Abstract
The diagnosis of ankle osteoarthritis (OA) is increasing as a result of advancements in non-invasive imaging modalities such as magnetic resonance imaging, improved arthroscopic surgical technology and heightened awareness among clinicians. Unlike OA of the knee, primary or age-related ankle OA is rare, with the majority of ankle OA classified as post-traumatic (PTOA). Ankle trauma, more specifically ankle sprain, is the single most common athletic injury, and no effective therapies are available to prevent or slow progression of PTOA. Despite the high incidence of ankle trauma and OA, ankle-related OA research is sparse, with the majority of clinical and basic studies pertaining to the knee joint. Fundamental differences exist between joints including their structure and molecular composition, response to trauma, susceptibility to OA, clinical manifestations of disease, and response to treatment. Considerable evidence suggests that research findings from knee should not be extrapolated to the ankle, however few ankle-specific preclinical models of PTOA are currently available. The objective of this article is to review the current state of ankle OA investigation, highlighting important differences between the ankle and knee that may limit the extent to which research findings from knee models are applicable to the ankle joint. Considerations for the development of new ankle-specific, clinically relevant animal models are discussed.
Keywords: PTOA, talocrural, sprain, osteochondral injury, preclinical model
Post-traumatic osteoarthritis (PTOA) develops secondary to joint trauma, with clinical signs of pain and dysfunction often lagging years or decades behind the initiating injury.1,2 By conservative estimates, approximately 12% of patients with symptomatic osteoarthritis (OA) had a traumatic incident to their joint as the inciting cause. This corresponds to roughly 5.6 million Americans affected by PTOA severe enough to be evaluated by an orthopedic surgeon.3
Specifically in the talocrural (TC; ankle) joint, trauma is the primary cause of OA. Unlike the knee and hip joints, where only 2–10% of OA is attributed to injury, up to 90% of arthritic change in the ankle is post-traumatic in nature.2–6 The ankle is the most commonly injured joint during sport activities, with >300,000 injuries per year reported in the US, and an estimated 52.3 ankle injuries per 1000 athletic exposures in high school-aged athletes.7 Ankle sprains are also the most common non-combat related injury with a 15% incidence rate in over 4000 military personnel evaluated.8 The true incidence of ankle sprains is likely much higher than reported; one prospective observational study of 10,393 basketball players found that over half of ankle injuries went unreported and were not treated by a healthcare professional.9 Individuals with a history of ankle sprain comprise 70–85% of patients undergoing surgery for end-stage ankle PTOA.10 The economic burden associated with ankle OA is demonstrated by the estimated 4400 total ankle replacements and 25,000 ankle fusions performed in the US in 2010.3,4 Additionally, patients with ankle PTOA are an average of 14 years younger at the time of diagnosis and progress more rapidly to end-stage disease compared to those with OA of other joints, resulting in increased duration of pain, loss of function, and associated economic burdens to society.3,6,11 These data collectively indicate that the incidence, as well as the aggregate treatment costs of ankle PTOA will increase as the population ages.
Currently, no effective therapeutics are available to prevent or slow the progression of OA,12 and increasing evidence suggests that interventions must occur early in order to modify the course of disease.13,14 Studies of PTOA present a unique opportunity for investigating targeted therapy, because unlike other forms of OA (e.g., idiopathic), PTOA has a defined start point. Likewise, in the pursuit of effective OA therapies, modeling PTOA enables the study of very early cellular and subcellular events that initiate and perpetuate cartilage degeneration. Despite the high incidence of ankle trauma and OA, ankle-specific OA research is sparse, with the majority of clinical and basic research pertaining to the knee and hip joints. A recent meta-analysis of risk factors associated with OA of the lower limb identified only 2 of 43 studies related to the ankle.2
Increasingly, evidence reveals fundamental differences in cartilage structure and biology between joints, suggesting distinct mechanisms of disease.14–27 For example, a recent study found significant differences in the rate of extracellular matrix turnover and collagen composition in knee versus hip OA.28 Therefore, research findings from other joints may not be applicable to the ankle. However, few ankle-specific preclinical models of PTOA currently exist. This gap may hinder progress in the study of pathomechanisms of talocrural OA as well as the development of therapeutic interventions to prevent the initiation and progression of PTOA. Therefore, the objectives of this article are to review ex vivo and in vivo (animal model) ankle PTOA research, to assess the extent to which currently available models may be applicable to the ankle joint, and to discuss considerations for the development of more translational models of ankle PTOA.
ETIOLOGY OF TALOCRURAL OA—THE ASSOCIATION BETWEEN CARTILAGE INJURY AND PTOA
Of the 70–90% of ankle OA classified as posttraumatic, the most commonly reported inciting events are severe sprain and intra-articular fracture.1–3,6,29,30
Chronically altered joint mechanics, including malalignment, instability, and incongruity are widely accepted as contributory factors in the development of ankle PTOA.4,6,30–34 The relative importance of these chronic abnormal loading conditions versus acute mechanical trauma to the articular surface at the time of injury remains unclear, and likely varies between ankle injury types.33 For example, one group found that elevated joint contact stresses from residual incongruity after repair of tibial plafond fractures could predict the development of POTA in patients.35 On the other hand, increasing evidence suggests the magnitude of injury to articular cartilage during initial trauma is the major predisposing factor in ankle PTOA development.13,36 For example, concomitant cartilage lesions are identified arthroscopically in 63–79% of acute ankle fractures.37,38 Although it is difficult to deconvolve chondral injury from fracture grade, long-term follow up of 109 ankle fracture patients found that initial cartilage damage is an independent predictor for the development of both clinical and radiographic PTOA.39
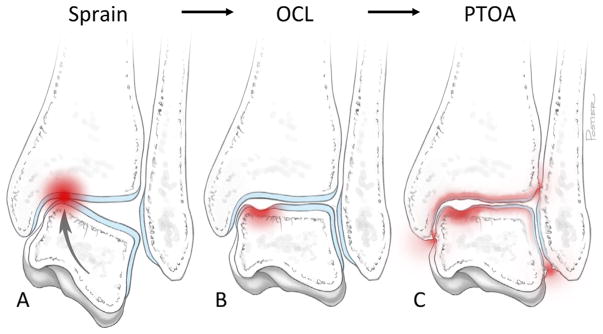
The importance of acute cartilage injury in the etiology of ankle OA is particularly evident for sprain-associated PTOA. During a typical severe ankle sprain, the medial tibial plafond is thought to impact the medial aspect of the talar dome, resulting in a talar osteochondral lesion (Fig. 1). This mechanism of injury is supported by the anatomic distribution of talar osteochondral lesions (OCLs), with the medial talus affected nearly twice as frequently as the lateral talus, and the mid-one third of the talar dome affected four times more commonly than the anterior and posterior thirds combined.40 Evidence suggest that the majority (as high as 95%) of severe ankle sprains result in OCLs and over half of patients with OCLs develop OA.41,42 Ligament injury resulting in chronic ankle instability is a common sequelae to severe sprain, and approximately 15% of ankle sprains are recurrences.43 However, instability alone cannot account for the incidence of resulting PTOA.10 In a population of patients presenting to orthopedic surgeons with severe ankle OA, approximately equal numbers of patients reported a single ankle sprain as those reporting recurrent sprains (i.e., chronic instability).3 Notably, one study reported that the mean latency time between injury and end-stage OA was 12 years shorter for patients who suffered a single ankle sprains than those who experienced chronic recurrent sprains. The authors speculate that the more rapid progression of PTOA in patients without chronic joint instability could be explained by the degree of cartilage damage sustained at the time of injury.11 Finally, no clinical study to date has demonstrated that any conservative or surgical therapies to stabilize the ankle joint after injury decrease the incidence of PTOA, further suggesting that the magnitude of the initial cartilage/subchondral bone injury is the primary inciting cause of ankle PTOA.10,44–47
Figure 1.
The proposed mechanism of posttraumatic osteoarthritis after a severe ankle sprain. (A) During a typical lateral ankle sprain (inversion) the medial aspect of the talus likely impacts the tibial plafond, which may result in (B) a talar osteochondral lesion (OCL). Direct trauma to the articular surface can initiate progressive, irreversible joint destruction culminating in (C) late-stage posttraumatic osteoarthritis (PTOA) years to decades after the original injury.
In patients presenting for ankle pain, MRI is the imaging modality of choice because of its ability to identify ligamentous injury, subchondral bone edema, and cartilage pathology.48–50 However, diagnosing subtle talar cartilage lesions and the early phases of PTOA remains challenging.45 In one study, 107 ankles in 101 patients (mean age 28.7 years) with chronic lateral ligament instability secondary to ankle sprain were examined arthroscopically to assess the articular cartilage prior to ligament reconstruction. In 99 ankles without abnormalities diagnosed on radiographs or MRI, 77% had chondral lesions identified during arthroscopy.51 In a recent study, 3T MRI of the ankle joint had a reported 71% sensitivity and 74% specificity for detecting talar dome articular cartilage defects (Outerbridge grades 3 and 4) that were confirmed on arthroscopy.49 A similar study reported a sensitivity of only 46% for the diagnosis of talar OCLs on 1.5T MRI.50 In one cohort of young, active individuals who had experienced an ankle sprain within 5 years of evaluation, T2 relaxation times were increased in injured ankles with and without instability relative to uninjured controls, indicating early subclinical cartilage degeneration.48 These findings suggest that while diagnostic imaging modalities continue to improve, the incidence of chondral lesions and early PTOA may be higher than previously recognized.
DIFFERENCES BETWEEN ANKLE AND KNEE JOINT
Differences between the ankle and knee, summarized in Table 1, are important considerations when extrapolating clinical or basic OA research knowledge of the knee to the ankle. Overall, the knee has an approximately ninefold higher incidence of clinical OA than the ankle, however the proportion of PTOA is at least sevenfold higher in the ankle.3 Improved understanding of the biological and mechanical factors underlying this disparity may contribute to the development of joint-specific therapies.
Table 1.
