Abstract
Aims
Non-invasive measures of cardiac mechanical function may have the potential to serve as markers of risk for heart failure; however, limited data exist regarding clinical correlates and heritability of these measures in the community.
Methods and results
We used speckle-tracking echocardiography to assess LV strain and synchrony in the Framingham Offspring Study (n = 2816; mean age 67 years, 54% women). In multivariable regression analyses, male gender (vs. female, P < 0.001), higher heart rate (P < 0.0001), and presence of cardiovascular disease (P < 0.001) were associated with worse global peak strains across all planes analysed (longitudinal, transverse, circumferential, and radial). Higher diastolic blood pressure and diabetes were associated with worse longitudinal strain (P < 0.01), and greater body mass index was associated with worse radial strain (P = 0.0004). Overall, however, clinical correlates accounted for only 4–19% of the variation in measures of LV mechanical function. Select measures of LV strain were heritable: longitudinal strain (h2 = 16%, P = 0.002), transverse strain (h2 = 15%, P = 0.006), and circumferential strain (h2 = 30%, P < 0.0001). Furthermore, in a subset of 1437 participants with parental data available, parental heart failure was associated with worse circumferential strain in the offspring free of heart failure (P = 0.01).
Conclusions
Our investigation in a large community-based sample identified heritablity and clinical correlates of LV mechanical function, and highlighted an association of parental heart failure with worse global circumferential strain in offspring.
Keywords: Myocardial strain, Heritability, Heart failure
Introduction
Substantial experimental and clinical data now indicate that, when applied to two-dimensional echocardiography, speckle-tracking-based measures of LV deformation—also known as myocardial tissue strains—can add prognostic information to conventional measures in a variety of clinical settings.1–5 Thus, echocardiographic measures of LV strain are now recognized as a non-invasive approach to assessing LV function with potentially broad applications.6 Because there are limited data on the clinical correlates of LV myocardial strain-based measures in the community, we performed speckle-tracking-based LV measures in a large community-based sample and examined their association with traditional cardiovascular disease (CVD) risk factors. Previous studies have observed that clinical characteristics generally account for only a modest portion of the variation in conventional measures of cardiac structure and function, with genetic factors contributing additional information.7–10 Additionally, limited data suggest that asymptomatic offspring with parental heart failure (HF) demonstrate subtle alterations in LV structure and function even though they are clinically free of HF.10,11 Therefore, we also investigated the degree to which strain-based measures of LV function are heritable. Furthermore, we evaluated if offspring individuals with a parental history of HF demonstrate altered LV strain compared with offspring individuals without a parental HF history.
Methods
Study sample
The selection criteria and sampling for the Framingham Offspring Study have been described.12,13 Of the 3021 participants who attended their eighth routine examination (2005–2008), 2888 underwent two-dimensional transthoracic echocardiography. Among the 2844 participants with images appropriate for strain analyses, 2816 participants had complete data on key clinical covariates, comprising the final sample for analyses. For investigation of the association between parental HF and LV mechanical function measures in Framingham Offspring Study participants, we identified 1437 individuals with both parents in the original cohort study. All study protocols were approved by the Institutional Review Board of Boston University Medical Center, and all participants provided written informed consent.
Echocardiography
Echocardiographic data were acquired digitally and conventional LV measures were performed offline according to standardized techniques (Supplementary material online) by readers completely blinded to all clinical data including parental HF status. Using an offline analysis program (2D Cardiac Performance Analysis v1.1, TomTec Imaging Systems, Unterschleißheim, Germany), speckle-tracking-based analyses of LV function were performed by our research team using a previously validated algorithm and according to a standardized protocol with demonstrated excellent reproducibility (Supplementary material online);14,15 this work was done in accordance with industry-wide efforts to maintain rigorous standards for performance of two-dimensional-speckle tracking echocardiography.16 The primary measures of LV mechanical function included global longitudinal strain (measured from the A2C and A4C views) and global circumferential strain (measured in the SAX view at the level of the mid ventricle). Secondary measures of LV mechanical function included global transverse strain and global radial strain, in addition to longitudinal and transverse segmental synchrony calculated as the standard deviation (SD) of time-to-peak longitudinal and transverse strains, respectively.6 Herein, we use the term ‘transverse’ strain to refer to radial strain measured in the apical (A2C and A4C) views and ‘radial’ strain to refer to radial strain measured in the SAX view.
