Abstract
Immune-activated microglia from aged mice produce exaggerated levels of cytokines. Despite high levels of microglial IL-10 in the aged brain, neuroinflammation was prolonged and associated with depressive-like deficits. Because astrocytes respond to IL-10 and, in turn, attenuate microglial activation, we investigated if astrocyte-mediated resolution of microglial activation was impaired with age. Here, aged astrocytes had a dysfunctional profile with higher GFAP, lower glutamate transporter expression, and significant cytoskeletal re-arrangement. Moreover, aged astrocytes had reduced expression of growth factors and IL-10 Receptor-1 (IL-10R1). Following in vivo LPS immune challenge, aged astrocytes had a molecular signature associated with reduced responsiveness to IL-10. This IL-10 insensitivity of aged astrocytes resulted in a failure to induce IL-10R1 and TGFβ and resolve microglial activation. Additionally, adult astrocytes reduced microglial activation when co-cultured ex vivo, while aged astrocytes did not. Consistent with the aging studies, IL-10RKO astrocytes did not augment TGFβ after immune challenge and failed to resolve microglial activation. Collectively, a major cytokine-regulatory loop between activated microglia and astrocytes is impaired in the aged brain.
Keywords: astrocyte, microglia, aging, neuroinflammation, interleukin-10, TGF-beta
1. Introduction
The bi-directional communication between the immune system and brain is altered with age (Corona, et al., 2012, Jurgens and Johnson, 2010). This neuroimmune communication is relevant because it is essential for mounting the appropriate immunological, physiological, and behavioral responses to pathogens. This communication results in the propagation of cytokines and chemokines by microglia (Dantzer, et al., 2008, Henry, et al., 2009, Nguyen, et al., 2002, Norden, et al., 2014) and the induction of sickness behavior (Dantzer, 2001, Kelley, et al., 2003). Altered neuroimmune communication in aging is significant because older individuals have an increased risk of infection with concomitant neurobehavioral deficits (Jackson, et al., 2004, Rockwood, et al., 1999), including depression (Koenig, et al., 1988, Penninx, et al., 1999) and cognitive impairment (Ahmed, et al., 2014, Dunn, et al., 2005, Iwashyna, et al., 2010). Thus, immune challenges in the elderly can be triggers for ongoing inflammatory processes that negatively affect mood and cognition.
The mechanism by which peripheral infection in the elderly causes neuropsychiatric complications is unknown, but it may be related to impaired regulation of microglia. For instance, immune challenge (LPS or E.coli) in aged rodents results in amplified glia-mediated neuroinflammation associated with neuropsychiatric complications (Barrientos, et al., 2010, Barrientos, et al., 2009, Godbout, et al., 2005, Godbout, et al., 2008, Wynne, et al., 2010). Several studies show that exaggerated microglial responses are related to impairments in regulatory systems making it difficult to resolve microglial activation (Jurgens and Johnson, 2010, Norden and Godbout, 2013). For example, microglia produce exaggerated levels of both pro- and anti-inflammatory cytokines, including Interleukin (IL)-10 (Henry, et al., 2009, Sierra, et al., 2007). This simultaneous induction of IL-1β and IL-10 is liked related to enhanced NF-κB activity, because it drives the transcription of both pro- and anti-inflammatory mediators (Cao, et al., 2006). Despite higher levels of IL-10 in the aged brain, microglial activation and sickness behavior was prolonged, and depression and acute cognitive impairments developed (Chen, et al., 2008, Godbout, et al., 2008, Huang, et al., 2008, Wynne, et al., 2010). Thus, anti-inflammatory responses to IL-10 may be impaired in the aged brain.
Astrocytes are potentially key to this IL-10 puzzle because they respond to IL-10 and inhibit microglial activation in a Transforming Growth Factor (TGF) β-dependent manner (Norden, et al., 2014). The IL-10 receptor has two necessary components for functional signaling: ligand binding domain (IL-10R1) and signaling domain (IL-10R2) (Moore, et al., 2001). While IL-10R2 is expressed constitutively by most cells, IL-10R1 expression is cell specific and low under homeostatic conditions (Moore, et al., 2001). IL-10 binding to IL-10R results in Jak1 and Tyk2 phosphorylation and subsequent STAT3 activation. STAT3 induces suppressor of cytokine signaling 3 (SOCS3), which inhibits IL-1β, IL-6, and TNFα. STAT3 also enhances anti-apoptotic and cell-cycle-progression genes including Myc, Cyclin-D1 (CCND1), and Cyclin-Dependent kinase inhibitors (CDKNs) (Donnelly, et al., 1999, Lin, et al., 2005, O’Farrell, et al., 2000). Furthermore, IL-10 has anti-inflammatory and neuroprotective roles within the CNS in EAE (Cua, et al., 2001), spinal cord injury (Bethea, et al., 1999, Ishii, et al., 2013), stroke (Frenkel, et al., 2005), and cytokine-induced sickness behavior (Bluthe, et al., 1999, Lynch, et al., 2004, Richwine, et al., 2009).
The induction of TGFβ by astrocytes after IL-10 (Norden, et al., 2014) is important because TGFβ has neuroprotective and anti-inflammatory effects in the CNS (Qian, et al., 2008). In microglia, increased pro-inflammatory mediators and cytokine expression initiated by LPS challenge was attenuated by TGFβ (Butovsky, et al., 2014, Herrera-Molina and von Bernhardi, 2005, Norden, et al., 2014, Tichauer, et al., 2014). Therefore, TGFβ produced by activated astrocytes can inhibit microglial activation.
Based on these findings, we surmise that key regulatory communication between microglia and astrocytes is impaired with age. Thus, the purpose of this study was to investigate the degree to which IL-10 and TGFβ cytokine interactions between microglia and astrocytes were impaired in the aged brain following an immune challenge. Here we provide novel evidence that aged astrocytes are less sensitive to IL-10, that this IL-10 insensitivity is related to IL-10R1, and that IL-10 insensitivity occurs concomitantly with decreased TGFβ feedback on active microglia.
2. Materials and Methods
2.1 Mice
Adult (3–4 months old) and aged (18–20 months old) BALB/c mice were purchased from the National Institute of Aging. Adult C57BL/6 and C57BL/6 IL-10R2−/− (IL-10RKO) mice were purchased from Jackson Laboratory and breeding pairs were established. Neonatal BALB/c or C57BL6 mice (postnatal day 1–3) and adult (3–4 months old) C57BL/6 and C57BL/6 IL-10R2−/− mice were obtained from our breeding colony kept in barrier-reared conditions in a specific-pathogen-free facility at The Ohio State University. Adult, aged, and IL-10RKO mice were individually housed in polypropylene cages and maintained at 25° C under a 12 h light/12 h dark cycle with ad libitum access to water and rodent chow. All procedures were in accordance with the National Institute of Health Guidelines for the Care and Use of Laboratory Animals and were approved by The Ohio State University Institutional Laboratory Animal Care and Use Committee.
2.2 Immune Challenge with Lipopolysaccharide
Adult and aged BALB/c mice were injected intraperitoneally (i.p.) with saline or Escherichia coli lipopolysaccharide (LPS) (0.33 mg/kg; serotype 0127:B8, Sigma, St. Louis, MO). Adult IL-10RWT and IL-10RKO C57BL6 mice were injected i.p. with 0.5 mg/kg LPS. These LPS dosages were selected because they elicit a pro-inflammatory cytokine response in the brain resulting in a transient sickness response in adult BALB/c mice (0.33 mg/kg) or adult C57BL6 mice (0.5 mg/kg) (Berg, et al., 2004, Godbout, et al., 2005, Wohleb, et al., 2012).
2.3 Immunohistochemistry for Iba-1 and GFAP
Iba-1 and GFAP labeling was performed as previously described (Fenn, et al., 2014). In brief, the brain was collected after transcardial perfusion with sterile PBS (PBS, pH 7.4) and 4% formaldehyde. Brains were post-fixed in 4% formaldehyde for 24 h and then cryoprotected in 20% sucrose for an additional 48 h. Preserved brains were frozen using dry-ice cooled isopentane (−165°C) and then sectioned (25 μm) using a Microm HM550 cryostat. Brain sections were identified by reference markers in accordance with the stereotaxic mouse brain atlas (Paxinos and Franklin, 2004). To label for Iba-1 or GFAP, sections were placed free-floating in cryoprotectant (30% polyethylene glycol, 30% ethylene glycol, 40% 0.2M phosphate buffer) until labeling. Next, sections were washed in PBS, then blocked (5% NGS, 1% BSA in PBS) and incubated with rabbit anti-mouse Iba-1 (1:1000; Wako Chemicals) or rabbit anti-mouse GFAP antibody (1:1000; Dako) overnight at 4°C. Next, sections were washed in PBS and incubated with a fluorochrome-conjugated secondary antibody (Alexa Fluor 594 or Alexa Fluor 488). Sections were mounted on slides, then cover-slipped with Fluoromount G (Beckman Coulter, Inc.), and stored at −20°C. Fluorescent images were visualized using an epifluorescent Leica DM5000B microscope and captured using a Leica DFC300 FX camera and imaging software.
2.4 Microglia and Astrocyte Analysis and Reconstruction
Glia Reconstruct was used to analyze GFAP and Iba-1 labeled images as previously described (Kongsui, et al., 2014a,Kongsui, et al., 2014b,Walker, et al., 2014) but with modifications. In brief, images were captured using a Leica DFC300 FX (20×) as described above. Representative hippocampal sections (3–5) per experimental mouse were used. Prior to analysis, the signal range was defined as occurring between X-Y that included all cellular material but no background, a cumulative spectra (CTS) analysis (Kongsui, et al., 2014b) was undertaken (Johnson and Walker, 2015). CTS analysis involves identifying the number of pixels within an image that occur at each of the 256 possible pixel intensities and then, expressing the number of pixels occurring at each pixel intensity as a percentage of the total number of pixels within the image. This method provides a significantly more transparent method for quantifying immuno-labeled material (Johnson and Walker, 2015). Following CTS analysis, which provides information only on shifts in the density of immune-labeled material, both astrocyte and microglial morphologies were digitally reconstructed using Matlab (The MathWorks, Inc.) executed algorithms that use variance minimization strategy to extract the signal from background. Size filtering was additionally used to eliminate non-cellular material (Radler, et al., 2015). Using this approach, the key morphological features of glia can be examined. For example, maximum cell perimeter, total cell length, cell body perimeter, number of primary processes, number of nodes (branch points), total length of all processes, and total volume of all processes can be measured. In addition, the area encompassed by the entire cell was measured as the convex hull area, determined from the polygon created from straight lines connecting the most distal points of the microglial processes. Pixel information was converted into μm values to provide information on relative size, area or volume of microglia and astrocytes.
2.5 Percoll-Enrichment of Microglia and Astrocytes
Microglia and astrocytes were isolated from brain homogenates using a modified Percoll density gradient as previously described (Norden et al., 2014). In brief, the brain was homogenized and cell pellets were re-suspended in 70% isotonic Percoll. A discontinuous Percoll density gradient was layered and centrifuged for 20 min at 2000×g. Enriched microglia were collected from the interphase between 70% and 45% Percoll. Of the cells recovered from this Percoll interphase, approximately 90% of the cells were CD11b+/CD45low microglia (Henry, et al., 2009). Less than 1.5% of the enriched cells were CD11b+/CD45high macrophages. Enriched astrocytes were collected from the interphase between 45% and 35% Percoll layers. Of the cells recovered from this Percoll gradient layer, 75–80% of the cells were GLAST-1+ astrocytes (Norden, et al., 2014, Norden, et al., 2016) (Fig. 7). These enriched astrocytes were washed and re-suspended in PBS for qPCR analysis or FACS buffer for flow cytometric analysis. The purity of the glia after Percoll experiments was consistent with our flow cytometric analysis of Percoll enriched glia (Henry, et al., 2009, Norden, et al., 2014, Sawicki, 2014). Notably, there is no contamination of the Percoll enrichments by endothelial cells (CD31+) (data not shown).
Figure 7. Impaired mRNA induction of IL-10R1 and TGFβ in aged astrocytes following peripheral immune challenge.

A) Adult (3mo) and aged (18 mo) BALB/c mice were injected with saline or LPS and 24 h later astrocytes were Percoll-enriched and mRNA levels were determined by qPCR (n=6). mRNA levels of B) IL-6, C) IL-1β, D) IL-10, E) IL-10R1, and F) TGFβ were determined. Bars represent the mean + SEM. Means with (*) are different from adult controls (p<0.05).
