Abstract
Lymph node metastasis in patients with urinary bladder cancer (UBC) is always associated with poor prognosis and is the determinant for tumor staging and the development of treatment regimens; however, its underlying mechanisms remain to be studied. Immunohistochemical staining of tumor sections from 62 UBC patients was performed using CCR7, D2-40 and CD34 antibodies. We showed that increased CCR7 expression was significantly associated with positive lymph node status (P=0.008), pT3-T4 tumor stage (P=0.015), tumor grade (P=0.010) and worse overall survival (OS, P<0.001) and that both CCR7 expression and lymph node metastasis were independent prognostic factors for OS (P=0.031 and P=0.001, respectively) based on multivariate analysis. We found that there was a significant association between MLVD and lymph node status (P=0.006), but this relation was not observed for MVD. Furthermore, we showed that increased CCR7 expression correlated significantly with higher MLVD (P=0.014) and MVD (P=0.002). Wound-healing and Matrigel Transwell assays indicated that activation of CCR7 with CCL21 significantly enhanced the invasion and migration abilities of UM-UC-3 cells, and this enhanced effect was significantly abrogated by CCR7 knockdown using siRNA. Western blot analysis revealed that the phospho-ERK1/2 level was markedly increased when UM-UC-3 cells were treated with CCL21 and significantly decreased when the CCR7 gene was silenced. MEK/ERK1/2 inhibition with PD98059 significantly suppressed the migration and invasion abilities of UM-UC-3 cells and also significantly abrogated the effects of CCL21/CCR7 on cell migration and invasion. Based on these results, we conclude that activation of the CCL21/CCR7 chemoaxis promotes lymph node metastasis of UBC in at least two ways. Firstly, although CCR7 is a promoting factor that induces both lymphangiogenesis and angiogenesis, it may promote lymph node metastasis through its lymphangiogenic effect rather than through its angiogenic effect. Secondly, the CCL21/CCR7 chemoaxis promotes the migration and invasion of UBC cells via the MEK/ERK1/2 signaling pathway rather than the PI3K/AKT pathway.
Keywords: urinary bladder cancer, lymph node metastasis, CCR7, CCL21, migration, invasion, angiogenesis, lymphangiogenesis, prognosis
Introduction
Urinary bladder cancer (UBC), which is mainly accompanied by symptoms of intermittent hematuria and vesical irritability, is the ninth most common type of cancer worldwide (1) and the second most common cancer of the genitourinary tract (2), with an estimated 76,960 newly diagnosed cases and 16,390 deaths in the United States in 2016 (3). Cigarette smoking is the most important risk factor for this heterogeneous cancer; other risk factors include schistosoma haematobium, occupational exposure to benzidine, β-naphthylamine, dyes or leather and physical trauma to the uroepithelium from infection, instrumentation or the presence of calculi. With respect to histopathology, transitional cell carcinomas account for >90% of all bladder cancers, whereas adenocarcinomas, squamous cell carcinomas (SCC) and undifferentiated bladder carcinomas constitute the remaining 10% of bladder cancers (4).
UBC displays the characteristic of frequent progression both at the time of diagnosis and after initial treatment. Such progression is the outcome of a tumorous natural history that includes the two closely related processes of invasion and metastasis. Tumor progression involves the migration of tumor cells and the invasion of other tissues and remains the most common cause of cancer-related deaths (5). Although non-muscle-invasive bladder cancer (NMIBC) accounts for ~70% of UBCs at initial presentation (6) and carries a 5-year survival rate of 90% (7), NMIBC tumors recur at a rate of 50–70% and progress at a rate of 15% in recurrent UBC patients (8). When NMIBC progresses to muscle-invasive bladder cancer (MIBC), which may display the characteristics of metastatic malignant tumors and subsequently lead to death, the 5-year survival rate falls to 60% (7). Even worse, ~50% of MIBC patients succumb to this disease despite optimal therapy (9), and ~80% of UBC patients with positive lymph node metastases die within the first 5 years after initial confirmed diagnosis (10,11). Tumor metastasis, which includes hematogenous and lymphatic metastasis, is one of the most critical aspects of tumor progression and contributes to ~90% of cancer-associated deaths (12). Despite advances in surgical techniques and in adjuvant chemotherapies and immunotherapies resulting from recent extensive studies of treatment options for advanced UBC with or without metastatic disease, the 5-year survival rate for patients with metastatic UBC was 5% (13), and that of patients with lymph node metastasis was <30% (14). Although conventional clinicopathological characteristics such as tumor grade and stage provide important prognostic information in UBC, they are of limited use in the prediction of tumor recurrence, progression, treatment response, and survival (15), partially due to the shortcomings of staging and grading subjectivity that can lead to high interobserver variability (16). Therefore, the investigation of novel molecular markers that are positively associated with tumor progression, metastasis and survival and determination of the molecular mechanisms of these markers in tumor progression are crucial for improving poor survival in UBC and developing a more exact prognosis-predictive and therapeutic strategy.
Various chemokines and their specific receptors have been shown to be involved in tumor progression, and some receptors have been particularly proposed as biomarkers for predicting survival, such as the CXCL12/CXCR7 axis in pancreatic (17), the CXCL12/CXCR4 axis in breast (18,19) and oral SCC (20), the CCL5/CCR1 axis in prostate (21) and the CCL21/CCR7 axis in gastric cancer (22). Among these, the most important chemokine/receptor axes are CXCL12/CXCR4 and CCL21/CCR7, mainly because of their determinant role in regulating the directional migration and organ-selective metastasis of tumor cells. An early breast cancer study showed significantly elevated CXCR4 and CCR7 expression in breast cancer cell lines and in tissue samples from patients and from mice in an established breast cancer model compared with normal controls (18). Given the preferential expression of CXCL12 in lung, liver and bone marrow and of CCL21 in lymph nodes found in the present study, the authors concluded that the CXCL12/CXCR4 axis may be responsible for metastasis to lung, liver and bone marrow and that CCL21 may be responsible for metastasis to lymph nodes (18). Notably, these hypotheses were confirmed by the results of subsequent experiments in vivo.
In the present study, we were most interested in the expression profile of CCR7 and the significance of the CCL21/CCR7 axis in progression, metastasis and survival in human UBC. Therefore, in the present study, we have initially evaluated the expression of CCR7 by immunohistochemical staining and determined its correlation with clinicopathological characteristics in 62 closely screened UBC patients. In addition, we investigated the microlymphatic vessel density (MLVD) and the microvessel density (MVD) in this cohort and determined their associations with CCR7 expression and clinicopathological parameters. These data provide preliminary evidence for a significant role of CCR7 in tumor progression, angiogenesis, lymphangiogenesis, survival and prognosis in UBC. In addition, the invasion and migration abilities of UBC cell lines with or without the intervention of CCL21 treatment or CCR7 gene silencing were tested in vitro. To further elucidate the mechanisms through which the CCL21/CCR7 axis regulates tumor behavior, the activities of the AKT and ERK-1/2 signaling pathways were also analyzed.
Materials and methods
Cell lines and reagents
The human bladder cancer cell lines T24, 5637, UM-UC-3 and RT4, as well as the SV-40 immortalized human uroepithelial cell line SV-HUC-1, which was used as a normal control, were purchased from the Cell Bank of the Chinese Academy of Sciences (Shanghai, China). The cells were cultured in Dulbecco's modified Eagle's medium (DMEM; Gibco, Grand Island, NY, USA) (UM-UC-3), RPMI-1640 medium (T24, 5637 and RT4) and F12K medium (SV-HUC-1) supplemented with 10% fetal bovine serum (FBS; Gibco), 100 units/ml penicillin, 100 μg/ml streptomycin and 2 mmol/l glutamine at 37°C in a humidified chamber supplemented with 5% CO2. When the cells reached 80–90% confluency, they were treated with various concentrations of recombinant human CCL21 purchased from PeproTech (Rocky Hill, NJ, USA) or with specific concentrations of CCL21 for 48 h to analyze the effect of activation of the CCL21/CCR7 axis on the invasion and migration capacity of UBC cells and the expression of possible related signaling pathway biomarkers.
Streptavidin-peroxidase (SP) ready-to-use kits, diaminobenzidine (DAB) tetrahydrochloride chromogenic reagent and mouse monoclonal antibody against CD34 were purchased from Beijing Zhongshan Golden Bridge Biotechnology (Beijing, China). Rabbit monoclonal antibody to CCR7, rabbit polyclonal antibody to total-ERK1/2 and rabbit monoclonal antibody to phospho-ERK1/2 were obtained from Abcam (Cambridge, MA, USA). Rabbit polyclonal antibody to AKT and rabbit polyclonal antibody to phospho-AKT were from ImmunoWay Biotechnology Inc., (Newark, DE, USA). Mouse monoclonal antibody to β-actin and horseradish peroxidase (HRP)-conjugated secondary antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Mouse monoclonal antibody to D2-40 was from Dako (Carpinteria, CA, USA). RIPA lysis buffer and the BCA protein assay kit were from Beyotime Institute of Biotechnology (Haimen, China). Sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) loading buffer was from Auragene Bioscience (Changsha, China). Lipofectamine 2000 was from Invitrogen (Carlsbad, CA, USA). Small interfering RNA (siRNA) oligonucleotides targeting the CCR7 gene and a control scrambled sequence siRNA were designed and synthesized by Shanghai GenePharma Co., Ltd. (Shanghai, China). PD98059 was obtained from Sigma-Aldrich (St. Louis, MO, USA).
Patient population and tissue specimen collection
After obtaining approval from the Xiangya Hospital Institutional Review Board, the clinical and pathological data of consecutive UBC patients who underwent radical cystectomy with bilateral pelvic and iliac lymphadenectomy at the authors' institution between June 2006 and June 2011 were reviewed. We included only patients for whom sufficient tissue and valid follow-up data were available and from whom written informed consent was obtained before they were enrolled in the study. We also excluded patients who received neoadjuvant chemotherapy or radiotherapy and those who exhibited synchronous or metachronous cancer in other organs, which finally left 62 patients for the analysis.
Tissue specimens were immediately formalin-fixed after surgery and then embedded in paraffin for immunohistochemical analysis. Sections from each case were stained with hematoxylin and eosin and investigated by light microscopy to confirm histopathology. The pathological tumor stage and grade were assigned according to the 2009 UICC-TNM classification system and the 2004 WHO system, respectively. Eight additional samples of normal bladder tissues were also obtained from sites apparently distant from the tumors. All procedures were performed in accordance with the ethical principles set forth in the Declaration of Helsinki. We had no access to information that could be used to identify individual participants during or after data collection.
Immunohistochemical staining and evaluation
UBC tissue samples were fixed in 10% formalin, embedded in paraffin, and cut into 4-μm slices. Immunohistochemical staining was performed on a single representative block from each case using the streptavidin-peroxidase method with an SP ready-to-use kit according to the manufacturer's instructions. Tissue blocks with at least 1 mm of peripheral tissue surrounding the tumor mass were selected. The tissue blocks were deparaffinized by incubating them twice in xylenol for 10 min at room temperature, dehydrated in a graded ethanol series (50, 75 and 100%), incubated in 3% hydrogen peroxide for 30 min to block endogenous peroxidases, subjected to antigen retrieval in 10 mmol/l citric acid buffer (pH 6.0) in a microwave oven for 10 min and allowed to cool at room temperature. Following three 5-min washes in phosphate-buffered saline (PBS), the sections were treated with normal goat serum for 10 min at room temperature to block non-specific antibody reactions. The sections were further incubated overnight at 4°C with the following diluted primary antibodies according to the manufacturer's instructions: rabbit monoclonal anti-CCR7 (1:200), mouse monoclonal anti-CD34 (1:200) and mouse monoclonal anti-D2-40 (1:50). The sections were then sequentially incubated with anti-goat polyvalent antibody for 10 min and with streptavidin-peroxidase for 10 min and subsequently visualized by incubation in 0.03% DAB tetrahydrochloride chromogenic reagent followed by counterstaining with hematoxylin. For negative controls, PBS was used as a substitute for the primary antibody. Human spleen tissues and normal lymph nodes were used as positive controls for CCR7 and D2-40 staining, respectively, as suggested by the manufacturer.
