Abstract
The effects of carvacrol, a natural biocide, on dual-species biofilms formed by Staphylococcus aureus and Salmonella enterica serovar Typhimurium were investigated with a constant-depth film fermentor. Biofilm development reached a quasi-steady state in 12 days at 25°C with S. aureus predominance (≈99%). Cryosectional analysis detected viable S. aureus and S. enterica serovar Typhimurium at depths of 320 and 180 μm from the film surface, respectively. Carvacrol pulses (1.0 mmol/h) inhibited S. aureus by 2.5 log CFU/biofilm during the early stages of film formation, ultimately causing a significant reduction (P < 0.001) of the staphylococcal population at quasi-steady state. Initial carvacrol pulsing elicited a 3 log CFU/biofilm reduction in viable S. enterica serovar Typhimurium, and additional periodic carvacrol pulses instigated significant inhibition of salmonellae (1 to 2 log CFU/biofilm) during biofilm development. Carvacrol pulsing reduced protein levels fivefold (P < 0.001) during initial biofilm development. Comparative studies with a peroxide-based commercial sanitizer (Spor-Klenz RTU) revealed that this commercial sanitizer was more biocidal than carvacrol during early biofilm development. When the biofilm reached quasi-steady state, however, periodic pulses with 1 mmol of carvacrol per h (P = 0.021) elicited a significantly higher inhibition than Spor-Klenz RTU (P = 0.772). Dual-species microcolonies formed under the influence of continuously fed low carvacrol concentrations (1.0 mmol/h) but failed to develop into a mature quasi-steady-state biofilm and did not reach any stage of film formation in the presence of high concentrations (5.0 mmol/h). These data show that carvacrol is an effective natural intervention to control dual-species biofilm formation.
Naturally occurring biofilms are generally composed of a number of microbial species (16). Microenvironments inside these matrices generate a range of habitats, providing niches for different types of organisms (37). These microenvironments change constantly in response to physical perturbation, such as film erosion and sloughing, and evolutionary pressure, such as niche invasion and nutrient pressure. However, the biofilm matures to form a quasi-steady state within the community, where global heterogeneity remains stable and the population acquires resilience to external influences, such as antimicrobial perturbation.
The control of food-borne pathogens such as Staphylococcus aureus and Salmonella enterica serovar Typhimurium has received a great deal of attention because these organisms can form resilient biofilms on a range of surfaces (14, 33). Eradication usually requires the use of alkaline or acidic detergents and/or iodophores (4). Though efficacious, issues such as corrosion, product contamination, and toxicity, combined with the rapid emergence of resistant microbial species, limit the use of these compounds. The use of natural antimicrobial agents can be an effective alternative or supplement for the control of microorganisms (26, 30).
Carvacrol is an essential oil component of oregano, thyme, marjoram, and summer savory (1, 20). Generally recognized as a safe food additive (CFR 172.515) (21), this natural phytochemical is used as a flavoring agent in several products, such as baked goods, candy, beverages, and chewing gum (11). Carvacrol is also considered a broad-spectrum antimicrobial, effective against bacteria, yeasts, and fungi (2, 6, 31, 34). Pathogens, including S. aureus and S. enterica serovar Typhimurium, are susceptible to carvacrol, to the extent that this agent could inactivate dried films of these pathogens on stainless steel (18, 19). Carvacrol is biocidal, resulting in bacterial membrane perturbations that lead to leakage of intracellular ATP and potassium ions and ultimately cell death (35). Despite their broad antimicrobial spectrum, the application of carvacrol and other essential oils in food preservation has been limited by their potent aromatic properties (29). However, their potential for use in cleaning, disinfection, and biofilm control has not been studied extensively.
In this study, we grew biofilms of S. aureus and S. enterica serovar Typhimurium with a constant-depth film fermentor and evaluated the antimicrobial activity of carvacrol. The effects of carvacrol, applied continuously and in pulses, during early biofilm formation and on quasi-steady-state biofilms were determined and compared to the effects of a peroxide-based commercial sanitizer, Spor-Klenz RTU.
MATERIALS AND METHODS
Antimicrobial agents.
For pulsing studies, 20 mM carvacrol (Sigma, Poole, United Kingdom) was formulated in deionized water containing 0.1% Tween 80 (vol/vol). For continuous-feeding experiments, tryptic soy broth (TSB) with 1% glucose (TSBG) was supplemented with carvacrol (20 mM) and 0.1% Tween 80 (vol/vol). Spor-Klenz RTU (Steris Ltd., Camberley, United Kingdom), a commercial disinfectant containing hydrogen peroxide (0.8%, wt/wt) and peroxyacetic acid (0.06%, wt/wt), was diluted with water to 20% (vol/vol) prior to use.
Bacteria and their cultivation.
All bacteriological media and diluents were from Oxoid (Basingstoke, United Kingdom), unless stated otherwise. Staphylococcus aureus NCTC 10788 and Salmonella enterica serovar Typhimurium NCTC 74 were maintained on glass beads in tryptic soy broth (TSB) containing 15% glycerol (TSBG) at −70°C. A primary inoculum was prepared by incubating each test strain individually in 10 ml of TSBG at 25°C for 30 h. Each primary inoculum was subsequently transferred to 100 ml of TSBG and further incubated at 25°C for 30 h. The cultures were aseptically transferred to a 500-ml feeder vessel, mixed thoroughly, and pumped into the constant-depth film fermentor at a flow rate of 13 ml/h for 15 h.
