Abstract
Filamentous fungi are well-established expression hosts often used to produce extracellular proteins of use in the food and pharmaceutical industries. The expression systems presently used in Aspergillus species rely on either strong constitutive promoters, e.g., that for glyceraldehyde-3-phosphate dehydrogenase, or inducible systems derived from metabolic pathways, e.g., glaA (glucoamylase) or alc (alcohol dehydrogenase). We describe for Aspergillus nidulans and Aspergillus niger a novel expression system that utilizes the transcriptional activation of the human estrogen receptor by estrogenic substances. The system functions independently from metabolic signals and therefore can be used with low-cost, complex media. A combination of positive and negative regulatory elements in the promoter drives the expression of a reporter gene, yielding a linear dose response to the inducer. The off status is completely tight, yet the system responds within minutes to induction and reaches a level of expression of up to 15% of total cell protein after 8 h. Both Aspergillus species are very sensitive to estrogenic substances, and low-cost inducers function in the picomolar concentration range, at which estrogenic substances also can be found in the environment. Given this high sensitivity to estrogens, Aspergillus cells carrying estrogen-responsive units could be used to detect xenoestrogens in food or in the environment.
In their natural environment, fungi use extracellular enzymes to gain access to complex, often water-insoluble carbon and nitrogen sources. Extracellular protein production by filamentous fungi usually is very efficient for homologous proteins, for which levels of grams per liter can be obtained. Aspergillus and Trichoderma strains have been reported to secrete up to 30 g of homologous proteins per liter in fermentation processes (e.g., glucoamylases) (11). The production of recombinant proteins of mammalian origin can be up to 4 orders of magnitude lower even when the same expression signals are used.
The reason for this difference is not completely understood (2). Along with mRNA stability, codon usage, and translational efficiency, overloading the secretory machinery with a protein that has a “foreign” structure appears to be a critical bottleneck (16, 34). High concentrations of such heterologous proteins can result in incorrect folding—leading to translational feedback inhibition by phosphorylation of the α subunit of eukaryotic initiation factor 2 (23) and subsequent intracellular degradation of the misfolded protein. Thus, strong but highly regulatable expression systems are needed to control the flow of proteins through the secretory pathway.
The use of strong promoters derived from housekeeping genes, such as those of the various fungal glyceraldehyde-3-phosphate dehydrogenase genes, has the disadvantage of continuous, growth-related expression levels (31). This problem may reduce yields of proteins susceptible to degradation or lead to cell death if the overexpressed foreign proteins are toxic to the host cells. Other promoters, e.g., the alcohol dehydrogenase (alcA) promoter from Aspergillus nidulans, have lower background expression levels and achieve very high expression levels under conditions of induction and glucose deprivation, but this system is not functional in the industrially relevant species Aspergillus niger (27).
Most promoters commonly used for industrial Aspergillus species are derived from the amylase (36), xylanase (7), and arabinase (12) genes. These genes are repressed by glucose, and carbon catabolite repression also can override induction (10, 46) and lead to the loss of inducibility. Moreover, it is not possible to obtain a linear dose response by altering the inducer concentration. The metabolic origin of these systems dictates their expression levels; when they are highly expressed, the secretory pathway may be overloaded, resulting in the misfolding and intracellular degradation of proteins (13).
In commercial applications, inducer compounds (applied in the millimolar range) are a major part of fermentation costs and can be toxic; e.g., alcohols require special measures for safe handling, storage, and disposal. The type of fermentation medium also affects the secretory capacity of a given expression host. Most importantly, the expression of extracellular proteases, such as those encoded by pepA, pepB, and pepF, which significantly affect secreted protein yield, are regulated by the types of carbon and nitrogen sources and by the pH of the medium (39, 41). Kurzatkowski et al. (22) have presented evidence that glucose also has a negative effect on the development of an efficient secretory apparatus. These data indicate that the use of promoters derived from metabolic genes requires a specific fermentation medium; therefore, most low-cost, complex media (containing multiple sugars and various nitrogen sources) cannot be used.
The human estrogen receptor (hERα) is a member of a family of nuclear receptors for small hydrophobic ligands (40) that regulate growth, differentiation, and homeostasis in vertebrate cells. hERα activity is regulated allosterically by ligand binding, which activates the protein and promotes nuclear entry, binding to high-affinity sites in chromatin, and subsequently transcriptional modulation (15). Expression systems based on hERα function in yeasts (25) and plants (49), but these organisms are not highly sensitive to estrogenic substances (in the micromolar range); for instance, in Saccharomyces cerevisiae, ERα induces high-level expression only when the PDR5 and SNQ2 genes encoding the ABC transporters are deleted (26).
In addition to the natural hormone, other compounds also can bind to ERα with a high affinity and either activate transcription or, by acting as antiestrogens, interfere with activation by estrogenic substances. Many plant-derived products contain highly active phytoestrogens, e.g., coumestrol, and many industrial and pharmaceutical compounds with xenohormone activity also are available (1).
The objective of this study was to develop a strong but metabolically independent, highly regulatable expression system for Aspergillus species. We show that Aspergillus species are highly sensitive to estrogenic compounds and that estrogen-responsive elements (EREs) activate the transcription of a reporter gene with a linear dose response.
MATERIALS AND METHODS
Strains.
The strains used in this study are listed in Table 1. Strain requests should be directed to B. Shoemaker, Contracts Coordinator, GlycoFi, Inc., 21 Lafayette St., Ste. 200, Lebanon, NH 03766.
TABLE 1.
