Abstract
Our aim was to characterize effectiveness and complications in children receiving oral midazolam alone, nasal midazolam alone, or oral midazolam with other sedatives. Children received oral midazolam alone, nasal midazolam, or oral midazolam in combination with other sedative medications. All subjects received a presedation history and physical examination and were sedated per protocol by any of 28 resident providers under attending supervision. Sedations were rated for success and complications by clinicians. Postoperative complications were assessed by trained staff up to 48 hours postoperatively. Seven hundred and one encounters, completed over 24 months, yielded 650 usable sedations. The majority of children were healthy (469; 68.2%) and 86% (532) weighed between 10 and 25 kg. Sedations were deemed successful in about 80% of cases. Planned treatment was completed in over 85% of encounters. Oral midazolam alone yielded the best behavior. Physical assessment factors of behavior and age were correlated (P = .035) with effectiveness. Hiccups and a positive medical history were significantly related (P = .049). Side effects of either nausea/vomiting, dysphoria, or hiccups occurred in less than 10% of cases. All 3 regimens were effective with minimal postoperative complications.
Key Words: Sedation, Midazolam, Morbidity, Complications
Dental caries is the most common chronic disease of childhood. By kindergarten, 40% of children are affected by early childhood caries.1 A portion of these young patients need pharmacologic intervention to complete dental treatment.2 When a patient has behavior problems that can't be resolved solely using communicative techniques, and general anesthesia can be avoided, conscious sedation may be beneficial. The decision to use sedation for treatment of early childhood caries includes consideration of the amount and difficulty of dental treatment, cost, parental preference, patient medical history, patient behavior, and psychological needs.
Sedation is an advanced pharmacologic behavior guidance technique, according to the American Academy of Pediatric Dentistry, aimed at providing safe and effective dental treatment.3 Oral sedation is the common route of administration of sedative agents by pediatric dentists and will be the method of sedation discussed in this paper. Sedation offers many advantages; however, one disadvantage is that its effectiveness varies because medications don't always achieve the desired degree of sedation, resulting in the patient still not being able to cooperate. Safety is another important consideration, because sedation is a continuum and a practitioner must be prepared to rescue a patient who falls into a level of sedation deeper than intended and experiences complications. Sedation is replacing more advanced procedures but may be decreasing in use overall.3
ORAL SEDATIVE AGENTS IN PEDIATRIC DENTISTRY
The ideal sedative agent or combination of agents reduces anxiety and mitigates uncooperative behavior while offering a wide margin of safety.4 Benzodiazepines have become a favored class of drugs for oral pediatric dental sedation because of relative safety at therapeutic doses.5 Benzodiazepines have the favorable effects of being amnestic, hypnotic, sedative, and anticonvulsant. Importantly, benzodiazepines can be reversed with flumazenil. However, as intravenous access is not generally provided by pediatric dentists, off-label intramuscular injection would be needed, and the proper dosage and time to reversal is not yet clear. Midazolam has an onset time of only 10–15 minutes, making it a desired agent in pediatric dentistry. Some of the best studies on effectiveness of sedation regimens involve midazolam. A 2014 study comparing midazolam dosages of 0.5 and 0.75 mg/kg concluded that 0.75 mg/kg enhanced sedation, cooperation, and parent satisfaction for patients whose cooperation could not be achieved with a 0.5 mg/kg dose.6 A 2006 study in which children were given a dose of 0.5 mg/kg oral midazolam had significantly lower heart rate and systolic blood pressure, and showed significantly more compliance during dental treatment and better amnesia after dental treatment compared to other midazolam regimens.7 A 2012 Cochrane review4 of dental sedation found that all placebo-controlled trials of midazolam reviewed reported significant levels of behavior improvement as compared to placebo.