Summary of Important Differences Between the Knee and Ankle Joints
| Ankle | Knee | |
|---|---|---|
| Prevalence of primary OA | Low | Higher (ninefold) |
| Proportion of OA secondary to trauma | High (80%) | Low (10%) |
| Anatomy and mechanics | ||
| Joint congruity | High | Low |
| Articular contact area | Low | Higher (threefold) |
| Cartilage thickness | Thin (0.7–1.62 mm) | Thick (1.5–2.6 mm) |
| Cartilage stiffness (compressive modulus) | High | Lower |
| ECM properties | ||
| sGAG content | Higher (twofold) | Lower |
| Water content | Lower | Higher |
| Collagen content | No difference | Similar |
| Metabolism | ||
| Basal PG synthesis and turnover | High | Low |
| Response to mechanical loading | ||
| Upregulation of collagen synthesis marker (CII) | Yes | No |
| Upregulation of aggrecan mRNA | Yes | No |
| Response to catabolic signals | ||
| Net response | Anabolic | Catabolic |
| IL-1 inhibition of PG synthesis | Low | Higher (eightfold) |
| IL-1 induced PG degradation | No | Yes |
| Fn-f induced PG loss | Low | High (30–50% loss) |
OA, osteoarthritis; sGAG, sulfated glycosaminoglycans; PG, proteoglycan; CII, type II collagen; IL-1, interleukin-1; Fn-F, fibronectin fragments. The ankle has a low prevalence of primary OA, but a high proportion of PTOA. The ankle joint is more congruent and has higher intrinsic stability than the knee. The extracellular matrix (ECM) properties of ankle cartilage may protect against primary OA and ankle chondrocytes may also have improved homeostatic mechanisms compared to that of knee chondrocytes.
Differences Between Ankle and Knee Joint Anatomy and Biomechanics
The articular surfaces of the talocrural joint are highly congruent resulting in intrinsic stability of the ankle based on bony anatomy alone52 (Fig. 2). Furthermore, during weight-bearing, redistribution of contact stresses over the tibiotalar articular surfaces increase ankle joint stability.52 Only at the extremes of the normal range of motion is stability of the ankle joint maintained by soft tissue structures including the anterior and posterior tibiofibular ligaments and the calcaneofibular ligament.53 In contrast, the rounded femoral condyles are incongruent with the flat surface of the tibial plateau, making the knee joint highly reliant on soft tissues such as menisci, collateral ligaments, and cruciate ligaments, to maintain stability during loading.54 The motion of the ankle joint is “rolling” with the center of rotation changing throughout a range of motion of 50 degrees plantar flexion to 20 degrees of dorsiflexion. The point of articulation is slightly oblique to the long axis of the tibia resulting in a slight (~3 degree) valgus conformation and an outward deviation of the foot with dorsiflexion and inward deviation with plantar flexion.55,56 In contrast, the normal motion of the knee joint is a combination of sliding, rolling, and rotation.54
Figure 2.
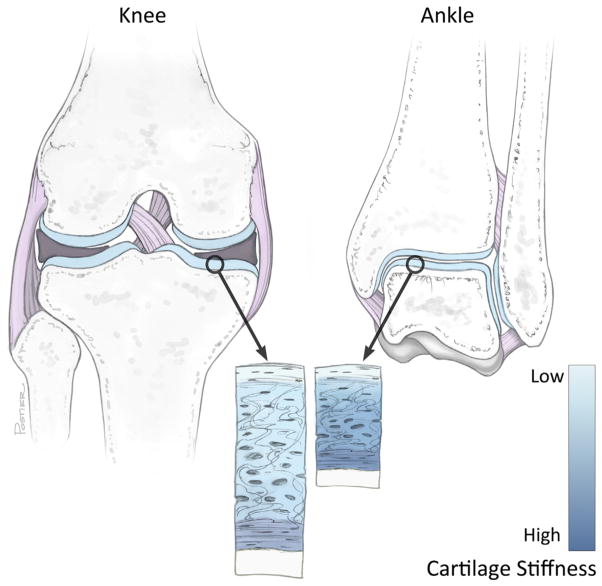
A comparison between the knee and ankle joints. The contact surface area of knee joint is approximately three times larger than the ankle. The ankle has more bony congruity than the knee, and is therefore less reliant on supporting soft tissues (pink-purple) to maintain stability. Ankle cartilage (blue) is approximately half the thickness of knee cartilage, although the superficial zone thickness is similar between joints. The extracellular matrix of ankle cartilage is more dense than that of the knee and has a higher dynamic stiffness and compressive modulus.
The articular cartilage in the ankle is approximately half the thickness of knee cartilage (Fig. 2), with a mean thickness of 0.7–1.62 mm in the ankle versus 1.5–2.6 mm in the knee.57,58 The relatively smaller size of the ankle joint results in a contact surface area approximately one-third that of the knee, which translates to higher force per area (stress) during loading.59 In plantar flexion, the contact area of the ankle joint decreases by greater than 40% with corresponding increases in peak stresses.60 This may partially explain the high incidence of ankle OA in retired ballet dancers61 and early subclinical disease in a cohort of active professional dancers.62 During eversion and inversion, contact area also decreases, and peak stresses increase.60 One study estimated that with 1 mm of lateral talar displacement, as might occur during a typical ankle sprain, contact area of the ankle joint is decreased by 42%.63 A computer simulation modeling study found that plantar flexion and inversion during forefoot loading increases the likelihood of ankle sprain,64 which is consistent with the high prevalence of sprains during jump landing in the sport of basketball.65 Accidental sprains incurred by subjects during controlled laboratory testing consistently accompanied internal rotation and rapid inversion, with or without plantar flexion.10
Differences Between Ankle and Knee Cartilage Extracellular Matrix Properties and Chondrocyte Distribution
The extracellular matrix (ECM) of articular cartilage, comprised mainly of proteoglycans and type 2 collagen, functions in load bearing and supports near-frictionless joint movement. In the ankle, the ECM is more dense than that of the knee, with lower water and higher glycosaminoglycan (GAG) concentrations.16 In compression, ankle cartilage has a higher dynamic stiffness and compressive modulus than knee cartilage16 (Fig. 2). These physical properties of ankle cartilage translate to an increased resistance to compressive loads, but do not necessarily explain why the ankle might be more resistant to mechanical damage than the knee. Recently, increased attention has been directed toward resolving depth-dependent mechanical properties of articular cartilage, revealing that the superficial layer is more compliant (lower compressive stiffness) and dissipates more shear energy than the deeper tissue.66–68 For example, the superficial 500 um of the articular surface has a shear modulus 2 orders of magnitude lower than that of the deep zone, and acts to dissipate nearly 90% of shear energy.69 Although ankle cartilage is roughly half as thick as knee cartilage, the superficial zone thickness is similar between joints, therefore the superficial zone comprises a relatively higher proportion of cartilage in the ankle than the knee (Fig. 2). This relative difference has been suggested to play an important protective role during physiologic loading in the ankle,16 and could have important implications in the development of ankle-specific therapies.
Chondrocytes are the sole cell type within articular cartilage and are responsible for maintaining the surrounding ECM.70 Cell density in full thickness ankle cartilage (41 ± 34 × 103 cells/mg) has been reported to be 48% higher than knee cartilage (28 ± 26 × 103 cells/mg).19 Interestingly, the spatial organization of superficial zone chondrocytes differ between the ankle and knee joints, and the understanding of these differences have evolved with improved imaging techniques.71,72 In the human ankle joint, superficial zone chondrocytes are predominantly arranged in pairs but in the in the femoral condyles of the knee, they are arranged in horizontally oriented strings. These distinct patterns are likely related to predominant collagen fiber orientation within the superficial zone, but it remains unclear if they are causally linked to local biomechanical forces or have implications in chondrocyte function or mechanotransduction. These predictable patterns of organization in the superficial zone chondrocytes change in early OA, both within areas of focal OA and in intact cartilage remote to focal lesions, indicating a coordinated response of chondrocytes to injury, and may serve as sensitive indicators of early preclinical OA and/or focal cartilage lesions elsewhere in the joint.72,73
Differences Between Ankle and Knee Cartilage Matrix Homeostasis and Response of Chondrocytes to Biochemical and Mechanical Stimulation
In matched pairs of ankle and knee cartilage from healthy cadaver joints, ankle chondrocytes had increased proteoglycan (PG) and collagen synthetic rates compared to knee chondrocytes, and these differences persisted throughout life.18,19 In diseased cartilage with surface fibrillations and fissuring consistent with early OA, markers of collagen synthesis (CPII) and aggrecan turnover (epitope 846) are increased in the ankle, but down-regulated in the knee. Markers consistent with collagen degradation (Col2-3/4C short) are higher in the knee than the ankle.25 These findings may explain the observation that age-related degeneration of the ECM does not occur in the ankle, or it happens at a much slower rate than in the knee.17
Ankle and knee cartilage also differ in their response to catabolic signals. Knee chondrocytes are approximately eight times more sensitive to inhibition of PG synthesis by the catabolic cytokine interleukin-1 (IL-1).18 The ankle is also less susceptible to fibronectin fragment (Fn-f)-mediated degradation.21,74 PG content was decreased by 30–50% in knee cartilage exposed to Fn-f for two weeks, whereas it was essentially unaffected in ankle cartilage after FN-f exposure for a month.74
More specifically with respect to PTOA, differences between ankle and knee cartilage are evident in their disparate response to injurious compression. In adult knee cartilage, injurious compression (65% fixed strain, 2 mm/s fixed velocity, strain rate approximately 400%/s) resulted in matrix damage in 46% of samples and a net loss of about 1.2% of total GAG in knee explants. Ankle cartilage subjected to the same magnitude of injury sustained little damage and no GAG loss, suggesting that ankle cartilage is more resistant to mechanical injury.22 An approximately twofold increase in aggrecan mRNA expression was observed in knee versus ankle chondrocytes in response to mechanical stimulation. Ankle chondrocytes express higher levels of integrin-associated proteins CD98, CD147, and galectin 3 than knee chondrocytes, suggesting differences in integrin-associated mechanotransduction and matrix remodeling.75 Collectively, these data suggest that ankle chondrocytes have superior homeostatic mechanisms compared to knee chondrocytes.
Despite this convincing body of literature indicating ankle chondrocytes are inherently more anabolic and less catabolic than knee chondrocytes, there is also interesting evidence to suggest otherwise. No differences in the synthetic capabilities or response to catabolic stimuli were detected in pellet cultures of chondrocytes from the ankle and knee of the same individual.76 In this study, chondrocytes from both joints were similar in expression of type 1 and 2 collagen mRNA. Pellet ECM contained equivalent concentrations of GAG and type II collagen and the synthetic rates of GAG and collagen were similarly decreased in response to IL-1 treatment.76 These findings suggest that characteristic differences between ankle and knee chondrocytes are lost once the cells are isolated from their native ECM and expanded ex vivo, implying that the native tissue environment may be more important in dictating the characteristic properties of ankle and knee chondrocytes than intrinsic differences between cell types.
Taken together, these findings suggest that major differences exist between joints, which are likely to influence disease pathomechanisms and affect clinical response to OA therapies. Therefore, ankle-specific and injury-specific modeling of PTOA may accelerate progress in basic and clinical research.