Strains used in this study
| Strain designation | Genotype | Strain description |
|---|---|---|
| argB pyrG | argB2 riboA1 pyrG89 | A. nidulans parent strain |
| hER | argB2 riboA1 | Parent strain + phERpyr4 |
| ERE-URA-nirA | riboA1 | hER + pERE-URA-nirA |
| ERE-URA-RS | riboA1 | hER + pERE-URA-RS |
| ERE-RS-nirA | riboA1 | hER + pERE-RS-nirA |
| alcA | riboA1 | hER + pRMalcA |
| A972a | cspA1 acrA brnA2 pyrG5 niaD2 | A. niger parent strain |
| 972 ERE-URA- nirA | cspA1 acrA1 brnA2 niaD2 | A972 + phERpyr4 and pERE-URA-nirA |
From the Fungal Genetic Stock Center (www.fgsc.net).
Plasmid construction. (i) phERpyr4.
A 2,941-bp BglII/PstI fragment from pAN52-1 (GenBank accession no. Z32697), containing the PgpdA-NcoI-BamHI-Ttrpc cassette, was inserted into pBluescript KS(+) (GenBank accession no. X52331) cut with BamHI/PstI. A 1,819-bp EcoRI fragment (end repaired by Klenow filling in) from plasmid Yep90-HEG0 (29), containing the complete coding sequence of hERα, was subsequently cloned into the NcoI site (end repaired by T4 polymerase) within the PgpdA-Ttrpc cassette. The Pgpd-hERα-Ttrpc cassette was released as a SpeI/ClaI fragment and cloned into a pBluescript KS(+) derivative cut with Spe/ClaI and already containing the pyr4 gene of Trichoderma reesei as a selectable marker. The pyr4 gene is located on a SalI fragment originating from pFG1 (14).
(ii) pRM2085.
A 274-bp PCR fragment containing the 3×ERE-PURA3 sequence (the 1× ERE sequence is 5′-GGTCACAGTGACC-3′) was amplified with primers (underlined sequences in primers represent bases introduced for cloning purposes) ERE-FW (5′-CGAATTCAGATCTCCATGCAGTTGGACG-3′) and URA3-RV (5′-TGGCAGCAACAGGACTAGGAT-3′) and with genomic DNA prepared from yeast strain YYM8 (24) as a template. A 108-bp nirA promoter fragment was amplified with primers NirA-RV (5′-TGGATCCATGGTAAATCAAGCCCAGACAGA-3′) and NirA-99 (5′-TCTACTGCAGGGAAACACGCCGAGC-3′) and with A. nidulans genomic DNA as a template. nirA codes for a transcriptional regulator mediating nitrate induction and is constitutively expressed at extremely low levels not detectable by Northern analysis (5). The 3×ERE-PURA3 sequence and the nirA promoter fragment were cut with EcoRI/PstI and BamHI/PstI, respectively, and fused at the unique PstI site (3×ERE-PURA3-PnirA) via subcloning in pBluescript KS(+). From the resulting plasmid, pZRM2059 (GenBank accession no. AY663843), a 284-bp BglII/BamHI fragment was released and cloned into the BamHI site of pAN923-42BBglII (31).
(iii) pERE-URA-RS.
The 101-bp BamHI/PstI nirA promoter fragment within pZRM2059 (3×ERE-PURA3-PnirA) was replaced with a 101-bp random sequence (RS) derived from the open reading frame (ORF) of the ampicillin resistance gene by PCR with primers RSnirA-BamF (5′-AAGGATCCATATCTTTTACTTTCACCAGCG-3′) and RSnirA-PstR (5′-TGACTGCAGAACATTTCCGTGTCGCCCTTATTC-3′) to obtain p2059-URA-RS (GenBank accession no. AY663844). Again, the chimeric promoter construct (3×ERE-PURA3-RS) was released (BamHI/BglII) and cloned into the BamHI site of pAN923-42BBglII.
(iv) pERE-RS-nirA.
The RS derived from the ORF of the ampicillin resistance gene was generated by PCR with primers RSURA3-PstF (5′-ATTCTGCAGAACCCACTCGTGCACCCAAC-3′) and RSURA3-R (5′-TTTCCGTGTCGCCCTTATTC-3′). The 3×ERE sequence was generated by annealing oligonucleotides ERE-BglII-F (5′-GGTCACTGTGACCGGTCACTGTGACCGGTCACTGTGACCAGATCTGGA-3′) and ERE-BglII-R (5′-TCCAGATCTGGTCACAGTGACCGGTCACAGTGACCGGTCACAGTGACC-3′). The two fragments were ligated and inserted into pRM2059 cut with PstI/BglII to replace the 183-bp 3×ERE-PURA3 fragment. The chimeric promoter construct (3×ERE-RS-PnirA) was excised with BamHI/BglII from the resulting plasmid, p2059-RS-nirA (GenBank accession no. AY663845), and cloned into the BamHI site of pAN923-42BBglII.
(v) pRMalcA.
pRM2085 was partially cut with XhoI and subsequently digested with BamHI. The 420-bp alcA promoter fragment was amplified by PCR with primers alcAXhoIF (5′-GTCCTCGAGCAGCTGAAAAAGCTGA-3′) and alcABamHIR (5′-TTGGATCCATTTTGAGGCGAGGTGA-3′), digested with BamHI/XhoI, and ligated into the vector backbone of pRM2085 to obtain vector pRMalcA.
Culture conditions, transformation, and Northern analysis.