Some pediatric patients are more anxious, more fearful, or less cooperative, or require a longer working time for dental sedation because of the amount of treatment to be completed. For these patients, agents such as nitrous oxide or midazolam may be inadequate.5 In these cases, a combination of sedation medications can help the clinician complete treatment, but brings increased risk of adverse events such as respiratory depression.8 Antihistamines are a popular class of sedative, generally used in combination with other sedatives or opioids, with desired effects of sedation, hypnosis, and nausea prevention, while having the advantage of not causing unconsciousness, respiratory depression, or cardiac depression like other sedation medications.9 Hydroxyzine and promethazine are the most commonly used agents in pediatric dental practice. When used in combination with other drugs such as meperidine, the antiemetic effect of these antihistamines helps to mitigate opioid-induced nausea and vomiting.10 The most common adverse effect of antihistamines is mild extrapyramidal symptoms such as motor restlessness, but this is rare with oral administration. Opioids are another option and they offer analgesic effects while enhancing sedation quality. This family of drugs may be an excellent choice for sedation during surgery that may elicit pain, although they do not replace local anesthetics, which are the primary analgesic during pediatric dental procedures. Opioids can produce respiratory and cardiovascular depression, and can lead to serious life-threatening complications including airway obstruction, hypoventilation, and hypotension10 as well as nausea and vomiting. They can, however, be reversed with naloxone, which can be administered intramuscularly or intranasally (IN) when intravenous access is not available.11
Route of administration is another area of sedation experiencing recent change. Oral administration has long been the preferred route in pediatric dentistry, but disadvantages include a long waiting period for effectiveness, unreliable absorption resulting in unpredictable efficacy, inability to titrate, and patient refusal.10 IN administration is a parenteral technique that has grown popular because a drug can be administered to a child who won't allow oral administration. Individual states, however, vary in allowing IN administration with a dental board permit for oral sedation. IN medications are absorbed via the nasal mucosa, avoiding first-pass metabolism and resulting in bioavailability similar to that of IV medication.12 Plasma levels peak at 10 minutes following IN administration.13 When used as the only sedation agent for a procedure, midazolam doses of 0.35 to 0.5 mg/kg are typically used.14 A 2001 study by Al-Rakaf et al15 compared 3 different dosages of IN midazolam and found that restorative dental treatment was completed for 79% of children receiving 0.3 mg/kg, 96% of children receiving 0.4 mg/kg, and 100% of children receiving 0.5 mg/kg IN. Dental behavior was significantly better in the 0.5 mg/kg group compared to the 2 lower dosages.
SAFETY CONSIDERATIONS AND COMPLICATIONS
Midazolam can evoke a paradoxical reaction in which a child becomes very agitated, hostile, angry, and even violent.16 This is distressing to parents, and sometimes patients who frequently do not have recall of these events, and treatment is usually not possible. Serious events such as hypoxemia, airway obstruction, laryngospasm, allergy, and even permanent neurologic damage and death are all possible with moderate sedation.17 These morbidities are more often associated with combinations of sedation medications.8 A 2014 study by Dosani et al18 evaluated postdischarge events 24 hours postsedation in patients receiving combinations of midazolam, hydroxyzine and meperidine. Following sedation, they found that motor imbalance was significantly associated with the addition of midazolam, 66% of children slept in the car, 30% were supervised by only 1 driver, and 12% of those children were difficult to awaken. A 2007 Danish study of oral midazolam in 687 children and adolescents reported the most common complications were double vision (6.1%) and paradoxical reaction (2.0%). Nausea or vomiting was reported in 0.5–1.0% of patients. Reduced respiration was found in 2 patients during treatment, but none posttreatment.19 Other studies report higher rates of some of these adverse effects. A review article of 16 pediatric dental sedation papers reported nausea and vomiting at 6% and paradoxical reaction at 3.8%.20 Another side effect of midazolam, regardless of route of administration, is hiccups, with incidence of 10–26%.21
Currently, research provides limited guidance on which medications, dosages, and techniques are most effective.22,23 A 2007 meta-analysis23 attempted to summarize relative efficacy of various sedation regimens, but was unable to reach a conclusion because of the variety of drug regimens and techniques used. The authors concluded it was difficult to isolate regimens for comparison and the studies reviewed were of poor quality and limited validity. A 2012 Cochrane review4 assessed 30 pediatric dentistry sedation clinical trials and found 83% at high risk of bias. Simple research studies investigating the dose-response relationship of the most common sedation techniques are few.
To provide information on the safety and efficacy of various regimens using midazolam, we conducted a retrospective analysis of over 600 oral sedation cases to determine effectiveness and frequency of complications for regimens used. Specifically, we were interested in the relationships between (a) sedation success and drug regimen, (b) factors identified in the patients' medical history and examination findings and sedation effectiveness, and (c) complications and drug regimens and medical factors.