For each tissue section, immunohistochemical detection of CCR7 expression was evaluated independently by two pathologists who were blinded to the patients' clinicopathological data. Expression was evaluated using a semi-quantitative scoring system that depended on the percentage of positively stained tumor cells and the staining intensity. The percentage of positively stained cells was graded on a semi-quantitative 5-point scale as follows: 0 (none); 1 (1–10%); 2 (11–50%); 3 (51–75%); and 4 (76–100%). The immunostaining intensity was graded on a semi-quantitative 4-point scale as follows: 0 (none, equivalent to the negative control); 1 (weak, slightly darker than the negative control); 2 (moderate, equivalent to an intensity between that of scores 1 and 3); and 3 (intense, equivalent to or darker than the positive control). Then, the percentage of positively stained cells and the staining intensity score were multiplied to yield a final score ranging from 0 to 12. Borderline cases were re-evaluated by joint review and discussion or by consultation with a third investigator familiar with immunohistochemical evaluation of pathology.
Evaluation of MLVD and MVD
For evaluation of MLVD and MVD, tissue sections were stained with D2-40 (a monoclonal antibody containing 166 amino acids that recognizes a sialoglycoprotein expressed in lymphatic endothelial cells with high specificity and affinity and thus is widely used for labeling microlymphatic vessels) and CD34 (a highly glycosylated transmembrane glycoprotein that is expressed in vascular endothelial cells and thus is widely used for labeling microvessels) antibodies, respectively. MLVD and MVD were assessed by two independent pathologists who had no prior knowledge of the patient information according to the following steps: Firstly, areas with the most intense vascularization (hot spots) were selected under low-power magnification (×40 or ×100); secondly, average MLVD/MVD was measured at high-power magnification (×200) in 3 randomized fields within the hot spots following agreement of the two pathologists. For each section, MLVD/MVD was defined as the average number of positively stained vessels per high-power field (HPF) in three HPFs within the selected hot spots. Hot spots within both the intratumoral area, which was defined as the area within the tumor mass, and peritumoral area, which was defined as the area within 1 mm of the tumor border, were analyzed. In addition to distinct stained vessels, single immunoreactive endothelial cells or clusters of immunoreactive endothelial cells (containing brown-colored particles due to the positive expression of CD34 or D2-40) that were clearly separated from the adjacent clusters or vessels, regardless of the presence of lumen, were also counted as one microvessel or as one microlymphatic vessel.
CCR7 gene silencing by siRNA
The target sequences of three siRNAs of CCR7 and one siRNA with a scrambled sequence, which served as a non-silencing control due to its random sequence that does not target any gene product, were as follows: CCR7 siRNA1 sense: 5′-GCGUCCUUCUCAUCAGCAAdTdT-3′ and antisense: 5′-UUGCUGAUGAGAAGGACGCdTdT-3′; CCR7 siRNA2 sense: 5′-GCUGGUCGUGUUGACCUAUdTdT-3′ and antisense: 5′-AUAGGUCAACACGACCAGCdTdT-3′; CCR7 siRNA3 sense: 5′-GAUGAGGUCACGGACGAUUdTdT-3′ and antisense: 5′-AAUCGUCCGUGACCUCAUCdTdT-3′; scrambled siRNA sense: 5′-AGUUCAACGACCAGUAGUCdTdT-3′ and antisense: 5′-GACUACUGGUCGUUGAACUdTdT-3′. Cells in 6-well culture plates were transfected with siRNA using Lipofectamine 2000 transfection reagent according to the manufacturer's instruction, and the transfection efficiency was assessed by western blot assay. Cells that showed effective depletion of CCR7 were selected for use in cell migration assays, cell invasion assays and western blot analysis.
Protein extraction and western blot analysis
Cells were lysed in RIPA lysis buffer for 30 min at 4°C and centrifuged at 130,00 rpm for 20 min at 4°C; the protein concentration of the lysate was measured using the BCA protein assay kit. Protein samples were mixed with an equal amount of SDS-PAGE loading buffer and separated using 10% SDS-PAGE. After electrophoresis, proteins were transferred to polyvinylidene fluoride membranes by semi-dry electrophoretic transfer. The membranes were blocked in 5% skim milk and incubated with primary antibodies to CCR7 (1:5,000), phospho-ERK (1:500), total-ERK (1:2,000), phospho-AKT (1:2,000), and total-AKT (1:2,000) overnight at 4°C. Monoclonal β-actin antibody (1:500) was used to provide a loading control. The membranes were washed five times in TBST and incubated with HRP-conjugated secondary antibodies. Immunoreactive bands were imaged with an EC3 300 Imaging System (UVP LLC, Upland, CA, USA), and the OD values corresponding to the image intensity were measured using Image-Pro Plus version 6.0 (IPP6.0; Media Cybernetics, Inc., Rockville, MD, USA) software.
Wound-healing assay
To assess the migration ability of human UBC cells, a wound-healing assay was performed. Cells were plated in 6-well culture dishes and incubated with DMEM containing 10% FBS with or without CCL21 treatment for 24 h at 37°C under 5% CO2. When the cells had formed a confluent monolayer, a scratch was made with a fine pipette tip, forming a linear wound in the central area of the cell monolayer, and the detached cells were carefully removed using PBS. The wound area was photographed at the beginning of the experiment and at 24 and 48 h after wounding the cell monolayer. Cell migration was assessed by measuring the size of the wound gap in at least six fields. The wound-healing assay was performed in triplicate.
Matrigel invasion assay
To assess the invasion ability of human UBC cells, a Matrigel invasion assay was performed using a polycarbonate membrane Transwell chamber containing a filter 6.5 mm in diameter with 8-μm pores (Corning Inc., Corning, NY, USA). The Transwell filter was precoated with basement membrane Matrigel (BD Biosciences, San Jose, CA, USA). Briefly, cells pretreated with or without CCL21 for 48 h were resuspended in serum-free DMEM medium, and 300 μl of the cell suspension (5×104 cells) was added to the upper chamber of the device. DMEM containing 10% FBS was added to the lower chamber as a chemoattractant. After incubation for 24 h at 37°C under 5% CO2, non-invasive cells on the upper surface of the filter were removed completely and carefully using cotton swabs, and the lower surface of the filter was fixed in 4% formaldehyde and stained with 0.5% crystal violet for 20 min. After washing the filter twice with PBS, the cells on the lower side of the filter were photographed using an inverted microscope (×100 magnification), and absorbance was measured at 570 nm. The Matrigel invasion assay was performed in triplicate.
Statistical analysis
Data are presented as the mean ± standard deviation (SD) of the values obtained in three independent experiments or as the median (minimum-maximum) for non-normally distributed data. Qualitative variables such as sex, histological grade, tumor stage and lymph node involvement are presented as frequencies or percentages. Qualitative variables were analyzed using the Chi-square test or Fisher's exact test as appropriate. Quantitative variables were compared using the independent t-test or one-way analysis of variance (ANOVA) to compare differences in 2 or more groups, and the least significant difference (LSD) t-test, the Dunnett's t-test or the Student-Newman-Keuls (SNK) q-test, as appropriate, were used for post hoc subgroup analysis. Survival curves were calculated using the Kaplan-Meier method, and differences in the survival curves were examined using the log-rank test. Cox's proportional hazard regression model was used for multivariate analysis to assess the effect of tumor variables on the prognosis of UBC. Statistical analysis was performed using SPSS statistical software, version 19.0 (IBM, Armonk, NY, USA). The significance of differences was accepted at P<0.05 (2-tailed).
Results
Correlation between CCR7 expression and clinicopathological parameters and prognosis in 62 UBC patients
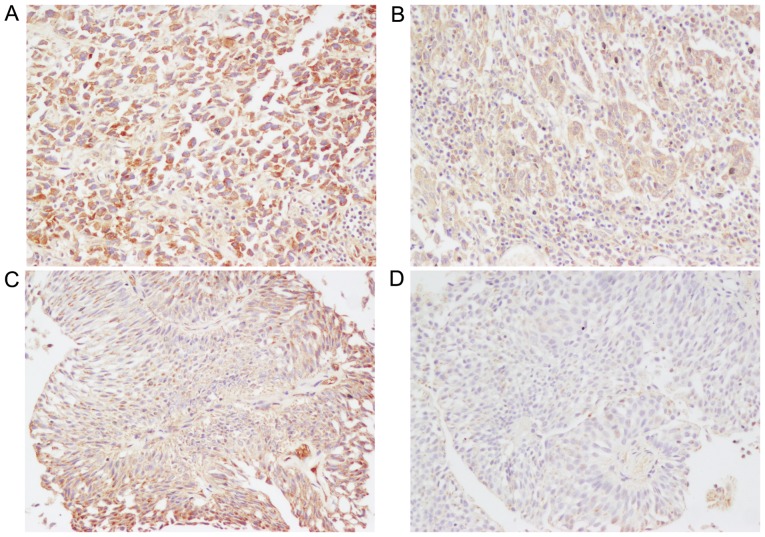
The expression and distribution of CCR7 protein in UBC tissues of different grades and stages, including papillary urothelial neoplasm of low malignant potential (PUNLMP), low-grade urothelial carcinoma (LGPUC) and high-grade urothelial carcinoma (HGPUC), as well as stage T1 (tumor invades the subepithelial connective tissue), stage T2 (tumor invades the muscularis propria bladder wall), stage T3 (tumor invades the perivesical tissue), and stage T4 (tumor invades any of the following: prostate, uterus, vagina, pelvic wall, or abdominal wall) cancerous tissues, were measured by immunohistochemical analysis in clinical samples obtained from a total of 62 patients (49 men and 13 women) with UBC. Fig. 1 shows representative immunostaining of CCR7 protein, which was positively stained only in the cytoplasm and cell membranes of UBC cells. The majority of cases showed a heterogeneous staining pattern that was characterized by variable staining intensity within the same section or field (Fig. 1C). The average CCR7 staining score for all 62 tumors was 5.80±0.04.
Figure 1.
Immunohistochemical detection of CCR7 protein in patients with urinary bladder cancer. Intense, moderate and weak CCR7 expression in urinary bladder cancer patients is shown in panels (A, B and D), respectively. (C) The urinary bladder cancer case that showed a heterogeneous staining pattern was characterized by intense staining within the tumor periphery and moderate staining within the tumor center in the same field. Original magnification, ×200.
The clinicopathological data of all cases are summarized in Table I. The follow-up time was defined as the interval between surgery and death or between surgery and the last observation for surviving patients. The median (range) follow-up time after operation was 43.5 months (6–74 months). Thirty-nine patients were alive at the last follow-up examination; the others died of metastasis, local tumor invasion, severely compromised immunity and other causes. The median age of the study subjects at the time of treatment was 60.5 years (range, 34–77 years).
Table I.