2.6 Flow Cytometric Assisted (FAC)-Sorting of Microglia and Astrocytes
Microglia and astrocytes were Percoll enriched as described above. Microglia and astrocyte fractions were pooled after isolation and were labeled with rat anti-mouse CD11b-FITC, CD45-PerCP-Cy5.5 (eBioscience) and GLAST-1-APC (Miltenyi Biotec) antibodies. Cells were sorted using a Becton-Dickinson FACSAria III cell sorter at the OSU Comprehensive Cancer core facility. Microglia were identified by GLAST-1−/CD11b+/CD45low expression and astrocytes were identified by GLAST-1+/CD11b−/CD45− expression. CD45high expressing peripherally derived macrophages were excluded when collecting microglia. Microglia and astrocytes (100,000–150,000 cells) were sorted into separate collection tubes. Cells were pelleted and lysed immediately in RNA lysis buffer.
2.7 NanoString and nCounter analysis of mRNA copy Number
FAC-sorted microglia were pelleted and lysed in RNA lysis buffer. Norgen Biotek Single Cell RNA Purification Columns were used to purify and concentrate total RNA from microglia. FAC-sorted astrocytes were pelleted and lysed in RA1 lysis buffer. The PrepEase Kit (USB, CA) was used to purify and concentrate total RNA from astrocytes. During the RNA isolation, astrocyte samples were treated with on-column Dnase digestion for 45 minutes at 37°C to eliminate contaminating genomic DNA. RNA quality and integrity was determined using Bioanalyzer 2100 (Agilent Technologies). RNA was analyzed by NanoString nCounter technology (www.nanostring.com) which allows expression analysis of multiple genes from a single sample. NanoString analysis was performed by the Nucleic acid core facility at OSU. nCounter multiplexed target profiling of 75 custom genes was performed. Customized plates were designed with selected astrocyte or microglia related genes that are relevant to inflammation, regulation, and growth/repair. Overall, the selected genes were categorized as glia signature genes (microglia: CX3CR1, CSF1R, CD11b, Iba-1 or astrocytes: EEAT2, GLAST-1, GFAP, Vimentin), genes associated with activation/inflammation (e.g., IL-1β, TNFα, CCL2), and genes associated with regulation/growth (e.g., TGFβ, IL-10R1/R2, BDNF, IGF1, GDNF). Microglia and astrocyte RNA samples were run on separate cassettes and, therefore, cannot be directly compared. Following technical normalization of positive and negative controls, RNA was normalized based on housekeeping expression of GAPDH, ACTB and OAZ1 expression. Data are expressed as mRNA copy numbers. For LPS comparisons, data are expressed as fold change.
2.8 RNA Isolation and RT-PCR
For cell cultures and coronal brain sections, total RNA was isolated using the Tri-Reagent protocol (Sigma-Aldrich). For Percoll isolated microglia and astrocytes, RNA was isolated using the PrepEase kit (USB, CA). RNA was reverse transcribed to cDNA and real-time (RT)-PCR was performed using the Applied Biosystems Taqman® Gene Expression assayAssay-on-Demand Gene Expression protocol. In brief, experimental cDNA was amplified by real-time PCR where a target cDNA (e.g., IL-1β, IL-6, CCL2) and a reference cDNA (glyceraldehyde-3-phosphate dehydrogenase; GAPDH) were amplified simultaneously using an oligonucleotide probe with a 5′ fluorescent reporter dye (6-FAM). Fluorescence was determined on an ABI PRISM 7300-sequence detection system (Applied Biosystems). Data were analyzed using the comparative threshold cycle (Ct) method and results are expressed as fold change.
2.9 IL-10 Receptor Surface Expression
In a separate cohort of mice, astrocytes were Percoll enriched as described above and were labeled with rat anti-mouse CD11b-FITC, IL-10R1-PE (eBioscience), and GLAST-1-APC (Miltenyi Biotec) antibodies (Norden, et al., 2014). Expression was determined using a Becton-Dickinson FACSCaliber four color Cytometer. Astrocytes were identified by GLAST-1+/CD11b− expression. In this experiment, CD11b was used to exclude both microglia and any peripherally derived myeloid cells from the astrocyte analysis. For each antibody, gating was determined based on appropriate negative isotype stained controls. Flow data were analyzed using FlowJo software (Tree Star, CA).
2.10 Primary microglia and astrocyte cultures
Primary microglia and astrocyte cultures were established from neonatal mice and maintained as previously described (Godbout, et al., 2004, Norden, et al., 2014). In brief, mixed glia cultures were shaken at 160rev/min and 37 °C for 3.5h to harvest microglia from the confluent cell layer. Remaining cells were treated with 50 mM l-leucine methyl ester (Sigma-Aldrich) for 45 minutes to deplete remaining microglia from the astrocyte layer. After l-leucine incubation, astrocytes recovered in growth medium supplemented with 0.1 mM l-leucine for 1–3 days (Phulwani, et al., 2008). Astrocytes were collected by incubating with 0.25% trypsin for 15 minutes, and then plated at a density of 50,000 cells/well in 24-well plates. Immediately before treatment, cells were washed with serum-free DMEM medium. Primary astrocytes were activated with 100 ng/mL LPS (stereotype 0127:B8, Sigma-Aldrich) for 1 h followed by IL-10 stimulation (10 ng/mL) (R&D Systems) for an additional 23 h. Following experimental treatments, RNA was isolated using Tri-Reagent.
2.11 Microglia-Astrocyte transwell co-cultures
Microglia-Astrocyte transwell co-cultures were established from mice using a protocol similar to one previously described (Norden, et al., 2014). In brief, primary microglia were plated at 50,000–75,000 cells/well in a 24-well transwell plate (Corning Life Sciences). After incubation for 3 hours, astrocytes were added to a removable 0.4 μm polycarbonate membrane at an equal number as microglia. Following incubation overnight, cells were washed with serum-free DMEM media prior to treatment. LPS was added to the shared medium at 10 ng/mL. After 1 hour, recombinant mouse IL-10 was suspended in PBS with 0.1%BSA and added at 10 ng/mL. Following experimental treatments, RNA was isolated from the microglia using Tri-Reagent.
2.12 Ex vivo microglia-astrocyte transwell co-cultures
Enriched microglia were isolated from saline (adult, aged) and LPS (adult) injected mice (4 h after injection) using Percoll density gradient separation. Microglia were plated at the bottom of poly-L-lysine coated 24-well plates (Corning Life Sciences). After 30 minutes, enriched astrocytes were added to a removable 0.4 μm polycarbonate membrane. In one experiment, an IL-10 neutralization antibody (10μg/mL, BD Pharmingen) or vehicle was added to wells where astrocytes were added. After an additional 4 h, conditioned media was collected and stored at −80°C and RNA was isolated from microglia using PrepEase kit (USB, CA).
2.13 Determination of IL-6 protein levels in conditioned media
IL-6 was determined from conditioned media using the BD OptEIA Mouse IL-6 ELISA according to the manufacturer’s instructions (BD Biosciences). In brief, 96-well enzyme immunoassay plates were coated with anti-mouse IL-6 capture antibody and incubated overnight at 4°C. Samples and IL-6 standards (0–1000 pg/ml) were added and incubated for 2 h at room temperature (RT). Plates were washed and incubated with biotinylated anti-mouse IL-6 antibody. Plates were washed and incubated with streptavidin-horseradish peroxidase conjugate. After 1 h incubation at RT, plates were washed and incubated with tetramethylbenzidine liquid substrate for 15 min. Reactions were terminated and absorbance was read at 450 nm using a Synergy HT Plate Reader (Bio-tek instruments). The assay was sensitive to 10 ng/ml IL-6 and the intra assay coefficients of variation were less than 10%.
2.14 Statistical Analysis
To ensure a normal distribution, data were subjected to the Shapiro-Wilk test using Statistical Analysis Systems (SAS) statistical software (Cary, NC). To determine significant main effects and interactions between main factors, data were analyzed using one-way (i.e., Pretreatment and Treatment) or two-way (i.e., Pretreatment × Treatment) ANOVA using the General Linear Model procedures of SAS. When appropriate, differences between treatment means were evaluated by an F-protected t-test using the Least-Significant Difference procedure of SAS. All data are expressed as treatment means ± standard error of the mean (SEM). Values were considered significant at p-values < 0.05 and a tendency at p-values ≤ 0.1.
3. Results
3.1 Age-Associated Remodeling and Cytoskeletal Re-organization of Microglia and Astrocytes
Because microglial activation is exaggerated and prolonged in the brain of aged mice after immune challenge (Henry, et al., 2009), we propose that critical anti-inflammatory pathways that regulate microglia are impaired with normal aging (Norden and Godbout, 2013). Our recent work provides evidence that during an inflammatory response astrocytes respond to IL-10, and in turn, produce TGFβ, which inhibits the activation of microglia (Norden, et al., 2014). Therefore, the purpose of this study was to determine if astrocyte-mediated resolution of microglial activation following immune challenge was impaired with age.
To begin to address age-related impairments in glial dynamics, mRNA levels of signature morphological genes for microglia (Iba-1) and astrocytes (GFAP, and vimentin) were determined in the cortex, cerebellum, and hippocampus of adult and aged mice. Fig. 1A shows that Iba-1 mRNA expression was similar in both adult and aged mice in the three regions examined. Fig. 1B shows that GFAP mRNA levels were increased in the hippocampus (p<0.02) and cerebellum (p<0.01) of aged mice compared to adults. GFAP mRNA expression, however, was not significantly increased by age in the cortex. Vimentin mRNA expression was selectively increased in the hippocampus of aged mice compared to adult controls (p<0.002, Fig. 1C).
Figure 1. Age-Associated Remodeling and Cytoskeletal Re-organization of Microglia and Astrocytes.

Adult (3 mo) and aged (18 mo) BALB/c mice were used and mRNA expression of A) Iba-1, B) GFAP, and C) vimentin were determined in the cortex (CX), hippocampus (HPC), and cerebellum (CB) (n=3). Bars represent the mean + SEM. In a separate study, adult and aged mice were used and Iba-1 and GFAP protein expression were determined in the hippocampus by Glia Reconstruct (n=5). D) Representative images of Iba-1 (red) and GFAP labeling (green) and the corresponding thresholded images (white) are shown. Several morphological parameters were evaluated in E) Iba-1+ microglia and F) GFAP+ astrocytes. Data are represented by the mean ± SEM. Means with (*) are different from adult controls (p<0.05).
Based on these mRNA data, morphological profiles of microglia and astrocytes (Glia Reconstruct) were evaluated in the hippocampus of adult and aged mice. First, cumulative spectra analysis was used to find the appropriate pixel intensity for Glia Reconstruct analysis (Kongsui, et al., 2014b). Images were then thresholded and analyzed. Fig. 1D shows representative labeling (left) and the corresponding thresholded images (right) for Iba-1 and GFAP from the CA1 region of the hippocampus for adult and aged mice. Fig. 1E shows Glia Reconstruct analysis of Iba-1+ microglia with cell number, perimeter, length, and convex hull area. There was no difference in the number of microglia in the HPC with age, but there were significant differences in microglial cell structure. For example, microglia in the HPC of aged mice had an increased perimeter (p<0.001), max length (p<0.001), and convex hull area (p<0.002) compared to microglia of adults. Notably, the morphological alterations in microglia identified by Iba-1 labeling were not reflected in the Iba-1 mRNA levels (Fig. 1A). Nonetheless, microglia in the HPC of aged mice had significant differences in cell structure.
Similar data were obtained with GFAP labeled astrocytes (Fig. 1F). Again, the number of astrocytes in the HPC was the same between adult and aged mice. In addition, aged astrocytes also had increased perimeter (p<0.02), max length (p<0.03), and convex hull area (p<0.001). For astrocytes, the increased GFAP mRNA levels in the HPC of aged mice were reflected in increased GFAP protein expression. Taken together, there is significant remodeling and cytoskeletal re-organization of microglia and astrocytes in the hippocampus with age.
