown). Comparison of sections of tumor tissue from the different groups of mice stained for NKp46 and CD8 also failed to detect any increase in the numbers of NK or CD8 T cells as a consequence of RABV infection (data not shown).
RABV infection of glioma- bearing mice has no impact on the tumor cell-specific humoral response
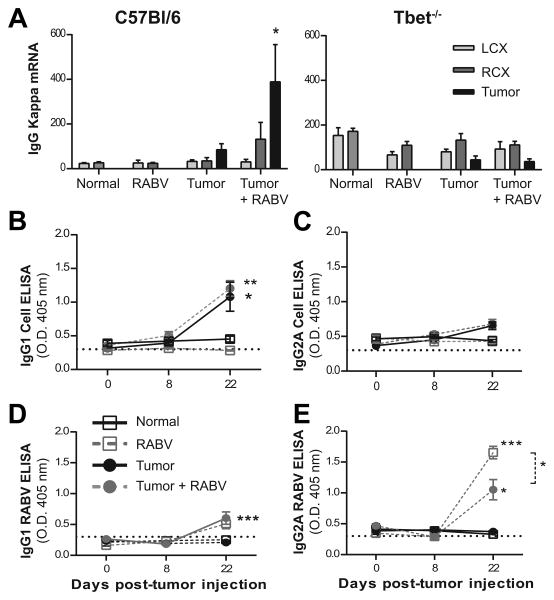
GL261-specific antibodies, which evidently contribute to immune protection against tumor growth in the mouse GL261 model (18), provide insight into the class and magnitude of the tumor-specific immune response. We therefore assessed GL261 and RABV- specific antibody production in the tumor-bearing mice following infection. Significantly higher levels of mRNA specific for the antibody κ-light chain were detected in tumor tissues from infected C57BL/6 mice by comparison with uninfected tumor-bearing animals 4 days after virus infection (12 days post tumor implantation) as well as both infected and uninfected tumor-bearing Tbet-/- mice (Fig. 5A). Levels of mRNA for the B cell marker CD19 were also increased in the tumor tissues of only RABV-infected C57BL/6 mice and staining of tumor sections revealed a slight increase in the numbers of CD19+ cells of these animals (data not shown). However, the development and isotypes of serum GL261-specific antibodies in GL261 tumor-bearing C57BL/6 mice, analyzed using a cell-based ELISA, were unaltered by infection with RABV, becoming significant 22 days after cell implantation and remaining predominantly IgG1 (Fig. 5B and C). On the other hand, the presence of tumor significantly reduced the levels of IgG2A RABV-specific antibodies elicited by virus infection without altering the relatively low levels of virus-specific IgG1 detectable 14 days after infection (Fig. 5D and E).
Figure 5. RABV infection of GL261-bearing mice does not enhance tumor-specific antibody production.
(A) qPCR analysis of CNS tissue was performed 12 d.p.t. in C57BL/6 and Tbet-/- mice, data expressed as mean ± SEM copies κ-L chain/ 100000 L13. Statistical differences between cohorts were not detected. (B) GL261 cell-reactive IgG1 and (C) IgG2A antibody measured via cell-based ELISA and presented as absorbance mean ± SEM in naïve mice, at infection time point, and 22 d.p.t. No statistically significant differences observed. (D) RABV- specific IgG1 and (E) IgG2A antibody isotyping performed via ELISA. Statistically significant differences between naïve and infected cohorts at 22 d.p.t. determined by Mann-Whitney t test with *p<.05, ***p<.01. n= 5 for mock tumor groups, n=10 for infected and uninfected tumor-bearing cohorts. Background absorbance indicated by dashed line.
RABV infection of tumor-bearing mice results in a Th1-dependent proinflammatory shift in the tumor microenvironment
The elevated numbers of infiltrating CD4+ T cells and increase in mRNA specific for antibody κ-light chain in tumor tissues suggests that there may be an impact on the immune status of the TME 4 days after RABV infection despite low levels of virus replication in CNS tissues at this time. To test this hypothesis, we first assessed tumor tissues from control and RABV-infected C57BL/6 and Tbet-/- mice for levels of immunologically-relevant mRNAs known to be induced in the CNS tissues of normal mice at the onset of rabies infection. At 12 days after tumor cell implantation we observed significantly higher levels of IFNγ mRNA expression in the tumor tissues of C57BL/6 mice that received RABV 4 days previously but not in tumor tissues from uninfected mice, Tbet-/- mice (Fig. 6A), or in the left cortex or right cortical tissues surrounding the tumors (data not shown). IFN-β mRNA levels were increased in tumor tissues from the same cohort of animals (Fig. 6B) as were levels of mRNA encoding CXCL10 (Fig. 6C) and TNFα (Fig. 6D). Of note, the levels of these mRNA in the tumor tissues from infected C57BL/6 mice are higher than those seen in similarly infected mice without tumors at this early time-point in the infection (data not shown). In contrast, levels of mRNA specific for the immunomodulatory cytokine TGF-β2 in tumor tissues were lowered by infection (Fig. 6E).