Correlations between clinicopathological characteristics and CCR7, MLVD and MVD in 62 urinary bladder cancer patients.
| Characteristics | No. of cases | CCR7 expression
|
MLVD
|
MVD
|
||||
|---|---|---|---|---|---|---|---|---|
| High (n=40) | Low (n=22) | P-value | MLVD/HPF (mean ± SD) | P-value | MVD/HPF (mean ± SD) | P-value | ||
| Age (years) | 0.618a | 0.872c | 0.632c | |||||
| <60 | 28 | 19 | 9 | 6.40±2.06 | 34.52±6.33 | |||
| ≥60 | 34 | 21 | 13 | 6.59±2.43 | 33.74±7.26 | |||
| Sex | 0.756b | 0.463c | 0.164c | |||||
| Male | 49 | 31 | 18 | 6.37±2.53 | 34.83±6.27 | |||
| Female | 13 | 9 | 4 | 7.02±2.09 | 31.32±7.56 | |||
| Primary tumor (pT)e | 0.015a | 0.027c | 0.016c | |||||
| pT1-T2 | 24 | 11 | 13 | 3.87±2.37 | 30.81±8.36 | |||
| pT3-T4 | 38 | 29 | 9 | 8.17±3.16 | 36.17±7.24 | |||
| Regional lymph nodes(pN)e | 0.008a | 0.006c | 0.385c | |||||
| pN0 | 40 | 21 | 19 | 4.03±2.03 | 33.43±7.82 | |||
| pN1-N3 | 22 | 19 | 3 | 11.00±2.64 | 35.30±6.59 | |||
| Classification of 2004 | 0.010a | 0.246d | 0.447d | |||||
| WHO grading system | ||||||||
| PUNLMP | 5 | 2 | 3 | 5.23±2.28 | 34.16±8.93 | |||
| LGPUC | 24 | 11 | 13 | 5.45±2.74 | 33.78±7.02 | |||
| HGPUC | 33 | 27 | 6 | 7.46±2.93 | 34.31±7.40 | |||
PUNLMP, papillary urothelial neoplasm of low malignant potential; LGPUC, low grade urothelial carcinoma; HGPUC, high grade urothelial carcinoma; MLVD, microlymphatic vessel density; MVD, microvessel density; HPF, high power fields.
Chi-square tests;
Fisher's exact test;
independent samples t-test;
one-way analysis of variance.
Staging was confirmed based on the 2009 UICC-TNM staging system.
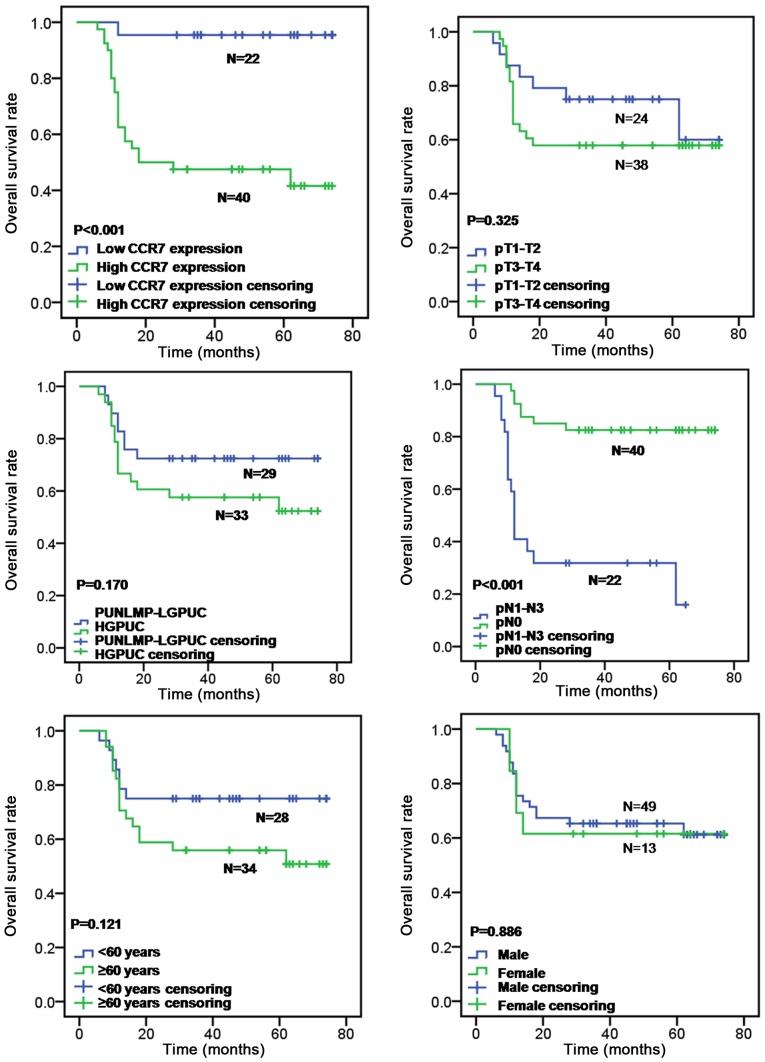
For statistical analyses, the mean CCR7 staining score was used as a cut-off to separate tissue sections with high CCR7 expression (final scores ranging from 6 to 12) from those with low CCR7 expression (final scores ranging from 0 to 4). The correlations between CCR7 immunoreactivity and the clinicopathological characteristics of the subjects are summarized in Table I. High expression of CCR7 protein was identified in 64.52% (40/62) of the tumors, and low expression was identified in 35.48% (22/62) of the tumors. CCR7 expression was significantly higher in patients with lymph node status of pN1-N3 than in patients with lymph node status of pN0 (P=0.008, Chi-square test) and was significantly associated with primary tumor stage (P=0.015; Chi-square test) and tumor grade (P=0.010; Chi-square test), whereas there was no significant correlation between CCR7 expression and patient age (P>0.05; Chi-square test) or sex (P>0.05; Fisher's exact test). Patients with high CCR7 expression exhibited a significantly worse overall survival rate than those with low CCR7 expression by the log-rank test (P<0.001; Fig. 2). Furthermore, lymph node metastasis was also correlated with overall survival rate (log-rank test, P<0.001; Fig. 2), whereas patient's age (log-rank test, P=0.121; Fig. 2), sex (log-rank test, P=0.886; Fig. 2), primary tumor stage (log-rank test, P=0.325; Fig. 2) and tumor grade (log-rank test, P=0.170; Fig. 2) had no prognostic significance for overall survival. Prognostic factors of UBC and their variable assignment as determined by the multivariate analysis are shown in Table II. By multivariate analysis based on the Cox's proportional hazard regression model, CCR7 protein expression level and pN were independent prognostic factors for overall survival in UBC patients (HR, 10.413, 95% CI, 1.242–87.313, P=0.031 and HR, 0.211, 95% CI, 0.083–0.534, P=0.001, respectively), whereas, the patient's sex, age, tumor grade and primary tumor stage were not independent prognostic factors (P=0.318, P=0.792, P=0.816 and P=0.351, respectively) (Table III). These results indicate that high levels of CCR7 expression may be associated with lymph node metastasis and poor overall survival in patients with UBC.
Figure 2.
Kaplan-Meier curves for overall survival in 62 patients with urinary bladder cancer. The patients with high CCR7 expression and positive lymph node metastasis showed a significantly worse long-term overall survival rate than those with low CCR7 expression and negative lymph node metastasis by the log-rank test (P<0.001), whereas age (P=0.121), sex (P=0.886), primary tumor stage (P=0.325) and tumor grade (P=0.170) had no prognostic significance for overall survival.
Table II.
Prognostic factors of urinary bladder cancer and their variable categorization.
| Prognostic factors | Variable names | Variable values |
|---|---|---|
| Age | X1 | 0=<60 years, 1=≥60 years |
| Sex | X2 | 0=male, 1=female |
| pT | X3 | 0=pT1-T2, 1=pT3-T4 |
| pN | X4 | 0=pN1-N3, 1=pN0 |
| Pathological grade | X5 | 0=PUNLMP-LGPUC, 1=HGPUC |
| CCR7 expression | X6 | 0=low, 1=high |
| Survival time | t | (months) |
| Survival status | Y | 0=censoring, 1=death |
PUNLMP, papillary urothelial neoplasm of low malignant potential; LGPUC, low grade urothelial carcinoma; HGPUC, high grade urothelial carcinoma.
Table III.
Cox multivariate analysis of the factors associated with overall survival.
| Variables | HR | 95% CI | P-value |
|---|---|---|---|
| Age (<60 vs. ≥60 years) | 0.854 | 0.264–2.761 | 0.792 |
| Sex (male vs. female) | 1.706 | 0.598–4.869 | 0.318 |
| pT (pT1-T2 vs. pT3-T4) | 1.583 | 0.603–4.157 | 0.351 |
| pN (pN1-N3 vs. pN0) | 0.211 | 0.083–0.534 | 0.001 |
| Pathological grade (PUNLMP-LGPUC vs. HGPUC) | 1.141 | 0.374–3.488 | 0.816 |
| CCR7 (low vs. high) | 10.413 | 1.242–87.313 | 0.031 |
PUNLMP, papillary urothelial neoplasm of low malignant potential; LGPUC, low grade urothelial carcinoma; HGPUC, high grade urothelial carcinoma. HR, hazard ratio; CI, confidence interval.
Identification of D2-40-positive lymphatic vessels and CD34-positive blood vessels and measurement of MVD/MLVD
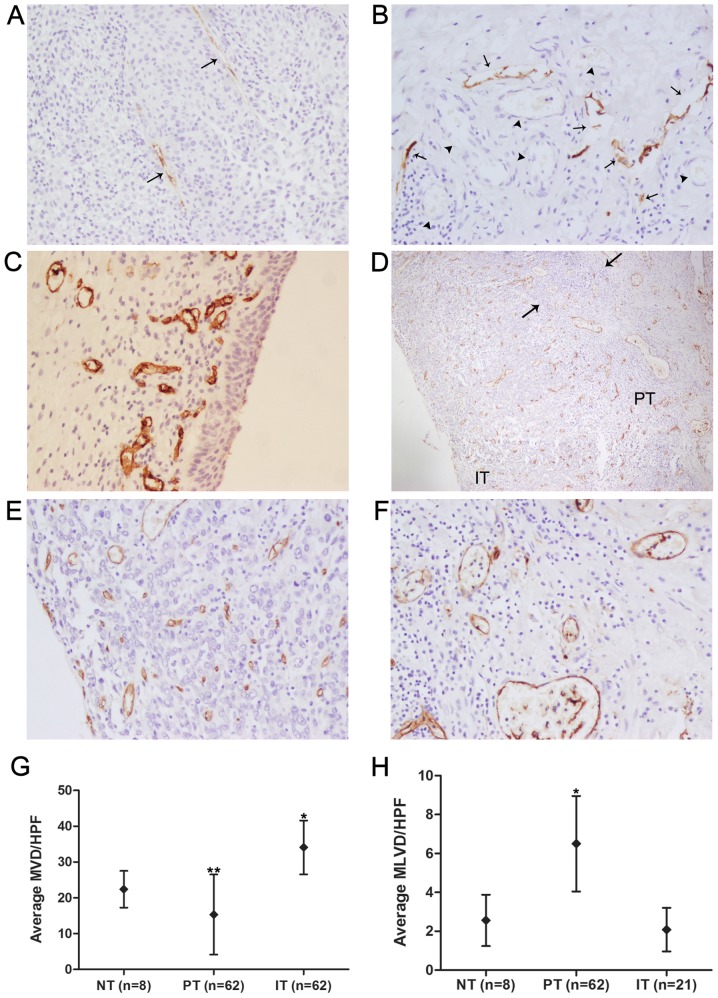
D2-40 positive staining was seen in thin-walled vessels devoid of erythrocytes, but no D2-40 staining was observed in tumor cells or blood vessels, indicating that D2-40 is specific for the lymphatic vasculature (Fig. 3A and B). D2-40-positive lymphatic vessels and CD34-positive blood vessels were detected in all tumor samples, and D2-40-positive lymphatic vessels were detected within peritumoral areas in 62 (100%) cases and within intratumoral areas in 21 (33.87%) cases. The morphological characteristics of D2-40-positive lymphatic vessels within intratumoral and peritumoral areas are shown in Fig. 3A and B. Peritumoral lymphatic vessels had more dilated lumina, were denser and more numerous, and showed a greater number of hotspots than intratumoral lymphatic vessels, which appeared collapsed and elongated. The anatomic locational correlation of peritumoral lymphatics with blood vessels was higher than that of intratumoral lymphatics (Fig. 3A and B). With regard to the eight normal bladder tissues obtained from obviously non-tumorous regions of bladder far from the tumor boundaries in patients who underwent radical cystectomy, both D2-40-positive lymphatic vessels and CD34-positive blood vessels were present in all normal control tissues, but were found only in the lamina propria and submucosa, and no epithelial expression was found (Fig. 3C). The average MLVD of normal bladder tissues was (2.56±1.08)/HPF, which was significantly lower than the average peritumoral MLVD [(6.50±2.01)/HPF; P<0.001, SNK q-test; Fig. 3H] and higher than the average intratumoral MLVD [(2.08±0.92)/HPF; P>0.05, SNK q-test; Fig. 3H], but the MLVD did not differ significantly between normal control tissues and intratumoral areas. Unlike the distribution of D2-40-positive vessels in serial tumor sections, CD34-positive blood vessels were homogeneously present in the peritumoral and intratumoral areas in the great majority of cases (Fig. 3D). The average MVDs of normal bladder tissues, peritumoral areas and intra-tumoral areas were (22.40±4.59)/HPF, (15.33±10.23)/HPF and (34.09±7.26)/HPF, respectively, and the differences between any two groups were significant (P<0.05, SNK q-test; Fig. 3G). Inflammatory infiltration by lymphocytes was frequently observed in the peritumoral area (Fig. 3D).