3.2 FAC-Sorted Microglia from Aged Mice have a Pro-Inflammatory mRNA Profile at Baseline
Next, specific astrocyte and microglia mRNA profiles from adult and aged mice were determined. In these studies, microglia and astrocytes were Percoll enriched, labeled, and FAC-sorted, and mRNA copy number of 75 genes was determined using a customized NanoString gene array (Fig. 2A). Fig. 2B shows relative mRNA expression of CD11b between FAC-sorted microglia and astrocytes. As expected, there was significant detection of CD11b mRNA in the FAC-sorted microglia but not the FAC-sorted astrocytes. Fig. 2C–E show mRNA copy number analyses of FAC-sorted microglia. Determined were several microglial signature genes (Fig. 2C), inflammatory genes (Fig. 2D), and regulatory/growth genes (Fig. 2C). An important aspect of mRNA copy number quantification is that the relative expression of genes can be compared to one another. For instance, the microglial signature gene fractalkine receptor (CX3CR1) was expressed at a high level with over 20,000 mRNA copies. In comparison, the mRNA copy numbers of cytokine genes, including IL-1, IL-6, and TNFα, were less than 20 mRNA copies each.
Figure 2. FAC-Sorted Microglia from Aged mice have a Pro-Inflammatory mRNA Profile at Baseline.

A) Adult (3 mo) and aged (18 mo) BALB/c mice were used and microglia and astrocytes were Percoll-enriched, labelled, FAC-sorted, and mRNA copy number was determined by NanoString analysis (n=4). For microglia, representative bivariate dot plots of CD11b and GLAST-1 labeling and CD11b and CD45 labeling are shown. Microglia (GLAST-1−/CD11b+/CD45low) were FAC-sorted and RNA was collected. B) Relative CD11b mRNA expression was determined in FAC-sorted microglia and astrocytes by qPCR (n=3). Microglial mRNA copy numbers of genes associated with C) microglial signature, D) inflammatory processes, and E) regulation and growth/support were determined. Tables represented the mean ± SEM. Means with (*) are different from adult controls (p<0.05) and means with (+) tend to be different from adult controls (p=0.06–0.10).
The mRNA copy number of several microglial signature genes in the order of highest to lowest mRNA copy number is represented in Fig. 2C. Independent of age, the three highest expressed genes by copy number in the FAC-sorted microglia were CX3CR1 (22,000 copies), CSF1R (15,000 copies), and TGFβR1 (5,000 copies, Fig. 2C&E). These three (CX3CR1, CSF1R, and TGFβR1) microglial signature genes and others, including TREM2, CD11b, CD200R1, Fc receptors (Fcgr1&3) and Iba-1, were not altered by age. The exception was CD45, which was increased in microglia from aged mice compared to adults (p<0.05). Notably, genes associated with an astrocyte signature, including EAAT2, GFAP and Aquaporin-4, had limited expression (fewer than 10 copies) in the FAC-sorted microglia (Fig. 2C).
The mRNA copy numbers of genes associated with inflammation are shown in Fig. 2D. These genes are sub-divided into groups associated with cytokines, cytokine receptors, chemokines and immune signaling. It is important to highlight the mRNA copy number for cytokines and chemokines was low at baseline in both age groups. Nonetheless, several of these inflammatory associated genes were increased in aged microglia compared to adults. For instance, there were age-related increases in microglia expression of pro-inflammatory cytokines: IL-1β (p<0.04), IL-6 (p<0.04), TNFα (p=0.06), cytokine receptors: IL-1R1 (p=0.1), IL-1RN (p<0.05), chemokines: CCL3 (p<0.03), CCL5 (p<0.05), CXCL10 (p<0.04), and immune signaling genes: IκBKβ (p=0.1), Caspase-1 (p<0.05), TLR1 (p<0.03), TRL2 (p=0.1), CD86 (p<0.03).
Fig. 2E shows the mRNA copy number of genes associated with regulation and growth. These genes are sub-divided into groups of genes associated with cytokines, cytokine receptors, immune signaling, repair, and growth/survival. The mRNA copy number of the majority of cytokines (IL-10 & IL-4) and growth factors (VEGFA, BDNF, GDNF, and IGF-1) in microglia were low in both age groups at baseline (Fig. 2E). The exceptions were relatively high copy number of TGFβ (1,000 copies), TGFβR1 (5,000 copies), and Jun (5,000 copies). Overall, few of these genes differed between adult and aged microglia. The differences were higher expression of IL-4Rα, MRC1, SMAD4 (common Smad) and SMAD7 (inhibitory Smad) in microglia of aged mice compared to adults (p<0.05, for each). Expression of Stat5a and CD163 also tended to be higher in aged microglia compared to adults (p=0.08, for each). Taken together, these data indicate that microglia from the brain of aged mice have a molecular signature associated with a higher inflammatory status.
3.3 FAC-sorted Astrocytes from the Aged Brain have an mRNA Profile Associated with Dysfunction
The mRNA profile of FAC-sorted astrocytes from adult and aged mice was also determined. The diagram in Fig. 3A shows the gating strategy for FAC-sorting the astrocytes (Glast-1+, CD11b−, CD45−). As expected, the astrocytes collected by FAC-sorting had high expression of GFAP and low expression of CD11b compared to the FAC-sorted microglia (Figs. 2B&3B). Fig. 3C–E shows the mRNA copy number of several astrocyte signature genes, inflammatory genes, and regulatory/growth genes. The mRNA copy number of astrocyte signature genes in the order of highest to lowest mRNA copy number is represented (Fig. 3C). Independent of age, the five highest expressed genes in astrocytes were Eaat2 (16,000 copies), Glast-1 (3,800 copies), Basigin (Bsg) (3,800 copies), S100B (1,100 copies) and Gfap (1,000 copies). The majority of these astrocyte signature genes were influenced by age. For example, mRNA expression of the two glutamate transport genes, Eaat2 (16,000 to 9,000 copies, p<0.03) and Glast-1 (Eaat1) (3,860 to 2,222 copies, p<0.05) were decreased in aged astrocytes compared to adults. In addition, S100A1 was also decreased in aged astrocytes compared to adults (p<0.02). GFAP (p<0.02) was increased and S100B tended to be increased (p=0.1) in aged astrocytes compared to adults (Fig. 3C). There was no difference in either vimentin (Vim) or aquaporin-4 (Aqp4) mRNA expression (Fig. 3C). Notably, genes associated with microglia signature CD11b (Fig. 2B) and Fcgr3 (Fig. 3C) had limited detection in the FAC-sorted astrocytes. Several myeloid-associated genes including CD86, CD14, Fcgr1, and P2RX7 showed some level of expression in the FAC-sorted astrocytes (Fig. 3C). This is, however, consistent with a previous genomic analysis study which showed that astrocytes express several immune/myeloid cell associated genes (Zamanian, et al., 2012).
Figure 3. FAC-sorted Astrocytes from the Aged Brain have an mRNA Profile Associated with Dysfunction.
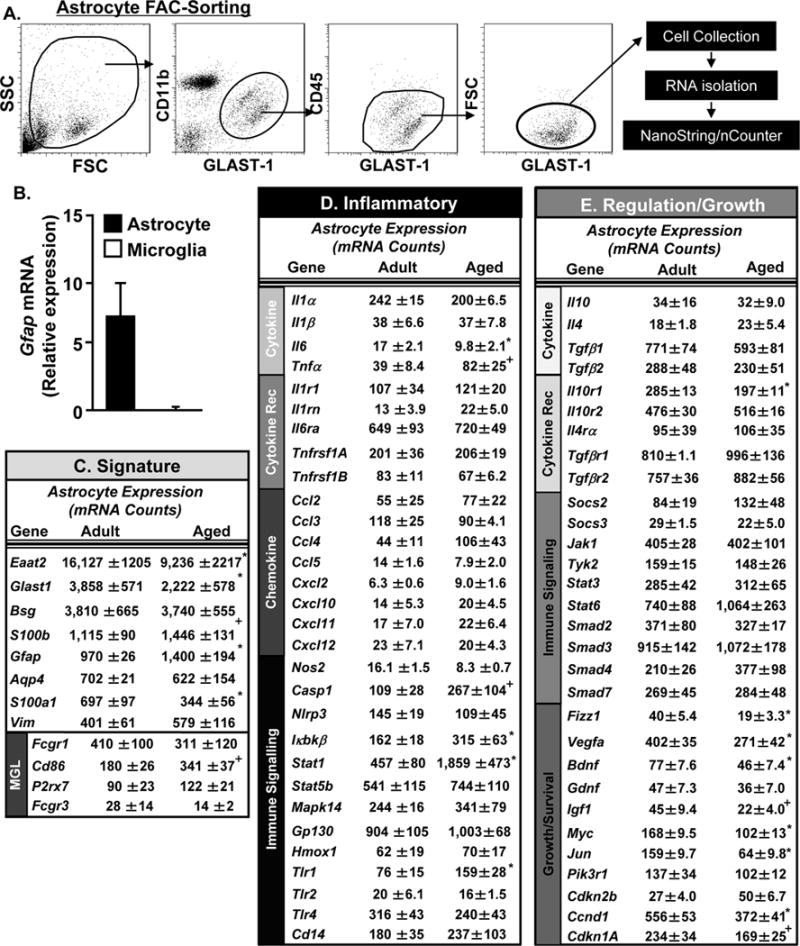
A) Adult (3 mo) and aged (18 mo) BALB/c mice were used and microglia and astrocytes were Percoll-enriched, labelled, FAC-sorted, and mRNA copy number was determined by NanoString analysis (n=4). For astrocytes, representative bivariate dot plots of Side scatter (SSC) and Forward scatter (FSC) and GLAST-1 and CD11b labeling are shown. Astrocytes (GLAST-1+/CD11b+) were FAC-sorted and RNA was collected. B) Relative GFAP mRNA expression was determined in FAC-sorted microglia and astrocytes by qPCR (n=3). Astrocyte mRNA copy numbers of genes associated with C) astrocyte signature, D) inflammatory processes, and E) regulation and growth support were determined. Data are represented by the mean ± SEM. Means with (*) are different from adult controls (p<0.05) and means with (+) tend to be different from adult controls (p=0.06–0.10).
Fig. 3D shows the mRNA copy number of genes associated with inflammation in FAC-sorted astrocytes from the brain of adult and aged mice. These genes are sub-divided into groups of genes associated with cytokines, cytokine receptors, chemokines and immune signaling. Similar to analysis of microglia, the mRNA copy number of cytokines and chemokines were low in both age groups (under 40 copies). Independent of age, astrocytes maintained relatively high expression of IL-6R, GP130, STAT1, and TLR4 (over 400 copies each). A limited number of inflammatory associated genes were increased in the astrocytes of aged mice. For example, there was higher expression of IκBKβ (p<0.05), TLR1 (p<0.03), STAT1 (p<0.02), and tended to be higher expression of Caspase-1 (p=0.1) and TNFα (p=0.1) in astrocytes with age.
Fig. 3E shows the mRNA copy number of genes associated with regulation and growth in FAC-sorted astrocytes from the brains of adult and aged mice. These genes are sub-divided into groups of genes associated with cytokines, cytokine receptors, immune signaling, growth and survival. In terms of regulatory or anti-inflammatory genes at baseline, there was no difference in cytokine expression. Aged astrocytes, however, had differential expression of the IL-10 receptor subunits. There was no difference in IL-10R2 (intracellular domain) expression, but there was a selective reduction in IL-10R1 (ligand binding domain) mRNA in astrocytes from aged mice (p<0.01, Fig. 3E). There was also a reduction in the copy number of several growth/repair associated factors in aged astrocytes compared to adults. For example, there was decreased expression of brain derived neurotrophic factor (BDNF, p<0.04), vascular endothelial growth factor A, (VEGFA, p<0.05), Resistin-like beta (RETNLB or Fizz1, p<0.02) and a tendency for reduced expression of insulin like growth factor-1 (IGF-1, p=0.1) in aged astrocytes compared to adults (Fig. 3E). In addition, other genes associated with growth and survival signaling pathways including Myc (p<0.02), Jun (p<0.01), Cyclin-D1 (CCND1, p<0.05) and Cyclin-Dependent kinase inhibitor 1A (CDKN1A, p=0.1) were reduced in aged astrocytes compared to adults. Overall, aged astrocytes had an mRNA profile associated with reduced neurotrophic support and increased inflammation.