Figure 6. The TME of C57BL/6 RABV- infected mice exhibit an early proinflammatory shift.
(A) IFNγ mRNA expression in C57BL/6 and Tbet-/- mice at 12 d.p.t. presented as mean ± SEM copies mRNA/100 L13. (B) IFN-β expression fold change shown as mean ± SEM normalized to L13. (C) CXCL10 mRNA expression as mean ± SEM copies/ 100 L13. (D) TNFα expression mean ± SEM mRNA copies/ 100000 L13. (E) TGF-β2 expression in tumors of infected and uninfected C57BL6 and Tbet-/- mice 12 d.p.t., represented as fold change. (F) iNOS mRNA expression presented as mean ± SEM copies mRNA/ 100000 L13. Statistically significant difference measured with qPCR data were determined with Mann-Whitney test and indicated by: *p<.05, **p<.01, ****p<.0001. (G) Fluorescent staining in tumors of C57BL/6 and (H) Tbet-/- mice 12 d.p.t. with and without RABV infection for iNOS (green) and DAPI (blue). (I) Fluorescent staining of iNOS (green) and F4/80 (red) and (J) iNOS (green) and CD11b (red) in tumors of C57BL/6 mice infected with RABV 12 d.p.t. Fluorescent Images representative of 6 sections per mouse and 2 mice per condition. Scale bar indicates 50μm.
Attenuated RABV infection is known to induce the expression of iNOS, an enzyme responsible for the production of nitric oxide and associated radicals, primarily by cells closely associated with the neurovascular unit as opposed to infiltrating M2 macrophages (33). iNOS mRNA levels appeared to be selectively elevated in the tumor tissues of RABV-infected C57BL/6 but not Tbet-/- mice (Fig. 6F). To provide further insight into the nature of the iNOS+ cells, sections from the cortex and tumor tissues of C57BL/6 and Tbet-/- mice, both uninfected or infected 4 days previously, were stained for iNOS and CD31, a marker that is normally found on endothelial cells but can be expressed by intratumoral macrophages (39). In the tumor bearing mice CD31+ cells can be seen throughout the sections from both mouse strains, but substantial iNOS staining is only seen in tissues from RABV-infected C57BL/6 mice, colocalizing with CD31+ vasculature in the cortex (not shown) and appearing in the cytoplasm and nucleus of CD31- cells in the tumor tissue (Fig. 6G-H). To determine whether or not macrophages are responsible for the iNOS expression in the tumors of RABV-infected C57BL/6 mice, tumor tissues from these animals were co-stained for iNOS and the macrophage markers F4/80 (Fig. 6I) and CD11b (Fig. 6J). While there was evidence of macrophage infiltration into tumor tissues at this early time point, iNOS expression localized to a different cell type, which we speculate based on morphology and the absence of macrophage markers for (CD11b and F4/80) is primarily the GL261 tumor cell. To investigate the possibility that the production of IFNγ in the TME of RABV-infected C57BL/6 mice may promote iNOS expression in GL261 cells, we incubated the cells with increasing concentrations of mouse recombinant IFNγ in vitro and stained the cells for iNOS. In culture, untreated GL261 cells exhibited low but detectable nuclear and cytoplasmic iNOS staining that increased in intensity with the addition of increasing concentrations of IFNγ (Supplemental Fig. 3).
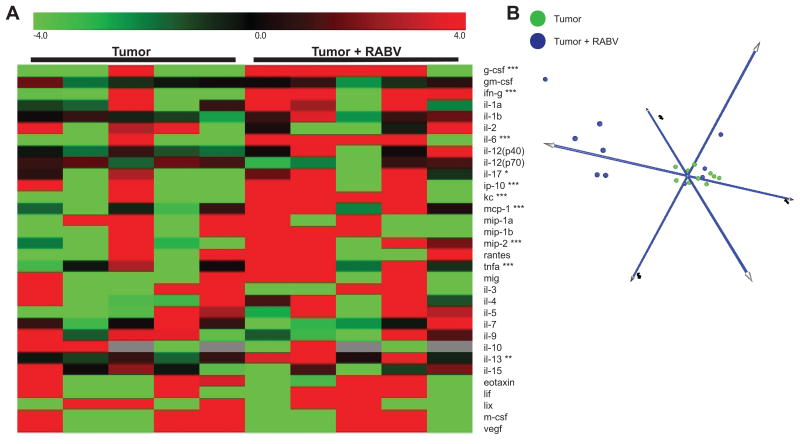
To provide further insight into the changes in the tumor tissues caused by cell infiltration and any functional changes in the GL261 tumor resident cells, supernatants from overnight cultures of dispersed cells from tumor tissues, excised from both 4-day infected and uninfected C57BL/6 mice 12 days post GL261 cell implantation, were analyzed for mouse cytokine/chemokine production by Luminex. A heat map representation and PCA of the data reveal distinct differences in the factors secreted by cells from the tumor tissues of infected versus uninfected mice, with those from the former producing significantly higher levels of a number of primarily type 1 cytokines including G-CSF, IFN-γ, IL-6, IL-17, CXCL10, MIP-2 and TNFα (Fig. 7A-B). Statistically significant differences cytokine/chemokine levels between sera obtained at the same time from infected and uninfected tumor-bearing mice were not detected (data not shown).