Constant-depth film fermentor.
The fermentor system (Garth Engineering Ltd., Taffs Well, United Kingdom) was designed according to Peters and Wimpenny (28), with modifications. Briefly, a stainless steel turntable housed 15 polytetrafluoroethylene (PTFE) pans, each containing six stainless steel plugs (grade 316; 5-mm diameter), recessed to a depth of 500 μm. Two PTFE scraper blades moved across the turntable surface to maintain the biofilms at a constant depth. The constant-depth film fermentor was encased in a glass housing section (Corning Limited, Stone, Staffordshire, United Kingdom) sealed with PTFE at the top and bottom. The top plate was fitted with several inlet ports: a sampling port, a medium inlet from which TSBG was fed into the constant-depth film fermentor, a seeding port, an air outlet filter, and a secondary feed port for introducing the antimicrobials. An anti-grow-back trap was placed at the medium inlet to prevent contamination of the TSBG reservoir. Waste was pumped out from the bottom of the constant-depth film fermentor via an outlet port. The steel turntable was rotated at 3 rpm, and the fermentor was aerated with 200 ml/min of sterile filtered air. The fermentor was maintained at 25 ± 0.4°C in a climatically controlled chamber (Sanyo Gallankamp, Loughborough, United Kingdom) monitored by a Monolog Datalogger (SIFAM Ltd., Torquay, England). Following seeding of the fermentor with the mixed culture described above, the feeder input was switched on at a rate of 50 ml/h, and the biofilms were allowed to develop.
Determination of viable mass in biofilms.
Biofilms were dispersed according to Gayford and Richards (13). Briefly, biofilm-bearing plugs from the constant-depth film fermentor were aseptically removed in triplicate and placed in 10 ml of deflocculant containing 0.01% Cirrasol PP811 (ICI Surfactants, Middlesborough, United Kingdom) and 0.01% sodium pyrophosphate (Sigma) with 5 g of glass beads (2.5-mm diameter; Fisher Scientific, Loughborough, United Kingdom). Bacteria in the biofilm were dispersed by vortexing the plugs for 90 s. The cell suspensions were serially diluted in maximum recovery diluent, plated on brilliant green agar supplemented with sulfamandelate for salmonellae and mannitol salt agar for staphylococci, incubated at 37°C, and enumerated after 24 h.
Biofilm mass was determined by protein analysis (22). Biofilm-bearing plugs were placed (in triplicate) in 1.5-ml microcentrifuge tubes containing 1 ml of deflocculant, vortexed as described above, and sonicated for 10 min to homogenize the biofilm suspension. Protein content of the biofilm was determined according to Lowry et al. (22) with bovine serum albumin as the standard.
Cryosectioning of biofilms.
Biofilms were removed from the fermentor and immersed in 25 ml of sterile 25% dextran (wt/vol) in TSBG at 25°C for 2 h. After dextran treatment, each biofilm was plunge-frozen in liquid nitrogen, mounted on an orientation device (Histological Equipment Ltd., Nottingham, United Kingdom) immersed in Cryo-M-Bed resin (Bright Instruments Company Ltd., Huntingdon, United Kingdom), and chilled to −70°C. The orientation device and biofilm were placed in a cryostat chamber (Pearse cold microtome cryostat-model HR M, SLEE, Mainz, Germany) and equilibrated to the slicing temperature of −15°C. Film sections were sliced horizontally to 20 μm in thickness. Sections were collected with sterile forceps, placed in 10 ml of deflocculant solution containing glass beads, and homogenized as described above. Bacteria from each section were enumerated on brilliant green agar and mannitol salt agar as described above.
Treatment of biofilms with biocides.
Biofilms were allowed to develop to quasi-steady state and subjected to daily pulses of emulsified carvacrol as follows. Medium input was stopped, and 20 mM carvacrol was pumped into the constant-depth film fermentor at a rate of 1.0 mmol/h. After 10 min of carvacrol exposure, biofilms were removed for viable-mass analysis (as described above) and replaced with new sterile plugs. The medium input was then restarted. This procedure was repeated on a daily basis for 5 days. A similar pulsing protocol was used to study the effect of carvacrol on developing biofilms, except that pulsing began immediately after seeding was complete, i.e., before a steady-state biofilm was formed. Additionally, the sample pans were removed and analyzed before and after each pulse for a period of 7 days. The effect of continuous exposure of developing biofilms to carvacrol was investigated by pumping carvacrol into the constant-depth film fermentor at 1.0 and 5.0 mmol/h immediately after seeding was complete.
A similar method of bacterial cultivation, treatment, and sampling of biofilms was performed for the 20% (vol/vol) Spor-Klenz RTU treatment group. All data were analyzed with the t test and analysis of variance on SigmaStat 3.0.
RESULTS
Dual-species biofilm formation.
S. aureus and S. enterica serovar Typhimurium formed a stable quasi-steady-state biofilm after about 12 days at 25°C in the constant-depth film fermentor. Quasi-steady state was defined as occurring when the viable populations and proteinaceous mass did not change significantly (P < 0.05) for several sampling points. S. aureus constituted ≈99% of the total viable numbers by day 12. The protein content of the biofilm increased during this 12-day period, reaching 450 ± 15 μg prior to the onset of the quasi-steady state (see Fig. 2C).