EX VIVO ANKLE CARTILAGE INJURY MODELS
In addition to the cartilage injury models mentioned above, several groups have used explanted ankle cartilage to investigate early disease mechanisms and response to therapeutic interventions. For example in one model, fresh cadaveric human tali were subjected to a single pressure-controlled impact injury (1Ns; up to 600 N within 2 ms).77 Explants were removed from the bone and cultured for up to 2 weeks. Chondrocyte viability was assessed using live-dead cell staining and apoptosis was assessed using a Tunnel assay. Histopathology was performed and a Modified Mankin score was used to assess cartilage injury. Explants were treated 1 h prior to injury or 48 h after injury with one of three cytoprotective drugs. The polaxamer surfactant P188, a plasma membrane stabilizer, was found to be superior to caspase-3 and caspase-9 inhibitors in preventing impact-induced chondrocyte death and the radial expansion of apoptosis from the site of impact.
A major strength of these types of ex vivo models is that they allow injury-induced cellular responses to be studied in situ (i.e., in chondrocytes within their native extracellular matrix) immediately after cartilage injury, and over time.78 Additionally, ex vivo models allow preliminary testing of putative early interventional therapies.79 A drawback of ex vivo models is that they fail to capture important aspects of disease pathogenesis including the inflammatory response, joint loading conditions, etc. Therefore, in vivo analogs of these injury- and joint-specific ex vivo models are important for preclinical testing of therapeutics.
NON-TRAUMATIC ANIMAL MODELS OF ANKLE OSTEOARTHRITIS
Several non-traumatic rodent models of ankle OA have been published. These models utilize the ankle joint to investigate cartilage degeneration secondary to acute joint inflammation, joint immobilization or spontaneous/age-associated OA. Despite limitations in the translatability of these models to clinical ankle PTOA, they may serve as useful tools to explore specific aspects of ankle OA pathophysiology. Furthermore, several of these studies have directly compared the ankle and knee joints within the same individual, and will therefore be reviewed.
Intraarticular Il-1β Model of Acute Ankle Inflammation in Rats
Scott et al.80 described injection of IL-1β into the ankle of rats as an acute model of joint inflammation. Biochemical changes in joint lavage fluid, gene expression changes in whole joint tissues, and histopathology were assessed up to 24 h after injection. They found that 100 ng of IL-1β caused joint swelling and hyperalgesia, as well as gene expression of pro-inflammatory and catabolic mediators, including IL-6, PTGS2, NOS2, TNFα, NFκB, ADAMTS5, and IL-1β. Biochemical analysis of joint lavage fluid revealed accumulation of GAG, IL-6 protein, and NO. Histopathology at 24 h showed evidence of synovitis. Although there was no histological evidence of cartilage destruction in this short time frame, the release of GAG into joint lavage fluids likely indicates early ECM degradation. Strengths of this model include the induction of reproducible joint inflammation and GAG release within 4 h. This model is potentially useful for the initial evaluation of antagonists of the IL-1 pathway. The utility of this model is limited by the short study duration and non-physiologic method of disease initiation.
Short-Term Ankle Immobilization Model of Cartilage Atrophy in Rats
Renner et al.81 evaluated the effect of a passive muscle stretching protocol on the articular cartilage of normal and previously immobilized rat ankles. One ankle in each mouse was immobilized non-invasively for 4 weeks and histology was performed at 7 weeks. Unilateral ankle immobilization caused increased cellularity and chondrocyte cloning in both the immobilized and non-immobilized limb over control animals. Similarly, proteoglycan depletion was present in both limbs of unilaterally immobilized mice, worse in the immobilized than the contralateral limb. No differences in cartilage thickness were observed.
When passive muscle stretching was instituted for 3 weeks following remobilization, higher cellularity was observed in treated ankles of the stretched group and chondrocyte cloning was observed in the contralateral limb. Notably, immobilized/stretched ankles had the highest PG loss of all the groups calling into question the utility of this modality or the methodology by which it was employed in this model. This model may be useful to investigate therapies to prevent cartilage atrophy following ankle immobilization, and in combination with an ankle destabilization model, could possibly provide insight into the relative importance of joint immobilization in the degenerative and healing processes after destabilizing ankle injuries.
Spontaneous Ankle OA Model in Guinea Pigs
Han et al. were the first to report OA-like lesions occurring spontaneously in the guinea pig ankle. They performed histologic examination and assessed collagen fiber orientation in knee and ankle pairs from male Dunkin–Hartley guinea pigs at 3 and 6 months of age. Changes in the ankles were evenly distributed between the tibial and talar joint surfaces. At 3 months of age, synovitis was present in all ankle joints and mild focal degenerative cartilage changes were present in some ankles, but histologic scores were not different than controls. At 6 months, moderate focal cartilage lesions, chondrocyte loss and loss of PG staining were present in all ankles. While ankle joint scores were only elevated at 6 months, knee joint scores were significantly elevated at 3 and 6 months. In areas of intact cartilage, changes in collagen fiber orientation were identified and correlated to PG loss indicating that remodeling of the ECM plays a role in early disease and may precede histologic changes this model of spontaneous OA.
STR/ORT Mouse Model of Spontaneous Ankle OA
The STR/ORT mouse is a well-established model of spontaneous knee OA, and its utility has been reviewed.82 Males are preferentially affected, and early calcification of periarticular soft tissue structures is a prominent feature of this model. Evans et al. described the radiographic changes and Collins et al. described the histopathological changes in the knees and ankles of STR/ORT mice from 3 to 10 months of age.83,84 The radiographic progression of OA in knee and ankle joints was different; in male mice, knee OA worsened directly with age, whereas ankle OA scores increased markedly at 5–6 months, then plateaued. Histology revealed extensive new bone formation in the entheses around the ankle and mineralization of the talar interosseous ligaments starting around 3 months of age. The development of knee and ankle OA was found to be independent within a single mouse.
The etiology of OA in STR/ORT mice is not entirely clear but recently, meta-analysis of transcription profiles revealed increased expression of genes related to endochondral ossification, increased MMP-13 and type X collagen expression as well as differential expression of regulators of tissue mineralization, suggesting an inherent chondrocyte defect related to endochondral growth.85 The excessive soft tissue calcification, which precedes cartilage degeneration in this model suggests that the pathophysiology is unlikely to be reflective of human ankle OA.82,86
BCBC/Y Mouse Model of Anklylosing Ankle OA
Yamamoto et al.87 described a mutant B6C3F1 mouse with a light coat color displaying progressive ankle swelling starting at around 9 months. By 10–20 months, these mice display an abnormal stance and gait, with progressive ankylosis of the tarsus on radiographs. On histologic examination, early cartilage lesions included chondrocyte necrosis, cartilage fibrillation and thinning. Later changes included full-thickness erosions in conjunction with a hyperplastic cartilage response.88 Severe osteophyte formation progressed to bridging ankylosis and finally complete joint fusion of the tarsal joints. Despite these dramatic changes, no synovial inflammation was identified. There is a strong sex predilection, with 87% of males affected and 21% of females. The disease mechanisms and underlying genetic basis of this atypical arthropathy, with a strong predilection for the ankle joints has not been identified.
Aging Model of Ankle OA in Rats
Although spontaneous knee OA is rarely reported in rats,89,90 Mohr and Lehman describe spontaneous ankle OA in 26-month-old CD/BR Sprague Dawley rats.91 Histology was performed on the talocrural and subtalar joints and morphologic changes were scored semi-quantitatively on a 40-point scale. Lesions ranged from focal chondrocyte necrosis, to loss of proteoglycan staining to fibrillation to partial and total loss of hyaline cartilage to full thickness lesions involving the calcified cartilage layer. Synovitis was rarely present. Although disease mechanisms were not investigated, changes were commonly present on opposing articular surfaces, suggesting localized increased contact pressures may play a role. Males had more severe lesions compared to females, which could be related to mechanical factors due to the higher body weight of males and/or endocrine factors.
This model was subsequently used to study the effect of meloxicam, a non-steroidal anti-inflammatory drug, on ankle OA.92 While no drug effect was found, this study demonstrated that the incidence and severity of OA changes are highest in the ankle compared to the hip and knee joints in this model. A consideration in the application of this model is that in humans, age-associated cartilage degeneration in the knee is consistently more severe than in the ankle of the same individual.26,93 A limitation of any of these spontaneous models of ankle OA is that in humans, primary ankle OA is uncommon.
MODELING ANKLE PTOA IN VIVO
Many preclinical PTOA models are available and have been well reviewed.94–101 Appropriate use of existing models and the development of preclinical models with improved translatability is an ongoing topic of discussion within and beyond the field of OA research.102–104 The majority of in vivo PTOA models utilize the knee joint, but the numerous anatomical, biomechanical, and biochemical differences between the ankle and the knee suggest that an ankle-specific model is appropriate when targeting therapy for ankle OA. Recently, two surgically induced models of ankle OA have been described.
Destabilization Models of Ankle OA in the Mouse
Recently, Change et al. described an aging model, as well as three destabilization models of mouse ankle OA.118 First, ankle and knee cartilage from 25-month-old mice were compared to cartilage obtained from humans undergoing joint replacement surgery. OARSI scores for tibiotalar cartilage were lower than for the medial compartment of the knee, indicating the mouse ankle is more resistant to age-associated cartilage degeneration than the knee, similar to humans. They also found that like humans, mouse talar cartilage is approximately half as thick, and talar subchondral bone was denser compared to the medial tibial plateau. This serves as an important baseline reference, and indicates that this model may have better translatability for the study of age-related ankle OA than those previously mentioned. One caveat to the interpretation of these findings is that normal, aged mouse tissues were compared to cartilage from end-stage OA joints in humans.
The authors go on to describe three methods of ankle joint destabilization in young mice. The medial model involved transection of the tibialis posterior tendon and deltoid ligament and incision of the medial joint capsule. This technique resulted in progressive cartilage degeneration in the talocrural joint over the 8 weeks following surgery. Increased MMP-13 and ADAMTS5 were detected on immunohistochemistry and chondrocyte apoptosis was identified on TUNEL staining. A lateral destabilization model resulted in subtalar OA changes, while a bilateral model resulted in both talocrural and subtalar OA.
The major strengths of the medial destabilization model is that it captures many important features of human disease, including progression of tibiotalar cartilage lesions, chondrocyte apoptosis and inflammatory cytokines, and also lacks the excessive osteophyte formation present in other mouse models. The differences in disease phenotype induced by the three surgical techniques highlights the importance of increased specificity in the type of injury used to study PTOA subtypes. In humans, injury to the lateral soft tissues are more commonly associated with ankle sprain and OA.105,106 In the mouse, complete transection of the major lateral stabilizing soft tissues and invasion of the joint capsule did not result in significant talocrural joint OA.