Figure 7. Differential cytokine levels are secreted by tumor cells of infected and uninfected mice.
(A). Heat map representation of cytokine levels from ex vivo tumor supernatant excised 12 d.p.t. from C57BL/6 mice with and without RABV infection, as measured by Luminex. Significantly different expression of individual cytokines determined by permutation test. Statistically significant differences indicated by *p<.05, **p<.01, ****p<.0001. (B) PCA projection of Luminex data clustered by sample groups for tumor supernatant of infected (blue) and uninfected (green) tumor bearing mice. N= 5 mice per condition, replicates averaged for heat map expression and biological replicates reported in PCA analyses.
RABV infection causes a shift in TAM from M2 to M1
At 15 days post-GL261 cell implantation similar numbers of CD206+ macrophages had accumulated in the tumor tissues regardless of whether or not the mice had been infected (data not shown). However when sections of tumors obtained from similar mice at 22 days post-implantation were stained for the macrophage markers CD11b and F4/80, the M2 marker CD206, and CD11c, which tends to be expressed at low levels on M2 macrophages and high levels on M1 macrophages, different patterns emerged for infected versus uninfected mice. While the majority of the CD11b+ cells apparent in the tumor tissues of uninfected C57BL/6 mice co-expressed CD206, the majority of the CD11b+ cells in tumors from infected C57BL/6 mice did not (Fig. 8A). CD11b and CD206 were expressed by different cell populations in tumor tissues from Tbet-/- mice but these were unchanged by infection (Fig.8B). When F4/80 was used to identify macrophages and CD11c used to examine the cell subsets, TAM in uninfected C57BL/6 mice were negative for CD11c, but positive for CD11c in tumor tissues from infected mice (Fig. 8C). In contrast, tumor tissues from uninfected Tbet-/- mice contained large numbers of F4/80+/CD11c+ populations with infection resulting in lower numbers of these cells and cohorts of cells that were either F4/80+ with low levels of CD11c or individually positive for each marker (Fig. 8D). Analysis of mRNA from tumor tissue at this late time point (22 days post- implantation) also showed significant reductions in the expression of genes associated with M2 cells including the phenotypic markers CD163 and CD206. The expression of genes encoding the M2 products restin like alpha (Retnla/FIZZ1) and Arg1, were also reduced although the reduction in Arg1 was not statistically significant. In contrast levels of expression of Ym1 which is also a product of M2 cells, were increased in tumors of infected mice. In addition to CD11c, mRNA encoding CD38, a marker expressed by a variety of activated immune cells including M1 macrophages, was found at significantly higher levels in tumors of RABV- infected mice (Supplemental Fig. 4).
Figure 8. RABV infection alters TAM polarization in GL261 tumors.
(A) IF staining 22 d.p.t. for CD206 (green), CD11b (red) and DAPI (blue) in tumors of infected and uninfected C57BL/6 and (B) Tbet-/- mice. (C) Tumors stained for CD11c (green), F4/80 (red) and DAPI (blue) in WT and (D) Tbet-/- infected and uninfected mice at 22 d.p.t. Images representative of 6 sections per mouse and 2 mice per condition. Scale bar indicates 50μm.
Discussion
Infection of normal mice with attenuated neurotropic RABV drives the production of proinflammatory type 1 cytokines, the expression of iNOS-dependent radicals, and the accumulation of CD4 Th1 cells in brain tissue, which are initially detectable 6 to 8 days after intranasal instillation of the virus (34). On the other hand, malignant gliomas, including those resulting from GL261 cell implantation, are characterized by the induction of type 2 immunity and the recruitment of anti-inflammatory M2 monocytes into the TME (3-5). In this study we show that infection of GL261 glioma-bearing C57BL/6 mice with attenuated rabies causes a profound change in the immune bias of the TME from type 2 to type 1 which is associated with extensive tumor necrosis and results in prolonged survival.
Because RABV does not spread to tumor tissues the TME alterations that occur as a consequence of RABV infection likely result from immunological signals originating in infected tissues distant to the tumor that trigger the production of proinflammatory cytokines in the tumor, ultimately leading to tumor cell death. We observe increased IFNy, TNFα and iNOS within the tumor tissue merely 4 days after RABV infection in C57BL/6 but not in Th1-deficient Tbet-/- mice. The outcome is consistent with prior reports that TNFα and IFNγ provide a therapeutic benefit in glioma, their expression promoting upregulation of iNOS which activates cell death cascades through the activity of its product nitric oxide (40-41). Accordingly, tumor-bearing uninfected C57BL/6 and uninfected as well as infected Tbet-/- mice, where there is minimal expression of proinflammatory cytokines, have lower iNOS expression and show little tumor necrosis. Whereas GL261 cells are positive for GFAP only at the tumor margin (data not shown and previously observed (42)) the iNOS-positive cells were scattered throughout the tumor parenchyma in infected mice generating a Th1 response. Astrocytes can be triggered to express iNOS by IFNγ and TNFα and iNOS-positive astrocytes have been observed in inflammatory lesions in mice with experimental allergic encephalomyelitis (43). Moreover, GL261 can be induced to express iNOS in vitro by IFNγ treatment. Our data therefore suggest that GL261 cells, transformed astrocytes, can also express iNOS in response to IFNy, and suggest that these are the predominant iNOS expressing cell in tumors undergoing necrosis as a consequence of the response to attenuated RABV.