Statistical methods
Measures of longitudinal and transverse segmental synchrony were logarithmically transformed due to skewed distributions. For each primary measure of LV mechanical function, we estimated age- and gender-adjusted Pearson correlation coefficients with the following key clinical covariates: body mass index, systolic blood pressure, diastolic blood pressure, antihypertensive medication therapy, diabetes mellitus, total/HDL cholesterol, natural log triglycerides, smoking status, resting heart rate, natural log C-reactive protein, and prevalent CVD (documented history of coronary heart disease, HF, or stroke). In stepwise linear regressions, we examined associations of LV mechanical function (separate models for each measure) with the above-listed clinical covariates. Covariates included in model selection were determined using the Schwarz Bayesian criterion. Given the number of primary strain measures (2) and clinical traits (13) analysed, we used a conservative Bonferroni-corrected P-value threshold of 0.002 (=0.05/26) for clinical correlates analyses.
In secondary analyses, we additionally investigated the association of estimated glomerular filtration rate (eGFR), physical activity, metabolic syndrome, and valve disease with the primary LV strain measures. We also estimated age- and gender-adjusted correlations between each LV mechanical function measure and conventional LV measures. We also performed multivariable linear regression analyses of the relation of each LV mechanical function measure with each conventional LV measure adjusting for significant clinical covariates (specific to each measure).
Heritability
For heritability estimates, we use standardized values of each LV strain measure and standardized values of the log-transformed LV synchrony measures. In analyses adjusting for age, gender, heart rate, and prevalent CVD (i.e. covariates, plus age and gender, that demonstrated associations with multiple LV mechanical function measures), we estimated heritability (h2) defined as the proportion of total variation explained by additive genetic effect—using the Sequential Oligogenic Linkage Analysis Routines (SOLAR, version 2.1.4).17
Parental heart failure
In the subset of individuals who had parents with known HF status, we repeated multivariable linear regression analyses to examine whether the presence of at least one parent (either mother or father) who developed HF (before age 70 years; independent variable) was associated with measures of LV mechanical function (dependent variables). Clinical covariates specific to each LV mechanical function measure were included. Parental HF diagnosis was based on available medical records and adjudicated by a panel of investigators according to the presence of at least two major or one major plus two minor criteria as previously described (major and minor criteria are based on select documented symptoms, physical exam findings, and diagnostic test results).18
All analyses were performed using SAS version 9.3 (SAS Institute, Cary, NC, USA).
Results
Characteristics of the study sample are shown in Table 1 and Supplementary material online, Table S1. A total of 95% of participants had conventionally defined ‘normal’ LV systolic function [i.e. fractional shortening (FS) ≥0.29]. Correlations between measures of LV mechanical function were small to moderate (see Supplementary material online, Table S2). The strongest correlations noted were between longitudinal and transverse strain (r = −0.52; P < 0.0001) and between circumferential and radial strain (r = −0.49; P < 0.0001).
Table 1.
Sample characteristics
| Total sample (n =2816) | |
|---|---|
| Clinical variables | |
| Age, years | 67 ± 9 |
| Women, n (%) | 1530 (54) |
| Body mass index, kg/m2 | 28.3 ± 5.4 |
| Systolic blood pressure, mmHg | 128 ± 17 |
| Diastolic blood pressure, mmHg | 73 ± 10 |
| Pulse pressure, mmHg | 55 ± 16 |
| Mean arterial pressure, mmHg | 92 ± 10 |
| Antihypertensive treatment, n (%) | 1502 (53) |
| Hypertension, n (%) | 1760 (63) |
| Diabetes, n (%) | 384 (14) |
| Current smoker, n (%) | 251 (9) |
| Total/HDL cholesterol ratio | 3.48 ± 1.06 |
| Triglycerides, mg/dL* | 102 (74, 141) |
| C-reactive protein, mg/dL* | 1.52 (0.76, 3.21) |
| Prevalent cardiovascular disease, n (%) | 436 (15) |
| Heart rate, b.p.m. | 62 ± 10 |
| Advanced echocardiographic measures | |
| LV longitudinal strain, % | −20.5 ± 3.5 |
| LV transverse strain, % | 29.5 ± 7.3 |
| LV circumferential strain, % | −31.8 ± 6.0 |
| LV radial strain, % | 43.3 ± 16.8 |
| Longitudinal segmental synchrony, ms* | 56.8 (43.3, 78.7) |
| Transverse segmental synchrony, ms* | 91.6 (63.9, 133.3) |
Values are shown as mean ± standard deviation or percentage frequency.
Median (25th, 75th percentiles).