3.4 Decreased Protein Expression of IL-10R1 on Aged Astrocytes
The mRNA copy number analysis revealed that astrocytes from aged mice had a significant reduction in IL-10R1 expression compared to adults. Thus, astrocytes were Percoll enriched from adult and aged mice and IL-10R1 protein expression was determined. Fig. 4A shows representative bivariate dot plots of Percoll enriched astrocytes (GLAST-1/CD11b) from adult and aged mice. There was no difference in the number of GLAST-1+ astrocytes collected by Percoll enrichment between the two age groups (Fig. 4B). There was, however, a reduced number of astrocytes that expressed the IL-10R1 (GLAST-1+/IL-10R1+). For instance, Fig. 4C&D shows a significant reduction in IL-10R1 surface expression in aged astrocytes (16% IL-10R1+) compared to adult astrocytes (23% IL-10R1+, p<0.03). Collectively, these data indicate that there is reduced IL-10R1 mRNA and protein expression in astrocytes of aged mice compared to adults.
Figure 4. Decreased Protein Expression of the IL-10 Receptor-1 on Aged Astrocytes.

Adult (3 mo) and aged (18 mo) BALB/c mice were used and astrocytes were Percoll-enriched and IL-10R1 protein expression was determined (n=8). A) Representative bivariate dot plots of CD11b and GLAST-1 labeling of astrocytes are shown. B) The percentage of astrocytes (GLAST-1/CD11b-) isolated from adult and aged mice. C) Representative bivariate dot plots of IL-10R1 labeling on GLAST-1+ astrocytes. D) The percentage of IL-10R1+ astrocytes in adult and aged mice. Bars represent the mean + SEM. Means with (*) are different from adult controls (p<0.05).
3.5 Exaggerated Neuroinflammation following Systemic Immune Challenge in the Aged
We have previously reported that microglia of the aged brain are hyperactive following immune challenge and produce high levels of both IL-1β and IL-10 (Henry, et al., 2009). In addition, the inflammatory response in aged microglia is prolonged compared to adults (Godbout, et al., 2005, Wynne, et al., 2010). To determine the degree to which anti-inflammatory regulation is altered in the aged brain, adult and aged mice were injected i.p. with LPS and 24 h later several pro-inflammatory (IL-1β and IL-6) and regulatory (IL-10 and TGFβ) cytokines and regulatory cytokine receptors (IL-10R1 and TGFβR1) were determined in a coronal brain section. The 24 h following LPS time point corresponds with transition from active sickness to resolution in adult mice, but it corresponds with prolonged sickness in aged mice (Godbout, et al., 2005, Wynne, et al., 2010).
Similar to previous reports (Godbout, et al., 2005, Richwine, et al., 2008, Wynne, et al., 2010), expression of IL-1β, IL-6 and IL-10 cytokines were higher in the aged brain 24 h after LPS than in the adult brain (p<0.02 for each, Fig. 5A–C). Expression of IL-10R1, was increased in the brain of adult mice 24 after LPS challenge (p<0.002), but was not increased in the brain of aged mice (Fig. 5D). Similar to IL-10R1, TGFβ mRNA expression was increased 24 h following LPS injection in the brain of adult mice, but not in the brain of aged mice (p<0.03, Fig. 5E). Moreover, there was an extended downregulation of TGFβR1 in the brain of aged mice compared to adults (p<0.02, Fig. 5F). These data indicate that inflammation in the aged brain is prolonged following LPS challenge and is associated with reduced mRNA expression of IL-10R1, TGFβ, and TGFβR1.
Figure 5. Prolonged Neuroinflammation following Peripheral Immune Challenge in the Aged.

Adult (3 mo) and aged (18 mo) BALB/c mice were injected i.p with LPS and a 1 mm coronal brain section (through the cortex) was collected 24 h later (n=6). mRNA levels of A) IL-6, B) IL-1β, C) IL-10, D) IL-10R1, E) TGFβ and F) TGFβR1 were determined. Bars represent the mean + SEM. Means with (*) are different from adult controls (p<0.05), means with (#) are different from Adult-LPS (p<0.05).
3.6 FAC-sorted Microglia from Aged Mice have a Hyper-active Profile following Peripheral Immune Challenge
Next, microglia were FAC-sorted 24 h after LPS i.p. injection and mRNA levels were determined for selected genes associated with signature, inflammatory processes, and regulation and growth (Fig. 6A–C). To focus on gene expression changes in microglia induced by LPS, the fold change of adult-LPS and aged-LPS groups relative to microglia from saline injected adults are represented in the graphs (Fig. 6). Microglia signature genes, including CX3CR1, CSF1R, TREM2 and CD200R were all reduced (from baseline) 24 h after LPS. These reductions in mRNA expression, however, were independent of age (Fig. 6A). Fig. 6B shows the expression of genes associated with inflammation in microglia collected 24 h after LPS challenge. There was robustly higher LPS-induced expression of pro-inflammatory cytokines including IL-1β (p<0.02) and TNFα (p<0.05) in microglia of aged mice compared to adults. There was also amplified expression of several chemokines (CCL4 and CCL5) and HMOX1 in aged microglia 24 h after LPS (p<0.05, for each, Fig. 6B). In addition, the expression of several cytokine (IL-1R1, TNFRSF1a, p<0.05) and Toll-like receptors (TLR1, TLR2, p=0.07) was higher in aged microglia than in adult microglia 24 h after LPS (Fig. 6B).
Figure 6. FAC-sorted microglia from aged mice have a hyper-active profile following peripheral immune challenge.
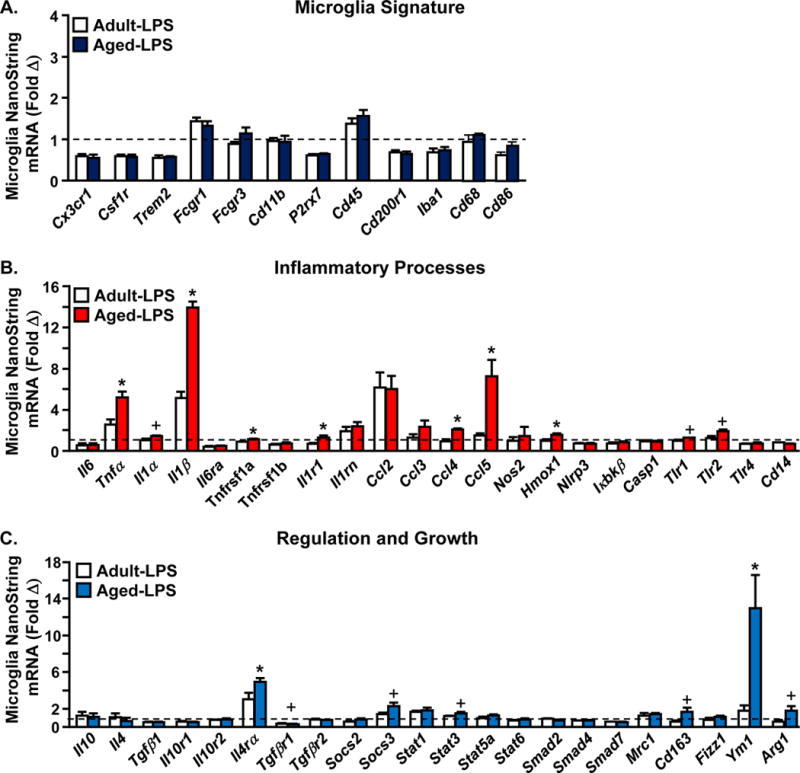
Adult (3mo) and aged (18 mo) BALB/c mice were injected with saline or LPS and microglia were Percoll-enriched, labelled, FAC-sorted, and mRNA copy number was determined by NanoString analysis (n=4). Fold change of mRNA copy number of genes associated with A) signature, B) inflammatory processes, and C) regulation and growth. Dotted line depicts fold change over baseline expression of control mice injected with saline. Data expressed as fold change from adult-saline. Means with (*) are different from adult LPS (p<0.05), means with (+) tend to be different from Adult-LPS (p=0.06–0.10).
Fig. 6C shows the mRNA expression of genes associated with regulation and growth in microglia collected 24 h after LPS challenge. Consistent with our previous study (Fenn, et al., 2012), several anti-inflammatory associated genes were higher in active microglia from the brain of aged mice compared to adults. For example, there was higher expression of IL-4Rα (p<0.02) and YM1 (p<0.02), and there tended to be higher expression of SOCS3 (p=0.09), Arg1 (p=0.1), and CD163 (p=0.07) in aged microglia compared to adult microglia 24 h after LPS (Fig. 6C). In addition, several genes associated with TGFβ signaling (SMAD2, SMAD3, SMAD4), were decreased in microglia 24 h following LPS injection, but these reductions were not further influenced by age. There was also a reduction of TGFβR1 in aged microglia 24 h after LPS compared to adults (tendency, p=0.08). Collectively, LPS injection causes an amplified neuroinflammatory response in microglia of aged mice.
3.7 Impaired mRNA Induction of IL-10R1 and TGFβ in Aged Astrocytes following Peripheral Immune Challenge
Our data indicate that microglial activation is exaggerated after peripheral LPS challenge, but it does not explain the failure to resolve their activation. We hypothesized that astrocytes have a reduced ability to provide negative feedback on microglia of the aged brain. Thus, adult and aged mice were challenged with LPS and mRNA levels of IL-6, IL-1β, IL-10, IL-10R1, and TGFβ were determined in Percoll enriched astrocytes collected 24 h later (Fig. 7B–F). Independent of LPS, IL-6 mRNA expression was not increased in astrocytes of either adult or aged mice 24 h following LPS (Fig. 7B). Fig. 7C shows that IL-1β expression was increased after LPS challenge (p<0.001, Fig. 7C), but this IL-1β induction in astrocytes was not influenced by age. IL-10 mRNA expression was also increased after LPS injection (p<0.02), but there was no effect of age (Fig. 7D). There were, however, age-related differences in the LPS associated induction of IL-10R1 and TGFβ in astrocytes. For instance, IL-10R1 and TGFβ mRNA levels were increased in enriched astrocytes from adult mice 24 h after LPS (p<0.02 for each). There was no induction of either of IL-10R1 and TGFβ in aged astrocytes 24 h after LPS (Figs. 7E&F). Taken together, there was limited induction of IL-10R1 and TGFβ in aged astrocytes following LPS challenge.
3.8 FAC-sorted Astrocytes from Aged mice have a Molecular Signature of Reduced IL-10 Sensitivity following Peripheral Immune Challenge
Our data may indicate a reduction in IL-10 sensitivity of aged astrocytes following induction of an immune response. To explore this further, GLAST-1+ astrocytes were FAC-sorted from adult and aged mice 24 h after LPS challenge. mRNA levels were determined for selected genes associated with astrocyte signature, inflammation, and regulation (IL-10 mediated focus). The fold change in gene expression of astrocytes from adult LPS and aged LPS mice are represented in the graphs (Fig. 8). Fig. 8A shows that several astrocyte signature genes (AQP4, EAAT1, Glast1, S100A1, BSG) were unchanged between adult and aged astrocytes after LPS challenge. S100B and GFAP mRNA levels were increased 24 h after LPS in astrocytes from aged mice compared to adults (p<0.05, Fig. 8A). Vimentin mRNA expression was increased after LPS in both groups, but astrocytes from aged mice had reduced vimentin expression compare to adult astrocytes 24 h after LPS (p<0.03, Fig. 8A).
Figure 8. FAC-sorted astrocytes from aged mice have a molecular signature of reduced IL-10 sensitivity following peripheral immune challenge.

Adult (3 mo) and aged (18 mo) BALB/c mice were injected with saline or LPS and astrocytes were Percoll-enriched, labelled, FAC-sorted, and mRNA copy number was determined by NanoString analysis (n=4). Fold change of mRNA copy number of genes associated with A) signature, B) inflammatory processes, and C) regulation and growth. Dotted line depicts fold change over baseline expression of controls injected with saline. Data expressed as fold change from adult-saline. Means with (*) are different from Adult-LPS (p<0.05) and means with (+) tend to be different from Adult-LPS (p=0.06–0.10).
Fig. 8B shows expression of genes associated with inflammation in astrocytes collected 24 h after LPS challenge. There was a modest maintenance of pro-inflammatory gene expression 24 h after LPS in astrocytes, but the differences as a function of age were limited (Fig. 8B). For example, caspase-1 was the only pro-inflammatory gene that was higher in aged astrocytes than adult astrocytes 24 h after LPS (p<0.04). There was also increased expression of several chemokines after LPS in astrocytes (CCL2, CCL3, CCL4 CCL5, CXCL2 and CXCL10), but none of these had exaggerated expression with age. In fact, chemokines CCL3 and CXCL10 were reduced in aged astrocytes compared to adult astrocytes 24 h after LPS (p<0.04, Fig. 8B).