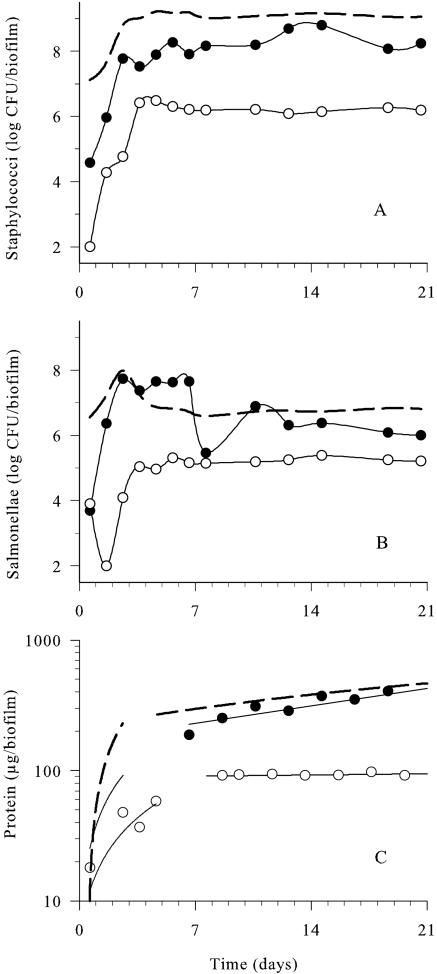
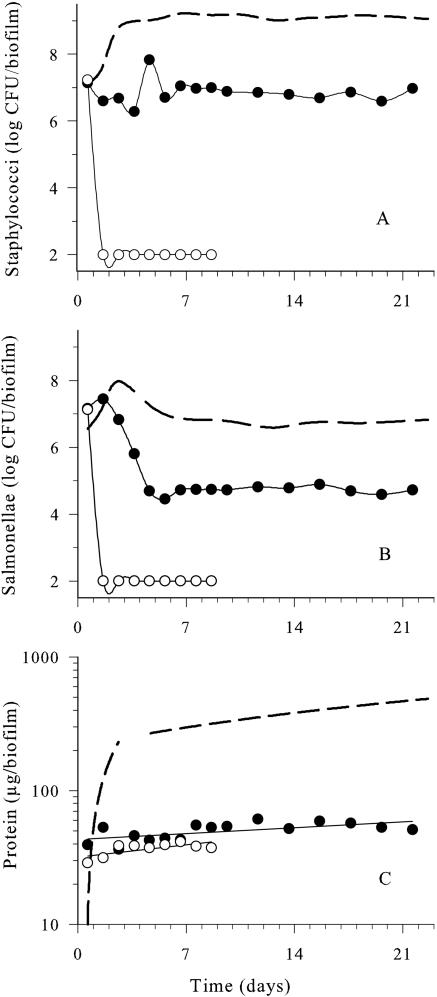
FIG. 2.
Effects of daily carvacrol (•, 1.0 mmol/h) and Spor-Klenz RTU (○, 20%) pulses on the development of dual-species biofilms compared to the untreated control (dotted line) at 25°C. A) Staphylococci. B) Salmonellae. C) Protein. Samples were collected ≤10 min after each antimicrobial pulse. Data points represent mean values of three independent experiments with standard deviations of <0.45 log CFU/biofilm for viable counts and <30 μg/biofilm for protein values. The experimental limit of detection was 2 log CFU/biofilm.
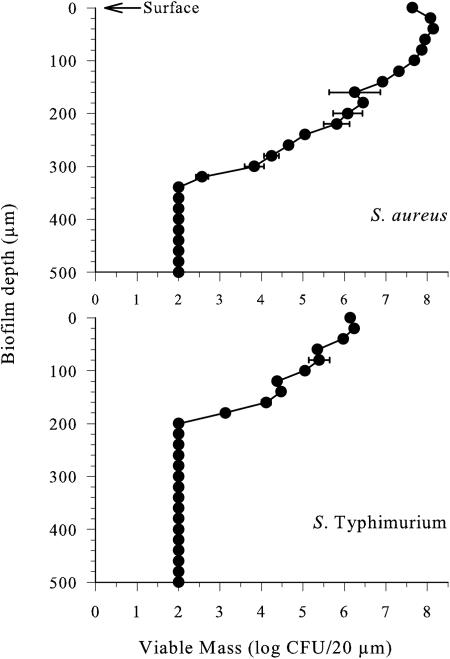
Cryosectional analysis of the 20-μm sections cut horizontally across the biofilm revealed that viable S. aureus were present at a level of 7 to 8 log CFU/section in the top 140 μm of the biofilm, constituting 93% of the total staphylococcal viable mass (Fig. 1). In the deeper strata of the biofilm, viable staphylococci decreased steadily to a depth of 300 to 320 μm, at which point there was a sharp decline in numbers to below the detection limit of the plate counting method. In contrast, salmonellae were present at a level of 6 log CFU/section in the top 40 μm of the biofilm, and this stratum accounted for 83% of the total viable mass of this organism. Viable numbers of salmonellae decreased steadily to a level of 3 log CFU/section at a depth of 180 μm and then dropped sharply to a level below the detection limit. Unlike staphylococci, salmonellae were not detected at depths between 180 and 320 μm from the biofilm surface. No viable organisms were detected in the strata up to 180 μm from the biofilm base, suggesting that this layer consisted primarily of cell detritus. Statistical analysis revealed significant differences (P < 0.05) between the numbers of staphylococci and salmonellae at each stratum down to 340 μm in depth. Therefore, the biofilm consisted of a cellular matrix dominated by staphylococci where a small but significant population of salmonellae persisted in the top strata.