Intraarticular Fracture Model of Ankle OA in the Yucatan Miniature Pig
Goetz et al. recently developed a porcine distal tibia intraarticular fracture/stabilization model to assess the effects of poor anatomic reconstruction on the development of ankle PTOA.107 Fractures of the distal tibia were created using an open joint approach and repaired using internal fixation with a bone plate. Synovial fluid analysis, radiographic monitoring and force plate analysis were performed after surgery and animals were sacrificed at 12 weeks. Osteochondral histology was performed and scored using automated Mankin scoring. By 12 weeks post-operatively, all fractures were healed and limb loading had returned to normal. Inflammatory cytokine concentrations in synovial fluid, including TNFα, IL1β, IL6, and IL8 were elevated transiently during the 2 weeks after fracture. Histology scores were worse in joints with articular incongruity compared to those that were anatomically reconstructed.
This is a well-validated model to investigate intra-articular fracture-induced PTOA and the effects of chronically altered ankle joint mechanics due to articular surface incongruity. Strengths of this model include a consistent fracture geometry, with reporting of the energy absorbed during fracture, and clinically relevant outcome measures including intra- and post-operative imaging and analysis, gait analysis to quantify pain/joint dysfunction, synovial fluid biomarkers and osteochondral histology, although synovial membrane was not assessed. Internal fixation techniques are similar to those used in human clinical patients. In this report, 27% (6/22) of the animals were lost post-operatively due to orthopedic complications. Longer-term follow up will be required if this model is to be used to test biological treatments to reduce the incidence of fracture-associated ankle PTOA.
CONSIDERATIONS FOR DEVELOPMENT OF NEW PRECLINICAL MODELS OF ANKLE PTOA
Method of OA Induction
When modeling PTOA, the induction method would ideally mimic that of naturally occurring disease. The majority of PTOA models utilize the knee (stifle) joint and rely on surgical destabilization of supporting soft tissue such as the meniscus or anterior cruciate ligament.94–99 PTOA models involving joint instability or the generation of an osteochondral fragment are valuable tools, however these models do not reflect the contributions of acute trauma to the articular cartilage at the time of injury. Although the specific injury parameters required to initiate clinical PTOA remain unclear, these mechanical thresholds have been studied in many model systems, and experimental evidence supports the importance of loading magnitude and rate as predictors of cartilage degeneration.13,22,31,108–111
To more specifically investigate mechanical overloading of the articular surface, single-impact load models are becoming more prevalent in PTOA research, and have been validated to initiate early OA-like lesions in the knee, but have not been applied to the ankle.112–114 This reductionist approach allows investigators to gain new insight into the cartilage-specific contributions to PTOA ex vivo, investigate mechanical thresholds for peracute cellular and subcellular responses to cartilage injury, and test targeted drugs to prevent PTOA in animal models. For example, a recent study examined microscale mechanics and corresponding chondrocyte death in articular cartilage following rapid impact injury.115 This new technique revealed that chondrocyte death is highly correlated with a threshold of 8% microscale strain. When the superficial layer of the cartilage was removed, cell death penetrated deeper into the cartilage, indicating a protective role for the superficial layer. Additionally, chondrocyte death developed within 2 h of impact, suggesting a narrow window for early therapeutic intervention after injury.115 In summary, an overly aggressive model with rapid progression to end-stage OA may not provide a sufficiently dynamic range of disease to evaluate therapeutic effects in a preclinical ankle model and a single, rapid impact model is most consistent with the likely etiopathogenesis of ankle PTOA.
Species Choice
Rodent models have the advantages of being low cost, genetically similar within a specific breed strain, and amenable to genetic manipulation. Rodent models have therefore been used extensively as screening tools for drug development and to investigate specific molecular pathways involved in OA pathogenesis.94,95,98,101,116,117 The most significant shortcomings of small animal models are the dissimilarities in cartilage structure and disparate loading compared to a human joint. An optimal preclinical model would be scaled appropriately to mimic joint size (Fig. 3), load, age and skeletal maturity of human clinical patients. The cartilage lesion should be located in an analogous location in ankle, and be of similar size, type and depth as clinically observed lesions, which is difficult or impossible to control in rodents. Recently, a mouse model of ankle OA was described based on surgical destabilization.118 Rabbit models are slightly larger and have been used in single impact studies of knee PTOA without the confounding variables of instability.112,119
Figure 3.
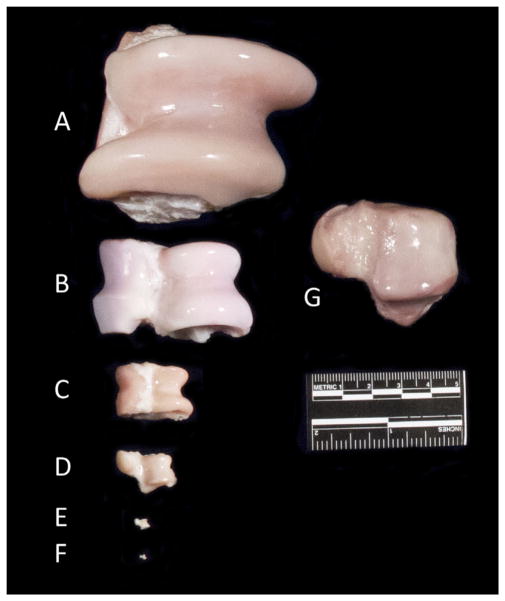
Comparative talus anatomy. Size comparison of left tali from species commonly used as animal models in osteoarthritis research; (A) horse, (B) pig, (C) sheep, (D) dog, (E) rat, (F) mouse, compared to the (G) human.
Common large animal species used in OA research include the dog, sheep, goat, pig and horse.98–101,103,114,120–123 A benefit common to these larger species is increased joint size, allowing OA outcome measures such as synovial fluid collection, clinical cartilage imaging modalities including MRI, quantitative gait analysis, arthroscopic joint examination, topographical evaluation within a single joint and ample tissue for histological, biochemical, biomechanical, and molecular analyses. Large joint size is a particularly important feature if precise anatomical placement of articular surface trauma is to be employed.
The dog knee has been widely used in preclinical models of knee OA, therefore validated outcome measures have been established in that joint.98,122 Most commonly, OA is induced by surgical destabilization, however single impact models have recently been described.114 Dogs are an athletic species and are prone to naturally occurring OA, however as a popular companion animal species, their use in biomedical research draws heightened scrutiny by the public. Sheep and goats have been used in several destabilization models of knee OA as well as a femoral condyle impact model.124 Sheep and goats have joints that are closer in size to the human ankle than dogs (Fig. 3), however naturally occurring OA is rare to non-existent, and these species may be less susceptible to OA after surgical induction, as ACL transection leads to joint instability but not significant OA in the goat knee.121,125 When considering the development of preclinical models, small ruminants (sheep and goats) have the particular disadvantage of being foregut fermenters, and therefore bioavailability of orally administered therapeutics differ significantly from monogastric species (i.e., humans, horses, dogs, pigs).
The horse is an established model organism for PTOA research and offers several advantages over other species. The horse is the largest model available and equine cartilage most closely approximates human cartilage thickness and biomechanical loading.126,127 Similar to humans, the cartilage of the equine talocrural joint has a higher GAG content, and is stiffer than that of the knee.128 The equine species is naturally prone to OA.129 Similar to the human ankle, the equine TC joint has a high degree of intrinsic bony stability and rarely suffers OA in the absence of injury.130,131 As in humans, equine TC PTOA does occur secondary to ligamentous injury, blunt trauma, OCLs and intraarticular fractures.130–132 The dimensions of the equine talocrural joint are well suited to arthroscopic examination and manipulation and it is among the most common arthroscopically approached joints in equine surgical practice. In addition to the potential to perform serial arthroscopic examinations, MRI is a well validated diagnostic modality to assess the equine TC joint, therefore the horse may be considered for studies where longitudinal evaluations of cartilage are needed.133–135
Outcome Measures
Histopathology remains the gold standard for assessing OA progression. Many systems have been used to evaluate OA changes, and these have been extensively reviewed.136 Commonly used scoring systems in animal research models are the Mankin Score,137 the OARSI scoring system138 and the ICRS score for cartilage repair.139 Recently, species-specific consensus scoring systems have been developed for the most important species used in OA research including dog, guinea pig, horse, mouse, rabbit, rat, and sheep/goat.100 To reduce the number of animal sacrifices at each time point, longitudinal outcome assessments are preferred including imaging, biochemical and genetic biomarkers, as well as assessments of pain, joint function and gait. Appropriate ankle-specific biomarkers will need to be identified and validated in order to develop translational PTOA models that more closely represent clinical subgroups of disease.140
MRI allows objective measures of soft tissue injuries and cartilage health in human and large animal veterinary patients, and its use and utility in preclinical animal models will continue to increase. Bone bruising is identified on MRI in 16–40% of patients after ankle sprain, and in up to 50% of patients with ligament injuries.141,142 Therefore, the assessment of subchondral bone should be included in the characterization of ankle injury models. Highly congruent joints with relatively thin articular cartilage are more challenging to assess using MRI, however steady advancements in imaging technology have allowed evaluation of subtle cartilage lesions in the ankle.143,144 Contemporary compositional MRI techniques including dGEMRIC and T1-rho, and T2 mapping have become increasingly useful for assessing cartilage degeneration and allow examination of biochemical or ultrastructural composition of articular cartilage relevant to OA research.144,145 Combining clinical and research data pertaining to the ankle may allow identification of preclinical disease. As discussed, evidence in patients with ankle injuries suggests that, in addition to advanced imaging, arthroscopic examination is particularly important in identifying early ankle PTOA. Therefore, the ideal model would allow serial arthroscopic examination of the talocrural joint.