Fig. 8C shows expression of genes associated with regulation and growth in astrocytes collected 24 h after LPS challenge. Overall, there were several age-related differences in astrocytes 24 h after LPS that corresponded with reduced IL-10 responsiveness. For example IL-10R1 and TGFβ were both increased in FAC-sorted astrocytes from adult mice after LPS but not in aged astrocytes (p<0.05). These data are consistent with the data from enriched astrocytes (Fig. 7). Moreover, several genes also associated with increased IL-10 signaling, including Jak1 (p<0.05), Myc (p=0.1), Tyk2 (p<0.05), PIK3R1 (p<0.02) and CDKN2B (p=0.1), were attenuated in aged astrocytes 24 h after LPS compared to adult astrocytes (Fig. 8C). Mapk14, another IL-10 responsive gene, is negatively regulated by IL-10. Consistent with this, Mapk14 tended to be higher in aged astrocytes compared to adult astrocytes (p=0.1). Thus, despite having higher levels of IL-10 present in the aged brain (Fig. 5C), there was significantly lower mRNA levels of several IL-10 responsive genes in aged astrocytes compared to adult astrocytes 24 h after LPS challenge (Fig. 11D). Taken together, these data indicate that there is decreased IL-10 signaling specifically in aged astrocytes after induction of an immune response.
Figure 11. Astrocytes from IL-10RKO Fail to Upregulate TGFβ after in vivo immune challenge.
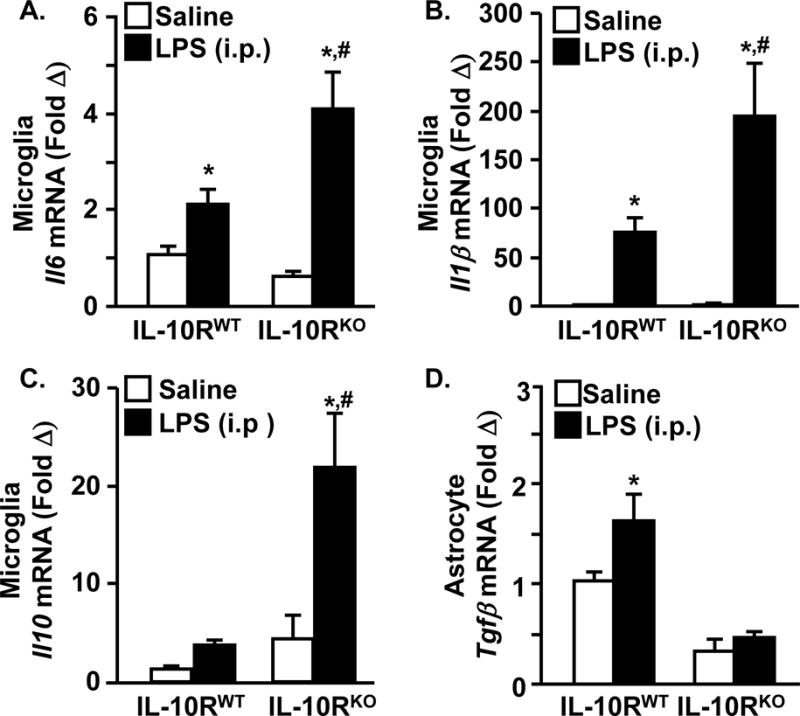
Adult IL-10RWT and IL-10RKO mice were injected i.p with LPS microglia and astrocytes were collected 4 h later. Microglial A) IL-6, B) IL-1β, and C) IL-10 mRNA expression and D) astrocyte TGFβ mRNA expression were determined (n=6). Means with (*) are different from WT-saline (p<0.05) and Means with (#) are different from WT-LPS.
3.9 Astrocytes from Aged Mice Fail to Directly Inhibit Activated Microglia
The data from FAC-sorted astrocytes indicate that aged astrocytes have an altered activation profile associated with diminished responsiveness to IL-10. Related to this, the activation of microglia from aged mice is prolonged after LPS challenge. Based on these findings, ex vivo transwell co-cultures were used to determine the direct effect of aged astrocytes on microglial activation. Adult mice were injected i.p. with LPS and at the apex of microglia cytokine expression (4 h), microglia were isolated and cultured ex vivo. Astrocytes from saline-injected adult and aged mice were added to a removable insert and co-cultured with the microglia (Fig. 9A). As a proof of principle, astrocytes were only used from saline injected adult or aged mice (not from LPS injected mice). This way, the astrocytes (resting) respond to the IL-10 produced by activated microglia. After 4 h in culture, mRNA from microglia and the conditioned media was collected. Fig. 9B shows that microglial cultured ex vivo 4 h after i.p. LPS injection had higher mRNA expression of IL-1β compared to microglia from saline injected mice (2-fold, p<0.05). The presence of adult astrocytes restored IL-1β mRNA expression in adult microglia back to baseline levels. The addition of aged astrocytes, however, had no effect on IL-1β mRNA expression in adult microglia and IL-1β remained significantly higher than controls (p<0.01). In a similar manner, IL-6 expression was higher in adult microglia cultured ex vivo 4 h after i.p. LPS injection (p<0.02, Fig. 9C). The presence of adult astrocytes again restored IL-6 mRNA expression in adult microglia back to baseline. The addition of aged astrocytes, however, had no effect on IL-6 expression in adult microglia and IL-6 remained significantly higher than saline controls (p<0.01, Fig. 9C). IL-6 protein levels were determined in the shared conditioned media from the ex vivo glial cultures. Fig. 9D confirms that the highest amounts of IL-6 protein was present when LPS activated microglia were cultured with aged astrocytes (p<0.001, from all). Thus, aged astrocytes potentiate the inflammatory environment in the ex vivo co-cultures. Taken together, astrocytes from adult mice directly attenuated microglial activation but astrocytes from aged mice were ineffective in resolving microglial activation.
Figure 9. Astrocytes from Aged Mice Fail to Inhibit Activated Microglia ex vivo.

A) Adult (3 mo) BALB/c mice were injected with saline or LPS and 4 h later microglia were Percoll-enriched and plated ex vivo. Astrocytes were Percoll enriched from adult (3 mo) and aged (18 mo) mice injected with saline and were added to the ex vivo glia. Microglia and conditioned media were collected from the ex vivo cultures 4 h later. Microglia mRNA expression of B) IL-1β and C) IL-6 was determined. D) IL-6 protein levels were determined in the conditioned media (n=8). Bars represent the mean + SEM. Means with (*) are different from adult controls (p<0.05).
3.10 Impaired Ability of IL-10RKO Astrocytes to Resolve Microglial Activation
Next, the potential link between impaired IL-10 signaling in aged astrocytes and the corresponding deficit in the resolution of microglial activation was examined using IL-10RKO mice. These are knock outs of IL-10R2, an intracellular component of the IL-10R (Moore, et al., 2001, Spencer, et al., 1998), but the result is the abrogation of IL-10 responses. In the first experiment, primary astrocyte cultures were established from IL-10RWT and IL-10RKO mice. These astrocytes were activated with LPS and then redirected with IL-10 (Fig. 10A). In primary IL-10RWT astrocytes, LPS stimulation increased TGFβ mRNA expression, and this was augmented by IL-10 (p<0.02 from all groups, Fig. 9A). In astrocytes from IL-10RKO mice, LPS increased TGFβ mRNA expression (p<0.001), but there was no augmentation by IL-10.
Figure 10. Impaired Ability of IL-10RKO Astrocytes to Resolve Microglial Activation.

A) Primary astrocytes cultured from IL-10RWT and IL-10RKO mice were activated with LPS, then stimulated with IL-10 and mRNA expression of TGFβ was determined 24 h later (n=12). Means with (*) are different from WT-Con (p<0.05) and Means with (#) are different from WT-LPS. In a separate study, B) microglia were co-cultured in a transwell system with either IL-10RWT or IL-10RKO astrocytes. Glia were activated by LPS and then stimulated with IL-10. IL-1β mRNA expression was determined in the microglia 5 h later (n=10) (*p<0.05). Adult (3 mo) BALB/c mice were injected with saline or LPS and 4 h later microglia were Percoll-enriched and plated ex vivo. Astrocytes were Percoll enriched from adult (3 mo) mice injected with saline and were added to the ex vivo glia. Vehicle or IL-10 neutralization antibody was added. Microglia and conditioned media were collected from the ex vivo cultures 4 h later. C) Microglia mRNA expression of IL-1β was determined. Means with (*) are different from controls (p<0.05).
In the second experiment, transwell glia co-cultures were established where microglia were plated at the bottom of the well and astrocytes from either IL-10RWT or IL-10RKO mice were added to a removable insert. Glia co-cultures were activated by LPS and then stimulated with IL-10. IL-1β mRNA was determined in the microglia collected 4 h after IL-10 stimulation. Fig. 10B shows that there was no effect of IL-10 on IL-1β expression in microglia that were cultured without astrocytes. The inclusion of astrocytes increased IL-1β expression in active microglia in the absence of IL-10. Nonetheless, IL-10 attenuated IL-1β expression in microglia when IL-10RWT astrocytes were present (p<0.004, Fig. 10B). When astrocytes from IL-10RKO mice were present, IL-10 did not attenuate the LPS-induced IL-1β mRNA expression in microglia (Fig. 10B).
In the third experiment, ex vivo transwell co-cultures were used to directly test the notion that the anti-inflammatory effects of astrocytes on microglia are mediated by IL-10 signaling. Adult mice were injected i.p. with LPS and after 4 h activated microglia were isolated and co-cultured ex vivo with astrocytes. Anti-IL-10 neutralizing antibody was added to block IL-10 signaling. Fig. 10C shows that activated microglia cultured ex vivo 4 h had higher mRNA expression of IL-1β compared to microglia from saline injected mice (p<0.05). The addition of adult astrocytes restored IL-1β mRNA expression in adult microglia back to similar levels as controls (p=0.10). Neutralization of IL-10 signaling, however, blocked the astrocyte-mediated reduction of IL-1β mRNA in microglia (p<0.01). Overall, these data confirm that intact IL-10 signaling is required for astrocytes to attenuate microglia activation.
3.11 Astrocytes from IL-10RKO Mice Fail to Upregulate TGFβ after in vivo immune challenge
To further establish that impaired IL-10 signaling in astrocytes leads to decreased TGFβ induction and deficits in microglial regulation, we examined this relationship in vivo using IL-10RKO mice. IL-10RWT and IL-10RKO mice were injected i.p. with LPS and enriched microglia and astrocytes were collected 4 h later. There was exaggerated microglial expression of IL-6, IL-1β, and IL-10 in IL-10RKO mice compared to IL-10RWT mice (p<0.01, Fig. 11A–C). This is consistent with a previous study (Richwine, et al., 2009). In addition, TGFβ mRNA was increased 2-fold in astrocytes from IL-10RWT mice 24 h after LPS (p<0.02 from all groups), but TGFβ mRNA was not induced in IL-10RKO mice (Fig. 11D). Collectively, these in vivo data indicate that functional IL-10 receptor signaling is required for astrocytes to augment TGFβ expression, which, in turn, attenuates microglial induction of IL-1β and IL-6.
4. Discussion
Novel data provided here indicate that astrocytes in the aged brain have a dysfunctional profile with decreased IL-10 receptor-1 expression, and this corresponds with reduced sensitivity to IL-10 redirection after immune challenge. For instance, FAC-sorting after LPS challenge revealed an astrocyte profile with reduced induction of IL-10-mediated genes, including TGFβ1. This failure to produce TGFβ1 after immune challenge was associated with prolonged microglial activation. Furthermore, aged astrocytes failed to directly resolve microglial activation ex vivo. The use of IL-10RKO mice confirmed that IL-10 insensitivity of astrocytes results in a failure to produce TGFβ1 and attenuate microglial activation. These findings provide a mechanism by which IL-10 insensitivity of astrocytes results in reduced TGFβ and extended microglial activation in the aged brain following induction of an immune response (Fig. 12).
Figure 12. Evidence of Impaired Astrocyte-Dependent Inhibition of Active Microglia in the Aged.