Corresponding with the early onset of tumor necrosis 4 days after infection 12 days following GL261 cell implantation, increased CD4+ T cells are observed in the tumor tissues of mice capable of generating a type 1 antiviral response and likely contribute to the antitumor effect and altered TME. The presence of these CD4+ cells in tumor but not surrounding tissue, along with their appearance merely four days after infection, suggests that they are not RABV-specific. In non-tumor bearing mice intranasally infected with attenuated RABV this is at the point when virus first appears in CNS tissues and it takes several additional days for antigen-specific cells to appear in the CNS. Moreover, virus spread to not only tumor tissues but also the surrounding parenchyma is limited. Therefore we consider that the CD4+ T cells are entering tumor tissues nonspecifically as a consequence of the local production of proinflammatory cytokines such as TNFα and the effects of free radical activity in the tumor, processes that have been implicated in the activation of vasculature and effector entry into CNS tissue (34, 44, 45). While the early onset of the CD4 cell entry, proinflammatory factor upregulation, and necrosis occur concomitantly and are likely related, there is a disparity between the high numbers of CD4+ T cells observed in the tumors and relatively low levels of CD4 mRNA in in tumor tissues. A similar phenomenon has been observed in Tbet-/- mice clearing attenuated RABV where there is substantial recruitment of Th2 CD4 cells to the CNS but the cells express low levels of activation markers and CD4 mRNA unlike the Th1 CD4 T cells that enter the CNS tissues of C57BL/6 in response to the virus (31). This phenomenon has not been reported for other neuroimmune processes which are generally associated with Th1 or Th17 cell infiltration. Consequently, we speculate that the current results may reflect a CD4 infiltrate that is predominantly Th2, as is the natural response to GL261 antigens (18), and a common mechanism where Th2 cells largely lose transcriptional activity in CNS tissues. While this concept is supported by the lack of Ki67 expression by the tumor infiltrating CD4 cells it remains to be validated in other models. While the activity of Th1 CD4 cells that enter tumor tissues is expected to be inhibited by the TME (46, 47) we expect that the anti-inflammatory mechanisms responsible are counteracted by the response to attenuated RABV. The fact that the therapeutic effect of attenuated rabies virus infection is only seen in mice that can mediate a Th1 response suggests that elements of the type 1 response are responsible, possibly including the activity of a limited subset of tumor antigen-specific Th1 cells in tumor tissues underlying more extensive Th2 accumulation. Alternatively, NK or CD8 T cells in the tumors of infected C57BL/6 mice, while not increased in number, may have acquired enhanced activity. Further experimentation is necessary to characterize the precise cell subsets involved.
A later effect of the high expression levels of type one factors in the TME of C57BL/6 mice infected with attenuated RABV, subsequent to the onset of necrosis, is a change in the TAM population. Approximately 10 days after the onset of tumor necrosis and the proinflammatory shift in the TME there is a profound reduction in expression by TAM of the M2 marker CD206+ and an increase in the expression of CD11c which is generally considered an M1 marker (48, 49). Together with the data from our studies of macrophage subset gene expression in tumor tissues, this suggests that RABV infection induces a shift in TAM away from a more typical M2 subset towards a less differentiated or M1 phenotype. Although the latter bear the M1 phenotype marker CD11c, we were unable to determine whether or not they express the functional M1 marker iNOS due to the extensive expression of this enzyme in the infected tissues. Tbet-/- mice, which have large populations of both CD206+ M2 and CD11c+ M1-like cells within their GL261 tumors, do not display any alterations in the polarization of these cells as a consequence of RABV infection reaffirming that this is a type 1-dependent process. Polarization of macrophages away from the M2 phenotype can block glioma progression. Specifically, administration of a CSF-1R inhibitor resulted in the loss of M2 cells, and though this was associated with an increase in macrophages with phagocytic functions, M1-related gene expression was not otherwise increased (16). M1 macrophages are known to produce certain of the type 1 factors that we observe in the infected animals, as well as iNOS which can directly contribute to tumor cell lysis through the production of nitric oxide and associated cytotoxic radicals (50). While iNOS is expressed in the tumor tissues 4 days after RABV infection of mice that had GL261 cells implanted 8 days previously, it does not appear to be expressed by the macrophages at this time. Consequently, TAMs are unlikely to be contributing to iNOS- dependent tumor necrosis at its onset.