A major challenge in developing appropriate preclinical animal models of OA is the ability to quantify pain as a clinical endpoint. Chronic pain is a hallmark of OA, and the ability to evaluate pain and joint dysfunction is integral to the relevance and utility of models in translational research. This is especially relevant for less severe models of OA, when the goal is to study and develop therapies targeted at early OA in humans.103 Numerous measures of pain and joint dysfunction that have been developed in multiple species, and it is not clear which of these will prove the most useful in ankle PTOA models. As an example, gait abnormalities are well-established indicators for pain. In horses and dogs, quantitative gait analysis has been used for over two decades to evaluate naturally occurring and experimental lameness. Studies have employed force plate, pressure plate, accelerometers and kinematic image analysis, and these outcome measures are well validated.146–153 In small animal models, pain and joint dysfunction have been less commonly reported outcome measures, although more recently these systems have been developed and validated for rodents.150,154,155 Quantitative gait analysis is possible in pigs, sheep, and goats, however their temperament is less amenable to pain assessment using standard techniques such as force plate analysis. New mechanisms of pain are being identified in OA patients, and appropriate outcome measures will need to be identified as new joint-specific and injury-specific PTOA models are developed.103,154,156,157
CONCLUSION
OA is the most common cause of chronic disability in the United States, and as the population ages, it will become increasingly burdensome to society. The ankle is the most commonly injured joint, and ankle PTOA disproportionately affects populations of young adults, athletes and military personnel. Laboratory and animals model studies will continue to reveal pathomechansisms of ankle PTOA. Remaining unmet needs related ankle OA include early interventional therapies (so called “point of injury care”) to prevent progression of early PTOA, as well as methods to treat established/late-stage OA. To address these knowledge gaps, an appropriate ankle-specific preclinical PTOA model would allow investigation of the early initiating events following talocrural cartilage injury, as well as longitudinal testing of targeted therapies in a clinically relevant species.
Acknowledgments
Grant sponsor: NIH; Grant numbers: 5T32OD011000-20, NIH 1K08AR068470.
The authors acknowledge Jim Postier for technical drawing. We thank David C. Jones for photography, Youichi Yasui for logistical support, and Margaret Goodale for technical support. MD was supported by NIH 5T32OD011000-20 and NIH 1K08AR068470.
Footnotes
AUTHORS’ CONTRIBUTIONS
All authors contributed intellectual content, provided critical revision of the manuscript, and approved the final version. MD and LF were responsible for conception of the manuscript and figures. MD was responsible for drafting of the article.
References
- 1.Kramer WC, Hendricks KJ, Wang J. Pathogenetic mechanisms of posttraumatic osteoarthritis: opportunities for early intervention. Int J Clin Exp Med. 2011;4:285–298. [PMC free article] [PubMed] [Google Scholar]
- 2.Richmond SA, Fukuchi RK, Ezzat A, et al. Are joint injury, sport activity, physical activity, obesity, or occupational activities predictors for osteoarthritis? A systematic review. J Orthop Sports Phys Ther. 2013;43:515–B19. doi: 10.2519/jospt.2013.4796. [DOI] [PubMed] [Google Scholar]
- 3.Brown TDT, Johnston RCR, Saltzman CLC, et al. Posttraumatic osteoarthritis: a first estimate of incidence, prevalence, and burden of disease. J Orthop Trauma. 2006;20:739–744. doi: 10.1097/01.bot.0000246468.80635.ef. [DOI] [PubMed] [Google Scholar]
- 4.Weatherall JM, Mroczek K, McLaurin T, et al. Post-traumatic ankle arthritis. Bull Hosp Jt Dis. 2013;71:104–112. [PubMed] [Google Scholar]
- 5.Wilson MG, Michet CJJ, Ilstrup DM, et al. Idiopathic symptomatic osteoarthritis of the hip and knee: a population-based incidence study. Mayo Clin Proc. 1990;65:1214–1221. doi: 10.1016/s0025-6196(12)62745-1. [DOI] [PubMed] [Google Scholar]
- 6.Valderrabano V, Horisberger M, Russell I, et al. Etiology of ankle osteoarthritis. Clin Orthop Relat Res. 2008;467:1800–1806. doi: 10.1007/s11999-008-0543-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nelson AJ, Collins CL, Yard EE, et al. Ankle injuries among United States high school sports athletes, 2005–2006. J Athl Train. 2007;42:381–387. [PMC free article] [PubMed] [Google Scholar]
- 8.Belmont PJJ, Goodman GP, Waterman B, et al. Disease and nonbattle injuries sustained by a U.S. army brigade combat team during operation iraqi freedom. Mil Med. 2010;175:469–476. doi: 10.7205/milmed-d-10-00041. [DOI] [PubMed] [Google Scholar]
- 9.McKay G, Goldie P, Payne WR, et al. A prospective study of injuries in basketball: a total profile and comparison by gender and standard of competition. J Sci Med Sport. 2001;4:196–211. doi: 10.1016/s1440-2440(01)80030-x. [DOI] [PubMed] [Google Scholar]
- 10.Gribble PA, Bleakley CM, Caulfield BM, et al. Evidence review for the 2016 International Ankle Consortium consensus statement on the prevalence, impact and long-term consequences of lateral ankle sprains. Br J Sports Med. 2016 doi: 10.1136/bjsports-2016-096188. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Valderrabano V, Hintermann B, Horisberger M, et al. Ligamentous posttraumatic ankle osteoarthritis. Am J Sport Med. 2006;34:612–620. doi: 10.1177/0363546505281813. [DOI] [PubMed] [Google Scholar]
- 12.Cheng DS, Visco CJ. Pharmaceutical therapy for osteoarthritis. PM&R. 2012;4:S82–88. doi: 10.1016/j.pmrj.2012.02.009. [DOI] [PubMed] [Google Scholar]
- 13.Anderson DD, Chubinskaya S, Guilak F, et al. Post-traumatic osteoarthritis: improved understanding and opportunities for early intervention. J Orthop Res. 2011;29:802–809. doi: 10.1002/jor.21359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lotz MK, Kraus VB. New developments in osteoarthritis. Posttraumatic osteoarthritis: pathogenesis and pharmacological treatment options. Arthritis Res Ther. 2010;12:211–211. doi: 10.1186/ar3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Quinn TM, Häuselmann H-J, Shintani N, et al. Cell and matrix morphology in articular cartilage from adult human knee and ankle joints suggests depth-associated adaptations to biomechanical and anatomical roles. Osteoarthr Cartil. 2013;21:1904–1912. [PubMed] [Google Scholar]
- 16.Treppo S, Koepp H, Quan EC, et al. Comparison of biomechanical and biochemical properties of cartilage from human knee and ankle pairs. J Orthop Res. 2000;18:739–748. doi: 10.1002/jor.1100180510. [DOI] [PubMed] [Google Scholar]
- 17.Aurich M, Poole AR, Reiner A, et al. Matrix homeostasis in aging normal human ankle cartilage. Arthritis Rheum. 2002;46:2903–2910. doi: 10.1002/art.10611. [DOI] [PubMed] [Google Scholar]
- 18.Eger W, Schumacher BL, Mollenhauer J, et al. Human knee and ankle cartilage explants: catabolic differences. J Orthop Res. 2002;20:526–534. doi: 10.1016/S0736-0266(01)00125-5. [DOI] [PubMed] [Google Scholar]
- 19.Huch K. Knee and ankle: human joints with different susceptibility to osteoarthritis reveal different cartilage cellularity and matrix synthesis in vitro. Arch Orthop Trauma Surg. 2001;121:301–306. doi: 10.1007/s004020000225. [DOI] [PubMed] [Google Scholar]
- 20.Huch K, Kuettner KE, Dieppe P. Osteoarthritis in ankle and knee joints. Semin Arthritis Rheum. 1997;26:667–674. doi: 10.1016/s0049-0172(97)80002-9. [DOI] [PubMed] [Google Scholar]
- 21.Dang Y, Cole AA, Homandberg GA. Comparison of the catabolic effects of fibronectin fragments in human knee and ankle cartilages. Osteoarthr Cartil. 2003;11:538–547. doi: 10.1016/s1063-4584(03)00085-2. [DOI] [PubMed] [Google Scholar]
- 22.Patwari P, Cheng DM, Cole AA, et al. Analysis of the relationship between peak stress and proteoglycan loss following injurious compression of human post-mortem knee and ankle cartilage. Biomech Model Mechanobiol. 2006;6:83–89. doi: 10.1007/s10237-006-0037-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hendren L, Beeson P. A review of the differences between normal and osteoarthritis articular cartilage in human knee and ankle joints. Foot (Edinb) 2009;19:171–176. doi: 10.1016/j.foot.2009.03.003. [DOI] [PubMed] [Google Scholar]
- 24.Aurich M, Hofmann GO, Rolauffs B, et al. Differences in injury pattern and prevalence of cartilage lesions in knee and ankle joints: a retrospective cohort study. Orthop Rev. 2014;6:5611. doi: 10.4081/or.2014.5611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Aurich M, Squires GR, Reiner A, et al. Differential matrix degradation and turnover in early cartilage lesions of human knee and ankle joints. Arthritis Rheum. 2005;52:112–119. doi: 10.1002/art.20740. [DOI] [PubMed] [Google Scholar]
- 26.Koepp H, Eger W, Muehleman C, et al. Prevalence of articular cartilage degeneration in the ankle and knee joints of human organ donors. J Orthop Sci. 1999;4:407–412. doi: 10.1007/s007760050123. [DOI] [PubMed] [Google Scholar]
- 27.Novakofski KD, Berg LC, Bronzini I, et al. Joint-dependent response to impact and implications for post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23:1130–1137. doi: 10.1016/j.joca.2015.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Catterall JB, Zura RD, Bolognesi MP, et al. Aspartic acid racemization reveals a high turnover state in knee compared with hip osteoarthritic cartilage. Osteoarthritis Cartilage. 2016;24:374–381. doi: 10.1016/j.joca.2015.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barg A, Pagenstert GI, Hügle T, et al. Ankle osteoarthritis: etiology, diagnostics, and classification. Foot Ankle Clin. 2013;18:411–426. doi: 10.1016/j.fcl.2013.06.001. [DOI] [PubMed] [Google Scholar]