Aspergillus strains were grown for 12 to 14 h at 37°C in liquid minimal medium (30) with appropriate supplements by shaking on a rotary shaker at 180 rpm. Estrogenic compounds were purchased from Sigma (St. Louis, Mo.) (diethylstilbestrol [DES] [catalog no. D-4628], 17-β-estradiol [catalog no. E-225717], and α-zearalanol [catalog no. Z-0292]) or from Fluka (Buchs, Switzerland) (coumestrol [catalog no. 27885]). Different inducer concentrations, types, and nutrients were tested by harvesting cultures by filtration and washing with two culture volumes of a 4°C Aspergillus minimal medium salt solution. Aliquots were transferred to fresh medium containing the inducer, and incubation was continued. After incubation, the mycelium was harvested by filtration and frozen in liquid N2. Transformation of Aspergillus strains and Northern analysis were carried out as described previously (5, 38).
Reporter enzyme assays.
Sodium phosphate buffer (50 mM Na3PO4, 1 mM EDTA [pH 7.0]) and glass beads (0.75 to 1.0 mm) were added to the frozen mycelium, and cells were disrupted by using a RiboLyser (Hybaid, Heidelberg, Germany). Cell debris was separated by centrifugation, and the supernatant was used immediately for the determination of protein concentrations and for β-galactosidase enzyme assays. Protein concentrations were determined by using a bicinchoninic acid assay (Pierce, Dallas, Tex.), and β-galactosidase specific activities were determined by using the protocol included in a protein expression kit from Invitrogen (Carlsbad, Calif.; catalog no. K1710-01). According to this protocol, β-galactosidase enzyme activity is calculated as 300,000 U of β-galactosidase per mg of protein, and the detection limit is ≥5 U.
RESULTS
Expression of hERα from the gpdA promoter.
We expressed hERα under the control of the strong constitutive A. nidulans gpdA promoter and the trpC terminator. Construct phERpyr4 carried the T. reesei pyr4 gene as a selectable marker. Several prototrophic transformants were analyzed by PCR and Southern blot analysis (data not shown). The strain designated hER carried a single copy of construct phERpyr4 in the A. nidulans pyrG89 argB2 genome. hER carried as a selectable marker argB−, which was used for the site-directed integration of a single copy of the reporter construct into the argB locus. By comparing only insertions at argB, differences in reporter gene expression due to integration into different genomic loci can be avoided. When the parent strain was compared with transformants expressing the hERα construct for their responses to the synthetic estrogenic compound DES in the standard minimal medium used in these experiments, we observed that all of the transformants were hypersensitive to DES. At DES concentrations of 100 nM, growth was strongly inhibited, and 1 μM prevented germination and growth. In contrast, growth and germination of the A. nidulans or A. niger parent strain were not affected by DES at levels of <10 mM (data not shown).
Induction of promoters carrying EREs by DES.
We constructed an expression vector based on A. nidulans vector pAN923-42BglII (42); the construct carries a mutated argB gene as the selectable marker for site-directed integration into the argB locus and the lacZ gene in front of the trpC terminator sequence. We combined a minimal promoter sequence derived from the S. cerevisiae URA3 gene containing the TATA box with three copies of the human ERE (29). To maintain the approximate distance between the URA3 TATA element and the ATG of the lacZ reporter gene, a 94-bp random “stuffer” fragment (RS) derived from the ORF of the Escherichia coli ampicillin resistance gene was introduced to complete the promoter. The final construct, termed pERE-URA-RS, was integrated as a single copy into the A. nidulans argB locus or randomly into A. niger.
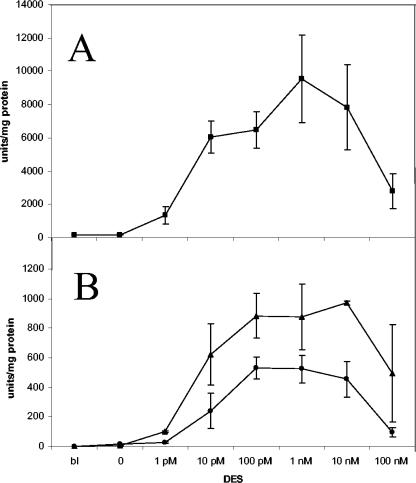
The hERα-ERE system is functional in Aspergillus species (Fig. 1A). We tested the effects of various DES concentrations in the medium and found that expression starts at a concentration of 1 pM (∼10% of the full level), reaches ∼50% of the full level at 10 pM DES, and is highest at 100 pM and 1 nM DES (∼10,000 U of β-galactosidase per mg of protein). Increasing the DES concentration to 10 or 100 nM resulted in reduced expression.
FIG. 1.
(A) β-Galactosidase activities of the A. nidulans ERE-URA-RS reporter strain at various final concentrations of DES. The strain was grown and shifted to induction medium, and the enzyme activity of the reporter was determined after 8 h. bI, before induction. Error bars indicate standard deviations of three independent experiments. (B) β-Galactosidase activities of the A. nidulans ERE-URA-nirA (triangles) and ERE-RS-nirA (circles) reporter strains at various final concentrations of DES. The strains were induced for 8 h, and the enzyme activities were measured.