METHODS
Sample
All patients seen for dental sedation at Nationwide Children's Hospital (NCH) Dental Clinic in Columbus, Ohio, between July 2012 and June 2014 were evaluated for inclusion. Patients were treated by any of 28 pediatric dentistry residents under supervision of state– and hospital–sedation-certified attending dental faculty. Per protocol, patients referred for dental sedation were at least 24 months of age, weighed at least 10 kg, and were not greater than the 99th percentile for body mass index. Referral was based on cooperative ability, extent of treatment to be completed, medical history, parental acceptance of sedation as a behavior guidance technique, and approval for sedation by calibrated faculty. Typically, sedation patients were rated as positive or negative in demonstrated behavior (ie, minimally cooperative or minimally uncooperative) on a 4-point Frankl-like scale at the presedation evaluation and had treatment that could be completed in 1 appointment. Generally, total treatment time of 20 minutes or less was used as this criterion. This included, for example, no more than 2 teeth requiring restorative care, extraction of 4 maxillary incisors, or 3 teeth in a single quadrant. Children exhibiting extremes of behavior on the 4-point scale may have been treated with sedation for a variety of reasons such as parental desire, amount of treatment, or socioeconomic factors. The NCH Institutional Review Board deemed the study exempt due its retrospective nature and deidentified data.
Data Collection
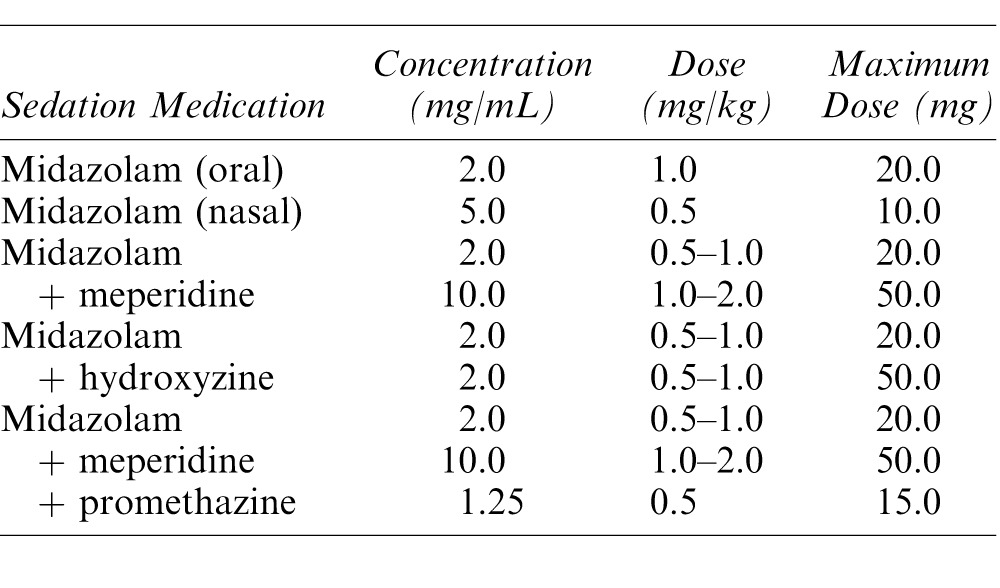
At all sedation appointments, medical history, medications, and allergies were reviewed with the parent, baseline vital signs obtained, nil per os status validated, and any recent history of illness or upper respiratory infection identified. A focused presedation history and physical examination was completed, including same-day height and weight measurement, visual assessment of the airway, and auscultation of breath sounds. The sedation regimen and route of administration were selected and dosages calculated based on same-day clinical findings. Dosages for the most common drug regimens, which comply with the NCH hospital maximum dosage guidelines, are listed in Table 1. Flumazenil was dosed and prepared for each midazolam sedation according to patient characteristics and administration protocol and ready for use if needed. No patients in this study required reversal.
Table 1. .
Dose Regimens Used in This Study
After a time-out, medication was administered by the resident, and the caregiver waited with the child in the operatory with door closed and dim lighting during the postadministration latency period while observed by staff. Prior to start of treatment, a blood pressure cuff and pulse oximeter were placed on the child's arm and finger/toe respectively to record preprocedure, intraprocedure (every 5 minutes), and postprocedure vital signs. Capnography was available if needed. Nitrous oxide in oxygen was titrated at the discretion of the operator as an adjunct to the sedation regimen, but its contribution to any particular sedation event was not identified or quantified. Administration ranged from 0 to 50% for part or most of the procedure. Supplemental oxygen was administered if clinically indicated when nitrous oxide in oxygen was not utilized. After dental treatment, the caregiver returned while the patient recovered and the child was discharged after meeting criteria.