FIG. 1.
Viable numbers of staphylococci and salmonellae in horizontal cross sections of a dual-species biofilm grown at 25°C for 12 days. Data points represent mean values of three independent experiments with standard deviation. The experimental limit of detection was 2 log CFU/20-μm section. Statistical analysis (t test) revealed significant differences (P > 0.05) between the staphylococcal and salmonellae populations throughout the biofilm.
Pulse addition of carvacrol alters biofilm development.
Developing dual-species biofilms were subjected to daily pulses of emulsified carvacrol solution (1.0 mmol/h every day for 21 days), where the medium was replaced with carvacrol for 10 min. Carvacrol was emulsified in 0.1% (vol/vol) Tween 80, which had previously been determined as having no antimicrobial effect against the dual-species biofilm (19). As shown in Fig. 2A, carvacrol elicited a 2.5 log CFU/biofilm reduction in S. aureus during the early stages (<2 days) of film formation compared with the untreated control. Following this initial reduction in viable numbers, the staphylococci proliferated at rates similar to those observed in the untreated control, but the final numbers between days 5 and 21 were about 1 log CFU/biofilm lower. In comparison, 20% Spor-Klenz RTU treatment reduced initial staphylococci by 4 log CFU/biofilm during early biofilm formation (<3 days) and was significantly more effective than carvacrol (P = 0.001). However, after 4 days, S. aureus viable cell numbers increased to 6.5 log CFU/biofilm. Both treatments altered the development of the staphylococcal population significantly (P < 0.001) compared to the untreated control.
During the initial 24 h of biofilm development, viable salmonellae were reduced by 3 log CFU/biofilm following carvacrol pulsing (Fig. 2B). However, there was a rapid recovery in viable cell numbers that exceeded and eventually equaled the cell numbers within the unperturbed biofilm (Fig. 2B). In contrast, Spor-Klenz RTU caused a reduction in numbers of 6 log CFU/biofilm on day 2, and the viable cell numbers recovered gradually by day 4 to reach quasi-steady state, which was 2 log CFU/biofilm below the control. Spor-Klenz RTU caused a significant decrease (P < 0.001) in salmonellae, but the effect of carvacrol was insignificant (P > 0.05).
The protein concentration of the untreated biofilm increased exponentially to 450 ± 15 μg to reach quasi-steady state by day 12 (Fig. 2C). Carvacrol pulses inhibited protein development during the initial 4 days of biofilm formation (P < 0.001). This was followed by a slow increase in protein to reach a quasi-steady state similar to the control. In contrast, Spor-Klenz RTU treatment reduced the initial protein levels to less than 50 μg/biofilm during early film formation (<5 days). This was followed by the onset of quasi-steady state by day 7, with protein concentrations at a level (90 μg/biofilm) that was fivefold lower than the control (P < 0.001).
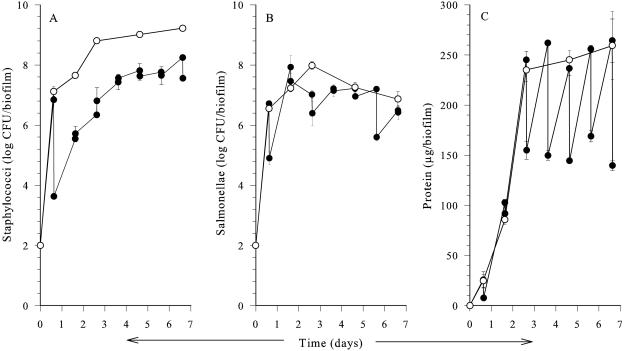
We then focused on the critical first week of biofilm development, where carvacrol had the greatest effect (Fig. 3). Carvacrol pulses of 1.0 mmol/h initially reduced both staphylococcal (3.2 log CFU/biofilm) and salmonella (2.0 log CFU/biofilm) counts compared to the control. Following this initial inactivation phase, both organisms resumed growth at a rate similar to that observed in the untreated control, but the absolute viable numbers of staphylococci remained at about 2 log CFU/biofilm below those in the untreated control until day 7 (Fig. 3A). Although the salmonellae were initially inactivated by carvacrol, by day 5 the numbers had reached levels similar to those in the untreated control (Fig. 3B). Statistical analysis confirmed that the reduction in numbers of staphylococci was significant (P < 0.027) throughout the 7-day experiment, but the change in salmonella numbers was significant on days 1 and 3 only (P = 0.003 and 0.017, respectively). Prior to each pulse, the protein levels of the carvacrol-treated biofilms were similar to the control (Fig. 3C). However, from day 3 onwards, carvacrol caused an immediate decrease in protein (100 μg/biofilm) after each pulse compared to the untreated biofilm.
FIG. 3.
Effects of daily carvacrol (•) pulses at 1.0 mmol/h on initial development of dual-species biofilm compared to the control (○) at 25°C. A) Staphylococci. B) Salmonellae. C) Protein. Samples were collected ≤10 min before and after each antimicrobial pulse. Data points represent mean values of three independent experiments with standard deviation. The experimental limit of detection was 2 log CFU/biofilm.