Age-related problems with regulation of microglia leads to prolonged neuroinflammation and corresponding neurobehavioral deficits after immune challenge. Following immune challenge, active microglia secrete IL-10 independent of age, but neuroinflammation persists in the aged. Aged astrocytes are more GFAP+ and have significant cellular re-organization. There are reduced levels of IL-10R1 on aged astrocytes and also a failure to increase IL-10R1 expression after immune challenge. Reduced IL-10R1 expression corresponds with a reduction in myriad IL-10 responses genes after immune challenge in aged astrocytes including TGFβ. IL-10 redirected adult astrocytes produce TGFβ, which inhibits microglia activation with reduced pro-inflammatory cytokine expression (IL-1β, IL-6, TNFα) and chemokine expression (CCL5). IL-10-insensitive aged astrocytes, however, do not have increased induction of TGFβ and microglial activation is unresolved.
Consistent with previous observations, we report robust cytoskeletal re-organization of glia from the hippocampus of aged mice (Rodriguez-Arellano, et al., 2015). Enhanced Iba-1 labeling in microglia of aged humans (Streit, et al., 2004) and enhanced Iba-1 and GFAP labeling in aged rodents (Cotrina and Nedergaard, 2002, VanGuilder, et al., 2011) has been reported previously. Here we report that astrocytes and microglia from the hippocampus of aged mice had increased cell body size, perimeter, and length. These increases indicate that aged glia were hypertrophic, swollen, and had longer/thicker processes. Notably these cellular changes in glia were not reflected by increased cell numbers. The degree to which these cytoskeletal re-arrangements represent activation or altered function is unclear (Norden, et al., 2016). Nonetheless, enlargement of the GFAP+ cytoskeleton was detected in models of neurodegeneration and stroke (Cekanaviciute, et al., 2014, Kraft, et al., 2013, Zamanian, et al., 2012). Therefore these structural differences in glia with age are interpreted to indicate an inflammatory environment within the hippocampus.
Previous studies indicate that the inflammatory status of the CNS is increased with age (Godbout, et al., 2005). The processes that lead to increased inflammation in the aged brain are unclear, but recent studies show a contribution of increased NFκB and inflammasome activation within the aged brain (Youm, et al., 2013, Zhang, et al., 2013). Here we used cell-specific enrichment and FAC-sorting approaches to determine mRNA profiles of microglia and astrocytes. Consistent with previous studies, aged microglia had a higher inflammatory cytokine mRNA profile compared to adults (Hickman, et al., 2013, Orre, et al., 2014, Sierra, et al., 2007, Ye and Johnson, 1999). This microglial profile included elevated expression of cytokine (IL-1β, IL-6, TNFα), chemokine (CCL3, CCL5, CXCL10), and immune-related (CD45, CD86, TLR2, Caspase-1, IL-4Rα) genes. At baseline, IL-10 mRNA expression was low in both adult and aged microglia. Notably, recent RNA sequencing data was interpreted to indicate that aged microglia were more neuroprotective because of enhanced expression of host defense genes (Hickman, et al., 2013). We interpret the current and previously published data on morphological/mRNA profiles and immune reactivity of aged microglia (Norden and Godbout, 2013) to indicate a more inflammatory profile.
We extend previous work on the aging brain to show that aged astrocytes have an mRNA profile associated with dysfunction (i.e., less supportive of growth, repair and regulation) (Rodriguez-Arellano, et al., 2015). For example, genes associated with astrocyte reactivity including GFAP and S100b (Cerutti and Chadi, 2000, Pekny and Pekna, 2004, Zamanian, et al., 2012) were increased with age. Also, astrocyte expression of glutamate transporters (EAAT2 and EAAT1) were decreased with age. Reductions in EAAT2 are consistent with inflammation detected after TBI, CNS viral infection, and neurodegeneration (Masliah, et al., 1996, van Landeghem, et al., 2006, Wang, et al., 2003). In fact, TNFα stimulation of astrocytes reduced expression of EAAT2, which was associated with decreased glutamate metabolism and decreased neuronal protection from excitotoxicity (Zou, et al., 2010). Moreover, key growth factors including BDNF, VEGFA, and IGF1 were reduced in aged astrocytes. These findings are in line with a report showing that aged astrocytes were less supportive of neuronal growth and neurogenesis compared to adult astrocytes (Miranda, et al., 2012). Overall, the increased GFAP, decreased glutamate transporters, and reduced neurotrophic factors in aged astrocytes reflect a CNS milieu that is more inflammatory and less supportive of cell growth and survival.
Determination of microglia and astrocyte profiles in concert allows for a better understanding of prolonged neuroinflammation that occurs in aged mice after immune challenge. Several groups have reported that IL-1β, IL-6, TNFα, and IL-10 mRNA expression is exaggerated and prolonged in the brain of aged rodents following peripheral immune challenge (for a review, see (Norden and Godbout, 2013). For example, we have previously shown increased IL-10 protein expression by aged microglia 4 hours after LPS injection (Henry, et al., 2009). Here, neuroinflammation after LPS was prolonged specifically by microglia with higher mRNA expression of IL-1β, TNFα, CCL4 and CCL5 at 24 h after LPS. Although adult astrocytes are responsive to peripheral immune challenge (Norden, et al., 2014, Zamanian, et al., 2012), aged astrocytes did not have an exaggerated pro-inflammatory (IL-1β, TNF) response 24 h after LPS. In fact, aged astrocytes had a reduced response to LPS with decreased CXCL10 and CCL3. Notably, decreased CXCL10 expression in the choroid plexus of aged mice was reported previously and interpreted to reflect increased IFN-1 signaling (Baruch, et al., 2014). Here, the higher STAT1 expression in astrocytes would support this premise of increased IFN-1 signaling in the aged brain. Overall, the prolonged cytokine expression in the aged brain after peripheral LPS challenge is perpetuated by microglia.
Recent work has begun to elucidate microglia-astrocyte interactions during inflammatory conditions (Liu, et al., 2011). Moreover, it is now recognized that astrocytes can have anti-inflammatory effects on microglial activation (Min, et al., 2006, Norden, et al., 2014, Sofroniew, 2015). A significant advance of the current study is that astrocytes were identified as the pivotal cell in the failure to resolve microglial activation in the aged brain following immune challenge. This was linked to IL-10 insensitivity of aged astrocytes. For instance, aged astrocytes had reduced IL-10R1 mRNA and protein at baseline. In addition, induction of IL-10 responsive genes including IL-10R1, TGFβ, and TGFβR1 were reduced 24 h after LPS in the aged brain even though IL-10 was highly expressed (Henry, et al., 2009). FAC-sorting shows that aged astrocytes 24 h after LPS had attenuated mRNA induction of myriad IL-10 responsive genes (Donnelly, et al., 1999, Lin, et al., 2005, Norden, et al., 2014, O’Farrell, et al., 2000), including IL-10R1, SOCS3, Jak1, Tyk2, PI3K, CDKN2B, and TGFβ. When interactions between active microglia and astrocytes were determined ex vivo, astrocytes from adult mice directly and effectively inhibited microglia IL-1β and IL-6 expression. This inhibition was not detected when astrocytes from aged mice were cultured ex vivo with active microglia. In fact, aged astrocytes augmented IL-6 secretion. Together, these data provide compelling evidence of deficient IL-10/IL-10R interactions in aged astrocytes that result in impaired inhibition of active microglia. Notably, there are several mechanisms by which microglia have exaggerated activation following immune challenge. It is clear that some of these are independent of age-related changes in astrocytes. For instance, aged microglia stimulated with LPS ex vivo have exaggerated expression of pro-inflammatory cytokines compared to adult microglia stimulated with LPS (Njie, et al., 2012). In these cultures, there were no astrocytes present. There are two main components that contribute to exaggerated microglial activation in the aged brain: 1) primed microglia have an amplified response to immune challenge and 2) primed microglia are resistant to anti-inflammatory feedback and have a prolonged inflammatory response (Norden and Godbout, 2013). The focus of this current study was on prolonged microglia activation in the aged brain. Here, we present several lines of evidence that this second component is associated with impaired anti-inflammatory feedback by astrocytes.
Another key aspect of this study was the evidence of impairments in a key regulatory cytokine loop of IL-10 (by microglia) and TFGβ (by astrocytes). TGFβ1 is a potent regulator of microglia, and its induction was associated with reduced IL-1β expression in activated microglia and the resolution of sickness behavior (Norden, et al., 2014, Wynne, et al., 2010). Indeed, other studies report decreased TGFβ expression and signaling in the aged brain following LPS injection or TBI (Kumar, et al., 2013, Tichauer, et al., 2014, Wynne, et al., 2010). Here, we provide novel data that astrocytes specifically are responsible for this lack of TGFβ induction. While the mRNA data indicates that aged astrocytes had decreased TGFβ production, a limitation was that corresponding TGFβ protein levels were not detectable. Nonetheless, the functional difference (i.e. prolonged microglia activation and reduced TGFβ mediated gene expression) suggest that TGFβ is reduced. Moreover, the aged astrocytes did not reverse microglia activation when cultured ex vivo 4 h after LPS compared to adult astrocytes, and we surmise these effects to be related to decreased TGFβ production. Related to this, an RNA sequencing study of microglia reported decreased TGFβR1 and TGFβ expression in aged microglia (Hickman, et al., 2013). While we did not detect this with age alone, reduced TGFβ signaling in aged microglia after immune challenge was evident. For instance, activated microglia from aged mice had exaggerated levels pro-inflammatory mediators that are negatively regulated by TGFβ, including IL-1β, TNF, CCL4, and CCL5 (Hu, et al., 1999, Norden, et al., 2014, Paglinawan, et al., 2003). In addition, microglia had decreased expression of TGFβR1 following LPS, and this downregulation was prolonged in aged microglia. This finding is consistent with reduced bioavailability of TGFβ because the presence of TGFβ augments TGFβR1 expression on microglia (Mitchell, et al., 2014). Importantly, aged microglia activated with LPS in vivo and treated ex vivo with TGFβ had decreased IL-1β expression after TGFβ treatment (data not shown). Therefore, we surmise that aged microglia are responsive to TGFβ if it were to be provided by the astrocytes. We expected that reduced TGFβ would result in less Smad related induction (SMAD2, 3, 4, 7) in microglia, but Smads are primarily controlled by post-translation modifications (Xu, et al., 2012) so mRNA changes may not be the best indicators of activation or inhibition. Collectively, decreased TGFβ induction by astrocytes in the aged brain corresponded with less TGFβ feedback on microglia.
The mechanism of impaired IL-10 signaling and decreased TGFβ induction by aged astrocytes after LPS challenge was confirmed using IL-10RKO mice. For instance, astrocytes from IL-10RKO mice failed to augment TGFβ. In vivo, this lack of IL-10 signaling was associated with higher microglial activation. This finding is consistent with a previous study where IL-10RKO mice had prolonged neuroinflammation and sickness behavior after LPS challenge (Richwine, et al., 2009). In addition, IL-10 attenuated microglial activation in co-cultures when IL-10RWT astrocytes were present but had no effect on microglia when IL-10RKO astrocytes were not present. In ex vivo cultures, the anti-inflammatory effect of astrocytes on microglia was blocked when IL-10 signaling was neutralized. Therefore, IL-10 redirected astrocytes provide negative feedback on activated microglia.
In summary, original and compelling evidence is provided that a key regulatory cytokine loop between microglia and astrocytes is impaired in the aged brain following a systemic immune challenge. There were age-related alterations in morphology and mRNA profiles consistent with inflammatory microglia and dysfunctional astrocytes. When microglia were activated following induction of an immune response, there was a failure to resolve this activation in a timely fashion. Critical to this was that aged astrocytes had reduced IL-10R1 expression, were insensitive to IL-10, and failed to resolve the activation of microglia in a TGFβ-dependent manner. Collectively, astrocytes were identified as pivotal cells in the failure to resolve microglial activation in the aged brain following immune challenge.
Acknowledgments
This research was supported by an NIA grant (R01-AG-033028) to J.P.G. D.M.N. was supported by the OSU Presidential Fellowship. We thank Dr. John Sheridan for the use of the FACSCaliber Cytometer and Dr. Kiecolt-Glaser for the use of the AB PRISM 7300-sequence detection system. We also thank the OSU Flow Cytometry core facility in the comprehensive cancer center for assistance with the cell sorting. Special thanks to Paolo Fadda at the OSU Nucleic Acid core facility for assistance with the NanoString and nCounter analysis.
Footnotes
Disclosure statement: The authors declare no competing financial interests.