A trained assistant also obtained demographic and contact information. In addition, type of restraint (if any) utilized, type of local anesthesia, provider rating of sedation effectiveness, and whether treatment planned was completed were recorded. Provider effectiveness was a personal judgment by the operator as to whether the encounter met safety and effectiveness goals. Within 48 hours of the sedation appointment, a trained assistant contacted the caregiver via phone to complete the postoperative complication and satisfaction questionnaire, including postoperative analgesic use, lip/cheek/tongue biting injuries, nausea and/or vomiting, hiccups, and any other complications. Parents were asked to estimate when, postoperatively, the child resumed eating, playing, and sleeping normally. Lastly, the caregivers were asked if they considered sedation successful, and if they would choose sedation again. Families who required an interpreter for the appointment were called by the assistant utilizing an interpreter. Patients whose caregivers could not be reached by phone were not included in this study's postoperative data analysis, but data from the sedation appointment itself were still included in the analyses.
For all patients seen for dental sedation during the specified time period, retrospective data were obtained from patients' electronic integrated medical-dental record (Epic). Data extracted for analysis included basic demographics, pertinent medical history, presedation history and physical findings, medication and dosages, perioperative information, and postoperative information. To obtain data from Epic, the data extraction tool SQL Developer was used to export data queries from Clarity (Epic's database) into an Excel database for each patient. The Epic Excel data set was merged with the Quality Assurance Survey data set that identified complications using v-lookups in Excel to create a comprehensive database for analysis.
Pearson's chi square test, Student t-test, Kruskal-Wallis test, or the Wilcoxon rank-sum test with Bonferroni-Holm method for multiple comparisons was used when appropriate for categorical and continuous variable comparisons when appropriate. Tests used for various comparisons are indicated below tables. All statistical analyses were performed using SAS 9.4 software. Descriptive statistics summarized the database. For each variable, a number of subjects had missing data. For instance, if patients could not be located using the query equation or any component of the medical history, presedation history, or physical exam was either incomplete or entered incorrectly, these data were not included in the analysis.
RESULTS
Analyses were completed for the 3 midazolam sedation regimens used at NCH: oral midazolam, IN midazolam, and oral midazolam combination (midazolam plus 1 or more other sedation medications), which included nitrous oxide when used (Table 1). Data from 701 sedation encounters were available, but data could not be matched to the sedation encounter for 51 subjects, resulting in a final sample size of 650 patients.
Demographics
The final sample was balanced, with 333 male subjects (51.2%) and 317 female subjects (48.8%). Age distribution included 125 subjects in the 2–3-year-old range, 161 in the 3–4-year-old range, 125 in the 4–5-year-old range, and 145 who were 6 or older. Weight data were available for 618 subjects and the majority of subjects (532; 86%) were 10–25 kg. The remaining subjects were over 25 kg. Approximately 68% (469) of children had no reported medical conditions. The overwhelming majority of patients were English speaking (553; 85%). The most common non-English first languages were Spanish (55; 8.5%) and Somali (15; 2.3%). Interpreters were used for 11.3% of sedation appointments. Race distribution had the most common being Caucasian (347; 54%), followed by African American (163; 25%), Latino/Hispanic (57; 8.8%) and biracial/multiracial (4; 7%).