Clinical correlates
Results of age- and gender-adjusted analyses are reported in the Supplementary material online. Estimated regression coefficients are represented by beta. In multivariable-adjusted analyses (Table 2; Supplementary material online, Tables S5 and S6), older age was significantly associated with better circumferential strain but worse longitudinal and transverse segmental synchrony (P < 0.001 for all). Women had better LV mechanical function than men, based on global peak strains in all planes (P < 0.001). However, women and men were similar regarding segmental synchrony (we had 80% power to detect gender difference if the true partial correlation was 0.053). Greater body mass index was associated with worse radial strain (β –1.26, P < 0.001) as well as worse longitudinal (β 0.06, P < 0.0001) and segmental (β 0.05, P < 0.0001) synchrony. Greater diastolic blood pressure and presence of diabetes were related to worse longitudinal strain (P ≤0.002 for both) as well as worse longitudinal synchrony (P < 0.001 for both). Notably, systolic blood pressure was not significantly associated with any LV mechanical function measure, whereas antihypertensive therapy was associated with better circumferential strain (β –1.07, P < 0.0001). All associations of higher heart rate and presence of CVD with LV mechanical function measures remained significant in multivariable analyses (Table 2 and Supplemental Tables 5 and 6). Log C-reactive protein remained unassociated with measures of mechanical LV function in these models. Results of secondary clinical correlates analyses are reported in the Supplementary material online.
Table 2.
Multivariable-adjusted clinical correlates of left ventricular strain measures*
| Predictor variables | Longitudinal strain, %
|
Circumferential strain, %
|
||
|---|---|---|---|---|
| Estimated β (SE) | P-value | Estimated β (SE) | P-value | |
| Age, years | −0.42 (0.12) | 0.0006 | ||
| Female gender | −1.92 (0.13) | <0.0001 | −2.87 (0.24) | <0.0001 |
| Body mass index, kg/m2 | ||||
| SBP, mmHg | ||||
| DBP, mmHg | 0.20 (0.06) | 0.002 | ||
| Antihypertensive therapy | −1.07 (0.25) | <0.0001 | ||
| Diabetes | 0.70 (0.18) | 0.0001 | ||
| Total/HDL cholesterol ratio | 0.34 (0.06) | <0.0001 | ||
| Log triglycerides | ||||
| Current smoker | ||||
| Heart rate, b.p.m. | 0.76 (0.06) | <0.0001 | 1.25 (0.12) | <0.0001 |
| Prevalent CVD | 1.69 (0.17) | <0.0001 | 2.75 (0.35) | <0.0001 |
| Log C-reactive protein | ||||
| Model R2 | 0.190 | 0.115 | ||
CVD, cardiovascular disease; DBP, diastolic blood pressure; SBP, systolic blood pressure.
In multivariable analyses, covariates included in model selection were determined using the Schwarz Bayesian criterion. Beta coefficients represent the estimated change in the dependent variable (strain measure) per 1 – SD change in the independent (continuous) variable or presence vs. absence of the independent (categorical) variable.
Overall, the clinical correlates included in multivariable analyses accounted for a relatively small portion of the variability in LV mechanical function measures: 19.0% for longitudinal strain, 3.9% for transverse strain, 11.5% for circumferential strain, 5.3% for radial strain, 8.3% for longitudinal synchrony, and 4.0% for transverse synchrony.
Heritability
The heritability (h2) of longitudinal strain was 16.0% (P = 0.002), transverse strain was 14.5% (P = 0.006), and circumferential strain was 29.7% (P < 0.0001). The remaining measures of LV mechanical function did not have significant heritability: radial strain (P = 0.27), longitudinal synchrony (P = 0.18), and transverse synchrony (P = 0.50).
Association with parental heart failure
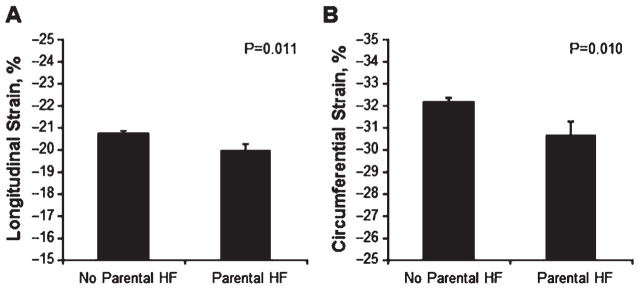
In 1437 individuals with available HF data on both parents, participants with compared to without parental HF appeared to have worse longitudinal and circumferential strain (Figure 1). Accordingly, in age- and gender-adjusted linear regression analyses, parental HF was significantly associated with worse longitudinal strain (β 0.21, P = 0.011) and circumferential strain (β 0.26, P = 0.006), but not with any other LV mechanical function measures (P > 0.10). In multivariable analyses adjusting for the significant clinical covariates identified above, the association of parental HF with longitudinal strain was no longer statistically significant (P = 0.15). However, the relationship of parental HF to circumferential strain remained statistically significant: β 0.23, P = 0.01. In the subset of individuals with parental HF data available, inclusion of parental HF in the fully adjusted multivariable model predicting abnormal circumferential strain (defined as greater than −20%)15 improved the C statistic from 0.80 to 0.83; however, this difference was not statistically significant in the sample size studied (P = 0.31).