TAM are a heterogeneous continuum of highly plastic macrophage subsets that carry out diverse functions and respond to changes within the tumor (5, 51). While we cannot rule out the possibility that M2 TAM selectively undergo cell death and are replaced by infiltrating M1 macrophages, our data is consistent with the concept that macrophage populations in the glioma TME can be re-educated by the local cytokine milieu, as described by other investigators (16, 52. 53). The precise mechanisms responsible for the TAM re-polarization observed here are unknown, yet are undoubtedly dependent on type 1 immune processes acting in the TME as TAM polarization occurs in the absence of any differences in serum cytokine levels between infected and uninfected tumor-bearing mice.
Interestingly, Th1- dependent TME modulation, iNOS expression, tumor necrosis, TAM polarization, and the increased survival of RABV-infected tumor-bearing C57BL/6 mice occurs without direct infection of the tumor, a requirement for oncolytic virus therapy. RABV replicates predominantly in the non- tumor bearing hemisphere and is largely excluded from the tumor, presumably due to antiviral factors secreted by GL261 cells. Also distinct from other immunotherapeutic strategies, the Th1-dependant changes in the TME are not accompanied by an enhanced Th1- biased glioma-specific peripheral response as measured by serum antibody. Instead, antibody isotype analysis indicates a shift in the RABV-specific response towards type 2 in the presence of a glioma. This bias has little impact on the pathogenicity of the virus as it is safe and readily cleared in mice that lack a range of immune components, including Tbet (31).
The failure of RABV infection to promote systemic Th1 anti-glioma humoral immunity may be due to the transient nature of the antiviral response. This may contribute to the fact that the mice eventually succumb to their tumor. However, we do not yet know whether tumor growth, which was severely curtailed, or other factors relevant to the anti-tumor immune response are responsible for the animals' demise. At death the extent of necrotic tissue in the brains of the animals was substantial. Conceivably, an adjustment in the timing of infection or concomitant vaccination with a type 1-biased glioma vaccine may improve the long-term outcome.
In summary, this study reports that infection of GL261 tumor- bearing mice with attenuated, neurotropic RABV prolongs survival and increases tumor necrosis via a Th1-dependent proinflammatory shift in the tumor microenvironment. Striking antitumor effects are achieved despite the limited ability of RABV to infect GL261 tumor cells. The change in the TME associated with the onset of tumor necrosis is accompanied by the expression of IFNγ, TNFα, and iNOS and followed by the replacement of tumor-supportive M2 with potentially destructive M1 cells. Mice lacking the Tbet transcription factor responsible for generating type 1 immunity did not exhibit increased survival, tumor necrosis, a proinflammatory TME modulation, or a beneficial change in the TAM population, indicating a Th1 dependent mechanism is responsible for these antitumor effects. Better understanding of how infection with attenuated, neurotropic RABV leads to modulation of the TME has therapeutic implications for brain tumor immunotherapy.
Supplementary Material
Acknowledgments
The authors thank Carla Portocarrero, Dr. Larry Harshyne and Dr. Aurore Lebrun for technical assistance, Rhonda Kean for review of the manuscript, and Drs. David Andrews and Bernhard Dietzschold for constructive suggestions.
This work was supported by National Institutes of Health Grant AI093369 (to D.C.H.).
Non-standard abbreviations
- BBB
Blood- brain barrier
- GBM
glioblastoma multiforme
- TAM
tumor associated macrophage
- TME
tumor microenvironment
- RABV
rabies virus
References
- 1.Reardon A, Wucherpfennig KW, Freeman G, Wu C, Chiocca EA, Wen PY, Curry WT, Mitchell DA, Fecci P, Sampson JH, Dranoff G. An update of vaccine therapy and other immunotherapeutic approaches for glioblastoma. Expert Rev Vaccines. 2013;12(6):597–615. doi: 10.1586/erv.13.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Parney IF, Waldron JS, Parsa AT. Flow cytometry and in vitro analysis of human glioma–associated macrophages: Laboratory investigation. J Neurosurg. 2009;110(3):572–82. doi: 10.3171/2008.7.JNS08475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur J Cancer. 2006;42(6):717–27. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence T, Natoli G. Transcriptional regulation of macrophage polarization: enabling diversity with identity. Nat Rev Immunol. 2011;11(11):750–61. doi: 10.1038/nri3088. [DOI] [PubMed] [Google Scholar]