Figure 3.
Immunohistochemical detection of D2-40 and CD34 proteins in patient with urinary bladder cancer. (A) Intratumoral lymphatic vessels (arrows) detected with D2-40 antibody in urinary bladder cancer samples presented collapsed and elongated size. (B) Peritumoral lymphatic vessels (arrows) detected with D2-40 antibody in urinary bladder cancer samples were found to be more dilated in the lumen, more numerous in vessel density and closer to anatomic locational correlation with blood vessels (arrowheads), which were identified by the characteristic of intraluminal erythrocytes compared with intratumoral lymphatics. (C) CD34-positive vessels representing blood vessels in normal control tissues were detected in the lamina propria and submucosa, while the epithelium expression was not found. (D) CD34-positive blood vessels were homogeneously present in the peritumoral (PT) and intratumoral areas (IT) of urinary bladder cancer cases, and inflammatory infiltration (the area between the two arrows) by lymphocytes was observed in the peritumoral area. Representative intratumoral and peritumoral blood vessels detected with CD34 antibody are shown, respectively (E and F). (G) Intratumoral MVD (n=62) was the highest, followed by the MVD of normal bladder tissues (NT, n=8) and peritumoral MVD (n=62). *P<0.05 compared to the PT group and the NT group, **P<0.05 compared to the IT group and the NT group (one-way ANOVA followed by the SNK q-test). (H) Peritumoral MLVD (n=62) was the highest, followed by the MLVD of normal bladder tissues (n=8) and intratumoral MLVD (n=21). *P<0.001 compared to the IT group and the NT group (one-way ANOVA followed by the SNK q-test). Original magnification, ×200 in A, B, C, E and F; ×40 in D.
Correlation of MVD/MLVD with clinicopathological parameters and CCR7 expression in 62 UBC patients
Microlymphatic vessel density per high-power field (MLVD/HPF) and microvessel density per high-power field (MVD/HPF) were assessed by immunohistochemical staining of 62 UBC tissues with antibodies against CD34 and D2-40. Due to the low detection rate and potentially non-functional characteristics of collapsed intratumoral lymphatic vessels, we only studied the relationship of peritumoral MLVD with clinical pathological parameters. As shown in Table I, a significantly higher MLVD/HPF was observed in patients with primary tumor stage of pT3-T4 (P=0.027) and lymph node status of pN1-N3 (P=0.006) than in patients with primary tumor stage of pT1-T2 and lymph node status of pN0. However, no obvious relationship was found between MLVD/HPF and patient age, sex or tumor grade (all P>0.05). In addition, the data also showed that higher MVD/HPF was significantly related to a primary tumor stage of pT3-T4 (P=0.016) rather than pT1-T2. MVD/HPF, however, was not significantly associated with patient age, sex, lymph node metastasis or tumor grade (all P>0.05).
For further analysis of the possible correlation between CCR7 expression and MLVD/MVD, the 62 UBC patients were grouped into a low-CCR7-expression group and a high-CCR7-expression group according to their CCR7 expression levels. Table IV shows that increased CCR7 expression was significantly correlated with higher MLVD/HPF (P=0.014) and higher MVD/HPF (P=0.002). These results imply that CCR7 is a promoting factor that induces both lymphangiogenesis and angiogenesis but that it may be correlated with lymph node metastasis by virtue of its lymphangiogenic role rather than its angiogenic role.
Table IV.
Correlations between CCR7 expression and MVD/MLVD in 62 urinary bladder cancer patients.
| CCR7 expression | No. of cases | MLVD
|
MVD
|
||
|---|---|---|---|---|---|
| MLVD/HPF (mean ± SD) | P-value | MVD/HPF (mean ± SD) | P-value | ||
| High | 40 | 8.11±2.39 | 0.014a | 37.03±8.03 | 0.002a |
| Low | 22 | 3.57±1.84 | 28.76±7.19 | ||
MLVD, microlymphatic vessel density; MVD, microvessel density; HPF, high power fields.
Independent samples t-test.
Influence of the CCL21/CCR7 axis on the migration and invasion capacity of UBC cells
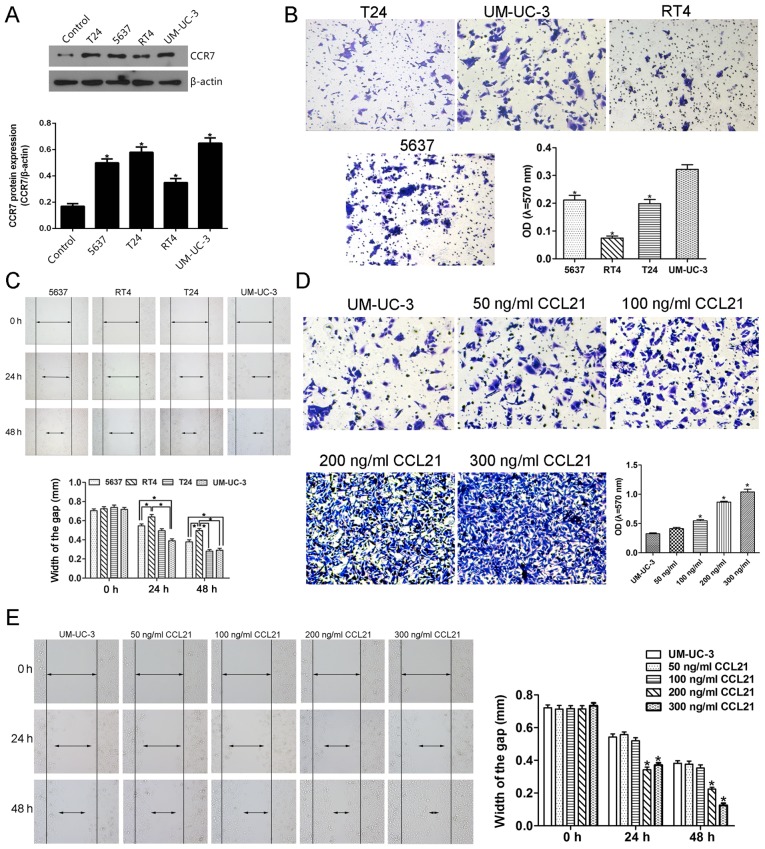
Prior to the investigation of the effect of the CCL21/CCR7 axis on the migration and invasion ability of UBC cells, we screened the constitutive expression of CCR7 in T24, 5637, UM-UC-3, RT4 UBC cells and SV-HUC-1 normal uroepithelial cells by western blot analysis. A significantly higher CCR7 expression level was observed in T24, 5637, UM-UC-3 and RT4 UBC cell lines than in SV-HUC-1 cell line which was used as the control cells (Fig. 4A). Furthermore, after treatment with 200 ng/ml CCL21, the migration ability of the UM-UC-3 cells assessed by the mean of wound-healing assay was significantly enhanced at 24 h compared with other cells, and that of UM-UC-3 and T24 cells was significantly enhanced at 48 h compared with other cells (Fig. 4C). Similarly, the invasive ability of UM-UC-3 cells determined by the mean of Matrigel invasion assay using a polycarbonate membrane Transwell chamber was significantly higher than that of other UBC cells (Fig. 4B). Therefore, based on the results that the migration and invasion abilities of UM-UC-3 UBC cells were significantly higher than that of other UBC cells, the UM-UC-3 UBC cell line was selected for the following in vitro study even though the T24, 5637 and UM-UC-3 cells show similar CCR7 protein expression level (Fig. 4A).
Figure 4.
The CCL21/CCR7 axis modulates the invasion and migration by UM-UC-3 cells in a dose- and time-dependent manner. (A) Western blotting was used to detect the CCR7 expression in SV-HUC-1 control cells and T24, 5637, UM-UC-3 and RT4 urinary bladder cancer cells. *P<0.05 compared to the control group (one-way ANOVA followed by Dunnett's t-test). (B) Matrigel Transwell assay was used to determine the invasion ability of T24, 5637, UM-UC-3 and RT4 urinary bladder cancer cells. *P<0.05 compared to UM-UC-3 cells (one-way ANOVA followed by Dunnett's t-test). (C) The wound-healing assay was used to assess the migration ability of T24, 5637, UM-UC-3 and RT4 urinary bladder cancer cells. *P<0.05 (one-way ANOVA followed by the SNK q-test). (D) Matrigel Transwell assay was used to determine the invasion ability of UM-UC-3 cells untreated or pretreated with 50 100, 200 and 300 ng/ml CCL21 for 48 h. *P<0.05 compared to untreated UM-UC-3 cells and to UM-UC-3 cells treated with 50 ng/ml CCL21 (one-way ANOVA followed by the LSD t-test). (E) The wound-healing assay was used to detect the migration ability of UM-UC-3 cells untreated or pretreated with 50, 100, 200 and 300 ng/ml CCL21 for 24 and 48 h. *P<0.05 compared to UM-UC-3 group and to UM-UC-3 cells pretreated with 50 or 100 ng/ml CCL21 (one-way ANOVA followed by the LSD t-test). Each bar represents the mean ± SD from three independent experiments.
The effect of CCL21 at various concentrations on the invasion and migration capacity of UM-UC-3 cells is shown in Fig. 4D and E. To determine whether CCL21 was able to modulate invasion ability in UM-UC-3 cells, the Matrigel invasion assay was used to evaluate the cell's invasion ability. As presented in Fig. 4D, the OD of the cell suspensions of CCL21-treated cells increased gradually and significantly as the concentration of CCL21 was increased from 100 to 300 ng/ml, indicating that CCL21 treatment significantly enhanced the invasion ability of UM-UC-3 cells in a dose-dependent manner (P<0.05). When the UM-UC-3 cells were treated with CCL21 at 50 and 100 ng/ml, the migration ability of the cells did not change significantly (Fig. 4E). However, as the treatment time increased and as the concentration of CCL21 protein was increased to 100 ng/ml and higher, an obvious effect of increased cell migration occurred. These results show that treatment of UM-UC-3 cells with CCL21 enhances their migration ability in a dose- and time-dependent manner.