Figure 1.
Myocardial strain in individuals with and without parental heart failure (HF). Values of longitudinal and circumferential strain are shown as unadjusted means ± standard errors.
Discussion
In a large community-based sample, we observed that variation in LV mechanical function is associated with key clinical characteristics including gender, heart rate, and prevalent CVD. However, clinical covariates accounted for only a modest proportion of the variation in measures of LV mechanical function. Therefore, we assessed the heritability of these measures and observed that circumferential, longitudinal, and transverse strains were among the most heritable of these traits. Furthermore, parental HF was associated with worse global circumferential strain in offspring who were free of HF. Together, our data indicate that certain aspects of LV mechanical function are determined by genetic as well as non-genetic factors, and suggest that offspring who are genetically predisposed to develop HF in later life (by virtue of parental history of the condition) demonstrate pre-clinical abnormalities of LV mechanical function.
Previous studies investigating the clinical correlates of LV mechanical function have predominantly evaluated modest sized referral samples, or used methods that provide information on myocardial mechanical function in only a single plane. Extending previous work, we analysed measures of LV mechanical function comprehensively, i.e. using data acquired from several sonographic views in a large sample of men and women living in the community. We identified certain clinical characteristics that were associated with LV mechanical function in some but not all planes; a select few clinical covariates were associated with mechanical function across all the longitudinal, transverse, circumferential, and radial planes investigated.
Women compared with men had consistently better global strains across all planes of LV mechanical function analysed. This finding is concordant with results from smaller studies19,20 and consistent with studies of conventionally measured LV function, which have reported higher FS and EF in women compared with men, particularly in older age groups.21,22 These results are probably related to the consistently noted gender-based differences in LV remodelling, for which women compared with men tend to have proportionately smaller LV cavity size and steeper increases in LV wall thickness with ageing.22 The degree to which hormonal, neurohormonal, and/or vascular factors contribute to the sexual dimorphism seen in analyses of both structure and mechanical function of the left ventricle is not yet known. Of note, there were no significant gender-based differences in segmental synchrony in fully adjusted analyses, despite adequate statistical power to detect modest differences between the men and women.
Higher heart rate was associated with worse LV strain in all planes. Smaller studies have shown similar results for speckle-tracking-based longitudinal strain in adults23 and Doppler-derived strain in children.24 Epidemiological studies have related higher resting heart rates to adverse cardiovascular outcomes, including coronary heart disease, cardiovascular mortality, and all-cause mortality.25–28 Whereas lower resting heart rate is considered a sign of physical fitness, higher resting heart rate may be a marker of autonomic dysfunction that could promote inefficient cardiac function even at rates lower than those typically associated with inducible cardiomyopathy.29
Previous studies have shown that speckle-tracking strain measures are sensitive for detecting myocardial ischaemia in the setting of suspected or known CVD.23,24 Thus, as expected, prevalent CVD was associated with worse LV strain in all planes as well as worse segmental synchrony in our community cohort. In analyses adjusting for prevalent CVD, certain cardiovascular risk factors were also related to select measures of mechanical function. Older age was associated with worse circumferential strain and segmental synchrony, consistent with data from cardiac magnetic resonance (CMR) studies;25 however, age was not related to longitudinal strain, suggesting that ageing-related myocardial changes may affect circumferential mesocardial fibre shortening more than longitudinal endocardial fibre shortening. In contrast, elevated diastolic blood pressure, diabetes, and hypercholesterolaemia were associated primarily with worse strain exclusively in the longitudinal plane; the additional relationship of diastolic blood pressure and diabetes to decreased longitudinal synchrony may suggest a greater propensity of these factors to influence longitudinal compared with mesocardial fibre function. These results are also concordant with CMR data indicating no significant relationships of diabetes or hypercholesterolaemia to circumferential strain.26 The relationship of antihypertensive therapy to better circumferential strain, however, suggests the possibility that circumferential fibre shortening can be responsive in a compensatory manner to elevated blood pressure severe enough to require treatment or to more chronic longstanding hypertensive stress.