Effects of carvacrol pulses on mature biofilms.
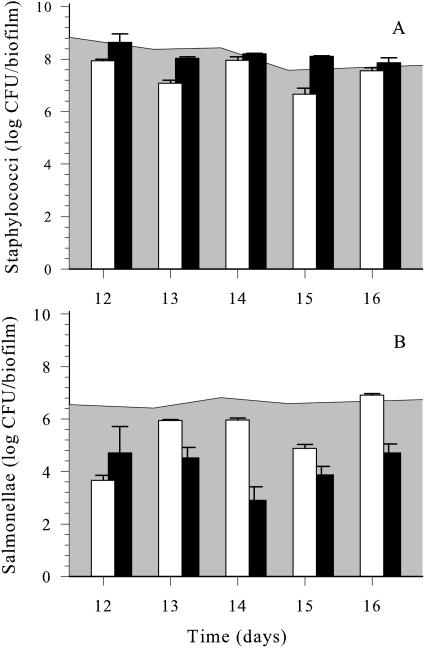
After reaching quasi-steady state (12 days), dual-species biofilms were exposed to carvacrol or Spor-Klenz RTU pulses (as described above) for 5 consecutive days (Fig. 4). Carvacrol elicited a significant reduction (P = 0.021) of S. aureus by 1 log CFU/biofilm compared to the control biofilm. In contrast, Spor-Klenz RTU failed to inhibit staphylococci (Fig. 4A). Salmonellae were more susceptible than staphylococci to both antimicrobial agents in mature biofilms (Fig. 4B). Carvacrol caused a 3 log CFU/biofilm reduction of salmonellae after the initial exposure and an average of 1 log CFU/biofilm reduction during subsequent pulses. Spor-Klenz RTU caused a 1.5 log CFU/biofilm decrease in salmonellae initially (P < 0.001) but was more effective than carvacrol after subsequent treatment (e.g., 3.5 log CFU/biofilm reduction on day 14). Biofilm analysis at shorter intervals (hourly sampling) revealed that after each carvacrol pulse, the viable numbers of both species recovered very slowly over a 24-h period (data not shown).
FIG. 4.
Effects of daily pulses of 1.0 mmol of carvacrol per h (white columns) and 20% Spor-Klenz RTU (black columns) on viable numbers of staphylococci (A) and salmonellae (B) in a quasi-steady-state dual-species biofilm at 25°C. Samples were collected ≤10 min after each antimicrobial pulse. Data points represent mean values of three independent experiments with standard deviation. The shaded background area represents counts in the untreated biofilm control.
Carvacrol elicited a pulse response on the viability of S. aureus and S. enterica serovar Typhimurium and affected the protein concentration of the film from the initial planktonic state to quasi-steady-state matrix formation (Table 1). Both bacterial species were sensitive to carvacrol during the planktonic stage. However, during microcolony formation and coalition stages, S. aureus acquired resistance but gradually became sensitized to carvacrol pulses when the biofilm reached quasi-steady state. In contrast, S. enterica serovar Typhimurium was sensitive to carvacrol during all stages except quasi-steady state.
TABLE 1.
Effect of carvacrol on viability and protein mass of dual-species biofilm formation by S. aureus and S. enterica serovar Typhimurium
| Biofilm stage | % Changea in biofilm viability (log CFU/biofilm) and mass (μg/biofilm)
|
||
|---|---|---|---|
| S. aureus | S. enterica serovar Typhimurium | Protein | |
| Planktonic | −99.5 | −98.5 | −29.2 |
| Adherent | 49.9 | −65.9 | −89.3 |
| Microcolony formation | 191.6 | −76.1 | −63.3 |
| Microcolony coalition | 35.6 | −14.9 | −57.1 |
| Proliferation | −34.3 | −46.0 | −61.0 |
| Maturity | −23.4 | −97.5 | −66.0 |
| Quasi-steady-state matrix | −79.5 | −13.9 | −52.8 |
Difference between viable counts or total protein content before and after pulsing with carvacrol. Biofilm stages were categorized based on visualization of morphological and developmental states during film formation on a steel surface. The data represent a conglomerated summary of the results obtained during this study.
Influence of continuous feeding of carvacrol on biofilm development.
Dual-species biofilm development in response to a continuous influx of 1.0 and 5.0 mmol/h carvacrol was investigated (Fig. 5). At 1.0 mmol/h, carvacrol reduced staphylococcal numbers by 3 log CFU/biofilm (P < 0.001) within 3 days of film development. However, after 4 days, staphylococcal numbers increased, reaching a quasi-steady state 7 days after the exposure began. Thereafter, staphylococcal counts remained 2 log CFU/biofilm lower than the control. Low levels (1.0 mmol/h) of carvacrol caused significant reductions (2.5 log CFU/biofilm) in salmonellae (P < 0.001) after the initial 4 days, followed by onset of quasi-steady state after 7 days, 2 log CFU/biofilm lower than the control (Fig. 5B). Within a day of continuous exposure to 5.0 mmol/h carvacrol, no viable staphylococci or salmonellae were detected in the dual-species biofilm (Fig. 5A and B). Protein concentration was reduced ninefold (P < 0.001) by low concentrations of carvacrol compared to the control (450 μg/biofilm). High doses of carvacrol prevented the build-up of proteinaceous mass (Fig. 5C). Protein analysis and bacterial viability data with low doses of carvacrol influx indicated a rapid approach to a quasi-steady state, culminating in the emergence of microcolony formation. Exposure to high doses of carvacrol resulted in total inhibition of dual-species biofilm formation.