- 5.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18(5):349–55. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 6.Lin EY, Li JF, Gnatovskiy L, Deng Y, Zhu L, Grzeski DA, Qian H, Xue X, Pollard JW. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Research. 2006;66(23):11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 7.Ahn G, Brown JM. Matrix metalloproteinase-9 is required for tumor vasculogenesis but not for angiogenesis: Role of bone marrow-derived myelomonocytic cells. Cancer Cell. 2008;13(3):193–205. doi: 10.1016/j.ccr.2007.11.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ostuni R, Kratochvill F, Murray PJ, Natoli G. Macrophages and cancer: from mechanisms to therapeutic implications. Trends Immunol. 2015;36(4):229–39. doi: 10.1016/j.it.2015.02.004. [DOI] [PubMed] [Google Scholar]
- 9.Kennedy BC, Showers RC, Anderson DE, Anderson L, Canoll P, Bruce JN, Anderson RCE. Tumor-Associated Macrophages in Glioma: Friend or Foe? J Oncol. 2013;2013 doi: 10.1155/2013/486912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bloch O, Crane CA, Kaur R, Safaee M, Rutkowski MJ, Parsa AT. Gliomas promote immunosuppression through induction of B7-H1 expression in tumor-associated macrophages. Clin Cancer Res. 2013;19(12):3165–75. doi: 10.1158/1078-0432.CCR-12-3314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chae M, Peterson TE, Balgeman A, Chen S, Zhang L, Renner DN, Johnson AJ, Parney IF. Increasing glioma-associated monocytes leads to increased intratumoral and systemic myeloid-derived suppressor cells in a murine model. Neuro-Oncol. 2014;17(7):978–91. doi: 10.1093/neuonc/nou343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhai H, Heppner FL, Tsirka SE. Microglia/macrophages promote glioma progression. Glia. 2011;59(3):472–85. doi: 10.1002/glia.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Prosniak M, Harshyne LA, Andrews DW, Kenyon LC, Bedelbaeva K, Apanasovich TV, Heber-Katz E, Curtis MT, Cotzia P, Hooper DC. Glioma Grade Is Associated with the Accumulation and Activity of Cells Bearing M2 Monocyte Markers. Clin Cancer Res. 2013;19(14):3776–86. doi: 10.1158/1078-0432.CCR-12-1940. [DOI] [PubMed] [Google Scholar]
- 14.Lu-Emerson C, Snuderl M, Kirkpatrick ND, Goveia J, Davidson C, Huang Y, Riedemann L, Taylor J, Ivy P, Duda DG, Ancukiewicz M, Plotkin SR, Chi AS, Gerstner ER, Eichler AF, Dietrich J, Stemmer-Rachamimov AO, Batchelor TT, Jain RK. Increase in tumor-associated macrophages after antiangiogenic therapy is associated with poor survival among patients with recurrent glioblastoma. Neuro-Oncol. 2013;15(8):1–9. doi: 10.1093/neuonc/not082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ye X, Xu S, Xin Y, Yu S, Ping Y, Chen L, Xiao H, Wang B, Yi L, Wang Q, Jiang X, Yang L, Zhang P, Qian C, Cui Y, Zhang X, Bian X. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J Immunol. 2012;189(1):444–53. doi: 10.4049/jimmunol.1103248. [DOI] [PubMed] [Google Scholar]
- 16.Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, Olson OC, Quick ML, Huse JT, Teijeiro V, Setty M, Leslie CS, Oei Y, Pedraza A, Zhang J, Brennan CW, Sutton JC, Holland EC, Daniel D, Joyce JA. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. 2013;19(10):1264–72. doi: 10.1038/nm.3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kosaka A, Ohkuri T, Okada H. Combination of an agonistic anti-CD40 monoclonal antibody and the COX-2 inhibitor celecoxib induces anti-glioma effects by promotion of type-1 immunity in myeloid cells and T-cells. Cancer Immunol Immunother. 2014;63(8):847–57. doi: 10.1007/s00262-014-1561-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Morin-Brureau M, Hooper KM, Prosniak M, Sauma S, Harshyne LA, Andrews DW, Hooper DC. Enhancement of glioma-specific immunity in mice by “NOBEL”, an insulin-like growth factor 1 receptor antisense oligodeoxynucleotide. Cancer Immunol Immunother. 2015;64(4):447–57. doi: 10.1007/s00262-015-1654-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Harshyne LA, Nasca BJ, Kenyon LC, Andrews DW, Hooper DC. Serum exosomes and cytokines promote a T-helper cell type 2 environment in the peripheral blood of glioblastoma patients. Neuro-Oncol. 2015;18(2):206–215. doi: 10.1093/neuonc/nov107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar R, Kamdar D, Madden L, Hills C, Crooks D, O'Brien D, Greenman J. Th1/Th2 cytokine imbalance in meningioma, anaplastic astrocytoma and glioblastoma multiforme patients. Oncol Rep. 2006;15(6):1513–1516. doi: 10.3892/or.15.6.1513. [DOI] [PubMed] [Google Scholar]
- 21.Szabo SJ, Kim ST, Costa GL, Zhang X, Fathman CG, Glimcher LH. A novel transcription factor, T-bet, directs Th1 lineage commitment. Cell. 2000;100(6):655–69. doi: 10.1016/s0092-8674(00)80702-3. [DOI] [PubMed] [Google Scholar]