Sedation Regimen Outcomes for Success
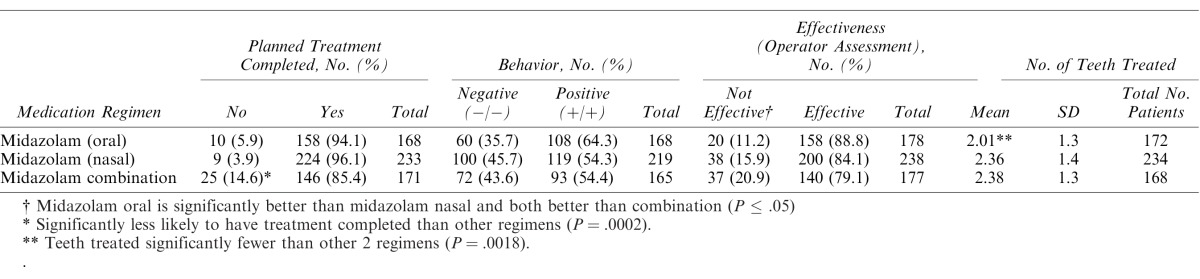
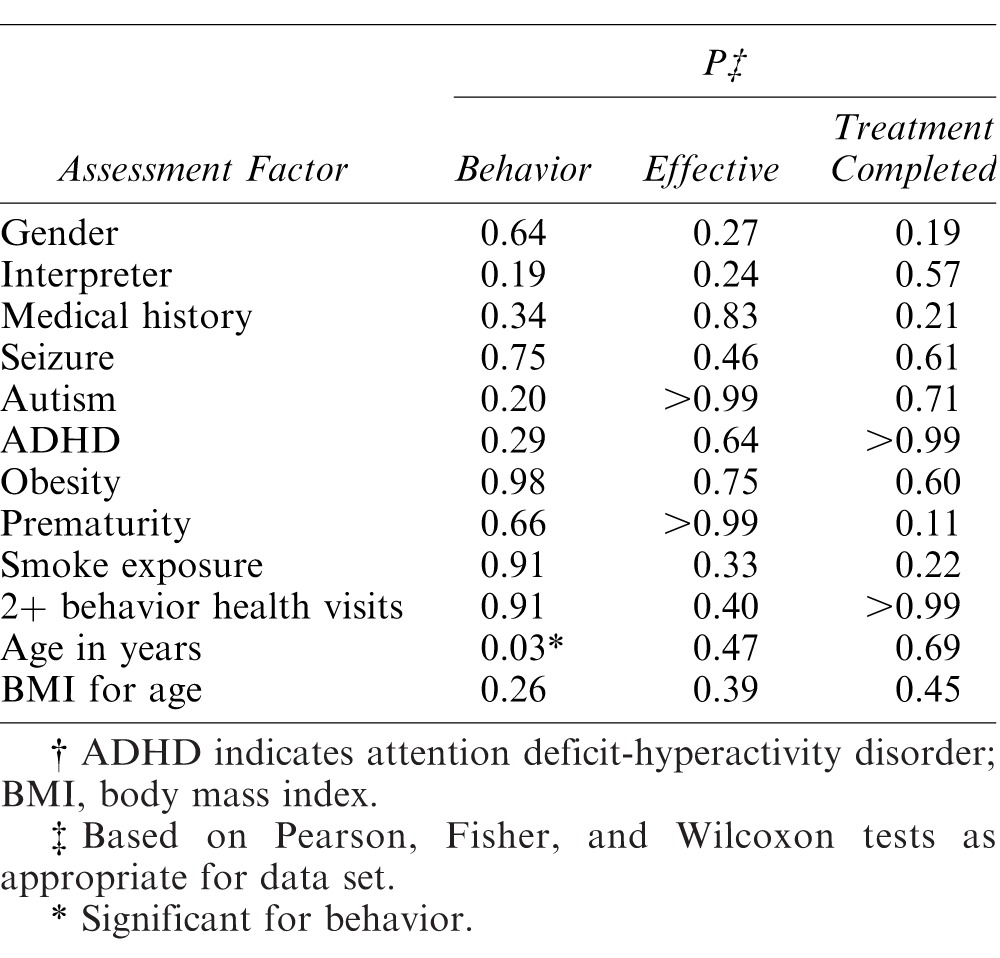
The 3 sedation regimens were compared for effectiveness using operator and clinical criteria including whether planned treatment was completed, behavioral rating for the sedation, overall sedation effectiveness, and number of teeth treated. Table 2 summarizes the findings for measures of success. The combination of midazolam and other agents was significantly less often associated with planned treatment completion, although all 3 regimens had rates of completion over 85%. Oral midazolam was more effective than nasal midazolam, which in turn surpassed the midazolam combinations when providers rated effectiveness. Behavior for the cumulative sedation was not as impressive as for provider-rated effectiveness, with oral midazolam alone having the most prominent effect and the other 2 regimens yielding almost equal poor and positive behaviors. Fewer teeth were treated when oral midazolam was used. When demographic data and medical history factors were assessed for relationship with behavior rating, operator-assessed effectiveness, and treatment completion, only age showed a significant relationship with behavior. No other comparisons were found to be significant (Table 3).