FIG. 5.
Development of a dual-species biofilm at 25°C in response to continuous exposure at low (•, 1.0 mmol/h) or high (○, 5.01 mmol/h) concentrations of carvacrol compared to untreated biofilm development (dotted line). A) Staphylococci. B) Salmonellae. C) Protein. The experimental limit of detection was 2 log CFU/biofilm. Samples were collected ≤10 min after each antimicrobial pulse. Data points represent mean values of three independent experiments with standard deviations of <0.3716 log CFU/biofilm for viable counts and <12.5 μg/biofilm for protein values.
DISCUSSION
This study elucidated the dynamics of dual-species biofilm formation by Staphylococcus aureus and Salmonella enterica serovar Typhimurium. The gram-positive staphylococci dominated the film matrix at 99% of the viable population, and quasi-steady state was reached after 12 days, as defined by viable counts and protein content. In contrast, monospecies biofilm formation by S. aureus is rapid and reaches quasi-steady state in <24 h (23). The role of nutrient and oxygen gradients in biofilm matrices is well established (15, 17, 36). Oxygen, in particular, due to its low water solubility and high rate of microbial utilization, may develop steep gradients of anoxic environments in deep regions of the biofilm matrix (37, 39). Cryosectional analysis performed in this study revealed a relationship between biofilm depth and bacterial viability. Thus, S. aureus and S. enterica serovar Typhimurium survived only down to depths of 320 and 180 μm, respectively. Restricted survival of dual species in the lower strata could possibly be due to unfavorable anoxic conditions. Accordingly, the majority of bacterial viability was limited to the top 100 μm of the biofilm.
Carvacrol selectively inhibited S. aureus during the early stages (<2 days) of biofilm development, which was comprised mainly of a planktonic, nonadherent population. Nostro et al. (27) reported carvacrol efficacy against planktonic staphylococci at a 1 to 2 mM concentration. In this study, S. aureus demonstrated resistance to higher concentrations of carvacrol (20 mM) due to the emergence of an adherent cell population in the biofilm. The selective evasion by salmonellae to initial carvacrol exposure could be attributed to the stress-adaptive mechanisms of S. enterica serovar Typhimurium (12). When exposed to carvacrol from the beginning of film development, mature biofilm-bound S. enterica serovar Typhimurium exhibited greater antimicrobial resistance than salmonellae in a mature biofilm previously unexposed to carvacrol. This suggests that cells in a previously treated mature biofilm acquired resistance to carvacrol.
In comparison to carvacrol, Spor-Klenz RTU demonstrated higher efficacy during the early stages (<3 days) of film formation and inhibited both staphylococci and salmonellae. This further confirms that hydrogen peroxide and peroxyacetic acid, the major active constituents of Spor-Klenz RTU, are potent sanitizing agents for biofilm control due to their ability to form highly biocidal reactive oxygen species (8, 32).
Microbial cell surface interaction resulting in leakage of intracellular constituents has been suggested as the biocidal mechanism for carvacrol-mediated antimicrobial activity (35). Exposure of S. aureus to carvacrol during the early stages of biofilm development led to potent inhibition of matrix formation, with shedding of proteinaceous mass after each antimicrobial pulse. Studies with lysostaphin, an agent with a mode of action similar to that of carvacrol, showed that this antimicrobial is biocidal against staphylococci in the biofilm and also dispersed the matrix that anchored the film (38). By analogy, the rapid killing of S. aureus by carvacrol also led to disruption of the proteinaceous matrix of the film. However, the shedding of such proteinaceous mass did not coincide with viability reductions of staphylococci in the biofilm, possibly due to continuous exfoliation of the matrix.
Studies of quasi-steady-state dual-species biofilm indicated a higher efficacy for carvacrol than Spor-Klenz RTU against S. aureus. However, both antimicrobial treatments demonstrated similar efficacy against S. enterica serovar Typhimurium. The exact mechanism of carvacrol-mediated inhibition of quasi-steady-state dual-species biofilms remains unclear. Despite its efficacy, carvacrol failed to eliminate the entire viable population within the biofilm.
Carvacrol was less effective against S. aureus as the biofilm matured and formed microcolonies. S. enterica serovar Typhimurium was sensitive throughout the maturation except at quasi-steady state. The resistance of the dual-species biofilm to biocides compared to its planktonic counterpart seems to be associated with the antimicrobial permeation blockade by the glycocalyx. The resistance exhibited by S. aureus coincided with an increase in matrix proliferation. However, staphylococci still proliferated after carvacrol treatment, suggesting that no single factor could be linked to this phenomenon (3, 24, 25). The physiological adaptation of film-borne microflora to antimicrobial agents could not be ruled out. Thus, specific physiological responses to active ingredients in Spor-Klenz RTU, especially oxidative stress responses against reactive oxygen intermediates such as H2O2 and peroxyacetic acid, are well-documented mechanisms of resistance development (7, 9).
Staphylococci demonstrated a greater tolerance to carvacrol than salmonellae, as reported previously (18, 19), although not within biofilms. The capsular polysaccharides of S. aureus, notably type 5 and type 8, have been suggested to block antimicrobial diffusion and protect the susceptible cytoplasmic target sites (5, 10).