References
- Ahmed S, Leurent B, Sampson EL. Risk factors for incident delirium among older people in acute hospital medical units: a systematic review and meta-analysis. Age and ageing. 2014;43(3):326–33. doi: 10.1093/ageing/afu022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Frank MG, Watkins LR, Maier SF. Memory impairments in healthy aging: Role of aging-induced microglial sensitization. Aging and disease. 2010;1(3):212–31. [PMC free article] [PubMed] [Google Scholar]
- Barrientos RM, Watkins LR, Rudy JW, Maier SF. Characterization of the sickness response in young and aging rats following E. coli infection. Brain, behavior, and immunity. 2009;23(4):450–4. doi: 10.1016/j.bbi.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baruch K, Deczkowska A, David E, Castellano JM, Miller O, Kertser A, Berkutzki T, Barnett-Itzhaki Z, Bezalel D, Wyss-Coray T, Amit I, Schwartz M. Aging. Aging-induced type I interferon response at the choroid plexus negatively affects brain function. Science. 2014;346(6205):89–93. doi: 10.1126/science.1252945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg BM, Godbout JP, Kelley KW, Johnson RW. Alpha-tocopherol attenuates lipopolysaccharide-induced sickness behavior in mice. Brain, behavior, and immunity. 2004;18(2):149–57. doi: 10.1016/S0889-1591(03)00113-2. [DOI] [PubMed] [Google Scholar]
- Bethea JR, Nagashima H, Acosta MC, Briceno C, Gomez F, Marcillo AE, Loor K, Green J, Dietrich WD. Systemically administered interleukin-10 reduces tumor necrosis factor-alpha production and significantly improves functional recovery following traumatic spinal cord injury in rats. Journal of neurotrauma. 1999;16(10):851–63. doi: 10.1089/neu.1999.16.851. [DOI] [PubMed] [Google Scholar]
- Bluthe RM, Castanon N, Pousset F, Bristow A, Ball C, Lestage J, Michaud B, Kelley KW, Dantzer R. Central injection of IL-10 antagonizes the behavioural effects of lipopolysaccharide in rats. Psychoneuroendocrinology. 1999;24(3):301–11. doi: 10.1016/s0306-4530(98)00077-8. [DOI] [PubMed] [Google Scholar]
- Butovsky O, Jedrychowski MP, Moore CS, Cialic R, Lanser AJ, Gabriely G, Koeglsperger T, Dake B, Wu PM, Doykan CE, Fanek Z, Liu L, Chen Z, Rothstein JD, Ransohoff RM, Gygi SP, Antel JP, Weiner HL. Identification of a unique TGF-beta-dependent molecular and functional signature in microglia. Nature neuroscience. 2014;17(1):131–43. doi: 10.1038/nn.3599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao S, Zhang X, Edwards JP, Mosser DM. NF-kappaB1 (p50) homodimers differentially regulate pro- and anti-inflammatory cytokines in macrophages. The Journal of biological chemistry. 2006;281(36):26041–50. doi: 10.1074/jbc.M602222200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cekanaviciute E, Fathali N, Doyle KP, Williams AM, Han J, Buckwalter MS. Astrocytic transforming growth factor-beta signaling reduces subacute neuroinflammation after stroke in mice. Glia. 2014;62(8):1227–40. doi: 10.1002/glia.22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerutti SM, Chadi G. S100 immunoreactivity is increased in reactive astrocytes of the visual pathways following a mechanical lesion of the rat occipital cortex. Cell biology international. 2000;24(1):35–49. doi: 10.1006/cbir.1999.0451. [DOI] [PubMed] [Google Scholar]
- Chen J, Buchanan JB, Sparkman NL, Godbout JP, Freund GG, Johnson RW. Neuroinflammation and disruption in working memory in aged mice after acute stimulation of the peripheral innate immune system. Brain, behavior, and immunity. 2008;22(3):301–11. doi: 10.1016/j.bbi.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corona AW, Fenn AM, Godbout JP. Cognitive and behavioral consequences of impaired immunoregulation in aging. Journal of neuroimmune pharmacology : the official journal of the Society on NeuroImmune Pharmacology. 2012;7(1):7–23. doi: 10.1007/s11481-011-9313-4. [DOI] [PubMed] [Google Scholar]
- Cotrina ML, Nedergaard M. Astrocytes in the aging brain. Journal of neuroscience research. 2002;67(1):1–10. doi: 10.1002/jnr.10121. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Hutchins B, LaFace DM, Stohlman SA, Coffman RL. Central nervous system expression of IL-10 inhibits autoimmune encephalomyelitis. J Immunol. 2001;166(1):602–8. doi: 10.4049/jimmunol.166.1.602. [DOI] [PubMed] [Google Scholar]
- Dantzer R. Cytokine-induced sickness behavior: mechanisms and implications. Annals of the New York Academy of Sciences. 2001;933:222–34. doi: 10.1111/j.1749-6632.2001.tb05827.x. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’;Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nature reviews Neuroscience. 2008;9(1):46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly RP, Dickensheets H, Finbloom DS. The interleukin-10 signal transduction pathway and regulation of gene expression in mononuclear phagocytes. Journal of interferon & cytokine research : the official journal of the International Society for Interferon and Cytokine Research. 1999;19(6):563–73. doi: 10.1089/107999099313695. [DOI] [PubMed] [Google Scholar]
- Dunn N, Mullee M, Perry VH, Holmes C. Association between dementia and infectious disease: evidence from a case-control study. Alzheimer Dis Assoc Disord. 2005;19(2):91–4. doi: 10.1097/01.wad.0000165511.52746.1f. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Gensel JC, Huang Y, Popovich PG, Lifshitz J, Godbout JP. Immune activation promotes depression 1 month after diffuse brain injury: a role for primed microglia. Biol Psychiatry. 2014;76(7):575–84. doi: 10.1016/j.biopsych.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenn AM, Henry CJ, Huang Y, Dugan A, Godbout JP. Lipopolysaccharide-induced interleukin (IL)-4 receptor-alpha expression and corresponding sensitivity to the M2 promoting effects of IL-4 are impaired in microglia of aged mice. Brain, behavior, and immunity. 2012;26(5):766–77. doi: 10.1016/j.bbi.2011.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frenkel D, Huang Z, Maron R, Koldzic DN, Moskowitz MA, Weiner HL. Neuroprotection by IL-10-producing MOG CD4+ T cells following ischemic stroke. Journal of the neurological sciences. 2005;233(1–2):125–32. doi: 10.1016/j.jns.2005.03.022. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Berg BM, Kelley KW, Johnson RW. alpha-Tocopherol reduces lipopolysaccharide-induced peroxide radical formation and interleukin-6 secretion in primary murine microglia and in brain. Journal of neuroimmunology. 2004;149(1–2):101–9. doi: 10.1016/j.jneuroim.2003.12.017. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Chen J, Abraham J, Richwine AF, Berg BM, Kelley KW, Johnson RW. Exaggerated neuroinflammation and sickness behavior in aged mice following activation of the peripheral innate immune system. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2005;19(10):1329–31. doi: 10.1096/fj.05-3776fje. [DOI] [PubMed] [Google Scholar]
- Godbout JP, Moreau M, Lestage J, Chen J, Sparkman NL, J OC, Castanon N, Kelley KW, Dantzer R, Johnson RW. Aging exacerbates depressive-like behavior in mice in response to activation of the peripheral innate immune system. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33(10):2341–51. doi: 10.1038/sj.npp.1301649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry CJ, Huang Y, Wynne AM, Godbout JP. Peripheral lipopolysaccharide (LPS) challenge promotes microglial hyperactivity in aged mice that is associated with exaggerated induction of both pro-inflammatory IL-1beta and anti-inflammatory IL-10 cytokines. Brain, behavior, and immunity. 2009;23(3):309–17. doi: 10.1016/j.bbi.2008.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrera-Molina R, von Bernhardi R. Transforming growth factor-beta 1 produced by hippocampal cells modulates microglial reactivity in culture. Neurobiology of disease. 2005;19(1–2):229–36. doi: 10.1016/j.nbd.2005.01.003. [DOI] [PubMed] [Google Scholar]
- Hickman SE, Kingery ND, Ohsumi TK, Borowsky ML, Wang LC, Means TK, El Khoury J. The microglial sensome revealed by direct RNA sequencing. Nature neuroscience. 2013;16(12):1896–905. doi: 10.1038/nn.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Chao CC, Ehrlich LC, Sheng WS, Sutton RL, Rockswold GL, Peterson PK. Inhibition of microglial cell RANTES production by IL-10 and TGF-beta. Journal of leukocyte biology. 1999;65(6):815–21. doi: 10.1002/jlb.65.6.815. [DOI] [PubMed] [Google Scholar]
- Huang Y, Henry CJ, Dantzer R, Johnson RW, Godbout JP. Exaggerated sickness behavior and brain proinflammatory cytokine expression in aged mice in response to intracerebroventricular lipopolysaccharide. Neurobiology of aging. 2008;29(11):1744–53. doi: 10.1016/j.neurobiolaging.2007.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishii H, Tanabe S, Ueno M, Kubo T, Kayama H, Serada S, Fujimoto M, Takeda K, Naka T, Yamashita T. ifn-gamma-dependent secretion of IL-10 from Th1 cells and microglia/macrophages contributes to functional recovery after spinal cord injury. Cell death & disease. 2013;4:e710. doi: 10.1038/cddis.2013.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwashyna TJ, Ely EW, Smith DM, Langa KM. Long-term cognitive impairment and functional disability among survivors of severe sepsis. Jama. 2010;304(16):1787–94. doi: 10.1001/jama.2010.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson JC, Gordon SM, Hart RP, Hopkins RO, Ely EW. The association between delirium and cognitive decline: a review of the empirical literature. Neuropsychology review. 2004;14(2):87–98. doi: 10.1023/b:nerv.0000028080.39602.17. [DOI] [PubMed] [Google Scholar]
- Johnson SJ, Walker FR. Strategies to improve quantitative assessment of immunohistochemical and immunofluorescence labelling. Scientific Reports. 2015 doi: 10.1038/srep10607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurgens HA, Johnson RW. Dysregulated neuronal-microglial cross-talk during aging, stress and inflammation. Experimental neurology. 2010 doi: 10.1016/j.expneurol.2010.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley KW, Bluthe RM, Dantzer R, Zhou JH, Shen WH, Johnson RW, Broussard SR. Cytokine-induced sickness behavior. Brain, behavior, and immunity. 2003;17(1 Suppl):112–8. doi: 10.1016/s0889-1591(02)00077-6. [DOI] [PubMed] [Google Scholar]
- Koenig HG, Meador KG, Cohen HJ, Blazer DG. Depression in elderly hospitalized patients with medical illness. Archives of internal medicine. 1988;148(9):1929–36. [PubMed] [Google Scholar]
- Kongsui R, Beynon SB, Johnson SJ, Mayhew J, Kuter P, Nilsson M, Walker FR. Chronic stress induces prolonged suppression of the P2X7 receptor within multiple regions of the hippocampus: a cumulative threshold spectra analysis. Brain, behavior, and immunity. 2014a;42:69–80. doi: 10.1016/j.bbi.2014.05.017. [DOI] [PubMed] [Google Scholar]
- Kongsui R, Beynon SB, Johnson SJ, Walker FR. Quantitative assessment of microglial morphology and density reveals remarkable consistency in the distribution and morphology of cells within the healthy prefrontal cortex of the rat. Journal of neuroinflammation. 2014b;11:182. doi: 10.1186/s12974-014-0182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraft AW, Hu X, Yoon H, Yan P, Xiao Q, Wang Y, Gil SC, Brown J, Wilhelmsson U, Restivo JL, Cirrito JR, Holtzman DM, Kim J, Pekny M, Lee JM. Attenuating astrocyte activation accelerates plaque pathogenesis in APP/PS1 mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(1):187–98. doi: 10.1096/fj.12-208660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Stoica BA, Sabirzhanov B, Burns MP, Faden AI, Loane DJ. Traumatic brain injury in aged animals increases lesion size and chronically alters microglial/macrophage classical and alternative activation states. Neurobiology of aging. 2013;34(5):1397–411. doi: 10.1016/j.neurobiolaging.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Q, Lai R, Chirieac LR, Li C, Thomazy VA, Grammatikakis I, Rassidakis GZ, Zhang W, Fujio Y, Kunisada K, Hamilton SR, Amin HM. Constitutive activation of JAK3/STAT3 in colon carcinoma tumors and cell lines: inhibition of JAK3/STAT3 signaling induces apoptosis and cell cycle arrest of colon carcinoma cells. The American journal of pathology. 2005;167(4):969–80. doi: 10.1016/S0002-9440(10)61187-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu W, Tang Y, Feng J. Cross talk between activation of microglia and astrocytes in pathological conditions in the central nervous system. Life sciences. 2011;89(5–6):141–6. doi: 10.1016/j.lfs.2011.05.011. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10–a role for IL-1 beta? Journal of neurochemistry. 2004;88(3):635–46. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- Masliah E, Alford M, DeTeresa R, Mallory M, Hansen L. Deficient glutamate transport is associated with neurodegeneration in Alzheimer’s disease. Annals of neurology. 1996;40(5):759–66. doi: 10.1002/ana.410400512. [DOI] [PubMed] [Google Scholar]