The levels of expression induced from this construct are of the same order of magnitude as those from the alcA-lacZ construct (∼35,000 U of β-galactosidase per mg of protein) transformed into the isogenic strain (also targeted to the argB locus). A strain that contains multiple copies of the pERE-URA-RS reporter construct even overrides (∼50,000 U of β-galactosidase per mg of protein) the activation potential of the alcA construct (data not shown). Thus, the expression level can be increased by increasing the number of copies of the ERE promoter, and the amount of activated hERα expressed from the gpdA promoter is not rate limiting. Background expression without DES in this strain (∼150 U of β-galactosidase per mg of protein) is only slightly above the background level seen in the isogenic strain carrying no reporter construct (∼20 U of β-galactosidase per mg of protein) and is considerably lower than the background level seen in A. nidulans with the noninduced, fully glucose-repressed alcA promoter (∼300 U of β-galactosidase per mg of protein) (data not shown).
Recognition of different estrogenic compounds by hERα in Aspergillus species.
We found (data not shown) that 17-β-estradiol was as active as DES. The growth hormone zearalanol (9) resulted in ∼40% the activation seen with DES, and coumestrol, a natural phytoestrogen, also showed significant induction at concentrations as low as 1 nM.
Sensitivity of hERα expression to carbon and nitrogen catabolites.
In an ideal expression system, there would be no medium constraints on an expression system for high-level protein production. However, most promoters used for protein expression are under the control of carbon catabolite repression and are sensitive to medium composition. Our constructs exhibited no such difficulties. We tested our constructs in a variety of synthetic media (Aspergillus minimal medium with various concentrations of glucose, arabinose, xylose, or fructose as the sole carbon source in the presence of ammonia as the sole nitrogen source and nitrate, urea, or ammonia as the sole nitrogen source in the presence of glucose as the sole carbon source) and complete media used for protein expression in Aspergillus species. In all media, the system was fully independent of both the nitrogen and the carbon sources, and the levels of expression differed no more than ±5% from those obtained with the standard medium (minimal medium with 1% glucose and 10 mM ammonia as the sole carbon and nitrogen sources, respectively) used in these experiments (data not shown).
Background and control of expression levels.
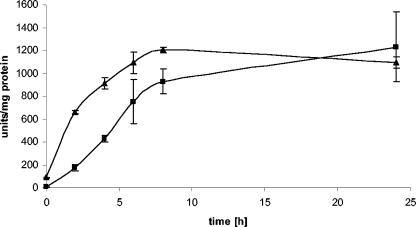
The pERE-URA-nirA construct, when integrated as a single copy into the argB locus, reduced background expression to a nondetectable level, while the induction response to DES was maintained (Fig. 1B and Table 2). However, the promoter strength was ∼10% that obtained with the original pERE-URA-RS construct, showing that the silencing function of the nirA promoter fragment was not fully counteracted by the hERα transactivator. If a longer piece of the nirA promoter (287 bp) was inserted instead of the 94-bp fragment, then a dominant repressing effect overrode the activation (data not shown), suggesting that either an additional negative element was introduced or that promoter spacing is critical to the function of the hormone response in Aspergillus species. When we compared A. nidulans strain ERE-URA-nirA with an A. niger strain carrying at least two ectopic integrations of the pERE-URA-nirA reporter construct, we found that absolute expression levels generally were higher under noninducing and inducing conditions in A. niger than in A. nidulans after 8 h (Fig. 2).
TABLE 2.
Dose responses of various reporter constructs combined with various DES inducer concentrationsa
| Strain designation | DES concn | β-Galactosidase activity | SD |
|---|---|---|---|
| hER | 1 nM | ND | NA |
| ERE-RS-nirA | 0 M | ND | NA |
| 1 pM | 25 | 6 | |
| 100 pM | 530 | 74 | |
| ERE-URA-nirA | 1 pM | 100 | 5 |
| 1 nM | 870 | 220 | |
| ERE-URA-RS | 0 M | 150 | 19 |
| 1 pM | 1,400 | 530 | |
| 2 pM | 2,500 | 1,500 | |
| 4 pM | 4,100 | 1,800 | |
| 6 pM | 5,800 | 30 | |
| 10 pM | 6,000 | 950 | |
| 100 pM | 6,500 | 1,100 | |
| 1 nM | 9,500 | 2,600 | |
| 10 nM | 7,800 | 2,600 | |
| 100 nM | 2,800 | 1,100 | |
| ERE-URA-RS (multicopy transformant) | 1 nM | 51,000 | 9,500 |
ND, not detectable (detection limit, ≥5 U of β-galactosidase). NA, not applicable. SDs were from three independent experiments.
FIG. 2.
Comparison of β-galactosidase activities of A. niger (triangles) and A. nidulans (squares). Both species carry the pERE-URA-nirA reporter construct. The inducer (DES) was added to a concentration of 1 nM (0-h control), and incubation proceeded for 2, 4, 6, 8, and 24 h before the determination of reporter enzyme activities. Error bars indicate standard deviations of three independent experiments.
Even though it is a repressing element, the nirA fragment can serve as a core promoter. We replaced the core URA3 sequence with an identical length of the ampicillin resistance gene RS, leaving the ERE equidistant from the nirA promoter fragment (construct pERE-RS-nirA). This construct has a background level of zero in the absence of the inducer but responds to DES (Fig. 1B). Promoter strength was reduced ∼25% by the removal of the URA3 sequence relative to that seen with pERE-URA-nirA. On the basis of these results, the three functional constructs combined with different concentrations of the inducer DES provided a linear dose response (Table 2) for the expression of a given gene, i.e., for the reporter used here, from 0 U to ∼50,000 U of β-galactosidase, the latter corresponding to approximately 15% of the total cellular protein.