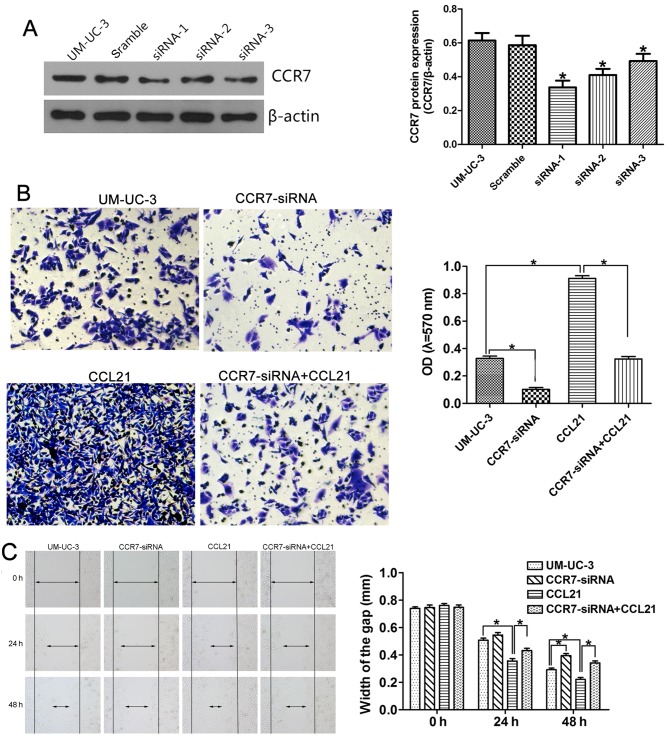
To confirm the influence of the CCL21/CCR7 axis on the migration and invasion capacity of UM-UC-3 cells, small interfering RNAs (siRNAs) targeting the CCR7 gene were used for CCR7 knockdown, and exogenous CCL21 was used for CCR7 activation. Fig. 5A shows that all three CCR7 siRNA sequences (siRNA-1, siRNA-2 and siRNA-3) significantly depleted CCR7 expression in the UM-UC-3 cell line, as determined by western blotting, compared with cells transfected with negative control siRNA. UM-UC-3 cells transfected with CCR7 siRNA-1 were selected for use in the following in vitro study. The effects of CCR7 knockdown on the invasion behavior of the cells, as represented by the OD values, are shown in Fig. 5B. The OD values were 0.328±0.028 in the control group, 0.102±0.024 in UM-UC-3 cells transfected with CCR7 siRNA-1 (CCR7-siRNA group; P<0.05 compared with the control group), 0.912±0.033 in UM-UC-3 cells pretreated with 200 ng/ml CCL21 for 48 h (CCL21 group; P<0.05 compared with the control group), and 0.324±0.032 in UM-UC-3 cells treated with 200 ng/ml CCL21 after transfection with CCR7 siRNA-1 (CCR7-siRNA+CCL21 group; P<0.05 compared with the CCL21 group). These results indicate that CCR7 knockdown attenuates the enhancement by CCL21 of the invasive behavior of UM-UC-3 cells. CCL21 treatment significantly enhanced cell migration ability, and CCR7 knockdown by siRNA significantly abrogated the enhanced effect of CCL21 on the migration of UM-UC-3 cells (Fig. 5C). After transfection with CCR7 siRNA-1 for 24 h, the difference in the migration ability of the CCR7-siRNA group and the control group was not statistically significant. However, when the UM-UC-3 cells were transfected for 48 h, the difference was significant.
Figure 5.
Gene silencing of CCR7 by siRNA inhibits migration and invasion by UM-UC-3 cells. UM-UC-3 cells were transfected with scrambled siRNA as a negative control or with CCR7 siRNA-1, siRNA-2 or siRNA-3. (A) CCR7 protein expression in transfected or non-transfected UM-UC-3 cells was evaluated using western blotting. *P<0.05 compared to non-transfected UM-UC-3 cells and to UM-UC-3 cells transfected with scrambled siRNA (one-way ANOVA followed by the LSD t-test). The invasion and migration capacities were measured using the Matrigel Transwell assay (B) and the wound-healing assay (C) in four independent groups consisting of a control group, a CCR7-siRNA group, a CCL21 group and a CCR7-siRNA+CCL21 group. *P<0.05 (one-way ANOVA followed by the LSD t-test). Each bar represents the mean ± SD from three independent experiments.
The ERK/AKT signaling pathway in the CCL21/CCR7 axis induces enhanced migration and invasion capacity of UBC cells
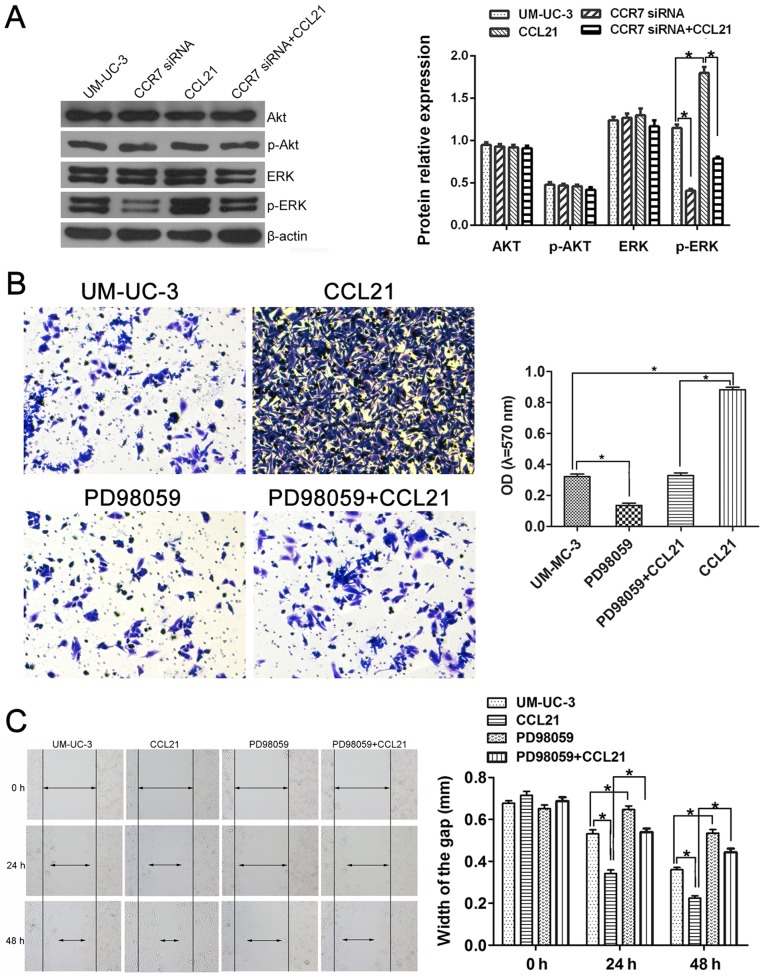
To investigate whether CCL21/CCR7 interaction enhanced the invasion and migration ability of UM-UC-3 cells via the MEK/ERK1/2 pathway or the PI3K/AKT pathway, the expression levels of total-ERK1/2, phospho-ERK1/2, total-AKT and phospho-AKT were first assessed using western blotting (Fig. 6A). The expression levels of total-ERK1/2, total-AKT and phospho-AKT in UM-UC-3 cells did not significantly change either after CCR7 gene knockdown or CCL21 treatment (P>0.05). However, the phospho-ERK1/2 protein level increased when the cells were treated with CCL21 and decreased when the CCR7 gene was silenced (P<0.05). These results indicate that CCL21/CCR7 interaction may modulate the action of the MEK/ERK signaling pathway but not that of the PI3K/AKT pathway in UM-UC-3 cells.
Figure 6.
The ERK1/2 pathway is involved in CCL21/CCR7 axis-induced enhanced migration and invasion by UM-UC-3 cells. (A) Total-ERK1/2, phospho-ERK1/2, total-AKT and phospho-AKT expression levels were detected by western blotting in the control group, the CCR7-siRNA group, the CCL21 group and the CCR7-siRNA+CCL21 group. *P<0.05 (one-way ANOVA followed by the LSD t-test). After sequential treatment with CCL21 and PD98059, UM-UC-3 cellular invasion and migration capacities were measured using Matrigel Transwell assays (B) and wound-healing assays (C). *P<0.05 (one-way ANOVA followed by the LSD t-test). Each bar represents the mean ± SD from three independent experiments.
To determine how CCL21/CCR7 interaction promoted the invasion and migration functions of UM-UC-3 cells via the MEK/ERK1/2 pathway, PD98059 was used to inhibit the activation of MEK. PD98059 significantly suppressed the invasion ability of UM-UC-3 cells and abrogated the enhancement of the invasion ability of UM-UC-3 cells by CCL21/CCR7 (P<0.05; Fig. 6B). Fig. 6C shows that inhibition of MEK (the upstream regulatory protein of ERK1/2) by PD98059 in UM-UC-3 cells led to significantly delayed wound healing at all times examined compared with the control group and significantly interfered with the rapid wound healing induced by CCL21 (P<0.05).
Discussion
The CC-chemokine receptor 7 (CCR7), which belongs to the Class A subfamily of G protein-coupled receptors with seven transmembrane domains, is widely expressed in various types of immune cells including naive, regulatory and central memory T cells, naive B cells, double-negative and single-positive thymocytes, and (semi-) mature dendritic cells (DCs) and is functionally involved in the homing of various subpopulations of T cells and antigen-presenting DCs to the T cell areas of lymphoid tissues upon activation by the ligand of CCL21 (23,24). Furthermore, aberrantly increased CCR7 expression has been reported in breast (18,25), gastric (22,26,27), colorectal (28–30), non-small cell lung (31,32), cervical (33), SCC of the head and neck (34), prostate (35,36), melanoma (37) and esophageal, oral and oropharyngeal SCC cancer (38–41) and is generally associated with lymph node metastasis and worse prognosis. In addition, a previous study conducted at our institution in which the CCR7 expression levels in 115 patients with UBC and 10 patients with benign prostatic hyperplasia (BPH) were compared found a significantly higher frequency of detectable CCR7 expression in the UBC group than in the BPH group and the clinical feasibility of CCR7 expression level as an independent predictive biomarker in the diagnosis of lymph node metastasis (42). However, little is known about the association of CCR7 expression with survival, prognosis, demographic factors or other clinicopathological features of UBC, such as tumor TNM staging and tumor differentiation, and the underlying mechanisms through which CCR7 affects the lymphatic metastasis of tumor cells remain obscure.
Our finding that high CCR7 expression was significantly correlated with lymph node metastasis and poor survival is consistent with the results of almost all related previous studies (22,27,30,33,39,40). However, whether the CCR7 expression level can be used as an independent predictive factor in tumor prognosis remained controversial. Ding et al (39) reported that the CCR7 expression level was not identified as an independent prognostic factor in esophageal SCC by multivariate analysis. In contrast, some investigators reported that CCR7 was an independent prognostic factor for overall survival in gastric cancer, colorectal carcinoma, and cervical cancer (27,28,33). It is unclear why these studies resulted in two completely different conclusions, and no explanations for this contradictory phenomenon are evident. We hypothesize that discrepancies in the weight ratio of CCR7 to other pro-metastatic factors, including MMP-9, IL-8, VEGF and EGFR, within different tumor environments as well as heterogeneity in tumor type, study population and study method may partially account for the above phenomena. Our multivariate analysis showed that high CCR7 expression and lymph node metastasis were independent prognostic factors for overall survival in UBC, consistent with the findings reported by Ma et al (27), Gunther et al (28) and Kodama et al (33). We suggest that UBC patients who have negative lymph node metastasis but detectable high CCR7 expression should still be closely monitored, even though emphasis has formerly been placed on patients with positive lymph node involvement.