- 30.Saltzman CL, Salamon ML, Blanchard GM, et al. Epidemiology of ankle arthritis: report of a consecutive series of 639 patients from a tertiary orthopaedic center. Iowa Orthop J. 2005;25:44–46. [PMC free article] [PubMed] [Google Scholar]
- 31.Buckwalter JA. The role of mechanical forces in the initiation and progression of osteoarthritis. HSS J. 2012;8:37–38. doi: 10.1007/s11420-011-9251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Blalock D, Miller A, Tilley M, et al. Joint instability and osteoarthritis. Clin Med Insights Arthritis Musculoskelet Disord. 2015;8:15–23. doi: 10.4137/CMAMD.S22147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.McKinley TO, Rudert MJ, Koos DC, et al. Incongruity versus instability in the etiology of posttraumatic arthritis. Clin Orthop Relat Res. 2004;423:44–51. doi: 10.1097/01.blo.0000131639.89143.26. [DOI] [PubMed] [Google Scholar]
- 34.Bischof JE, Spritzer CE, Caputo AM, et al. Journal of biomechanics. J Biomech. 2010;43:2561–2566. doi: 10.1016/j.jbiomech.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson DD, Van Hofwegen C, Marsh JL, et al. Is elevated contact stress predictive of post-traumatic osteoarthritis for imprecisely reduced tibial plafond fractures? J Orthop Res. 2010;29:33–39. doi: 10.1002/jor.21202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.McKinley TO, Borrelli J, D’Lima DD, et al. Basic science of intra-articular fractures and posttraumatic osteoarthritis. J Orthop Trauma. 2010;24:567–570. doi: 10.1097/BOT.0b013e3181ed298d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hintermann B, Regazzoni P, Lampert C, et al. Arthroscopic findings in acute fractures of the ankle. J Bone Joint Surg Br. 2000;82:345–351. doi: 10.1302/0301-620x.82b3.10064. [DOI] [PubMed] [Google Scholar]
- 38.Loren GJ, Ferkel RD. Arthroscopic assessment of occult intra-articular injury in acute ankle fractures. Arthroscopy. 2002;18:412–421. doi: 10.1053/jars.2002.32317. [DOI] [PubMed] [Google Scholar]
- 39.Stufkens SA, Knupp M, Horisberger M, et al. Cartilage lesions and the development of osteoarthritis after internal fixation of ankle fractures: a prospective study. J Bone Joint Surg Am. 2010;92:279–286. doi: 10.2106/JBJS.H.01635. [DOI] [PubMed] [Google Scholar]
- 40.Raikin SM, Elias I, Zoga AC, et al. Osteochondral lesions of the talus: localization and morphologic data from 424 patients using a novel anatomical grid scheme. Foot Ankle Int. 2007;28:154–161. doi: 10.3113/FAI.2007.0154. [DOI] [PubMed] [Google Scholar]
- 41.Taga I, Shino K, Inoue M, et al. Articular cartilage lesions in ankles with lateral ligament injury. An arthroscopic study. Am J Sport Med. 1993;21:120–126. doi: 10.1177/036354659302100120. discussion 126–7. [DOI] [PubMed] [Google Scholar]
- 42.Klammer G, Maquieira GJ, Spahn S, et al. Natural history of nonoperatively treated osteochondral lesions of the talus. Foot Ankle Int. 2015;36:24–31. doi: 10.1177/1071100714552480. [DOI] [PubMed] [Google Scholar]
- 43.Swenson DM, Collins CL, Fields SK, et al. Epidemiology of U.S. high school sports-related ligamentous ankle injuries, 2005/06-2010/11. Clin J Sport Med. 2013;23:190–196. doi: 10.1097/JSM.0b013e31827d21fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Marsh JL, Buckwalter J, Gelberman R, et al. Articular fractures: does an anatomic reduction really change the result? J Bone Joint Surg Am. 2002;84A:1259–1271. [PubMed] [Google Scholar]
- 45.O’Loughlin PF, Heyworth BE, Kennedy JG. Current concepts in the diagnosis and treatment of osteochondral lesions of the ankle. Am J Sport Med. 2010;38:392–404. doi: 10.1177/0363546509336336. [DOI] [PubMed] [Google Scholar]
- 46.Lynch SA, Renström PA. Treatment of acute lateral ankle ligament rupture in the athlete. Conservative versus surgical treatment. Sports Med. 1999;27:61–71. doi: 10.2165/00007256-199927010-00005. [DOI] [PubMed] [Google Scholar]
- 47.Petersen W, Rembitzki IV, Koppenburg AG, et al. Treatment of acute ankle ligament injuries: a systematic review. Arch Orthop Trauma Surg. 2013;133:1129–1141. doi: 10.1007/s00402-013-1742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Golditz T, Steib S, Pfeifer K, et al. Functional ankle instability as a risk factor for osteoarthritis: using T2-mapping to analyze early cartilage degeneration in the ankle joint of young athletes. Osteoarthr Cartil. 2014;22:1377–1385. doi: 10.1016/j.joca.2014.04.029. [DOI] [PubMed] [Google Scholar]
- 49.Gatlin CC, Matheny LM, Ho CP, et al. Diagnostic accuracy of 3.0 Tesla magnetic resonance imaging for the detection of articular cartilage lesions of the talus. Foot Ankle Int. 2015;36:288–292. doi: 10.1177/1071100714553469. [DOI] [PubMed] [Google Scholar]
- 50.Roemer FW, Jomaah N, Niu J, et al. Ligamentous injuries and the risk of associated tissue damage in acute ankle sprains in athletes: a cross-sectional mri study. Am J Sport Med. 2014;42:1549–1557. doi: 10.1177/0363546514529643. [DOI] [PubMed] [Google Scholar]
- 51.Sugimoto K, Takakura Y, Okahashi K, et al. Chondral injuries of the ankle with recurrent lateral instability: an arthroscopic study. J Bone Joint Surg Am. 2009;91:99–106. doi: 10.2106/JBJS.G.00087. [DOI] [PubMed] [Google Scholar]
- 52.Tochigi Y, Rudert MJ, Saltzman CL, et al. Contribution of articular surface geometry to ankle stabilization. J Bone Joint Surg Am. 2006;88:2704–2713. doi: 10.2106/JBJS.E.00758. [DOI] [PubMed] [Google Scholar]
- 53.Fong DT, Chan Y-Y, Mok K-M, et al. Understanding acute ankle ligamentous sprain injury in sports. Sports Med Arthrosc Rehabil Ther Technol. 2009;1:14. doi: 10.1186/1758-2555-1-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Madeti BK, Chalamalasetti SR. Bolla pragada SKSSR. Biomechanics of knee joint—A review. Front Mech Eng. 2015;10:176–186. [Google Scholar]
- 55.Deland JT, Morris GD, Sung IH. Biomechanics of the ankle joint. A perspective on total ankle replacement. Foot Ankle Clin. 2000;5:747–759. [PubMed] [Google Scholar]
- 56.Stauffer RN, Chao EY, Brewster RC. Force and motion analysis of the normal, diseased, and prosthetic ankle joint. Clin Orthop Relat Res. 1977:189–196. [PubMed] [Google Scholar]
- 57.Shepherd DE. Thickness of human articular cartilage in joints of the lower limb. Ann Rheum Dis. 1999;58:27. doi: 10.1136/ard.58.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Adam C, Eckstein F, Milz S, et al. The distribution of cartilage thickness within the joints of the lower limb of elderly individuals. J Anat. 1998;193:203–214. doi: 10.1046/j.1469-7580.1998.19320203.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.DeSmet AA, Dalinka MK, Alazraki N, et al. Chronic ankle pain. American college of radiology. ACR appropriateness criteria. Radiology. 2000;215:321–332. [PubMed] [Google Scholar]
- 60.Calhoun JH, Li F, Ledbetter BR, et al. A comprehensive study of pressure distribution in the ankle joint with inversion and eversion. Foot Ankle Int. 1994;15:125–133. doi: 10.1177/107110079401500307. [DOI] [PubMed] [Google Scholar]
- 61.van Dijk CN, Lim LS, Poortman A, et al. Degenerative joint disease in female ballet dancers. Am J Sport Med. 1995;23:295–300. doi: 10.1177/036354659502300307. [DOI] [PubMed] [Google Scholar]
- 62.Angioi M, Maffulli GD, McCormack M, et al. Early signs of osteoarthritis in professional ballet dancers: a preliminary study. Clin J Sport Med. 2014;24:435–437. doi: 10.1097/JSM.0000000000000035. [DOI] [PubMed] [Google Scholar]
- 63.Ramsey PL, Hamilton W. Changes in tibiotalar area of contact caused by lateral talar shift. J Bone Joint Surg Am. 1976;58:356–357. [PubMed] [Google Scholar]
- 64.Wright IC, Neptune RR, van den Bogert AJ, et al. The influence of foot positioning on ankle sprains. J Biomech. 2000;33:513–519. doi: 10.1016/s0021-9290(99)00218-3. [DOI] [PubMed] [Google Scholar]
- 65.McKay GD, Goldie PA, Payne WR, et al. Ankle injuries in basketball: injury rate and risk factors. Br J Sports Med. 2001;35:103–108. doi: 10.1136/bjsm.35.2.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Buckley MR, Bonassar LJ, Cohen I. Localization of viscous behavior and shear energy dissipation in articular cartilage under dynamic shear loading. J Biomech Eng. 2013;135:031002. doi: 10.1115/1.4007454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Silverberg JL, Barrett AR, Das M, et al. Structure-Function relations and rigidity percolation in the shear properties of articular cartilage. Biophysj. 2014;107:1721–1730. doi: 10.1016/j.bpj.2014.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Korhonen RK, Wong M, Arokoski J, et al. Importance of the superficial tissue layer for the indentation stiffness of articular cartilage. Med Eng Phys. 2002;24:99–108. doi: 10.1016/s1350-4533(01)00123-0. [DOI] [PubMed] [Google Scholar]
- 69.Silverberg JL, Dillavou S, Bonassar L, et al. Anatomic variation of depth-dependent mechanical properties in neonatal bovine articular cartilage. J Orthop Res. 2012;31:686–691. doi: 10.1002/jor.22303. [DOI] [PubMed] [Google Scholar]
- 70.Poole AR, Kojima T, Yasuda T, et al. Composition and structure of articular cartilage: a template for tissue repair. Clin Orthop Relat Res. 2001;391:S26–S33. doi: 10.1097/00003086-200110001-00004. [DOI] [PubMed] [Google Scholar]
- 71.Schumacher BL, Su J-L, Lindley KM, et al. Horizontally oriented clusters of multiple chondrons in the superficial zone of ankle, but not knee articular cartilage. Anat Rec. 2002;266:241–248. doi: 10.1002/ar.10063. [DOI] [PubMed] [Google Scholar]
- 72.Aicher WK, Rolauffs B. The spatial organisation of joint surface chondrocytes: review of its potential roles in tissue functioning, disease and early, preclinical diagnosis of osteoarthritis. Ann Rheum Dis. 2014;73:645–653. doi: 10.1136/annrheumdis-2013-204308. [DOI] [PubMed] [Google Scholar]