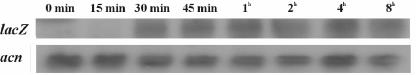
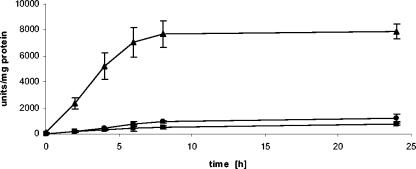
Induction kinetics.
Induction of the reporter gene transcript was evident as soon as 15 min following the addition of DES and reached the maximum level after 30 min. This level was maintained for at least 8 h (Fig. 3). Reporter enzyme induction lagged by approximately 60 min, and saturation of the intracellular reporter enzyme concentration was reached after 8 h (Fig. 4). Despite continuous transcription, no further increase in intracellular enzyme levels occurred, even when incubation times were extended to 24 h, probably as a result of intracellular reporter protein degradation.
FIG. 3.
Northern blot analysis of the A. nidulans ERE-URA-nirA reporter strain induced with 1 nM DES. RNA was prepared at the indicated time points. The blot was hybridized once with the lacZ gene, stripped, and reprobed with the A.nidulans acnA (actin-encoding) gene as a loading control.
FIG. 4.
Saturation of intracellular reporter enzyme activity. β-Galactosidase was measured after induction with 1 nM DES for various times in ERE construct strains ERE-URA-RS (triangles), ERE-URA-nirA (circles), and ERE-RS-nirA (squares). Error bars indicate standard deviations of three independent experiments.
DISCUSSION
We have shown that hERα functions efficiently in Aspergillus species. A. nidulans and A. niger wild-type strains were not affected by <10 mM DES in the medium. Transformants expressing the receptor under the control of the gpdA promoter were extremely sensitive to this compound. In S. cerevisiae, cells expressing the hERα protein at high levels grow in the presence of 1 μM estrogen (29). In A. nidulans, this concentration, at least in the form of DES, prevents colony formation, and growth is impaired at concentrations as low as 10 nM DES in plate assays. It is not clear why Aspergillus species are so much more sensitive to large amounts of activated hERα, but the toxic effect could result from the ability of hERα to recruit coactivators (28) that can modify local nucleosome positioning or alter large-scale chromatin structure. Alternatively, high concentrations of activated hERα in Aspergillus species could bind, via the AF-1 and AF-2 domains, to the TATA-binding protein (35) and/or to TATA-binding protein-associated factors (17, 18). Such binding would prevent these proteins from participating in RNA polymerase II complexes and polymerase II-dependent transcription. Finally, Aspergillus species might take up estrogens more efficiently and/or have less capacity to extrude the compounds via efflux pumps such as ABC transporters. Consistent with this explanation is the toxicity of 100 μM DES for S. cerevisiae ΔPDR5 ΔSNQ2 cells lacking these transporters, whereas PDR5 SNQ2+ strains are viable at this DES concentration. Toxicity for these yeast cells is independent of whether or not they express hERα (26; our unpublished observations). The high sensitivity of Aspergillus hERα-expressing strains to DES could enable these strains to be used as novel genetic screening tools.
There are concerns about using DES in large quantities in industrial fermentations. Therefore, we tested the responses of the expression modules to other estrogenic compounds. For instance, the nonsteroidal mycotoxin zearalenone induces the Aspergillus hERα-ERE promoter at a concentration of 10 nM (data not shown). This amount of zearalenone is ∼20-fold lower than the guideline level for zearalenone concentrations in wheat (200 nmol/kg) intended for human consumption in Austria (26). Zearalanol, a derivative of zearalenone marketed as Ralgro, is an anabolic growth-promoting compound used for finishing cattle in feed lots in the United States. Zearalanol has been found at up to 5 μg/liter (15 nM) in urine samples (21, 48) or up to 0.1 μg/kg in beef tissue samples (6). Zearalanol activates the Aspergillus system to about 40% the DES induction capacity. Based on these numbers, a concentration of 0.5 nM in beef tissue samples would be readily detected by an Aspergillus reporter strain (calculated as 800 U of β-galactosidase at 1 pM zearalanol). 17-β-Estradiol, an active ingredient in oral contraceptives, is fully equivalent to DES in activation potential; i.e., transcriptional induction can be seen at 1 pM. These concentrations (several nanograms per liter) commonly are found in surface waters or effluents of sewage treatment plants (3). Other endocrine disrupters with estrogenic capabilities, e.g., 4-nonylphenol, which were not tested in our assays, might be present in environmental samples at levels of up to several hundred nanograms per liter.
For the formulation of a fermentation medium, a potentially interesting natural estrogen is coumestrol, which can activate the Aspergillus system at nanomolar concentrations. This phytoestrogen is present at levels of milligrams per kilogram in soy sprouts (32), and the addition of soy flour in the range of parts per billion would lead to a fully functional induction medium. It is clear that the extremely high sensitivity of Aspergillus cells to synthetic or natural estrogens makes the expression modules suitable for biotechnological applications and the transformed strains potential bioreporting microorganisms for detecting estrogenic compounds in food or environmental samples.
A panoply of expression systems have been described for various organisms; they include common metabolic signals, metal-induced systems (45), antibiotic-induced systems (4), and light-induced gene switches (37). For filamentous fungi, no metabolically independent expression systems have yet been developed. Thus, fungal biotechnologists have had to adapt the fermentation medium to conditions under which an expression system functions optimally. For Aspergillus glaA, which encodes glucoamylase (43), or exlA, which encodes exoxylanase (7), glucose and fructose (the preferred fungal carbon sources) must be absent or present at limiting concentrations, and specific inducers, such as starch, xylan, cellulose, or derivatives of these compounds, must be supplied. Similarly, the regulation and activities of extracellular proteases which are of major importance in yield optimization are both carbon and nitrogen source sensitive (19) and dependent on ambient pH (44). These constraints on medium composition often prevent the use of cost-effective raw materials in commercial fermentations.