Speckle-tracking echocardiography provides detailed information regarding LV mechanical function in systole— including the extent to which the LV shortens in the long axis (longitudinal strain), the LV cavity size decreases in circumference (circumferential strain), and the LV walls thicken (transverse and radial strains). Therefore, the components of LV deformation, as represented by strains measured in distinct planes, may reflect changes in myocardial tissue function resulting from different types of stress on the myocardium. Accordingly, we observed that distinct LV strain components (e.g. longitudinal vs. circumferential) were differentially associated with individual CVD risk factors. Notably, we observed a greater number of clinical correlates for strain measured in some planes compared with others. This finding may be related to transverse and radial strains representing aggregate rather than specific aspects of deformation in the endocardial, mesocardial, and epicardial layers of the myocardium. Further work is needed to investigate the unique relationships of individual risk factors to different aspects of LV mechanical function.
In our study, clinical covariates overall accounted for <20% of the interindividual variation in measures of LV mechanical function, suggesting the potential influence of genetic factors. In heritability analyses, we observed that additive genetic factors accounted for 16, 15, and 30% of the unexplained variation in global longitudinal, transverse, and circumferential strains, respectively. Although numerous studies report similar estimates for heritability of LV structural traits,7,27–31 particularly LV mass, there are no data on the heritability of LV function to our knowledge. Based on sensitive and detailed measures of LV function, our results indicate that certain aspects of LV mechanical function are dependent on genetic as well as acquired attributes.
In the subset of individuals with available parental history, having at least one parent with HF was associated with having significantly worse global circumferential strain. Notably, circumferential strain was the most heritable strain measure studied. We observed a modest, non-significant increase in the C statistic from the addition of parental HF to models for abnormal circumferential strain, suggesting that parental HF may be more important for understanding potential underlying biological pathways to subclinical LV dysfunction rather than serving as a marker for risk prediction per se. Because this trait reflects shortening around the circumference of the LV, the finding is consistent with reports of parental HF in association with LV systolic dysfunction defined as FS <0.2910 and eccentric LV geometry.11 Among individuals with normal FS and normal LV geometry, worse circumferential strain could represent an impaired ability to compensate for decrements in other aspects of LV mechanical function (e.g. longitudinal strain) that tend to decline with chronic exposure to traditional risk factors.32 The potential ability of myocardial function in different planes to serve complementary and, when required, compensatory roles has been referred to as planar heterogeneity and planar compensation, respectively. Importantly, previous investigators have observed that asymptomatic carriers of sarcomeric mutations associated with dilated cardiomyopathy had worse circumferential strain compared with normal controls, despite normal LV size and EF, on echocardiography.33 Similarly, decrements in global circumferential strain in our large community-based study sample may signal a predisposition for the future development of more overt cardiac dysfunction. The extent to which variation in circumferential strain may represent a risk marker specifically for HF with reduced or preserved EF, whether it signals greater risk for HF in earlier or later adulthood, and whether it may serve as a method for screening individuals at risk for HF warrants additional detailed and prospective investigation in the community setting. Further studies are also needed to evaluate the extent to which screening and targeting interventions for abnormal circumferential strain could represent an effective strategy for HF prevention.
Study limitations and strengths
Several limitations of the current study merit consideration. We analysed parental HF as a surrogate for genetic predisposition for HF, and data were unavailable to perform pre-HF strain measures for parents of the offspring participants studied. Although we were able to examine the association of left atrial diameter with myocardial strain, comprehensive measures of diastolic function were not available for the current analyses. Our myocardial data did not include measurement of longitudinal post-systolic shortening, which may provide additional information regarding systolic performance and reserve.34 In addition, biomarkers of cardiac stress such as natriuretic peptides were not available for the present study and warrant investigation in future large studies of myocardial strain. Given the lack of data on persons receiving treatment for active cancer in our cohort, the potential associations of chemotherapy with strain measures could not be assessed. Because our study sample included middle-age to older men and women, who were exclusively Caucasian of predominantly European ancestry, the extent to which our results may apply to other age or racial/ethnic groups remains unknown. Given the observational design of our study, conclusions regarding causality and directionality of reported associations cannot be made from our results. Notwithstanding these limitations, ours is the largest study to date of both clinical correlates and heritability of strain measures in a community-based cohort. Additional large studies are needed to validate our findings and further clarify the extent to which both clinical and genetic factors influence variation in myocardial strain.
In summary, our study of clinical correlates and heritability of LV mechanical function represents the largest to date in a community-based sample. We have shown significant associations of LV strain measures with select clinical traits including gender, heart rate, and prevalent CVD. Other risk factors were differentially associated with strain assessed in various imaging planes, suggesting planar heterogeneity in the impact of these risk factors. We also observed that heritable factors contribute equivalent or greater variability in longitudinal, transverse, and circumferential strain than the standard clinical factors that we evaluated. Furthermore, parental history of HF was significantly associated with worse circumferential strain, raising the possibility that impaired circumferential strain may reflect a familial predisposition to the eventual development of symptomatic cardiac dysfunction. Additional prospective investigations of multiethnic samples are warranted to investigate the mechanisms underlying our cross-sectional observations and to elucidate the potential implications of these findings for the prognosis and management of HF risk in the community.