- 22.Deligne C, Metidji A, Fridman WH, Teillaud JL. Anti-CD20 therapy induces a memory Th1 response through the IFN-γ/IL-12 axis and prevents protumor regulatory T-cell expansion in mice. Leukemia. 2015;29(4):947–57. doi: 10.1038/leu.2014.275. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura T, Nakui M, Sato M, Iwakabe K, Kitamura H, Sekimoto M, Ohta A, KOda T, Nishimura S. The critical role of Th1-dominant immunity in tumor immunology. Cancer Chemother Pharmacol. 2000;46(1):52–61. doi: 10.1007/pl00014051. [DOI] [PubMed] [Google Scholar]
- 24.Nagarkatti M, Clary SR, Nagarkatti PS. Characterization of tumor-infiltrating CD4+ T cells as Th1 cells based on lymphokine secretion and functional properties. J Immunol. 1990;144(12):4898–905. [PubMed] [Google Scholar]
- 25.Haabeth OA, Bogen B, Corthay A. A model for cancer-suppressive inflammation. OncoImmunology. 2012;1(7):1146–55. doi: 10.4161/onci.21542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akasaki Y, Liu G, Chung NHC, Ehtesham M, Black KL, Yu JS. Induction of a CD4+ T Regulatory Type 1 Response by Cyclooxygenase-2-Overexpressing Glioma. J Immunol. 2004;173(7):4352–9. doi: 10.4049/jimmunol.173.7.4352. [DOI] [PubMed] [Google Scholar]
- 27.Liu Y, Ehtesham M, Samoto K, Wheeler CJ, Thompson RC, Villarreal LP, Black KL, Yu JS. In situ adenoviral interleukin 12 gene transfer confers potent and long-lasting cytotoxic immunity in glioma. Cancer Gene Ther. 2002;9(1):9–15. doi: 10.1038/sj.cgt.7700399. [DOI] [PubMed] [Google Scholar]
- 28.Wollmann G, Ozduman K, van den Pol AN. Oncolytic virus therapy for glioblastoma multiforme: Concepts and candidates. Cancer J. 2012;18(1):69–81. doi: 10.1097/PPO.0b013e31824671c9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Parker JN, Gillespie GY, Love CE, Randall S, Whitley RJ, Markert JM. Engineered herpes simplex virus expressing IL-12 in the treatment of experimental murine brain tumors. Proc Natl Acad Sci. 2000;97(5):2208–13. doi: 10.1073/pnas.040557897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Faber M, Faber ML, Papaneri A, Bette M, Weihe E, Dietzschold B, Schnell MJ. A single amino acid change in rabies virus glycoprotein increases virus spread and enhances virus pathogenicity. J Virol. 2005;79(22):14141–8. doi: 10.1128/JVI.79.22.14141-14148.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lebrun A, Portocarrero C, Kean RB, Barkhouse DA, Faber M, Hooper DC. T-bet Is required for the rapid clearance of attenuated rabies virus from central nervous system tissue. J Immunol. 2015;195(9):4358–68. doi: 10.4049/jimmunol.1501274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hooper DC, Morimoto K, Bette M, Weihe E, Koprowski H, Dietzschold B. Collaboration of antibody and inflammation in clearance of rabies virus from the central nervous system. J Virol. 1998;72(5):3711–9. doi: 10.1128/jvi.72.5.3711-3719.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fabis MJ, Phares TW, Kean RB, Koprowski H, Hooper DC. Blood–brain barrier changes and cell invasion differ between therapeutic immune clearance of neurotrophic virus and CNS autoimmunity. Proc Natl Acad Sci. 2008;105(40):15511–6. doi: 10.1073/pnas.0807656105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Phares TW, Fabis MJ, Brimer CM, Kean RB, Hooper DC. A peroxynitrite-dependent pathway is responsible for blood-brain barrier permeability changes during a central nervous system inflammatory response: TNF-α is neither necessary nor sufficient. J Immunol. 2007;178(11):7334–43. doi: 10.4049/jimmunol.178.11.7334. [DOI] [PubMed] [Google Scholar]
- 35.Barkhouse DA, Garcia SA, Bongiorno EK, Lebrun A, Faber M, Hooper DC. Expression of Interferon Gamma by a Recombinant Rabies Virus Strongly Attenuates the Pathogenicity of the Virus via Induction of Type I Interferon. J Virol. 2015;89(1):312–22. doi: 10.1128/JVI.01572-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hooper DC, Roy A, Barkhouse DA, Li J, Kean RB. Rabies virus clearance from the central nervous system. In: Jackson AC, editor. Advances in Virus Research. Academic Press; San Diego, CA: 2011. pp. 55–71. [DOI] [PubMed] [Google Scholar]
- 37.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, Braisted J, Klapa M, Thiagarajan M, Sturn A, Snuffin M, Rezantsev A, Popov D, Ryltsov A, Kostukovich E, Borisovsky I, Lui Z, Vinsavich A, Trush V, QUackenbush J. TM4: a free, open-source system for microarray data management and analysis. BioTechniques. 2003;34(2):374–8. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 38.Phares TW, Kean RB, Mikheeva T, Hooper DC. Regional differences in blood-brain barrier permeability changes and inflammation in the apathogenic clearance of virus from the central nervous system. J Immunol. 2006;176(12):7666–75. doi: 10.4049/jimmunol.176.12.7666. [DOI] [PubMed] [Google Scholar]