In our system, expression from EREs is independent of medium components, and the induction functions equally well in complex media or minimal media with various carbon and nitrogen sources. Aspergillus species show a linear dose response to 3 log concentrations of estrogen and as much as 300-fold induction. The possibility of fine-tuning expression levels further through the modular combination of various inducer concentrations is another advantage of this system.
The expression of a heterologous protein in a fungal host usually results in protein yields much lower than those seen for homologous secreted proteins, such as Aspergillus glucoamylase or Trichoderma cellulase (13, 20). The native enzymes can accumulate in the fermentation medium at rates of up to 30 g/liter; levels of heterologous proteins usually are in the range of milligrams per liter. The fungal secretory pathway is thought to be the bottleneck for the efficient secretion of heterologous proteins due to misfolding or incorrect glycosylation, either or both of which could evoke the unfolded-protein response and induce protein degradation pathways. The overproduction of foldases and chaperones in some cases can (33) and in others cannot (8) compensate for the lack of appropriate posttranslational modifications. Thus, to achieve optimum expression levels for the secretion of foreign proteins, the secretory pathway should not be overloaded through the hyperexpression of “your favorite gene” (47). The ability to adjust expression levels in Aspergillus species with the hERα-ERE system from 0 to ∼15% of total cell protein provides a means for systematically addressing optimal expression levels in heterologous protein expression.
Acknowledgments
We thank Pierre Chambon for providing yeast strains and estrogen receptor plasmid Yep90-HEG0 and Jeffrey A. Mullen and David F. Steele for critically reading the manuscript.
This work was supported by Austrian Science Fund grant START-Y114MOB to J.S. and by Austrian Genome Research Program GEN-AU.
REFERENCES
- 1.Aravindakshan, J., M. Gregory, D. J. Marcogliese, M. Fournier, and D. G. Cyr. 2004. Consumption of xenoestrogen-contaminated fish during lactation alters adult male reproductive function. Toxicol. Sci. 81:179-189. [DOI] [PubMed] [Google Scholar]
- 2.Archer, D. B., and J. F. Peberdy. 1997. The molecular biology of secreted enzyme production by fungi. Crit. Rev. Biotechnol. 17:273-306. [DOI] [PubMed] [Google Scholar]
- 3.Belfroid, A. C., A. Van der Horst, A. D. Vethaak, A. J. Schafer, G. B. Rijs, J. Wegener, and W. P. Cofino. 1999. Analysis and occurrence of estrogenic hormones and their glucuronides in surface water and waste water in The Netherlands. Sci. Total Environ. 225:101-108. [DOI] [PubMed] [Google Scholar]
- 4.Blau, H. M., and F. M. Rossi. 1999. Tet B or not tet B: advances in tetracycline-inducible gene expression. Proc. Natl. Acad. Sci. USA 96:797-799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger, G., J. Strauss, C. Scazzocchio, and B. F. Lang. 1991. nirA, the pathway-specific regulatory gene of nitrate assimilation in Aspergillus nidulans, encodes a putative GAL4-type zinc finger protein and contains four introns in highly conserved regions. Mol. Cell. Biol. 11:5746-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chichila, T. M., D. Silvestre, T. R. Covey, and J. D. Henion. 1988. Distribution of zeranol in bovine tissues determined by selected ion monitoring capillary gas chromatography/mass spectrometry. J. Anal. Toxicol. 12:310-318. [DOI] [PubMed] [Google Scholar]
- 7.de Graaff, L. H., H. C. van den Broeck, A. J. van Ooijen, and J. Visser. 1994. Regulation of the xylanase-encoding xlnA gene of Aspergillus tubigensis. Mol. Microbiol. 12:479-490. [DOI] [PubMed] [Google Scholar]
- 8.Dorner, A. J., L. C. Wasley, and R. J. Kaufman. 1992. Overexpression of GRP78 mitigates stress induction of glucose regulated proteins and blocks secretion of selective proteins in Chinese hamster ovary cells. EMBO J. 11:1563-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Egan, C. L., L. L. Wilson, T. R. Drake, W. R. Henning, E. W. Mills, S. D. Meyer, and D. C. Kenison. 1993. Effects of different doses of zeranol on growth, hemoglobin, and carcass traits in veal calves. J. Anim. Sci. 71:1081-1087. [DOI] [PubMed] [Google Scholar]
- 10.Felenbok, B., M. Flipphi, and I. Nikolaev. 2001. Ethanol catabolism in Aspergillus nidulans: a model system for studying gene regulation. Prog. Nucleic Acids Res. Mol. Biol. 69:149-204. [DOI] [PubMed] [Google Scholar]
- 11.Finkelstein, D. B., J. Rambosek, M. S. Crawford, C. L. Soliday, P. C. McAda, and J. Leach. 1989. Protein secretion in A. niger, p. 295-300. In C. L. Hershberger, S. W. Queener, and G. Hegeman (ed.), Genetics and molecular biology of industrial microorganisms. American Society for Microbiology, Washington, D.C.