Supplementary Material
Representative curves for left ventricular strain are shown for the long axis and short axis views.
Conventional left ventricular measures in the study sample.
Correlations between left ventricular mechanical function measures.
Age- and sex-adjusted correlates of primary left ventricular strain measures.
Age- and sex-adjusted correlates of secondary left ventricular strain measures.
Multivariable-adjusted additional clinical correlates of primary left ventricular strain measures.
Multivariable-adjusted clinical correlates of secondary left ventricular strain measures.
Multivariable-adjusted echocardiographic correlates of primary left ventricular strain measures.
Multivariable-adjusted echocardiographic correlates of secondary left ventricular strain measures.
Multivariable-adjusted electrocardiographic correlates of left ventricular synchrony measures.
Acknowledgments
Funding
This work was supported by the Ellison Foundation (to S.C.), the National Heart, Lung and Blood Institute’s Framingham Heart Study (contract no. N01-HC-25195), and the following grants: R00HL107642 (to S.C.) and R01HL093328 (to R.S.V.).
Footnotes
Conflict of interest: none declared.
References
- 1.Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging. 2009;2:356–364. doi: 10.1161/CIRCIMAGING.109.862334. [DOI] [PubMed] [Google Scholar]
- 2.Bertini M, Ng AC, Antoni ML, Nucifora G, Ewe SH, Auger D, Marsan NA, Schalij MJ, Bax JJ, Delgado V. Global longitudinal strain predicts long-term survival in patients with chronic ischemic cardiomyopathy. Circ Cardiovasc Imaging. 2012;5:383–391. doi: 10.1161/CIRCIMAGING.111.970434. [DOI] [PubMed] [Google Scholar]
- 3.Mignot A, Donal E, Zaroui A, Reant P, Salem A, Hamon C, Monzy S, Roudaut R, Habib G, Lafitte S. Global longitudinal strain as a major predictor of cardiac events in patients with depressed left ventricular function: a multicenter study. J Am Soc Echocardiogr. 2010;23:1019–1024. doi: 10.1016/j.echo.2010.07.019. [DOI] [PubMed] [Google Scholar]
- 4.Nahum J, Bensaid A, Dussault C, Macron L, Clemence D, Bouhemad B, Monin JL, Rande JL, Gueret P, Lim P. Impact of longitudinal myocardial deformation on the prognosis of chronic heart failure patients. Circ Cardiovasc Imaging. 2010;3:249–256. doi: 10.1161/CIRCIMAGING.109.910893. [DOI] [PubMed] [Google Scholar]
- 5.Woo JS, Kim WS, Yu TK, Ha SJ, Kim SY, Bae JH, Kim KS. Prognostic value of serial global longitudinal strain measured by two-dimensional speckle tracking echocardiography in patients with ST-segment elevation myocardial infarction. Am J Cardiol. 2011;108:340–347. doi: 10.1016/j.amjcard.2011.03.052. [DOI] [PubMed] [Google Scholar]
- 6.Mor-Avi V, Lang RM, Badano LP, Belohlavek M, Cardim NM, Derumeaux G, Galderisi M, Marwick T, Nagueh SF, Sengupta PP, Sicari R, Smiseth OA, Smule-vitz B, Takeuchi M, Thomas JD, Vannan M, Voigt JU, Zamorano JL. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications endorsed by the Japanese Society of Echocardiography. Eur J Echocardiogr. 2011;12:167–205. doi: 10.1093/ejechocard/jer021. [DOI] [PubMed] [Google Scholar]
- 7.Bielen E, Fagard R, Amery A. The inheritance of left ventricular structure and function assessed by imaging and Doppler echocardiography. Am Heart J. 1991;121:1743–1749. doi: 10.1016/0002-8703(91)90021-9. [DOI] [PubMed] [Google Scholar]
- 8.Verhaaren HA, Schieken RM, Mosteller M, Hewitt JK, Eaves LJ, Nance WE. Bivariate genetic analysis of left ventricular mass and weight in pubertal twins (the Medical College of Virginia twin study) Am J Cardiol. 1991;68:661–668. doi: 10.1016/0002-9149(91)90361-n. [DOI] [PubMed] [Google Scholar]
- 9.Schunkert H, Brockel U, Hengstenberg C, Luchner A, Muscholl MW, Kurzidim K, Kuch B, Doring A, Riegger GA, Hense HW. Familial predisposition of left ventricular hypertrophy. J Am Coll Cardiol. 1999;33:1685–1691. doi: 10.1016/s0735-1097(99)00050-9. [DOI] [PubMed] [Google Scholar]