Table 2. .
Effectiveness of Sedation According to Planned Treatment Completion, Behavior Within Sedation, Operator Assessment, and Number of Teeth Treated for 3 Regimens
Table 3. .
Relationship of Demographic Data/Medical History to Sedation Effectiveness (Based on Measures of Behavior, Operator-Assessed Effectiveness, and Treatment Completion)†
Complications
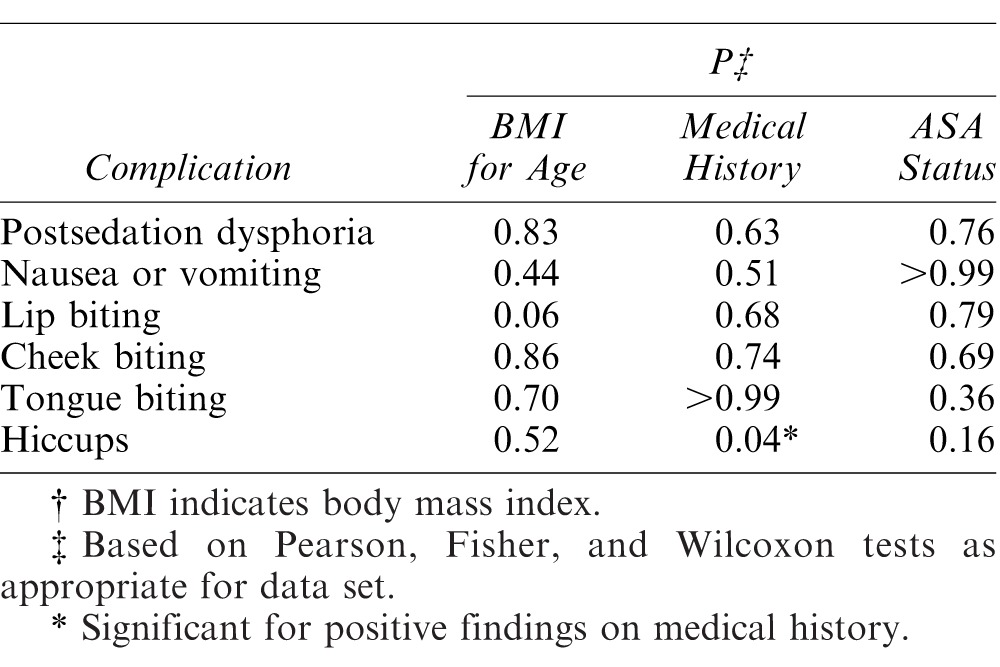
Postoperative contact was made with 350 caregivers. Very few complications were noted intraoperatively by providers or postoperatively when caregivers were contacted. Nausea and/or vomiting were noted in fewer than 4% of patients (13/350) available for postoperative evaluation, but the combination sedative regimens proved significantly higher than either nasal or oral midazolam alone, with 8 patients reporting this complication (P = .04) shown in Table 4. Paradoxical reaction or “angry-child syndrome” was reported in only 36 of 589 patients (6.1%) and the event was not significantly different across sedation regimens. Hiccups was a condition that also occurred across regimens, and was significantly related to a positive medical history, which meant an affirmative response to any of a list of health items (Table 4). Time to resumption of play and eating was not significantly different among the 3 regimens.
Table 4. .
Relationship of Patient Characteristics/Physical Assessment to Intraoperative and Postoperative Complications†
DISCUSSION
This retrospective study was undertaken to look at midazolam when used alone or in combination with other sedatives in pediatric dentistry, a growing area of interest within sedation because of the safety and positive sedative aspects of this medication. The large number of sedations using midazolam in various ways allowed comparison in 2 areas important to clinicians: effectiveness and safety across all 3 regimens. Overall, midazolam in the 3 regimens used in this study proved safe and effective for procedural sedation for minor dental procedures in children, duplicating findings in other studies.6,7
The data in this current study came from over 600 cases performed under conditions dictated by an institutional protocol that specifies preoperative, intraoperative, and postoperative procedures, including thresholds and ranges for doses of medications used as well as criteria for sedation readiness and postoperative discharge. At NCH, sedation cases are reviewed by an independent third party for quality assessment, which supports the consistency of sedations used in this review, in spite of patient variation and multiple providers. As in any retrospective study of this kind, limitations of subject selection consistency, operator variability, and intraoperative behavior management, to name a few variables, must be considered as well. For example, a characteristic of light procedural sedation, as described by S. Wilson (written communication, May 2016) is the ability to use communicative behavior management, which will vary by clinician, based on experience and other factors. Additionally, nitrous oxide as a sedation additive was not controlled, so some patients may not have had its benefit, and when used, it may have been administered with a wide range of concentrations and in varying effectiveness, as permitted by a child's movement. The potential but unknown effect of nitrous oxide on outcomes should be noted as a limitation in extending the results of this study.