MVD, as assessed by immunohistochemistry using antibodies against CD34, CD31 or factor VIII-related antigen markers to identify vasculature, has been widely used as a surrogate marker for angiogenesis, which is characterized by neovascularization and is required for tumors to grow beyond a certain size and to metastasize to new sites (43). Of all these specific endothelial markers used for quantitative angiogenic studies, CD34 is widely accepted as the optimal marker especially in the invasive tumors due to its robustness and ease of use (44,45), and thus, the antibody against CD34 was selected in this study. It is known that MVD is associated with tumor progression and survival in UBC (46–49). In addition, several studies have reported that altered MVD is also associated with lymph node metastasis and decreased survival rate in UBC (50–52). In contrast to the extensive studies on tumor-induced angiogenesis, little is known about MLVD and tumor-induced lymphangiogenesis, which has been evaluated using similiar methods as for MVD except that the antibodies used were LYVE-1-, D2-40/podoplanin- or VEGFR-3-specific. In our literature review, we found that the use of VEGFR-3 as a marker for lymphatic vessels is controversial. A considerable amount of evidence shows that VEGFR-3 expression in tumors was detectable in the endothelium of lymphatic vessels, and VEGFR-3-positive vessels were counted when measuring MLVD (53–55). Additionally, however, it was reported that VEGFR-3 lacks specificity for lymphatic vessels in human tumors; tumoral VEGFR-3 expression was also present in the endothelium of blood vessels, and VEGFR-3 stained vessels were therefore used for MVD determination (56–58). The high specificity and sensitivity of D2-40 as a lymphatic endothelial marker to quantitatively assess lymphangiogenesis were verified by means of the immunohistochemical single staining using a single antibody to D2-40 (59), the immunohistochemical double staining using antibodies to D2-40 and CD34 (60) and the ultrastructure study using electron microscopy (61). Therefore, the antibody against D2-40 was selected as the specific lymphatic marker in this study. MLVD has been widely used as a marker for lymphangiogenesis characterized by the de novo formation of lymphatic vessels. With regard to the clinical significance and prognostic value of MLVD, a considerable number of studies reported that increased MLVD was statistically significantly correlated with lymph node metastasis and survival in head and neck SCC (62–64), breast (65), gastric cancer (66), prostate adenocarcinoma (67), cutaneous malignant melanoma (68,69) and UBC (53,70). There is compelling evidence that pro-angiogenic and pro-lymphangiogenic factors (such as agents in the vascular growth factor family) trigger angiogenesis and lymphangiogenesis, respectively, and are related to adverse tumor outcomes. However, the role of CCR7 as a pro-angiogenic or pro-lymphangiogenic factor was unclear. Based on the notably significant relationship of lymph node metastasis to MLVD/MVD and CCR7 expression in human cancers, as discussed above, we speculate that CCR7 may play a pivotal role in lymph node metastasis and poor prognosis of UBC by acting as a pro-angiogenic or pro-lymphangiogenic factor to induce angiogenesis or lymphangiogenesis.
Our finding that MLVD was significantly higher in peritumoral areas than in normal control tissue suggested that at least some of the peritumoral lymphatics were indeed derived from the newly formed lymphatic vessels by lymphangiogenesis rather than being pre-existing vessels. The lower MLVD in intratumoral areas compared with the normal and peritumoral compartments is consistent with observations reported in other studies (62,71–73), as are the collapsed and elongated morphological characteristics of intratumoral lymphatics. These observations suggest a possible mechanism for the formation of intratumoral lymphatics in which the early-stage rapidly growing tumor grows into pre-existing lymphatic vessels to form partitions that ultimately surround the whole lymphatic vessel; the increased intratumoral pressure resulting from the tumor merisis then further compresses the separated lymphatics into a collapsed and elongated shape. Although this hypothetical non-lymphangiogenic pattern for the formation of intratumoral lymphatics requires further functional studies in vitro and in vivo to verify its validity, we speculate that intratumoral non-lymphangiogenic lymphatic vessels are probably non-functional for tumor progression. Reports by others (74–76) that no lymphatic vessels were detected within the intratumoral areas of malignant tumors of the female genital system further support our hypothesis. In view of the poor function of intratumoral lymphatics in tumor metastasis, the clinical significance of peritumoral lymphangiogenesis and peritumoral MLVD was analyzed. Our results showed that regional lymph node invasion was significantly associated with increased levels of peritumoral MLVD rather than MVD, consistent with the findings of other studies (53,62,65–69) and suggesting that peritumoral lymphangiogenesis rather than angiogenesis is involved in the process of regional lymph node metastasis. On the other hand, in view of the significant correlation between MVD and tumor stage that was observed in this study (Table I), we presumed that tumor-associated angiogenesis is mainly involved in tumor growth and progression by acting as a pipeline for the transpor nutrients required for tumor cell growth. This presumption is consistent with our finding that MVD was highest within the intratumoral areas, followed by normal controls and peritumoral areas (Fig. 3G), indicating a greater demand for blood supply and nutrients within the tumor. Further analysis of correlations between CCR7 and MVD/MLVD revealed that high CCR7 expression level was associated with both increased MVD and MLVD, suggesting a promoting role of CCR7 in angiogenesis and lymphangiogenesis. Based on these findings, we concluded that, as a pro-angiogenic and pro-lymphangiogenic factor, CCR7 expression correlates with lymph node metastasis due to its lymphangiogenic role rather than its angiogenic role. A recent study reported the interesting finding that the CCL21/CCR7 axis is indirectly involved in breast cancer-induced lymphangiogenesis through regulation of the expression of VEGF-C (77). Whether the CCL21/CCR7 axis induces tumor lymphangiogenesis through a direct role as a pro-lymphangiogenic factor like the VEGF/VEGFR family or through indirect regulation of VEGF-C or VEGF-D remains an interesting question for future scientific research.
Several studies have shown that CCR7 activity is associated with migration and invasion by certain cancer cell lines (22,27,78–81). However, whether the upregulation and downregulation of CCR7 expression modulates the biological behavior of UBC cell lines in vitro is poorly defined, despite the fact that a higher CCR7 level was observed in UBC tissues than in normal controls (42). Our results demonstrate that CCL21/CCR7 interaction significantly enhances the migration and invasion capacity of human UM-UC-3 cells in a dose- and time-dependent manner. Moreover, knock down of the CCR7 gene by small interfering RNAs targeting CCR7 sequences was shown in the present study to significantly suppress the enhancement of migration and invasion by human UM-UC-3 cells that was induced by treatment of the cells with CCL21. These results further confirmed that the activation of CCR7 is responsible for the increased migration and invasion abilities of human UBC cells. Some previous studies reported that the CCL21/CCR7 axis enhances the migration and invasion of cancer cells via the ERK1/2 pathway (78), whereas others found that the CCL21/CCR7-induced enhanced behavior of cancer cells were mediated by the PI3K/AKT pathway (81). The present study shows that CCR7 activation by CCL21 treatment result in a significant increase in ERK1/2 phosphorylation but not AKT phosphorylation in UBC cells, and CCR7 knockdown by efficacious siRNA transfection significantly decreases ERK1/2 phosphorylation only. Furthermore, PD98059, a specific inhibitor of MEK, the upstream activator protein of ERK1/2, remarkably suppressed the enhanced invasion and migration abilities of UBC cells induced by CCL21. Based on these results, we came to a conclusion that the PI3K/AKT and ERK pathways play a critical role in the carcinogenesis and progression of UBC (78,81–83), but the ERK pathway may play a more important role in the CCL21/CCR7-induced progression of UBC than the AKT pathway.
This study is limited by its retrospective design and by the semi-quantitative immunohistochemical methodology used in the analysis of clinical tumor samples. Although immunohistochemistry has been widely used in the study of protein expression, it has inherent defects, including high variability in technical procedures and staining scoring standards in different studies. Another limitation of the present study is the isolation of the tumor cells from their microenvironment in our in vitro experiments. Animal models, especially the orthotopic xenograft model, which most closely mimics human primary tumor carcinogenesis, remain a crucial connection between cell-based experiments and the translation of novel agents into cancer therapeutics, and our results lack testing with in vivo experiments or appropriate animal models that provide a realistic microenvironment for tumor survival. A statistical methological defect of our research is the small sample size of 62 subjects that may result in the reduced test power (1-β) and the increased type II error (β).
In conclusion, this study provides novel insights into the multifaceted role played by the CCL21/CCR7 chemoaxis in the complex mechanics of lymph node metastasis in patients with UBC. On the one hand, CCR7 is a promoting factor that induces both lymphangiogenesis and angiogenesis; however, it may correlate with lymph node metastasis due to its lymphangiogenic role rather than due to its angiogenic role. On the other hand, the CCL21/CCR7 chemoaxis promotes the migration and invasion by UBC cells via the MEK/ERK1/2 signaling pathway rather than the PI3K/AKT pathway. These insights into the mechanisms of CCL21/CCR7-mediated migration, invasion and lymph node metastasis in UBC enable us to inhibit the molecular processes involved in these events and thereby deliver therapeutic benefit to patients with advanced disease.
Glossary
Abbreviations
- MLVD
microlymphatic vessel density
- MVD
microvessel density
- CCL21
chemokine (C-C motif) ligand 21
- CCR7
C-C chemokine receptor 7
- ERK
extracellular signal-regulated kinase
- siRNA
small interfering RNA
References
- 1.Ploeg M, Aben KK, Kiemeney LA. The present and future burden of urinary bladder cancer in the world. World J Urol. 2009;27:289–293. doi: 10.1007/s00345-009-0383-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Johansson SL, Cohen SM. Epidemiology and etiology of bladder cancer. Semin Surg Oncol. 1997;13:291–298. doi: 10.1002/(SICI)1098-2388(199709/10)13:5<291::AID-SSU2>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- 3.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66:7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 4.Metts MC, Metts JC, Milito SJ, Thomas CR., Jr Bladder cancer: A review of diagnosis and management. J Natl Med Assoc. 2000;92:285–294. [PMC free article] [PubMed] [Google Scholar]