- 10.Lee DS, Pencina MJ, Benjamin EJ, Wang TJ, Levy D, O’Donnell CJ, Nam BH, Larson MG, D’Agostino RB, Vasan RS. Association of parental heart failure with risk of heart failure in offspring. N Engl J Med. 2006;355:138–147. doi: 10.1056/NEJMoa052948. [DOI] [PubMed] [Google Scholar]
- 11.Lam CS, Liu X, Yang Q, Larson MG, Pencina MJ, Aragam J, Redfield MM, Benjamin EJ, Vasan RS. Familial aggregation of left ventricular geometry and association with parental heart failure: the Framingham Heart Study. Circ Cardiovasc Genet. 2010;3:492–498. doi: 10.1161/CIRCGENETICS.110.941088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dawber TR, Meadors GF, Moore FE., Jr Epidemiological approaches to heart disease: the Framingham Study. Am J Public Health Nations Health. 1951;41:279–281. doi: 10.2105/ajph.41.3.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham Offspring Study. Am J Epidemiol. 1979;110:281–290. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 14.Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, Aragam J, Benjamin EJ, Solomon SD, Vasan RS. Reproducibility of speckle-tracking-based strain measures of left ventricular function in a community-based study. J Am Soc Echocardiogr. 2013;26:1258–1266. doi: 10.1016/j.echo.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cheng S, Larson MG, McCabe EL, Osypiuk E, Lehman BT, Stanchev P, Aragam J, Benjamin EJ, Solomon SD, Vasan RS. Age- and sex-based reference limits and clinical correlates of myocardial strain and synchrony: the Framingham Heart Study. Circ Cardiovasc Imaging. 2013;6:692–699. doi: 10.1161/CIRCIMAGING.112.000627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Byrd BF., 3rd A unique collaboration to advance strain imaging. J Am Soc Echocardiogr. 2013;26:21A–22A. doi: 10.1016/j.echo.2013.09.010. [DOI] [PubMed] [Google Scholar]
- 17.Almasy L, Blangero J. Multipoint quantitative-trait linkage analysis in general pedigrees. Am J Hum Genet. 1998;62:1198–1211. doi: 10.1086/301844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–1446. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 19.Dalen H, Thorstensen A, Aase SA, Ingul CB, Torp H, Vatten LJ, Stoylen A. Segmental and global longitudinal strain and strain rate based on echocardiography of 1266 healthy individuals: the HUNT study in Norway. Eur J Echocardiogr. 2010;11:176–183. doi: 10.1093/ejechocard/jep194. [DOI] [PubMed] [Google Scholar]
- 20.Sun JP, Lee AP, Wu C, Lam YY, Hung MJ, Chen L, Hu Z, Fang F, Yang XS, Merlino JD, Yu CM. Quantification of left ventricular regional myocardial function using two-dimensional speckle tracking echocardiography in healthy volunteers—a multi-center study. Int J Cardiol. 2013;167:495–501. doi: 10.1016/j.ijcard.2012.01.071. [DOI] [PubMed] [Google Scholar]
- 21.Redfield MM, Jacobsen SJ, Burnett JC, Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289:194–202. doi: 10.1001/jama.289.2.194. [DOI] [PubMed] [Google Scholar]
- 22.Cheng S, Xanthakis V, Sullivan LM, Lieb W, Massaro J, Aragam J, Benjamin EJ, Vasan RS. Correlates of echocardiographic indices of cardiac remodeling over the adult life course: longitudinal observations from the Framingham Heart Study. Circulation. 2010;122:570–578. doi: 10.1161/CIRCULATIONAHA.110.937821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Winter R, Jussila R, Nowak J, Brodin LA. Speckle tracking echocardiography is a sensitive tool for the detection of myocardial ischemia: a pilot study from the catheterization laboratory during percutaneous coronary intervention. J Am Soc Echocardiogr. 2007;20:974–981. doi: 10.1016/j.echo.2007.01.029. [DOI] [PubMed] [Google Scholar]
- 24.Gjesdal O, Hopp E, Vartdal T, Lunde K, Helle-Valle T, Aakhus S, Smith HJ, Ihlen H, Edvardsen T. Global longitudinal strain measured by two-dimensional speckle tracking echocardiography is closely related to myocardial infarct size in chronic ischaemic heart disease. Clin Sci (Lond) 2007;113:287–296. doi: 10.1042/CS20070066. [DOI] [PubMed] [Google Scholar]