Over 100 sedations involved meperidine. The relative contribution of the analgesic effect of this opioid to sedation success is difficult to determine. Across all 3 regimens, about 82% involved use of local anesthesia, further complicating the analysis.
The success rate of the 3 regimens was impressive, as was the lack of complications or comorbidities. When looking at sedation success, the one metric that appeared inconsistent with the other 3 was overall sedation behavior as rated by the clinician. Because this was a summative assessment, it must be looked at with the understanding that behavior can change over the course of a sedation visit, particularly when using a short-acting medication such as midazolam with a rapid peak blood level. The inconsistency of the behavior rating with the other measures of success is most likely the product of pediatric dental intervention, which often has the clinician completing even when behavior remains a problem intraoperatively. The relatively small number of average teeth treated, resulting in relatively short appointments corresponding somewhat closely to midazolam's clinical effective sedation duration, likely also contributed to the high success rate.
The rarity of complications as well as the consistency of complications identified in this study with those previously reported to be associated with midazolam19–21 should be seen as a positive for clinicians choosing to use midazolam. Paradoxical reaction and hiccups were noted in this study, the former often a concern of parents whose child may never have behaved in that manner. The findings of this study reinforce the best practice of alerting parents to these complications during the informed consent process to relieve anxiety and prepare them for postoperative care of the child.
Finally, the role of patient selection must be seen as a factor in the outcomes of this study. At our institution, children needing minor dental procedures with a likelihood of a positive outcome of the sedation are the ones most often selected for procedural sedation. Aggressive behavior, extensive treatment needs, and medical complications are contraindications for sedation, and these children are most often treated under general anesthesia. The high success rate in this study's sample may be reflective of good patient selection, as anxious rather than aggressive children are usually sedated, only a small amount of treatment is needed and thus treatment is likely to be accomplished quickly, and largely healthy children with lesser risk of complications receive sedative medications.
In this large retrospective study, we conclude that midazolam, administered orally, nasally, and orally in combination with other medications, (a) proved effective for minor procedural sedation for pediatric dentistry procedures and (b) had minimal postoperative complications. Medical history and clinical evaluation were not associated with either success of sedation or postoperative complications, except for age and behavior and positive medical history for hiccups, respectively.
REFERENCES
- 1. Casamassimo PS, Thikkurissy S, Edelstein BL, Maiorini E. . Beyond the dmft: the human and economic cost of early childhood caries. J Am Dent Assoc. 2009; 140: 650– 657. [DOI] [PubMed] [Google Scholar]
- 2. Wilson S. . Pharmacological management of the pediatric dental patient. Pediatr Dent. 2004; 26: 131– 136. [PubMed] [Google Scholar]
- 3. American Academy of Pediatric Dentistry. Guideline for monitoring and management of pediatric patients during and after sedation for diagnostic and therapeutic procedures. Reference manual. Pediatr Dent. 2014–2015; 36: 209– 225. [Google Scholar]
- 4. Lourenço-Matharu L, Ashley PF, Furness S. . Sedation of children undergoing dental treatment. Cochrane Database Syst Rev. 2012; 3:CD003877. [DOI] [PubMed] [Google Scholar]