- 73.Rolauffs B, Rothdiener M, Bahrs C, et al. Onset of preclinical osteoarthritis: the angular spatial organization permits early diagnosis. Arthritis Rheumatism. 2011;63:1637–1647. doi: 10.1002/art.30217. [DOI] [PubMed] [Google Scholar]
- 74.Kang Y, Koepp H, Cole AA, et al. Cultured human ankle and knee cartilage differ in susceptibility to damage mediated by fibronectin fragments. J Orthop Res. 1998;16:551–556. doi: 10.1002/jor.1100160505. [DOI] [PubMed] [Google Scholar]
- 75.Orazizadeh M, Cartlidge C, Wright MO, et al. Mechanical responses and integrin associated protein expression by human ankle chondrocytes. Biorheology. 2006;43:249–258. [PubMed] [Google Scholar]
- 76.Candrian C, Bonacina E, Frueh JA, et al. Intra-individual comparison of human ankle and knee chondrocytes in vitro: relevance for talar cartilage repair. Osteoarthritis Cartilage. 2009;17:489–496. doi: 10.1016/j.joca.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 77.Garrido CP, Hakimiyan AA, Rappoport L, et al. Anti-apoptotic treatments prevent cartilage degradation after acute trauma to human ankle cartilage. Osteoarthritis Cartilage. 2009;17:1244–1251. doi: 10.1016/j.joca.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kurz B, Lemke AK, Fay J, et al. Pathomechanisms of cartilage destruction by mechanical injury. Ann Anat. 2005;187:473–485. doi: 10.1016/j.aanat.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 79.Chubinskaya S, Wimmer MA. Key pathways to prevent posttraumatic arthritis for future molecule-Based therapy. Cartilage. 2013;4:S13–21. doi: 10.1177/1947603513487457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Scott I, Midha A, Rashid U, et al. Correlation of gene and mediator expression with clinical endpoints in an acute interleukin-1b-driven model of joint pathology. Osteoarthritis Cartilage. 2009;17:790–797. doi: 10.1016/j.joca.2008.09.016. [DOI] [PubMed] [Google Scholar]
- 81.Renner AF, Carvalho E, Soares E, et al. The effect of a passive muscle stretching protocol on the articular cartilage. Osteoarthritis Cartilage. 2006;14:196–202. doi: 10.1016/j.joca.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 82.Mason RM, Chambers MG, Flannelly J, et al. The STR/ort mouse and its use as a model of osteoarthritis. Osteoarthritis Cartilage. 2001;9:85–91. doi: 10.1053/joca.2000.0363. [DOI] [PubMed] [Google Scholar]
- 83.Collins C, Evans RG, Ponsford F, et al. Chondro-osseous metaplasia, bone density and patellar cartilage proteoglycan content in the osteoarthritis of STR/ORT mice. Osteoarthritis Cartilage. 1994;2:111–118. doi: 10.1016/s1063-4584(05)80061-5. [DOI] [PubMed] [Google Scholar]
- 84.Evans RG, Collins C, Miller P, et al. Radiological scoring of osteoarthritis progression in STR/ORT mice. Osteoarthritis Cartilage. 1994;2:103–109. doi: 10.1016/s1063-4584(05)80060-3. [DOI] [PubMed] [Google Scholar]
- 85.Staines KA, Madi K, Mirczuk SM, et al. Endochondral growth defect and deployment of transient chondrocyte behaviors underlie osteoarthritis onset in a natural murine model. Arthritis Rheumatol. 2016;68:880–891. doi: 10.1002/art.39508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Das-Gupta EP, Lyons TJ, Hoyland JA, et al. New histological observations in spontaneously developing osteoarthritis in the STR/ORT mouse questioning its acceptability as a model of human osteoarthritis. Int J Exp Pathol. 1993;74:627–634. [PMC free article] [PubMed] [Google Scholar]
- 87.Yamamoto H, Iwase N. Spontaneous osteoarthritic lesions in a new mutant strain of the mouse. Exp Anim. 1998;47:131–135. doi: 10.1538/expanim.47.131. [DOI] [PubMed] [Google Scholar]
- 88.Yamamoto H, Iwase N, Kohno M. Histopathological characterization. Exp Toxicol Pathol. 1999;51:15–20. doi: 10.1016/S0940-2993(99)80051-7. [DOI] [PubMed] [Google Scholar]
- 89.Gerwin N, Bendele AM, Glasson S, et al. The OARSI histopathology initiative. Osteoarthritis Cartilage. 2010;18:S24–34. doi: 10.1016/j.joca.2010.05.030. [DOI] [PubMed] [Google Scholar]
- 90.Moriyama H, Kanemura N, Brouns I, et al. Effects of aging and exercise training on the histological and mechanical properties of articular structures in knee joints of male rat. Biogerontology. 2012;13:369–381. doi: 10.1007/s10522-012-9381-8. [DOI] [PubMed] [Google Scholar]
- 91.Mohr W, Lehmann H. Osteoarthrosis of the ankle joints in old rats. Z Rheumatol. 1992;51:35–40. [PubMed] [Google Scholar]
- 92.Mohr W, Lehmann H, Engelhardt G. Chondroneutrality of meloxicam in rats with spontaneous osteoarthrosis of the ankle joint. Z Rheumatol. 1997;56:21–30. doi: 10.1007/s003930050017. [DOI] [PubMed] [Google Scholar]
- 93.Kuettner KE, Cole AA. Cartilage degeneration in different human joints. Osteoarthritis Cartilage. 2005;13:93–103. doi: 10.1016/j.joca.2004.11.006. [DOI] [PubMed] [Google Scholar]
- 94.Little CB, Hunter DJ. Post-traumatic osteoarthritis: from mouse models to clinical trials. Nat Rev Rheumatol. 2013;9:485–497. doi: 10.1038/nrrheum.2013.72. [DOI] [PubMed] [Google Scholar]
- 95.Little CB, Smith MM. Animal models of osteoarthritis. Curr Rheumatol Rev. 2008;4:175–182. [Google Scholar]
- 96.Bendele AM. Animal models of osteoarthritis. J Musculoskelet Neuronal Interact. 2001;1:363–376. [PubMed] [Google Scholar]
- 97.Mastbergen SC, Lafeber FP. Animal models of osteoarthritis—why choose a larger model. US Musculoskelet Rev. 2009:11–17. [Google Scholar]
- 98.Gregory MH, Capito N, Kuroki K, et al. A review of translational animal models for knee osteoarthritis. Arthritis. 2012;2012:1–14. doi: 10.1155/2012/764621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lampropoulou-Adamidou K, Lelovas P, Karadimas EV, et al. Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol. 2013;24:263–271. doi: 10.1007/s00590-013-1205-2. [DOI] [PubMed] [Google Scholar]
- 100.Aigner T, Cook JL, Gerwin N, et al. Histopathology atlas of animal model systems. Osteoarthritis Cartilage. 2010;18:S2–S6. doi: 10.1016/j.joca.2010.07.013. [DOI] [PubMed] [Google Scholar]
- 101.McCoy AM. Animal models of osteoarthritis: comparisons and key considerations. Vet Pathol. 2015;52:803–818. doi: 10.1177/0300985815588611. [DOI] [PubMed] [Google Scholar]
- 102.Malfait A-M, Little CB. On the predictive utility of animal models of osteoarthritis. Arthritis Res Ther. 2015;17:225. doi: 10.1186/s13075-015-0747-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Poole R, Blake S, Buschmann M, et al. Recommendations for the use of preclinical models in the study and treatment of osteoarthritis. Osteoarthritis Cartilage. 2020;18:S10–S16. doi: 10.1016/j.joca.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 104.Wendler A, Wehling M. The translatability of animal models for clinical development: biomarkers and disease models. Curr Opin Pharmacol. 2010;10:601–606. doi: 10.1016/j.coph.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 105.Czajka CM, Tran E, Cai AN, et al. Ankle sprains and instability. Med Clin NA. 2014;98:313–329. doi: 10.1016/j.mcna.2013.11.003. [DOI] [PubMed] [Google Scholar]
- 106.Kerkhoffs GM, Blankevoort L, van Poll D, et al. Anterior lateral ankle ligament damage and anterior talocrural-joint laxity: an overview of the in vitro reports in literature. Clin Biomech (Bristol, Avon) 2001;16:635–643. doi: 10.1016/s0268-0033(01)00054-7. [DOI] [PubMed] [Google Scholar]
- 107.Goetz JE, Fredericks D, Petersen E, et al. A clinically realistic large animal model of intra-articular fracture that progresses to post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2015;23:1797–1805. doi: 10.1016/j.joca.2015.05.022. [DOI] [PubMed] [Google Scholar]
- 108.Buckwalter JA, Brown TD. Joint injury, repair, and remodeling. Clin Orthop Relat Res. 2004;423:7–16. [PubMed] [Google Scholar]
- 109.Ewers BJ, Dvoracek-Driksna D, Orth MW, et al. The extent of matrix damage and chondrocyte death in mechanically traumatized articular cartilage explants depends on rate of loading. J Orthop Res. 2001;19:779–784. doi: 10.1016/S0736-0266(01)00006-7. [DOI] [PubMed] [Google Scholar]
- 110.Diestelmeier BW, Rudert MJ, Tochigi Y, et al. An instrumented pendulum system for measuring energy absorption during fracture insult to large animal joints in vivo. J Biomech Eng. 2014;136:064502. doi: 10.1115/1.4025113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Bonnevie ED, Delco ML, Fortier LA, et al. Characterization of tissue response to impact loads delivered using a hand-Held instrument for studying articular cartilage injury. Cartilage. 2015;6:226–232. doi: 10.1177/1947603515595071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Alexander PG, McCarron JA, Levine MJ, et al. An In vivo lapine model for impact-induced injury and osteoarthritic degeneration of articular cartilage. Cartilage. 2012;3:323–333. doi: 10.1177/1947603512447301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rickey EJ, Cruz AM, Trout DR, et al. Evaluation of experimental impact injury for inducing post-traumatic osteoarthritis in the metacarpophalangeal joints of horses. Am J Vet Res. 2012;73:1540–1552. doi: 10.2460/ajvr.73.10.1540. [DOI] [PubMed] [Google Scholar]
- 114.Brimmo O, Pfeiffer F, Bozynski C, et al. Development of a novel canine model for posttraumatic osteoarthritis of the knee. J Knee Surg. 2016;29:235–241. doi: 10.1055/s-0035-1549026. [DOI] [PubMed] [Google Scholar]