- 5.Xu X, Chen H, Lin Y, Hu Z, Mao Y, Wu J, Xu X, Zhu Y, Li S, Zheng X. MicroRNA-409-3p inhibits migration and invasion of bladder cancer cells via targeting c-Met. Mol Cells. 2013;36:62–68. doi: 10.1007/s10059-013-0044-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Babjuk M, Burger M, Zigeuner R, Shariat SF, van Rhijn BW, Compérat E, Sylvester RJ, Kaasinen E, Böhle A, Palou Redorta J, et al. European Association of Urology EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder: Update 2013. Eur Urol. 2013;64:639–653. doi: 10.1016/j.eururo.2013.06.003. [DOI] [PubMed] [Google Scholar]
- 7.Luke C, Tracey E, Stapleton A, Roder D. Exploring contrary trends in bladder cancer incidence, mortality and survival: Implications for research and cancer control. Intern Med J. 2010;40:357–362. doi: 10.1111/j.1445-5994.2009.01980.x. [DOI] [PubMed] [Google Scholar]
- 8.Zuiverloon TC, Nieuweboer AJ, Vékony H, Kirkels WJ, Bangma CH, Zwarthoff EC. Markers predicting response to bacillus Calmette-Guérin immunotherapy in high-risk bladder cancer patients: A systematic review. Eur Urol. 2012;61:128–145. doi: 10.1016/j.eururo.2011.09.026. [DOI] [PubMed] [Google Scholar]
- 9.Abdollah F, Gandaglia G, Thuret R, Schmitges J, Tian Z, Jeldres C, Passoni NM, Briganti A, Shariat SF, Perrotte P, et al. Incidence, survival and mortality rates of stage-specific bladder cancer in United States: A trend analysis. Cancer Epidemiol. 2013;37:219–225. doi: 10.1016/j.canep.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Meeks JJ, Bellmunt J, Bochner BH, Clarke NW, Daneshmand S, Galsky MD, Hahn NM, Lerner SP, Mason M, Powles T, et al. A systematic review of neoadjuvant and adjuvant chemotherapy for muscle-invasive bladder cancer. Eur Urol. 2012;62:523–533. doi: 10.1016/j.eururo.2012.05.048. [DOI] [PubMed] [Google Scholar]
- 11.Itesako T, Seki N, Yoshino H, Chiyomaru T, Yamasaki T, Hidaka H, Yonezawa T, Nohata N, Kinoshita T, Nakagawa M, et al. The microRNA expression signature of bladder cancer by deep sequencing: The functional significance of the miR-195/497 cluster. PLoS One. 2014;9:e84311. doi: 10.1371/journal.pone.0084311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gupta GP, Massagué J. Cancer metastasis: Building a framework. Cell. 2006;127:679–695. doi: 10.1016/j.cell.2006.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Siegel R, Ma J, Zou Z, Jemal A. Cancer statistics, 2014. CA Cancer J Clin. 2014;64:9–29. doi: 10.3322/caac.21208. [DOI] [PubMed] [Google Scholar]
- 14.Shariat SF, Karakiewicz PI, Palapattu GS, Amiel GE, Lotan Y, Rogers CG, Vazina A, Bastian PJ, Gupta A, Sagalowsky A, et al. Nomograms provide improved accuracy for predicting survival after radical cystectomy. Clin Cancer Res. 2006;12:6663–6676. doi: 10.1158/1078-0432.CCR-06-0372. [DOI] [PubMed] [Google Scholar]
- 15.Habuchi T, Marberger M, Droller MJ, Hemstreet GP, III, Grossman HB, Schalken JA, Schmitz-Dräger BJ, Murphy WM, Bono AV, Goebell P, et al. Prognostic markers for bladder cancer: International Consensus Panel on bladder tumor markers. Urology. 2005;66(Suppl 1):64–74. doi: 10.1016/j.urology.2005.08.065. [DOI] [PubMed] [Google Scholar]
- 16.Bol MG, Baak JP, Buhr-Wildhagen S, Kruse AJ, Kjellevold KH, Janssen EA, Mestad O, Øgreid P. Reproducibility and prognostic variability of grade and lamina propria invasion in stages Ta, T1 urothelial carcinoma of the bladder. J Urol. 2003;169:1291–1294. doi: 10.1097/01.ju.0000055471.78783.ae. [DOI] [PubMed] [Google Scholar]
- 17.Guo JC, Li J, Zhou L, Yang JY, Zhang ZG, Liang ZY, Zhou WX, You L, Zhang TP, Zhao YP. CXCL12-CXCR7 axis contributes to the invasive phenotype of pancreatic cancer. Oncotarget. 2016;7:62006–62018. doi: 10.18632/oncotarget.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Müller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, et al. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–56. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 19.Yasuoka H, Tsujimoto M, Yoshidome K, Nakahara M, Kodama R, Sanke T, Nakamura Y. Cytoplasmic CXCR4 expression in breast cancer: Induction by nitric oxide and correlation with lymph node metastasis and poor prognosis. BMC Cancer. 2008;8:340. doi: 10.1186/1471-2407-8-340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yu T, Wu Y, Helman JI, Wen Y, Wang C, Li L. CXCR4 promotes oral squamous cell carcinoma migration and invasion through inducing expression of MMP-9 and MMP-13 via the ERK signaling pathway. Mol Cancer Res. 2011;9:161–172. doi: 10.1158/1541-7786.MCR-10-0386. [DOI] [PubMed] [Google Scholar]
- 21.Kato T, Fujita Y, Nakane K, Mizutani K, Terazawa R, Ehara H, Kanimoto Y, Kojima T, Nozawa Y, Deguchi T, et al. CCR1/CCL5 interaction promotes invasion of taxane-resistant PC3 prostate cancer cells by increasing secretion of MMPs 2/9 and by activating ERK and Rac signaling. Cytokine. 2013;64:251–257. doi: 10.1016/j.cyto.2013.06.313. [DOI] [PubMed] [Google Scholar]
- 22.Mashino K, Sadanaga N, Yamaguchi H, Tanaka F, Ohta M, Shibuta K, Inoue H, Mori M. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–2941. [PubMed] [Google Scholar]
- 23.Comerford I, Harata-Lee Y, Bunting MD, Gregor C, Kara EE, McColl SR. A myriad of functions and complex regulation of the CCR7/CCL19/CCL21 chemokine axis in the adaptive immune system. Cytokine Growth Factor Rev. 2013;24:269–283. doi: 10.1016/j.cytogfr.2013.03.001. [DOI] [PubMed] [Google Scholar]
- 24.Förster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: Balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 25.Cabioglu N, Yazici MS, Arun B, Broglio KR, Hortobagyi GN, Price JE, Sahin A. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 26.Ishigami S, Natsugoe S, Nakajo A, Tokuda K, Uenosono Y, Arigami T, Matsumoto M, Okumura H, Hokita S, Aikou T. Prognostic value of CCR7 expression in gastric cancer. Hepatogastroenterology. 2007;54:1025–1028. [PubMed] [Google Scholar]
- 27.Ma H, Gao L, Li S, Qin J, Chen L, Liu X, Xu P, Wang F, Xiao H, Zhou S, et al. CCR7 enhances TGF-β1-induced epithelial-mesenchymal transition and is associated with lymph node metastasis and poor overall survival in gastric cancer. Oncotarget. 2015;6:24348–24360. doi: 10.18632/oncotarget.4484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Günther K, Leier J, Henning G, Dimmler A, Weissbach R, Hohenberger W, Förster R. Prediction of lymph node metastasis in colorectal carcinoma by expressionof chemokine receptor CCR7. Int J Cancer. 2005;116:726–733. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- 29.Yu S, Duan J, Zhou Z, Pang Q, Wuyang J, Liu T, He X, Xinfa L, Chen Y. A critical role of CCR7 in invasiveness and metastasis of SW620 colon cancer cell in vitro and in vivo. Cancer Biol Ther. 2008;7:1037–1043. doi: 10.4161/cbt.7.7.6065. [DOI] [PubMed] [Google Scholar]
- 30.Malietzis G, Lee GH, Bernardo D, Blakemore AI, Knight SC, Moorghen M, Al-Hassi HO, Jenkins JT. The prognostic significance and relationship with body composition of CCR7-positive cells in colorectal cancer. J Surg Oncol. 2015;112:86–92. doi: 10.1002/jso.23959. [DOI] [PubMed] [Google Scholar]
- 31.Koizumi K, Kozawa Y, Ohashi Y, Nakamura ES, Aozuka Y, Sakurai H, Ichiki K, Doki Y, Misaki T, Saiki I. CCL21 promotes the migration and adhesion of highly lymph node metastatic human non-small cell lung cancer Lu-99 in vitro. Oncol Rep. 2007;17:1511–1516. [PubMed] [Google Scholar]
- 32.Takanami I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: Correlation with lymph node metastasis. Int J Cancer. 2003;105:186–189. doi: 10.1002/ijc.11063. [DOI] [PubMed] [Google Scholar]
- 33.Kodama J, Hasengaowa, Kusumoto T, Seki N, Matsuo T, Ojima Y, Nakamura K, Hongo A, Hiramatsu Y. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann Oncol. 2007;18:70–76. doi: 10.1093/annonc/mdl342. [DOI] [PubMed] [Google Scholar]
- 34.Wang J, Xi L, Hunt JL, Gooding W, Whiteside TL, Chen Z, Godfrey TE, Ferris RL. Expression pattern of chemokine receptor 6 (CCR6) and CCR7 in squamous cell carcinoma of the head and neck identifies a novel metastatic phenotype. Cancer Res. 2004;64:1861–1866. doi: 10.1158/0008-5472.CAN-03-2968. [DOI] [PubMed] [Google Scholar]
- 35.Yousefieh N, Hahto SM, Stephens AL, Ciavarra RP. Regulated expression of CCL21 in the prostate tumor microenvironment inhibits tumor growth and metastasis in an orthotopic model of prostate cancer. Cancer Microenviron. 2009;2:59–67. doi: 10.1007/s12307-009-0021-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Heresi GA, Wang J, Taichman R, Chirinos JA, Regalado JJ, Lichtstein DM, Rosenblatt JD. Expression of the chemokine receptor CCR7 in prostate cancer presenting with generalized lymphadenopathy: Report of a case, review of the literature, and analysis of chemokine receptor expression. Urol Oncol. 2005;23:261–267. doi: 10.1016/j.urolonc.2005.02.004. [DOI] [PubMed] [Google Scholar]
- 37.Mori T, Kim J, Yamano T, Takeuchi H, Huang S, Umetani N, Koyanagi K, Hoon DS. Epigenetic up-regulation of C-C chemokine receptor 7 and C-X-C chemokine receptor 4 expression in melanoma cells. Cancer Res. 2005;65:1800–1807. doi: 10.1158/0008-5472.CAN-04-3531. [DOI] [PubMed] [Google Scholar]
- 38.Ishida K, Iwahashi M, Nakamori M, Nakamura M, Yokoyama S, Iida T, Naka T, Nakamura Y, Yamaue H. High CCR7 mRNA expression of cancer cells is associated with lymph node involvement in patients with esophageal squamous cell carcinoma. Int J Oncol. 2009;34:915–922. doi: 10.3892/ijo_00000217. [DOI] [PubMed] [Google Scholar]
- 39.Ding Y, Shimada Y, Maeda M, Kawabe A, Kaganoi J, Komoto I, Hashimoto Y, Miyake M, Hashida H, Imamura M. Association of CC chemokine receptor 7 with lymph node metastasis of esophageal squamous cell carcinoma. Clin Cancer Res. 2003;9:3406–3412. [PubMed] [Google Scholar]
- 40.Shi M, Chen D, Yang D, Liu XY. CCL21-CCR7 promotes the lymph node metastasis of esophageal squamous cell carcinoma by up-regulating MUC1. J Exp Clin Cancer Res. 2015;34:149. doi: 10.1186/s13046-015-0268-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shang ZJ, Liu K, Shao Z. Expression of chemokine receptor CCR7 is associated with cervical lymph node metastasis of oral squamous cell carcinoma. Oral Oncol. 2009;45:480–485. doi: 10.1016/j.oraloncology.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 42.Chen J, Cui YU, Liu L, Li C, Tang Y, Zhou XU, Qi L, Zu X. CCR7 as a predictive biomarker associated with computed tomography for the diagnosis of lymph node metastasis in bladder carcinoma. Oncol Lett. 2016;11:735–740. doi: 10.3892/ol.2015.3939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Elfiky AA, Rosenberg JE. Targeting angiogenesis in bladder cancer. Curr Oncol Rep. 2009;11:244–249. doi: 10.1007/s11912-009-0034-2. [DOI] [PubMed] [Google Scholar]
- 44.Vermeulen PB, Gasparini G, Fox SB, Toi M, Martin L, McCulloch P, Pezzella F, Viale G, Weidner N, Harris AL, et al. Quantification of angiogenesis in solid human tumours: An international consensus on the methodology and criteria of evaluation. Eur J Cancer. 1996;32A:2474–2484. doi: 10.1016/S0959-8049(96)00379-6. [DOI] [PubMed] [Google Scholar]