- 5. Wilson S. . Management of child patient behavior: quality of care, fear and anxiety, and the child patient. Pediatr Dent. 2013; 35: 170– 174. [PubMed] [Google Scholar]
- 6. Peretz B, Kharouba J, Somri M. . A comparison of two different dosages of oral midazolam in the same pediatric dental patients. Pediatr Dent. 2014; 36: 228– 232. [PubMed] [Google Scholar]
- 7. Wan K, Jing Q, Zhao JZ. . Evaluation of oral midazolam as conscious sedation for pediatric patients in oral restoration. Chin Med Sci J. 2006; 21: 163– 166. [PubMed] [Google Scholar]
- 8. Coté CJ, Karl HW, Notterman DA, Weinberg JA, McCloskey C. . Adverse sedation events in pediatrics: analysis of medications used for sedation. Pediatrics. 2000; 106: 633– 644. [DOI] [PubMed] [Google Scholar]
- 9. Malamed SF. . Emergency medicine in pediatric dentistry: preparation and management. J Calif Dent Assoc. 2003; 31: 749– 755. [PubMed] [Google Scholar]
- 10. Malamed S. . Sedation: A Guide to Patient Management. 5th ed. St Louis, Mo: Elsevier; 2009. [Google Scholar]
- 11. Yasny JS, Asgari A. . Considerations for the use of enteral sedation in pediatric dentistry. J Clin Pediatr Dent. 2008; 32: 85– 93. [DOI] [PubMed] [Google Scholar]
- 12. Rey E, Delaunay L, Pons G, et al. Pharmacokinetics of midazolam in children: comparative study of intranasal and intravenous administration. Eur J Clin Pharmacol. 1991; 41: 355– 357. [DOI] [PubMed] [Google Scholar]
- 13. Walbergh EJ, Wills RJ, Eckhert J. . Plasma concentrations of midazolam in children following intranasal administration. Anesthesiology. 1991; 74: 233– 235. [DOI] [PubMed] [Google Scholar]
- 14. Gobeaux D, Sardnal F, Cohn H, Lequoy O. . Intranasal midazolam in pediatric ophthalmology [in French]. Cah Anesthesiol. 1991; 39: 34– 36. [PubMed] [Google Scholar]
- 15. al-Rakaf H, Bello LL, Turkustani A, Adenubi JO. . Intra-nasal midazolam in conscious sedation of young paediatric dental patients. Int J Paediatr Dent. 2001; 11: 33– 40. [DOI] [PubMed] [Google Scholar]
- 16. Weinbroum AA, Szold O, Ogorek D, Flaishon R. . The midazolam-induced paradox phenomenon is reversible by flumazenil. Epidemiology, patient characteristics and review of the literature. Eur J Anaesthesiol. 2001; 18 12: 789– 797. [DOI] [PubMed] [Google Scholar]
- 17. Coté CJ, Notterman DA, Karl HW, Weinberg JA, McCloskey C. . Adverse sedation events in pediatrics: a critical incident analysis of contributing factors. Pediatrics. 2000; 105 4 Pt 1: 805– 814. [DOI] [PubMed] [Google Scholar]
- 18. Dosani FZ, Flaitz CM, Whitmire HC, Vance BJ, Hill JR. . Postdischarge events occurring after pediatric sedation for dentistry. Pediatr Dent. 2014; 36: 411– 416. [PubMed] [Google Scholar]
- 19. Uldum B, Hallonsten AL, Poulsen S. . Midazolam conscious sedation in a large Danish municipal dental service for children and adolescents. Int J Paediatr Dent. 2008; 18: 256– 261. [DOI] [PubMed] [Google Scholar]
- 20. Papineni A, Lourenço-Matharu L, Ashley PF. . Safety of oral midazolam sedation use in paediatric dentistry: a review. Int J Paediatr Dent. 2014; 24: 2– 13. [DOI] [PubMed] [Google Scholar]
- 21. Marhofer P, Glaser C, Krenn CG, Grabner CM, Semsroth M. . Incidence and therapy of midazolam induced hiccups in paediatric anaesthesia. Paediatr Anaesth. 1999; 9: 295– 298. [DOI] [PubMed] [Google Scholar]
- 22. Padmanabhan MY, Pandey RK, Saksena AK, Chandra G. . A comparative evaluation of agents producing analgo-sedation in pediatric dental patients. J Clin Pediatr Dent. 2009; 34: 183– 188. [DOI] [PubMed] [Google Scholar]
- 23. Malamed SF. . Sedation and safety: 36 years of perspective. Alpha Omegan. 2006; 99: 70– 74. [DOI] [PubMed] [Google Scholar]
- 24. Matharu LL, Ashley PF. . What is the evidence for paediatric dental sedation? J Dent. 2007; 35: 2– 20. [DOI] [PubMed] [Google Scholar]