- 115.Bartell LR, Fortier LA, Bonassar LJ, et al. Measuring microscale strain fields in articular cartilage during rapid impact reveals thresholds for chondrocyte death and a protective role for the superficial layer. J Biomech. 2015;48:3440–3446. doi: 10.1016/j.jbiomech.2015.05.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Christiansen BA, Guilak F, Lockwood KA, et al. Non-invasive mouse models of post-traumatic osteoarthritis. Osteoarthritis and Cartilage. 2015;23:1627–1638. doi: 10.1016/j.joca.2015.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Chang S, Yasui T, Tanaka S, et al. Establishment of surgical destabilization model of mouse ankle osteoarthritis. Osteoarthritis Cartilage. 2015;23:A291–292. [Google Scholar]
- 118.Chang SH, Yasui T, Taketomi S, et al. Comparison of mouse and human ankles and establishment of mouse ankle osteoarthritis models by surgically-induced instability. Osteoarthritis Cartilage. 2016;24:688–697. doi: 10.1016/j.joca.2015.11.008. [DOI] [PubMed] [Google Scholar]
- 119.Alexander PG, Song Y, Taboas JM, et al. Development of a spring-Loaded impact device to deliver injurious mechanical impacts to the articular cartilage surface. Cartilage. 2012;4:52–62. doi: 10.1177/1947603512455195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.McIlwraith CW, Frisbie DD, Kawcak CE, et al. The OARSI histopathology initiative. Osteoarthritis Cartilage. 2020;18:S93–S105. doi: 10.1016/j.joca.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 121.Little CB, Smith MM, Cake MA, et al. The OARSI histopathology initiative. Osteoarthritis Cartilage. 2010;18:S80–92. doi: 10.1016/j.joca.2010.04.016. [DOI] [PubMed] [Google Scholar]
- 122.Cook JL, Kuroki K, Visco D, et al. The OARSI histopathology initiative. Osteoarthritis Cartilage. 2010;18:S66–79. doi: 10.1016/j.joca.2010.04.017. [DOI] [PubMed] [Google Scholar]
- 123.Boyce MK, Trumble TN, Carlson CS, et al. Nonterminal animal model of post-traumatic osteoarthritis induced by acute joint injury. Osteoarthritis Cartilage. 2013;21:746–755. doi: 10.1016/j.joca.2013.02.653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hurtig M, Chubinskaya S, Dickey J, et al. BMP-7 protects against progression of cartilage degeneration after impact injury. J Orthop Res. 2009;27:602–611. doi: 10.1002/jor.20787. [DOI] [PubMed] [Google Scholar]
- 125.Rorvik AM, Teige J. Unstable stifles without clinical or radiographic osteoarthritis in young goats: an experimental study. Acta Vet Scand. 1996;37:265–272. doi: 10.1186/BF03548093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Malda J, de Grauw JC, Benders KEM, et al. Of mice, men and elephants: the relation between articular cartilage thickness and body mass. PLoS ONE. 2013;8:e57683. doi: 10.1371/journal.pone.0057683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Frisbie DD, Cross MW, McIlwraith CW. A comparative study of articular cartilage thickness in the stifle of animal species used in human pre-clinical studies compared to articular cartilage thickness in the human knee. Vet Comp Orthop Traumatol. 2006;19:142–146. [PubMed] [Google Scholar]
- 128.Garcia-Seco E, Wilson DA, Cook JL, et al. Measurement of articular cartilage stiffness of the femoropatellar, tarsocrural, and metatarsophalangeal joints in horses and comparison with biochemical data. Vet Surg. 2005;34:571–578. doi: 10.1111/j.1532-950X.2005.00090.x. [DOI] [PubMed] [Google Scholar]
- 129.McIlwraith CW, Frisbie DD, Kawcak CE. The horse as a model of naturally occurring osteoarthritis. Bone Joint Res. 2012;1:297–309. doi: 10.1302/2046-3758.111.2000132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fleck SKV, Dyson SJ. Lameness associated with tarsocrural joint pathology in 17 mature horses 1997–2010) Equine Vet Edu. 2012;24:628–638. [Google Scholar]
- 131.Barker WHJ, Smith MRW, Minshall GJ, et al. Soft tissue injuries of the tarsocrural joint: a retrospective analysis of 30 cases evaluated arthroscopically. Equine Vet J. 2012;45:435–441. doi: 10.1111/j.2042-3306.2012.00685.x. [DOI] [PubMed] [Google Scholar]
- 132.Lamb L, Zubrod C, Hague B, et al. Clinical outcome of collateral ligament injuries of the tarsus. Can Vet J. 2012;53:518–524. [PMC free article] [PubMed] [Google Scholar]
- 133.Latorre R, Arencibia A, Gil F, et al. Correlation of magnetic resonance images with anatomic features of the equine tarsus. Am J Vet Res. 2006;67:756–761. doi: 10.2460/ajvr.67.5.756. [DOI] [PubMed] [Google Scholar]
- 134.Raes EV, Bergman EHJ, van der Veen H, et al. Comparison of cross-sectional anatomy and computed tomography of the tarsus in horses. Am J Vet Res. 2011;72:1209–1221. doi: 10.2460/ajvr.72.9.1209. [DOI] [PubMed] [Google Scholar]
- 135.Chu CR, Szczodry M, Bruno S. Animal models for cartilage regeneration and repair. Tissue Eng Part B Rev. 2010;16:105–115. doi: 10.1089/ten.teb.2009.0452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Rutgers M, van Pelt MJP, Dhert WJA, et al. Review evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18:12–23. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 137.Mankin HJ, Dorfman H, Lippiello L, et al. Biochemical and metabolic abnormalities in articular cartilage from osteo-arthritic human hips. II. Correlation of morphology with biochemical and metabolic data. J Bone Joint Surg Am. 1971;53:523–537. [PubMed] [Google Scholar]
- 138.Pritzker K, Gay S, Jimenez S, et al. Osteoarthritis cartilage histopathology: grading and staging 1, 2. Osteoarthritis Cartilage. 2006;14:13–29. doi: 10.1016/j.joca.2005.07.014. [DOI] [PubMed] [Google Scholar]
- 139.Mainil-Varlet P, Aigner T, Brittberg M, et al. Histological assessment of cartilage repair: a report by the Histology Endpoint Committee of the International Cartilage Repair Society (ICRS) J Bone Joint Surg Am. 2003;85-A:45–57. [PubMed] [Google Scholar]
- 140.Dell’Isola A, Allan R, Smith SL, et al. Identification of clinical phenotypes in knee osteoarthritis: a systematic review of the literature. BMC Musculoskeletal Disorders. 2016;17:425. doi: 10.1186/s12891-016-1286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Pinar H, Akseki D, Kovanlikaya I, et al. Bone bruises detected by magnetic resonance imaging following lateral ankle sprains. Knee Surg Sports Traumatol Arthrosc. 1997;5:113–117. doi: 10.1007/s001670050036. [DOI] [PubMed] [Google Scholar]
- 142.Labovitz JM, Schweitzer ME. Occult osseous injuries after ankle sprains: incidence, location, pattern, and age. Foot Ankle Int. 1998;19:661–667. doi: 10.1177/107110079801901003. [DOI] [PubMed] [Google Scholar]
- 143.Welsch GH, Mamisch TC, Weber M, et al. High-resolution morphological and biochemical imaging of articular cartilage of the ankle joint at 3.0 T using a new dedicated phased array coil: in vivo reproducibility study. Skeletal Radiol. 2008;37:519–526. doi: 10.1007/s00256-008-0474-z. [DOI] [PubMed] [Google Scholar]
- 144.Lee S, Yoon YC, Kim JH. T2 mapping of the articular cartilage in the ankle: correlation to the status of anterior talofibular ligament. Clin Radiol. 2021;68:e355–e361. doi: 10.1016/j.crad.2013.01.023. [DOI] [PubMed] [Google Scholar]
- 145.Guermazi A, Alizai H, Crema MD, et al. Compositional MRI techniques for evaluation of cartilage degeneration in osteoarthritis. Osteoarthr Cartil. 2015;23:1639–1653. doi: 10.1016/j.joca.2015.05.026. [DOI] [PubMed] [Google Scholar]
- 146.Pfau T, Robilliard JJ, Weller R, et al. Assessment of mild hindlimb lameness during over ground locomotion using linear discriminant analysis of inertial sensor data. Equine Vet J. 2010;39:407–413. doi: 10.2746/042516407x185719. [DOI] [PubMed] [Google Scholar]
- 147.Keegan KG, Wilson DA, Kramer J, et al. Comparison of a body-mounted inertial sensor system-based method with subjective evaluation for detection of lameness in horses. Am J Vet Res. 2013;74:17–24. doi: 10.2460/ajvr.74.1.17. [DOI] [PubMed] [Google Scholar]
- 148.Keegan KG, Kramer J, Yonezawa Y, et al. Assessment of repeatability of a wireless, inertial sensor-based lameness evaluation system for horses. Am J Vet Res. 2011;72:1156–1163. doi: 10.2460/ajvr.72.9.1156. [DOI] [PubMed] [Google Scholar]
- 149.McCracken MJ, Kramer J, Keegan KG, et al. Comparison of an inertial sensor system of lameness quantification with subjective lameness evaluation. Equine Vet J. 2012;44:652–656. doi: 10.1111/j.2042-3306.2012.00571.x. [DOI] [PubMed] [Google Scholar]
- 150.Zumwalt AC, Hamrick M, Schmitt D. Force plate for measuring the ground reaction forces in small animal locomotion. J Biomech. 2006;39:2877–2881. doi: 10.1016/j.jbiomech.2005.10.006. [DOI] [PubMed] [Google Scholar]
- 151.McLaughlin RM. Kinetic and kinematic gait analysis in dogs. Vet Clin North Am Small Anim Pract. 2001;31:193–201. doi: 10.1016/s0195-5616(01)50045-5. [DOI] [PubMed] [Google Scholar]
- 152.Gillette RL, Angle TC. Recent developments in canine locomotor analysis: a review. Vet J. 2008;178:165–176. doi: 10.1016/j.tvjl.2008.01.009. [DOI] [PubMed] [Google Scholar]
- 153.Keegan KG. Evidence-based lameness detection and quantification. Vet Clin North Am Equine Pract. 2007;23:403–423. doi: 10.1016/j.cveq.2007.04.008. [DOI] [PubMed] [Google Scholar]
- 154.Piel MJ, Kroin JS, van Wijnen AJ, et al. Pain assessment in animal models of osteoarthritis. Gene. 2014;537:184–188. doi: 10.1016/j.gene.2013.11.091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Blaker CL, Clarke EC, Little CB. Using mouse models to investigate the pathophysiology, treatment, and prevention of post-traumatic osteoarthritis. J Orthop Res. 2016 doi: 10.1002/jor.23343. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 156.Neogi T, Guermazi A, Roemer F, et al. Association of joint inflammation with pain sensitization in knee osteoarthritis: the multicenter osteoarthritis study. Arthritis Rheumatol. 2016;68:654–661. doi: 10.1002/art.39488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Lee AS, Ellman MB, Yan D, et al. A current review of molecular mechanisms regarding osteoarthritis and pain. Gene. 2013;527:440–447. doi: 10.1016/j.gene.2013.05.069. [DOI] [PMC free article] [PubMed] [Google Scholar]