- 39.McKenney JK, Weiss SW, Folpe AL. CD31 expression in intratumoral macrophages: a potential diagnostic pitfall. Am J Surg Pathol. 2001;25(9):1167–73. doi: 10.1097/00000478-200109000-00007. [DOI] [PubMed] [Google Scholar]
- 40.Ehtesham M, Samoto K, Kabos P, Acosta FL, Gutierrez MA, Black KL, Yu JS. Treatment of intracranial glioma with in situ interferon-gamma and tumor necrosis factor-alpha gene transfer. Cancer Gene Ther. 2002;9(11):925–34. doi: 10.1038/sj.cgt.7700516. [DOI] [PubMed] [Google Scholar]
- 41.Kwak JY, Han MK, Choi KS, Park IH, Park SY, Sohn MH, Kim UH, McGregor JR, Samlowski WE, Yim CY. Cytokines Secreted by Lymphokine-activated killer cells induce endogenous nitric oxide synthesis and apoptosis in DLD-1 colon cancer cells. Cell Immunol. 2000;203(2):84–94. doi: 10.1006/cimm.2000.1682. [DOI] [PubMed] [Google Scholar]
- 42.Wainwright DA, Sengupta S, Han Y, Ulasov IV, Lesniak MS. The presence of IL-17A and T helper 17 cells in experimental mouse brain tumors and human glioma. PLOS One. 2010;5(10):e15390. doi: 10.1371/journal.pone.0015390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Tran EH, Hardin-Pouzet H, Verge G, Owens T. Astrocytes and microglia express inducible nitric oxide synthase in mice with experimental allergic encephalomyelitis. J Neuroimmunol. 1997;74(1-2):121–129. doi: 10.1016/s0165-5728(96)00215-9. [DOI] [PubMed] [Google Scholar]
- 44.Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, Klapproth K, Schäkel K, Garbi N, Jäger D, Weitz J, Schmitz-Winnenthal H, Hämmerling GJ, Bechhove P. Low-dose irradiation programs macrophage differentiation to an iNOS+/M1 phenotype that orchestrates effective T cell immunotherapy. Cancer Cell. 2013;24(5):589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- 45.Abadier M, Jahromi NH, Alves LC, Boscacci R, Vestweber D, Barnum S, Deutsch U, Engelhardt B, Lyck R. Cell surface levels of endothelial ICAM-1 influence the transcellular or paracellular T-cell diapedesis across the blood–brain barrier. Eur J Immunol. 2015;45(4):1043–58. doi: 10.1002/eji.201445125. [DOI] [PubMed] [Google Scholar]
- 46.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, Roche PC, Lu J, Zhu G, Tamada K, Lennon VA, Celis E, Chen L. Tumor-associated B7-H1 promotes T-cell apoptosis: A potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 47.Hadrup S, Donia M, thor Straten P. Effector CD4 and CD8 T cells and their role in the tumor microenvironment. Cancer Microenviron. 2012;6(2):123–33. doi: 10.1007/s12307-012-0127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Movahedi K, Laoui D, Gysemans C, Baeten M, Stange G, Van de Bossche J, Mack M, Pipeleers D, In't Veld P, De Baetselier P, Van Ginderachter J. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Research. 2010;70(14):5728–39. doi: 10.1158/0008-5472.CAN-09-4672. [DOI] [PubMed] [Google Scholar]
- 49.Peterson TE, Kirkpatrick ND, Huang Y, Farrar CT, Marijt KA, Kloepper J, Datta M, Amoozgar Z, Seano G, Jung K, et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc Natl Acad Sci. 2016;113:4470–4475. doi: 10.1073/pnas.1525349113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lizotte PH, Baird JR, Stevens CA, Lauer P, Green WR, Brockstedt DG, Fiering SN. Attenuated Listeria monocytogenes reprograms M2-polarized tumor-associated macrophages in ovarian cancer leading to iNOS-mediated tumor cell lysis. OncoImmunology. 2014;3(5):e28926. doi: 10.4161/onci.28926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Murray PJ, Allen JE, Biswas SK, Fisher EA, Gilroy DW, Goerdt S, Gordon S, Hamilton JA, Ivashkiv LB, Lawrence T, Locati M, Mantovani A, Martinez FO, Mege J, Mosser DM, Natoli G, Saeij JP, Schultze JL, Shirey KA, Sica A, Suttles J, Udalova I, van Ginderachter JA, Vogel SN, Wynn TA. Macrophage activation and polarization: Nomenclature and experimental guidelines. Immunity. 2014;41(1):14–20. doi: 10.1016/j.immuni.2014.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fujiwara Y, Komohara Y, Ikeda T, Takeya M. Corosolic acid inhibits glioblastoma cell proliferation by suppressing the activation of signal transducer and activator of transcription-3 and nuclear factor-kappa B in tumor cells and tumor-associated macrophages. Cancer Sci. 2011;102(1):206–11. doi: 10.1111/j.1349-7006.2010.01772.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Komohara Y, Fujiwara Y, Ohnishi K, Takeya M. Tumor-associated macrophages: Potential therapeutic targets for anti-cancer therapy. Adv Drug Deliv Rev. 2016;99:180–5. doi: 10.1016/j.addr.2015.11.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.