- 45.Vermeulen PB, Gasparini G, Fox SB, Colpaert C, Marson LP, Gion M, Beliën JA, de Waal RM, Van Marck E, Magnani E, et al. Second international consensus on the methodology and criteria of evaluation of angiogenesis quantification in solid human tumours. Eur J Cancer. 2002;38:1564–1579. doi: 10.1016/S0959-8049(02)00094-1. [DOI] [PubMed] [Google Scholar]
- 46.Dickinson AJ, Fox SB, Persad RA, Hollyer J, Sibley GN, Harris AL. Quantification of angiogenesis as an independent predictor of prognosis in invasive bladder carcinomas. Br J Urol. 1994;74:762–766. doi: 10.1111/j.1464-410X.1994.tb07122.x. [DOI] [PubMed] [Google Scholar]
- 47.Bochner BH, Cote RJ, Weidner N, Groshen S, Chen SC, Skinner DG, Nichols PW. Angiogenesis in bladder cancer: Relationship between microvessel density and tumor prognosis. J Natl Cancer Inst. 1995;87:1603–1612. doi: 10.1093/jnci/87.21.1603. [DOI] [PubMed] [Google Scholar]
- 48.Goddard JC, Sutton CD, Furness PN, O'Byrne KJ, Kockelbergh RC. Microvessel density at presentation predicts subsequent muscle invasion in superficial bladder cancer. Clin Cancer Res. 2003;9:2583–2586. [PubMed] [Google Scholar]
- 49.Canoğlu A, Göğüş C, Bedük Y, Orhan D, Tulunay O, Baltaci S. Microvessel density as a prognostic marker in bladder carcinoma: Correlation with tumor grade, stage and prognosis. Int Urol Nephrol. 2004;36:401–405. doi: 10.1007/s11255-004-8869-9. [DOI] [PubMed] [Google Scholar]
- 50.Chaudhary R, Bromley M, Clarke NW, Betts CD, Barnard RJ, Ryder WD, Kumar S. Prognostic relevance of micro-vessel density in cancer of the urinary bladder. Anticancer Res. 1999;19:3479–3484. [PubMed] [Google Scholar]
- 51.Jaeger TM, Weidner N, Chew K, Moore DH, Kerschmann RL, Waldman FM, Carroll PR. Tumor angiogenesis correlates with lymph node metastases in invasive bladder cancer. J Urol. 1995;154:69–71. doi: 10.1016/S0022-5347(01)67230-6. [DOI] [PubMed] [Google Scholar]
- 52.Shariat SF, Youssef RF, Gupta A, Chade DC, Karakiewicz PI, Isbarn H, Jeldres C, Sagalowsky AI, Ashfaq R, Lotan Y. Association of angiogenesis related markers with bladder cancer outcomes and other molecular markers. J Urol. 2010;183:1744–1750. doi: 10.1016/j.juro.2010.01.018. [DOI] [PubMed] [Google Scholar]
- 53.Zhou M, He L, Zu X, Zhang H, Zeng H, Qi L. Lymphatic vessel density as a predictor of lymph node metastasis and its relationship with prognosis in urothelial carcinoma of the bladder. BJU Int. 2011;107:1930–1935. doi: 10.1111/j.1464-410X.2010.09725.x. [DOI] [PubMed] [Google Scholar]
- 54.Jacquemier J, Mathoulin-Portier MP, Valtola R, Charafe-Jauffret E, Geneix J, Houvenaeghel G, Puig B, Bardou VJ, Hassoun J, Viens P, et al. Prognosis of breast-carcinoma lymphagenesis evaluated by immunohistochemical investigation of vascular-endothelial-growth-factor receptor 3. Int J Cancer. 2000;89:69–73. doi: 10.1002/(SICI)1097-0215(20000120)89:1<69::AID-IJC11>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 55.Zeng Y, Opeskin K, Baldwin ME, Horvath LG, Achen MG, Stacker SA, Sutherland RL, Williams ED. Expression of vascular endothelial growth factor receptor-3 by lymphatic endothelial cells is associated with lymph node metastasis in prostate cancer. Clin Cancer Res. 2004;10:5137–5144. doi: 10.1158/1078-0432.CCR-03-0434. [DOI] [PubMed] [Google Scholar]
- 56.Longatto Filho A, Martins A, Costa SM, Schmitt FC. VEGFR-3 expression in breast cancer tissue is not restricted to lymphatic vessels. Pathol Res Pract. 2005;201:93–99. doi: 10.1016/j.prp.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 57.Valtola R, Salven P, Heikkilä P, Taipale J, Joensuu H, Rehn M, Pihlajaniemi T, Weich H, deWaal R, Alitalo K. VEGFR-3 and its ligand VEGF-C are associated with angiogenesis in breast cancer. Am J Pathol. 1999;154:1381–1390. doi: 10.1016/S0002-9440(10)65392-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Partanen TA, Alitalo K, Miettinen M. Lack of lymphatic vascular specificity of vascular endothelial growth factor receptor 3 in 185 vascular tumors. Cancer. 1999;86:2406–2412. doi: 10.1002/(SICI)1097-0142(19991201)86:11<2406::AID-CNCR31>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 59.Kahn HJ, Bailey D, Marks A. Monoclonal antibody D2-40, a new marker of lymphatic endothelium, reacts with Kaposi's sarcoma and a subset of angiosarcomas. Mod Pathol. 2002;15:434–440. doi: 10.1038/modpathol.3880543. [DOI] [PubMed] [Google Scholar]
- 60.Afonso J, Santos LL, Amaro T, Lobo F, Longatto-Filho A. The aggressiveness of urothelial carcinoma depends to a large extent on lymphovascular invasion - the prognostic contribution of related molecular markers. Histopathology. 2009;55:514–524. doi: 10.1111/j.1365-2559.2009.03425.x. [DOI] [PubMed] [Google Scholar]
- 61.Braun M, Flucke U, Debald M, Walgenbach-Bruenagel G, Walgenbach KJ, Höller T, Pölcher M, Wolfgarten M, Sauerwald A, Keyver-Paik M, et al. Detection of lymphovascular invasion in early breast cancer by D2-40 (podoplanin): A clinically useful predictor for axillary lymph node metastases. Breast Cancer Res Treat. 2008;112:503–511. doi: 10.1007/s10549-007-9875-2. [DOI] [PubMed] [Google Scholar]
- 62.Franchi A, Gallo O, Massi D, Baroni G, Santucci M. Tumor lymphangiogenesis in head and neck squamous cell carcinoma: A morphometric study with clinical correlations. Cancer. 2004;101:973–978. doi: 10.1002/cncr.20454. [DOI] [PubMed] [Google Scholar]
- 63.Beasley NJ, Prevo R, Banerji S, Leek RD, Moore J, van Trappen P, Cox G, Harris AL, Jackson DG. Intratumoral lymphangiogenesis and lymph node metastasis in head and neck cancer. Cancer Res. 2002;62:1315–1320. [PubMed] [Google Scholar]
- 64.Maula SM, Luukkaa M, Grénman R, Jackson D, Jalkanen S, Ristamäki R. Intratumoral lymphatics are essential for the metastatic spread and prognosis in squamous cell carcinomas of the head and neck region. Cancer Res. 2003;63:1920–1926. [PubMed] [Google Scholar]
- 65.Nakamura Y, Yasuoka H, Tsujimoto M, Imabun S, Nakahara M, Nakao K, Nakamura M, Mori I, Kakudo K. Lymph vessel density correlates with nodal status, VEGF-C expression, and prognosis in breast cancer. Breast Cancer Res Treat. 2005;91:125–132. doi: 10.1007/s10549-004-5783-x. [DOI] [PubMed] [Google Scholar]
- 66.Wang XL, Fang JP, Tang RY, Chen XM. Different significance between intratumoral and peritumoral lymphatic vessel density in gastric cancer: A retrospective study of 123 cases. BMC Cancer. 2010;10:299. doi: 10.1186/1471-2407-10-299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Roma AA, Magi-Galluzzi C, Kral MA, Jin TT, Klein EA, Zhou M. Peritumoral lymphatic invasion is associated with regional lymph node metastases in prostate adenocarcinoma. Mod Pathol. 2006;19:392–398. doi: 10.1038/modpathol.3800546. [DOI] [PubMed] [Google Scholar]
- 68.Dadras SS, Paul T, Bertoncini J, Brown LF, Muzikansky A, Jackson DG, Ellwanger U, Garbe C, Mihm MC, Detmar M. Tumor lymphangiogenesis: A novel prognostic indicator for cutaneous melanoma metastasis and survival. Am J Pathol. 2003;162:1951–1960. doi: 10.1016/S0002-9440(10)64328-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Dadras SS, Lange-Asschenfeldt B, Velasco P, Nguyen L, Vora A, Muzikansky A, Jahnke K, Hauschild A, Hirakawa S, Mihm MC, et al. Tumor lymphangiogenesis predicts melanoma metastasis to sentinel lymph nodes. Mod Pathol. 2005;18:1232–1242. doi: 10.1038/modpathol.3800410. [DOI] [PubMed] [Google Scholar]
- 70.Ma Y, Hou Y, Liu B, Li X, Yang S, Ma J. Intratumoral lymphatics and lymphatic vessel invasion detected by D2-40 are essential for lymph node metastasis in bladder transitional cell carcinoma. Anat Rec (Hoboken) 2010;293:1847–1854. doi: 10.1002/ar.21217. [DOI] [PubMed] [Google Scholar]
- 71.Fernández MI, Bolenz C, Trojan L, Steidler A, Weiss C, Alken P, Grobholz R, Michel MS. Prognostic implications of lymphangiogenesis in muscle-invasive transitional cell carcinoma of the bladder. Eur Urol. 2008;53:571–578. doi: 10.1016/j.eururo.2007.08.030. [DOI] [PubMed] [Google Scholar]
- 72.Miyata Y, Kanda S, Ohba K, Nomata K, Hayashida Y, Eguchi J, Hayashi T, Kanetake H. Lymphangiogenesis and angiogenesis in bladder cancer: prognostic implications and regulation by vascular endothelial growth factors-A, -C, and -D. Clin Cancer Res. 2006;12:800–806. doi: 10.1158/1078-0432.CCR-05-1284. [DOI] [PubMed] [Google Scholar]
- 73.Liang P, Hong JW, Ubukata H, Liu HR, Watanabe Y, Katano M, Motohashi G, Kasuga T, Nakada I, Tabuchi T. Increased density and diameter of lymphatic microvessels correlate with lymph node metastasis in early stage invasive colorectal carcinoma. Virchows Arch. 2006;448:570–575. doi: 10.1007/s00428-006-0166-9. [DOI] [PubMed] [Google Scholar]
- 74.Williams CS, Leek RD, Robson AM, Banerji S, Prevo R, Harris AL, Jackson DG. Absence of lymphangiogenesis and intratumoural lymph vessels in human metastatic breast cancer. J Pathol. 2003;200:195–206. doi: 10.1002/path.1343. [DOI] [PubMed] [Google Scholar]
- 75.Birner P, Schindl M, Obermair A, Plank C, Breitenecker G, Kowalski H, Oberhuber G. Lymphatic microvessel density in epithelial ovarian cancer: Its impact on prognosis. Anticancer Res. 2000;20:2981–2985. [PubMed] [Google Scholar]
- 76.Schoppmann SF, Birner P, Stöckl J, Kalt R, Ullrich R, Caucig C, Kriehuber E, Nagy K, Alitalo K, Kerjaschki D. Tumor-associated macrophages express lymphatic endothelial growth factors and are related to peritumoral lymphangiogenesis. Am J Pathol. 2002;161:947–956. doi: 10.1016/S0002-9440(10)64255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Tutunea-Fatan E, Majumder M, Xin X, Lala PK. The role of CCL21/CCR7 chemokine axis in breast cancer-induced lymphangiogenesis. Mol Cancer. 2015;14:35. doi: 10.1186/s12943-015-0306-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Redondo-Muñoz J, José Terol M, García-Marco JA, García-Pardo A. Matrix metalloproteinase-9 is up-regulated by CCL21/CCR7 interaction via extracellular signal-regulated kinase-1/2 signaling and is involved in CCL21-driven B-cell chronic lymphocytic leukemia cell invasion and migration. Blood. 2008;111:383–386. doi: 10.1182/blood-2007-08-107300. [DOI] [PubMed] [Google Scholar]
- 79.Li F, Zou Z, Suo N, Zhang Z, Wan F, Zhong G, Qu Y, Ntaka KS, Tian H. CCL21/CCR7 axis activating chemotaxis accompanied with epithelial-mesenchymal transition in human breast carcinoma. Med Oncol. 2014;31:180. doi: 10.1007/s12032-014-0180-8. [DOI] [PubMed] [Google Scholar]
- 80.Xia X, Liu K, Zhang H, Shang Z. Correlation between CCR7 expression and lymph node metastatic potential of human tongue carcinoma. Oral Dis. 2015;21:123–131. doi: 10.1111/odi.12228. [DOI] [PubMed] [Google Scholar]
- 81.Yang J, Wang S, Zhao G, Sun B. Effect of chemokine receptors CCR7 on disseminated behavior of human T cell lymphoma: clinical and experimental study. J Exp Clin Cancer Res. 2011;30:51. doi: 10.1186/1756-9966-30-51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Calderaro J, Rebouissou S, de Koning L, Masmoudi A, Hérault A, Dubois T, Maille P, Soyeux P, Sibony M, de la Taille A, et al. PI3K/AKT pathway activation in bladder carcinogenesis. Int J Cancer. 2014;134:1776–1784. doi: 10.1002/ijc.28518. [DOI] [PubMed] [Google Scholar]
- 83.Islam SS, Mokhtari RB, Akbari P, Hatina J, Yeger H, Farhat WA. Simultaneous targeting of bladder tumor growth, Survival, and epithelial-to-mesenchymal transition with a novel therapeutic combination of acetazolamide (AZ) and sulforaphane (SFN) Target Oncol. 2016;11:209–227. doi: 10.1007/s11523-015-0386-5. [DOI] [PubMed] [Google Scholar]