Carvacrol influx at low concentrations (1.0 mmol/h) prevented the build-up of protein mass but allowed bacterial populations to reach quasi-steady state, arresting at the microcolony formation stage. This finding is in contrast with previous observations that antimicrobial treatment increases polysaccharide production, which is probably an adaptive response (21). In an earlier study, we demonstrated that 2 mM carvacrol was nonbiocidal to S. aureus in dried films (18). The present study showed that continuous exposure of this organism to nonbiocidal concentrations of carvacrol could disrupt normal development of the biofilm. At high concentrations (5.0 mmol/h), carvacrol caused total inactivation of both bacteria and prevented coaggregation of cells; presumably, viable cells were killed prior to the initial attachment of bacteria.
In conclusion, this study has demonstrated the inhibitory effects of carvacrol, a natural antimicrobial, in a dual-species biofilm at various stages of maturation. Pulse and continuous-exposure studies showed that carvacrol was as effective as Spor-Klenz RTU, a commercial-grade sanitizing agent, against biofilms. The results also demonstrated the resilient nature and regenerative capacity of biofilm matrices.
Acknowledgments
We are indebted to Julian Wimpenny for the loan of the fermentor systems, the SLEE cryostat, and stimulating discussions. We are grateful to Tony Hope, Steve Jones, and Chris Merridan for excellent technical assistance.
This work was supported by a doctoral studentship from South Bank University, London, United Kingdom.
REFERENCES
- 1.Arrebola, M. L., M. C. Navarro, J. Jimenez, and F. A. Ocana. 1994. Yield and composition of the essential oil of Thymus serpylloides subsp. serpylloides. Phytochemistry 36:67-72. [Google Scholar]
- 2.Beuchat, L. R. 1994. Antimicrobial properties of spices and their essential oils, p. 167-179. In V. M. Dillon and R. G. Board (ed.), Natural antimicrobial systems and food preservation. CAB International, Wallingford, United Kingdom.
- 3.Brown, M. R. W., and P. Gilbert. 1993. Sensitivity of biofilms to antimicrobial agents. J. Appl. Bacteriol. Symp. Suppl. 74:87S-97S. [DOI] [PubMed] [Google Scholar]
- 4.Corrieu, G. 1981. State of the art of cleaning surfaces, p. 90-114. In B. Hallstrom, B. D. Lund, and C. Tragardh (ed.), Fundamentals and applications of surface phenomena associated with fouling and cleaning in food processing, Lund University, Lund, Sweden.
- 5.Cross, A. S. 1990. The biological significance of bacterial encapsulation, p. 86-95. In K. Jann and B. Kann (ed.), Current topics in microbiology and immunology vol. 150, bacterial capsules. Springer-Verlag, Berlin, Germany. [DOI] [PubMed]
- 6.Davidson, P. M., and A. S. Naidu. 2000. Phytophenols, p. 265-294. In A. S. Naidu (ed.), Natural food antimicrobial systems. CRC Press, Boca Raton, Fla.
- 7.Demple, B. 1991. Regulation of bacterial oxidative stress genes. Annu. Rev. Genet. 25:315-337. [DOI] [PubMed] [Google Scholar]
- 8.Elkins, J. G., D. J. Hassett, P. S. Stewart, H. P. Schweizer, and T. R. McDermott. 1999. Protective role of catalase in Pseudomonas aeruginosa biofilm resistance to hydrogen peroxide. Appl. Environ. Microbiol. 65:4594-4600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farr, S., B., and T. Kogoma. 1991. Oxidative stress responses in Escherichia coli and Salmonella typhimurium. Microbiol. Rev. 55:561-585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fattom, A. I., J. Sarwar, L. Basham, S. Ennifar, and R. Naso. 1998. Antigenic determinants of Staphylococcus aureus type 5 and type 8 capsular polysaccharide vaccines. Infect. Immun. 66:4588-4592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fenaroli, G. 1995. Fenaroli's handbook of flavor ingredients, 3rd ed. CRC Press, Boca Raton, Fla.
- 12.Foster, J. W., and M. P. Spector. 1995. How salmonella survive against the odds? Annu. Rev. Microbiol. 49:145-174. [DOI] [PubMed] [Google Scholar]
- 13.Gayford, C. G., and J. P. Richards. 1970. Isolation and enumeration of aerobic heterotrophic bacteria in activated sludge. J. Appl. Bacteriol. 33:342-350. [DOI] [PubMed] [Google Scholar]
- 14.Hood, S. K., and E. A. Zottola. 1997. Adherence to stainless steel by foodborne microorganisms during growth in model food systems. Int. J. Food Microbiol. 37:145-153. [DOI] [PubMed] [Google Scholar]
- 15.Huang, C.-T., F. P. Yu, G. A. McFeters, and P. S. Stewart. 1995. Nonuniform spatial patterns of respiratory activity within biofilms during disinfection. Appl. Environ. Microbiol. 61:2252-2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James, G. A., L. Beaudette, and J. W. Costerton. 1995. Interspecies bacterial interactions in biofilms. J. Ind. Microbiol. 15:257-262. [Google Scholar]
- 17.Kinniment, S. 1992. Use of a constant depth film fermentor to study the growth, physiology and effects of antimicrobials on biofilm derived from metalworking fluids. Ph.D. thesis. Cardiff University, Cardiff, Wales.