- 25.Cheng S, Fernandes VR, Bluemke DA, McClelland RL, Kronmal RA, Lima JA. Age-related left ventricular remodeling and associated risk for cardiovascular outcomes: the Multi-Ethnic Study of Atherosclerosis. Circ Cardiovasc Imaging. 2009;2:191–198. doi: 10.1161/CIRCIMAGING.108.819938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rosen BD, Saad MF, Shea S, Nasir K, Edvardsen T, Burke G, Jerosch-Herold M, Arnett DK, Lai S, Bluemke DA, Lima JA. Hypertension and smoking are associated with reduced regional left ventricular function in asymptomatic: individuals the Multi-Ethnic Study of Atherosclerosis. J Am Coll Cardiol. 2006;47:1150–1158. doi: 10.1016/j.jacc.2005.08.078. [DOI] [PubMed] [Google Scholar]
- 27.Post WS, Larson MG, Myers RH, Galderisi M, Levy D. Heritability of left ventricular mass: the Framingham Heart Study. Hypertension. 1997;30:1025–1028. doi: 10.1161/01.hyp.30.5.1025. [DOI] [PubMed] [Google Scholar]
- 28.Arnett DK, Hong Y, Bella JN, Oberman A, Kitzman DW, Hopkins PN, Rao DC, Devereux RB. Sibling correlation of left ventricular mass and geometry in hypertensive African Americans and whites: the HyperGEN study. Hypertension Genetic Epidemiology Network Am J Hypertens. 2001;14:1226–1230. doi: 10.1016/s0895-7061(01)02200-2. [DOI] [PubMed] [Google Scholar]
- 29.Bella JN, MacCluer JW, Roman MJ, Almasy L, North KE, Best LG, Lee ET, Fabsitz RR, Howard BV, Devereux RB. Heritability of left ventricular dimensions and mass in American Indians: the Strong Heart Study. J Hypertens. 2004;22:281–286. doi: 10.1097/00004872-200402000-00011. [DOI] [PubMed] [Google Scholar]
- 30.Chien KL, Hsu HC, Su TC, Chen MF, Lee YT. Heritability and major gene effects on left ventricular mass in the Chinese population: a family study. BMC Cardiovasc Disord. 2006;6:37. doi: 10.1186/1471-2261-6-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Palatini P, Krause L, Amerena J, Nesbitt S, Majahalme S, Tikhonoff V, Valentini M, Julius S. Genetic contribution to the variance in left ventricular mass: the Tecumseh Offspring Study. J Hypertens. 2001;19:1217–1222. doi: 10.1097/00004872-200107000-00006. [DOI] [PubMed] [Google Scholar]
- 32.Borg AN, Ray SG. A unifying framework for understanding heart failure? Response to ‘Left ventricular torsion by two-dimensional speckle tracking echocardiography in patients with diastolic dysfunction and normal ejection fraction’. J Am Soc Echocardiogr. 2009;22:318–320. doi: 10.1016/j.echo.2008.11.026. author reply 321–312. [DOI] [PubMed] [Google Scholar]
- 33.Lakdawala NK, Thune JJ, Colan SD, Cirino AL, Farrohi F, Rivero J, McDonough B, Sparks E, Orav EJ, Seidman JG, Seidman CE, Ho CY. Subtle abnormalities in contractile function are an early manifestation of sarcomere mutations in dilated cardiomyopathy. Circ Cardiovasc Genet. 2012;5:503–510. doi: 10.1161/CIRCGENETICS.112.962761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Voigt JU, Lindenmeier G, Exner B, Regenfus M, Werner D, Reulbach U, Nixdorff U, Flachskampf FA, Daniel WG. Incidence and characteristics of segmental postsystolic longitudinal shortening in normal, acutely ischemic, and scarred myocardium. J Am Soc Echocardiogr. 2003;16:415–423. doi: 10.1016/s0894-7317(03)00111-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Representative curves for left ventricular strain are shown for the long axis and short axis views.
Conventional left ventricular measures in the study sample.
Correlations between left ventricular mechanical function measures.
Age- and sex-adjusted correlates of primary left ventricular strain measures.
Age- and sex-adjusted correlates of secondary left ventricular strain measures.
Multivariable-adjusted additional clinical correlates of primary left ventricular strain measures.
Multivariable-adjusted clinical correlates of secondary left ventricular strain measures.
Multivariable-adjusted echocardiographic correlates of primary left ventricular strain measures.
Multivariable-adjusted echocardiographic correlates of secondary left ventricular strain measures.
Multivariable-adjusted electrocardiographic correlates of left ventricular synchrony measures.