- Min KJ, Yang MS, Kim SU, Jou I, Joe EH. Astrocytes induce hemeoxygenase-1 expression in microglia: a feasible mechanism for preventing excessive brain inflammation. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26(6):1880–7. doi: 10.1523/JNEUROSCI.3696-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miranda CJ, Braun L, Jiang Y, Hester ME, Zhang L, Riolo M, Wang H, Rao M, Altura RA, Kaspar BK. Aging brain microenvironment decreases hippocampal neurogenesis through Wnt-mediated survivin signaling. Aging cell. 2012;11(3):542–52. doi: 10.1111/j.1474-9726.2012.00816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell K, Shah JP, Tsytsikova LV, Campbell AM, Affram K, Symes AJ. LPS antagonism of TGF-beta signaling results in prolonged survival and activation of rat primary microglia. Journal of neurochemistry. 2014;129(1):155–68. doi: 10.1111/jnc.12612. [DOI] [PubMed] [Google Scholar]
- Moore KW, de Waal Malefyt R, Coffman RL, O’Garra A. Interleukin-10 and the interleukin-10 receptor. Annual review of immunology. 2001;19:683–765. doi: 10.1146/annurev.immunol.19.1.683. [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Julien JP, Rivest S. Innate immunity: the missing link in neuroprotection and neurodegeneration? Nature reviews Neuroscience. 2002;3(3):216–27. doi: 10.1038/nrn752. [DOI] [PubMed] [Google Scholar]
- Njie EG, Boelen E, Stassen FR, Steinbusch HW, Borchelt DR, Streit WJ. Ex vivo cultures of microglia from young and aged rodent brain reveal age-related changes in microglial function. Neurobiology of aging. 2012;33(1):195 e1–12. doi: 10.1016/j.neurobiolaging.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Fenn AM, Dugan A, Godbout JP. TGFbeta produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62(6):881–95. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Godbout JP. Review: microglia of the aged brain: primed to be activated and resistant to regulation. Neuropathology and applied neurobiology. 2013;39(1):19–34. doi: 10.1111/j.1365-2990.2012.01306.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norden DM, Trojanowski PJ, Villanueva E, Navarro E, Godbout JP. Sequential activation of microglia and astrocyte cytokine expression precedes increased iba-1 or GFAP immunoreactivity following systemic immune challenge. Glia. 2016;64(2):300–16. doi: 10.1002/glia.22930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Farrell AM, Parry DA, Zindy F, Roussel MF, Lees E, Moore KW, Mui AL. Stat3-dependent induction of p19INK4D by IL-10 contributes to inhibition of macrophage proliferation. J Immunol. 2000;164(9):4607–15. doi: 10.4049/jimmunol.164.9.4607. [DOI] [PubMed] [Google Scholar]
- Orre M, Kamphuis W, Osborn LM, Melief J, Kooijman L, Huitinga I, Klooster J, Bossers K, Hol EM. Acute isolation and transcriptome characterization of cortical astrocytes and microglia from young and aged mice. Neurobiology of aging. 2014;35(1):1–14. doi: 10.1016/j.neurobiolaging.2013.07.008. [DOI] [PubMed] [Google Scholar]
- Paglinawan R, Malipiero U, Schlapbach R, Frei K, Reith W, Fontana A. TGFbeta directs gene expression of activated microglia to an anti-inflammatory phenotype strongly focusing on chemokine genes and cell migratory genes. Glia. 2003;44(3):219–31. doi: 10.1002/glia.10286. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Franklin K. The mouse brain in stereotaxic coordinates. 2nd 2004. [Google Scholar]
- Pekny M, Pekna M. Astrocyte intermediate filaments in CNS pathologies and regeneration. J Pathol. 2004;204(4):428–37. doi: 10.1002/path.1645. [DOI] [PubMed] [Google Scholar]
- Penninx BW, Geerlings SW, Deeg DJ, van Eijk JT, van Tilburg W, Beekman AT. Minor and major depression and the risk of death in older persons. Archives of general psychiatry. 1999;56(10):889–95. doi: 10.1001/archpsyc.56.10.889. [DOI] [PubMed] [Google Scholar]
- Phulwani NK, Esen N, Syed MM, Kielian T. TLR2 expression in astrocytes is induced by TNF-alpha- and NF-kappa B-dependent pathways. J Immunol. 2008;181(6):3841–9. doi: 10.4049/jimmunol.181.6.3841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Wei SJ, Zhang D, Hu X, Xu Z, Wilson B, El-Benna J, Hong JS, Flood PM. Potent anti-inflammatory and neuroprotective effects of TGF-beta1 are mediated through the inhibition of ERK and p47phox-Ser345 phosphorylation and translocation in microglia. J Immunol. 2008;181(1):660–8. doi: 10.4049/jimmunol.181.1.660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radler ME, Wright BJ, Walker FR, Hale MW, Kent S. Calorie restriction increases lipopolysaccharide-induced neuropeptide Y immunolabeling and reduces microglial cell area in the arcuate hypothalamic nucleus. Neuroscience. 2015;285:236–47. doi: 10.1016/j.neuroscience.2014.11.014. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Parkin AO, Buchanan JB, Chen J, Markham JA, Juraska JM, Johnson RW. Architectural changes to CA1 pyramidal neurons in adult and aged mice after peripheral immune stimulation. Psychoneuroendocrinology. 2008;33(10):1369–77. doi: 10.1016/j.psyneuen.2008.08.003. [DOI] [PubMed] [Google Scholar]
- Richwine AF, Sparkman NL, Dilger RN, Buchanan JB, Johnson RW. Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide. Brain, behavior, and immunity. 2009;23(6):794–802. doi: 10.1016/j.bbi.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rockwood K, Cosway S, Carver D, Jarrett P, Stadnyk K, Fisk J. The risk of dementia and death after delerium. Age and ageing. 1999;28:551–6. doi: 10.1093/ageing/28.6.551. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Arellano JJ, Parpura V, Zorec R, Verkhratsky A. Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience. 2015 doi: 10.1016/j.neuroscience.2015.01.007. [DOI] [PubMed] [Google Scholar]
- Sawicki CM, McKim D, Wohleb ES, Jarrett BL, Norden DM, Godbout JP, Sheridan JF. Social defeat promotes a reactive endothelium in a brain region-dependent manner with increased expression of key adhesion molecules, selectins and chemokines associated with the recruitment of myeloid cells to the brain. Journal of Neuroscience. 2014 doi: 10.1016/j.neuroscience.2014.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sierra A, Gottfried-Blackmore AC, McEwen BS, Bulloch K. Microglia derived from aging mice exhibit an altered inflammatory profile. Glia. 2007;55(4):412–24. doi: 10.1002/glia.20468. [DOI] [PubMed] [Google Scholar]
- Sofroniew MV. Astrocyte barriers to neurotoxic inflammation. Nature reviews Neuroscience. 2015;16(5):249–63. doi: 10.1038/nrn3898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SD, Di Marco F, Hooley J, Pitts-Meek S, Bauer M, Ryan AM, Sordat B, Gibbs VC, Aguet M. The orphan receptor CRF2-4 is an essential subunit of the interleukin 10 receptor. The Journal of experimental medicine. 1998;187(4):571–8. doi: 10.1084/jem.187.4.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streit WJ, Sammons NW, Kuhns AJ, Sparks DL. Dystrophic microglia in the aging human brain. Glia. 2004;45(2):208–12. doi: 10.1002/glia.10319. [DOI] [PubMed] [Google Scholar]
- Tichauer JE, Flores B, Soler B, Eugenin-von Bernhardi L, Ramirez G, von Bernhardi R. Age-dependent changes on TGFbeta1 Smad3 pathway modify the pattern of microglial cell activation. Brain, behavior, and immunity. 2014;37:187–96. doi: 10.1016/j.bbi.2013.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Landeghem FK, Weiss T, Oehmichen M, von Deimling A. Decreased expression of glutamate transporters in astrocytes after human traumatic brain injury. Journal of neurotrauma. 2006;23(10):1518–28. doi: 10.1089/neu.2006.23.1518. [DOI] [PubMed] [Google Scholar]
- VanGuilder HD, Bixler GV, Brucklacher RM, Farley JA, Yan H, Warrington JP, Sonntag WE, Freeman WM. Concurrent hippocampal induction of MHC II pathway components and glial activation with advanced aging is not correlated with cognitive impairment. Journal of neuroinflammation. 2011;8:138. doi: 10.1186/1742-2094-8-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker FR, Beynon SB, Jones KA, Zhao Z, Kongsui R, Cairns M, Nilsson M. Dynamic structural remodelling of microglia in health and disease: a review of the models, the signals and the mechanisms. Brain, behavior, and immunity. 2014;37:1–14. doi: 10.1016/j.bbi.2013.12.010. [DOI] [PubMed] [Google Scholar]
- Wang Z, Pekarskaya O, Bencheikh M, Chao W, Gelbard HA, Ghorpade A, Rothstein JD, Volsky DJ. Reduced expression of glutamate transporter EAAT2 and impaired glutamate transport in human primary astrocytes exposed to HIV-1 or gp120. Virology. 2003;312(1):60–73. doi: 10.1016/s0042-6822(03)00181-8. [DOI] [PubMed] [Google Scholar]
- Wohleb ES, Fenn AM, Pacenta AM, Powell ND, Sheridan JF, Godbout JP. Peripheral innate immune challenge exaggerated microglia activation, increased the number of inflammatory CNS macrophages, and prolonged social withdrawal in socially defeated mice. Psychoneuroendocrinology. 2012;37(9):1491–505. doi: 10.1016/j.psyneuen.2012.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynne AM, Henry CJ, Huang Y, Cleland A, Godbout JP. Protracted downregulation of CX(3)CR1 on microglia of aged mice after lipopolysaccharide challenge. Brain, behavior, and immunity. 2010;24(7):1190–201. doi: 10.1016/j.bbi.2010.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu P, Liu J, Derynck R. Post-translational regulation of TGF-beta receptor and Smad signaling. FEBS letters. 2012;586(14):1871–84. doi: 10.1016/j.febslet.2012.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye SM, Johnson RW. Increased interleukin-6 expression by microglia from brain of aged mice. Journal of neuroimmunology. 1999;93(1–2):139–48. doi: 10.1016/s0165-5728(98)00217-3. [DOI] [PubMed] [Google Scholar]
- Youm YH, Grant RW, McCabe LR, Albarado DC, Nguyen KY, Ravussin A, Pistell P, Newman S, Carter R, Laque A, Munzberg H, Rosen CJ, Ingram DK, Salbaum JM, Dixit VD. Canonical Nlrp3 inflammasome links systemic low-grade inflammation to functional decline in aging. Cell metabolism. 2013;18(4):519–32. doi: 10.1016/j.cmet.2013.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamanian JL, Xu L, Foo LC, Nouri N, Zhou L, Giffard RG, Barres BA. Genomic analysis of reactive astrogliosis. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32(18):6391–410. doi: 10.1523/JNEUROSCI.6221-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G, Li J, Purkayastha S, Tang Y, Zhang H, Yin Y, Li B, Liu G, Cai D. Hypothalamic programming of systemic ageing involving IKK-beta, NF-kappaB and GnRH. Nature. 2013;497(7448):211–6. doi: 10.1038/nature12143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zou J, Wang YX, Dou FF, Lu HZ, Ma ZW, Lu PH, Xu XM. Glutamine synthetase down-regulation reduces astrocyte protection against glutamate excitotoxicity to neurons. Neurochemistry international. 2010;56(4):577–84. doi: 10.1016/j.neuint.2009.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]