- 12.Flipphi, M. J., J. Visser, P. van der Veen, and L. H. de Graaff. 1994. Arabinase gene expression in Aspergillus niger: indications for coordinated regulation. Microbiology 140:2673-2682. [DOI] [PubMed] [Google Scholar]
- 13.Gouka, R. J., P. J. Punt, and C. A. van den Hondel. 1997. Efficient production of secreted proteins by Aspergillus: progress, limitations and prospects. Appl. Microbiol. Biotechnol. 47:1-11. [DOI] [PubMed] [Google Scholar]
- 14.Gruber, F., J. Visser, C. P. Kubicek, and L. H. de Graaff. 1990. The development of a heterologous transformation system for the cellulolytic fungus Trichoderma reesei based on a pyrG-negative mutant strain. Curr. Genet. 18:71-76. [DOI] [PubMed] [Google Scholar]
- 15.Hanstein, B., S. Djahansouzi, P. Dall, M. W. Beckmann, and H. G. Bender. 2004. Insights into the molecular biology of the estrogen receptor define novel therapeutic targets for breast cancer. Eur. J. Endocrinol. 150:243-255. [DOI] [PubMed] [Google Scholar]
- 16.Harding, H. P., M. Calfon, F. Urano, I. Novoa, and D. Ron. 2002. Transcriptional and translational control in the mammalian unfolded protein response. Annu. Rev. Cell Dev. Biol. 18:575-599. [DOI] [PubMed] [Google Scholar]
- 17.Ing, N. H., J. M. Beekman, S. Y. Tsai, M. J. Tsai, and B. W. O'Malley. 1992. Members of the steroid hormone receptor superfamily interact with TFIIB (S300-II). J. Biol. Chem. 267:17617-17623. [PubMed] [Google Scholar]
- 18.Jacq, X., C. Brou, Y. Lutz, I. Davidson, P. Chambon, and L. Tora. 1994. Human TAFII30 is present in a distinct TFIID complex and is required for transcriptional activation by the estrogen receptor. Cell 79:107-117. [DOI] [PubMed] [Google Scholar]
- 19.Jarai, G., and F. Buxton. 1994. Nitrogen, carbon, and pH regulation of extracellular acidic proteases of Aspergillus niger. Curr. Genet. 26:238-244. [DOI] [PubMed] [Google Scholar]
- 20.Keranen, S., and M. Penttila. 1995. Production of recombinant proteins in the filamentous fungus Trichoderma reesei. Curr. Opin. Biotechnol. 6:534-537. [DOI] [PubMed] [Google Scholar]
- 21.Kleinova, M., P. Zollner, H. Kahlbacher, W. Hochsteiner, and W. Lindner. 2002. Metabolic profiles of the mycotoxin zearalenone and of the growth promoter zeranol in urine, liver, and muscle of heifers. J. Agric. Food Chem. 50:4769-4776. [DOI] [PubMed] [Google Scholar]
- 22.Kurzatkowski, W., A. Torronen, J. Filipek, R. L. Mach, P. Herzog, S. Sowka, and C. P. Kubicek. 1996. Glucose-induced secretion of Trichoderma reesei xylanases. Appl. Environ. Microbiol. 62:2859-2865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ma, Y., and L. M. Hendershot. 2003. Delineation of a negative feedback regulatory loop that controls protein translation during endoplasmic reticulum stress. J. Biol. Chem. 278:34864-34873. [DOI] [PubMed] [Google Scholar]
- 24.Mahe, Y., Y. Lemoine, and K. Kuchler. 1996. The ATP binding cassette transporters Pdr5 and Snq2 of Saccharomyces cerevisiae can mediate transport of steroids in vivo. J. Biol. Chem. 271:25167-25172. [DOI] [PubMed] [Google Scholar]
- 25.Metzger, D., J. H. White, and P. Chambon. 1988. The human oestrogen receptor functions in yeast. Nature 334:31-36. [DOI] [PubMed] [Google Scholar]
- 26.Mitterbauer, R., H. Weindorfer, N. Safaie, R. Krska, M. Lemmens, P. Ruckenbauer, K. Kuchler, and G. Adam. 2003. A sensitive and inexpensive yeast bioassay for the mycotoxin zearalenone and other compounds with estrogenic activity. Appl. Environ. Microbiol. 69:805-811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nikolaev, I., M. Mathieu, P. van de Vondervoort, J. Visser, and B. Felenbok. 2002. Heterologous expression of the Aspergillus nidulans alcR-alcA system in Aspergillus niger. Fungal Genet. Biol. 37:89-97. [DOI] [PubMed] [Google Scholar]
- 28.Nye, A. C., R. R. Rajendran, D. L. Stenoien, M. A. Mancini, B. S. Katzenellenbogen, and A. S. Belmont. 2002. Alteration of large-scale chromatin structure by estrogen receptor. Mol. Cell. Biol. 22:3437-3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pierrat, B., D. M. Heery, Y. Lemoine, and R. Losson. 1992. Functional analysis of the human estrogen receptor using a phenotypic transactivation assay in yeast. Gene 119:237-245. [DOI] [PubMed] [Google Scholar]
- 30.Pontecorvo, G., J. A. Roper, L. M. Hemmons, K. D. MacDonald, and A. W. J. Bufton. 1953. The genetics of Aspergillus nidulans. Adv. Genet. 5:141-238. [DOI] [PubMed] [Google Scholar]
- 31.Punt, P. J., N. D. Zegers, M. Busscher, P. H. Pouwels, and C. A. van den Hondel. 1991. Intracellular and extracellular production of proteins in Aspergillus under the control of expression signals of the highly expressed Aspergillus nidulans gpdA gene. J. Biotechnol. 17:19-33. [DOI] [PubMed] [Google Scholar]
- 32.Reinli, K., and G. Block. 1996. Phytoestrogen content of foods-a compendium of literature values. Nutr. Cancer 26:123-148. [DOI] [PubMed] [Google Scholar]
- 33.Robinson, A. S., V. Hines, and K. D. Wittrup. 1994. Protein disulfide isomerase overexpression increases secretion of foreign proteins in Saccharomyces cerevisiae. Bio/Technology 12:381-384. [DOI] [PubMed] [Google Scholar]
- 34.Rutkowski, D. T., and R. J. Kaufman. 2004. A trip to the ER: coping with stress. Trends Cell Biol. 14:20-28. [DOI] [PubMed] [Google Scholar]
- 35.Sadovsky, Y., P. Webb, G. Lopez, J. D. Baxter, P. M. Fitzpatrick, E. Gizang-Ginsberg, V. Cavailles, M. G. Parker, and P. J. Kushner. 1995. Transcriptional activators differ in their responses to overexpression of TATA box-binding protein. Mol. Cell. Biol. 15:1554-1563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sakaguchi, K., M. Takagi, H. Horiuchi, and K. Gomi. 1992. Fungal enzymes used in oriental food and beverage industries, p. 54-99. In J. R. Kinghorn and G. Turner (ed.), Applied molecular genetics of filamentous fungi. Blackie, London, England.