- 18.Knowles, J. R., and S. Roller. 2001. Efficacy of chitosan, carvacrol and a hydrogen peroxide-based biocide against foodborne microorganisms in suspension and adhered to stainless steel. J. Food Prot. 64:1542-1548. [DOI] [PubMed] [Google Scholar]
- 19.Knowles, J. R. 2002. Microbial adhesion and its control using natural and synthetic biocides. Ph.D. thesis. South Bank University, London, United Kingdom.
- 20.Lagouri, V., G. Blekas, M. Tsimidou, S. Kokkini, and D. Boskou. 1993. Composition and antioxidant activity of essential oils from oregano plants grown wild in Greece. Z. Lebens. Unter. Fors. 197:20-23. [Google Scholar]
- 21.Leriche, V., and B. Carpentier. 1995. Viable but non-culturable Salmonella Typhimurium in single- and binary-species biofilms in response to chlorine treatment. J. Food Prot. 58:1186-1191. [DOI] [PubMed] [Google Scholar]
- 22.Lowry, O. H. 1951. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 193:265-275. [PubMed] [Google Scholar]
- 23.Luppens, S. B., M. W. Reij, R. W. van der Heijden, F. M. Rombouts, and T. Abee. 2002. Development of a standard test to assess the resistance of Staphylococcus aureus biofilm cells to disinfectants. Appl. Environ. Microbiol. 68:4194-4200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mah, T. F., and G. A. O'Toole. 2001. Mechanisms of biofilm resistance to antimicrobial agents. Trends Microbiol. 9:34-39. [DOI] [PubMed] [Google Scholar]
- 25.Morton, L., D. Greenway, C. Gaylarde, and S. Surman. 1998. Consideration of some implications of the resistance of biofilms to biocides. Int. Biodeter. Biodeg. 41:247-259. [Google Scholar]
- 26.Naidu, A. S. 2000. Natural food antimicrobial systems. CRC Press, Boca Raton, Fla.
- 27.Nostro, A., A. R. Blanco, M. A. Cannatelli, V. Enea, G. Flamini, I. Morelli, A. Sudano Roccaro, and V. Alonzo. 2004. Susceptibility of methicillin-resistant staphylococci to oregano essential oil, carvacrol and thymol. FEMS Microbiol. Lett. 230:191-195. [DOI] [PubMed] [Google Scholar]
- 28.Peters, A. C., and J. W. T. Wimpenny. 1988. A constant depth laboratory model film fermentor. Biotechnol. Bioeng. 32:263-270. [DOI] [PubMed] [Google Scholar]
- 29.Roller, S. 1995. The quest for natural antimicrobials as novel means of food preservation: Status report on a European research project. Int. Biodeter. Biodeg. 36:333-345. [Google Scholar]
- 30.Roller, S. 2003. Natural antimicrobials for the minimal processing of foods. Woodhead Publishing Ltd., Cambridge, United Kingdom.
- 31.Sivropoulou, A., E. Papanikolaou, C. Nikolaou, S. Kokkini, T. Lanaras, and M. Arsenakis. 1996. Antimicrobial and cytotoxic activities of origanum essential oils. J. Agric. Food Chem. 44:1202-1205. [Google Scholar]
- 32.Stewart, P. S., F. Roe, J. Rayner, J. G. Elkins, Z. Lewandowski, U. A. Ochsner, and D. J. Hassett. 2000. Effect of catalase on hydrogen peroxide penetration into Pseudomonas aeruginosa biofilms. Appl. Environ. Microbiol. 66:836-838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thomas, C. J., and T. A. McMeekin. 1980. Contamination of broiler carcass skin during commercial processing procedures: an electron microscopic study. Appl. Environ. Microbiol. 41:492-503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Thompson, D. P. 1990. Influence of pH on the fungitoxic activity of naturally occurring compounds. J. Food Prot. 53:412-415. [DOI] [PubMed] [Google Scholar]
- 35.Ultee, A., E. P. W. Kets, and E. J. Smid. 1999. Mechanisms of action of carvacrol on the food-borne pathogen Bacillus cereus. Appl. Environ. Microbiol. 65:4606-4610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Vroom, J. M., K. J. De Grauw, H. C. Gerritsen, D. J. Bradshaw, P. D. Marsh, G. K. Watson, J. J. Birmingham, and C. Allison. 1999. Depth penetration and detection of pH gradients in biofilms by two-photon excitation microscopy. Appl. Environ. Microbiol. 65:3502-3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wimpenny, J. W. T. 1994. The spatial organisation of biofilms, p. 1-6. In J. W. T. Wimpenny, W. Nichols, D. Stickler, and H. Lappin-Scott (ed.), Bacterial biofilms and their control in medicine and industry. Bioline, Cardiff, United Kingdom.
- 38.Wu, J. A., C. Kusuma, J. J. Mond, and J. F. Kokai-Kun. 2003. Lysostaphin disrupts Staphylococcus aureus and Staphylococcus epidermidis biofilms on artificial surfaces. Antimicrob. Agents Chemother. 47:3407-3414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Xu, K. D., P. S. Stewart, F. Xia, C. T. Huang, and G. A. McFeters. 1998. Spatial physiological heterogeneity in Pseudomonas aeruginosa biofilm is determined by oxygen availability. Appl. Environ. Microbiol. 64:4035-4039. [DOI] [PMC free article] [PubMed] [Google Scholar]