- 37.Shimizu-Sato, S., E. Huq, J. M. Tepperman, and P. H. Quail. 2002. A light-switchable gene promoter system. Nat. Biotechnol. 20:1041-1044. [DOI] [PubMed] [Google Scholar]
- 38.Strauss, J., M. I. Muro-Pastor, and C. Scazzocchio. 1998. The regulator of nitrate assimilation in ascomycetes is a dimer which binds a nonrepeated, asymmetrical sequence. Mol. Cell. Biol. 18:1339-1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Swift, R. J., A. Karandikar, A. M. Griffen, P. J. Punt, C. A. van den Hondel, G. D. Robson, A. P. Trinci, and M. G. Wiebe. 2000. The effect of organic nitrogen sources on recombinant glucoamylase production by Aspergillus niger in chemostat culture. Fungal Genet. Biol. 31:125-133. [DOI] [PubMed] [Google Scholar]
- 40.Tsai, M. J., and B. W. O'Malley. 1994. Molecular mechanisms of action of steroid/thyroid receptor superfamily members. Annu. Rev. Biochem. 63:451-486. [DOI] [PubMed] [Google Scholar]
- 41.van den Hombergh, J. P., P. J. van de Vondervoort, L. Fraissinet-Tachet, and J. Visser. 1997. Aspergillus as a host for heterologous protein production: the problem of proteases. Trends Biotechnol. 15:256-263. [DOI] [PubMed] [Google Scholar]
- 42.van Gorcom, R. F., P. J. Punt, P. H. Pouwels, and C. A. van den Hondel. 1986. A system for the analysis of expression signals in Aspergillus. Gene 48:211-217. [DOI] [PubMed] [Google Scholar]
- 43.Verdoes, J. C., A. D. van Diepeningen, P. J. Punt, A. J. Debets, A. H. Stouthamer, and C. A. van den Hondel. 1994. Evaluation of molecular and genetic approaches to generate glucoamylase overproducing strains of Aspergillus niger. J. Biotechnol. 36:165-175. [DOI] [PubMed] [Google Scholar]
- 44.Wallis, G. L., R. J. Swift, R. Atterbury, S. Trappe, U. Rinas, F. W. Hemming, M. G. Wiebe, A. P. Trinci, and J. F. Peberdy. 2001. The effect of pH on glucoamylase production, glycosylation and chemostat evolution of Aspergillus niger. Biochim. Biophys. Acta 1527:112-122. [DOI] [PubMed] [Google Scholar]
- 45.Winge, D. R., L. T. Jensen, and C. Srinivasan. 1998. Metal-ion regulation of gene expression in yeast. Curr. Opin. Chem. Biol. 2:216-221. [DOI] [PubMed] [Google Scholar]
- 46.Withers, J. M., R. J. Swift, M. G. Wiebe, G. D. Robson, P. J. Punt, C. A. van den Hondel, and A. P. Trinci. 1998. Optimization and stability of glucoamylase production by recombinant strains of Aspergillus niger in chemostat culture. Biotechnol. Bioeng. 59:407-418. [PubMed] [Google Scholar]
- 47.Wittrup, K. D., A. S. Robinson, R. N. Parekh, and K. J. Forrester. 1994. Existence of an optimum expression level for secretion of foreign proteins in yeast. Ann. N. Y. Acad. Sci. 745:321-330. [DOI] [PubMed] [Google Scholar]
- 48.Zollner, P., J. Jodlbauer, M. Kleinova, H. Kahlbacher, T. Kuhn, W. Hochsteiner, and W. Lindner. 2002. Concentration levels of zearalenone and its metabolites in urine, muscle tissue, and liver samples of pigs fed with mycotoxin-contaminated oats. J. Agric. Food Chem. 50:2494-2501. [DOI] [PubMed] [Google Scholar]
- 49.Zuo, J., Q. W. Niu, and N. H. Chua. 2000. Technical advance: an estrogen receptor-based transactivator XVE mediates highly inducible gene expression in transgenic plants. Plant J. 24:265-273. [DOI] [PubMed] [Google Scholar]