Abstract
Malignant tumors, including breast cancers, are frequently infiltrated with innate immune cells and tumor-associated macrophages (TAMs) represent the major inflammatory component in stroma of many tumors. In this study, we examined the immunoreactivity of the macrophage markers CD68 and CD163 as well as the hormone receptors estrogen receptor α (ERα), progesterone receptor (PR), estrogen receptor β1 (ERβ1), human epidermal growth factor receptor 2 (HER-2), matrix metalloproteinase 9 (MMP-9), urokinase-type plasminogen activator receptor (uPAR) and the proliferations marker Ki67 in 17 breast cancer biopsies. The quantitative score for CD68+ and CD163+ strongly indicate M2 phenotype dominance in the currently investigated biopsies. We found that an increasing level of macrophages was negatively associated with ERα or PR, whereas a positive association was observed for Ki-67 or uPAR. No significant association could be seen between the level of macrophage and HER-2, ERβ1 or MMP-9 expression. Effect of conditioned media (CM) generated from cultured human M1 and M2 macrophage phenotypes were investigated on the proliferation and expression of selected markers in the T47D breast cancer cell line. We found that in contrast to the in vivo situation, in particularly the CM from M1 macrophages decreased the growth and Ki67 expression in T47D, and significantly increased ERβ1 mRNA levels. Moreover, in accordance to the in vivo situation the CM from the macrophages decreased the expression of ERα protein as well as ERα or PR mRNA. In conclusion our results show that macrophages alone have the capability to decrease the tumor cell expression of ERα and PR in vitro. In the tumor environment in vivo macrophages also contribute to an increase in tumor cell expression of uPAR and Ki67, suggesting that macrophages are involved in impairing the prognosis for breast cancer patients.
Keywords: breast cancer, breast cancer cell line, T47D, estrogen receptors, progesterone receptor, M1 macrophages, M2 macrophages, urokinase-type plasminogen activator receptor
Introduction
Breast cancer, the most frequently diagnosed cancer and the leading cause of cancer deaths in women worldwide (1), is a heterogeneous disease with different biological hallmarks, and thereby varying prognostic and therapeutic characteristics. Tumors are classified into different subtypes based on the immunohistochemical expression of estrogen receptor α (ERα), progesterone receptor (PR), human epidermal growth factor receptor-2 (HER-2) and Ki67 which also provides prognostic and predictive information on response to hormonal or targeted therapies (2). Approximately 20–30% of all breast cancers overexpress HER-2 leading to an uncontrolled growth of cancer cells (3). Estrogen and progesterone are steroid hormones that play an essential role for normal mammary gland growth and development as well as for breast cancer progression. Most of their effects are mediated by ERs and PR which are intracellular receptors which constitute members of the nuclear receptor superfamily of transcription factors. Approximately 75% of malignant breast tumors are ERα positive and more than half of these tumors also express PR (4,5). For decades, ERα was thought to be the only ER present in mammary epithelial cells until the identification of a second estrogen receptor, ERβ, in 1996 (6). ERβ is the most widely expressed ER in normal, mammary tissue. Five different isoforms (ERβ1-ERβ5) exist in humans, though ERβ1 is considered the only fully functional isoform. The role for ERβ1 and other splice variants in breast cancer is still being investigated and may not be consistent among different breast cancer subtypes (7,8).
A solid, malignant tumor consists of cancer cells within a tumor stroma of essentially fibroblasts, endothelial cells, smooth muscle cells and immune cells (9). Of the latter, macrophages appear to play a significant role in carcinogenesis (10). Thus, the association between inflammation and cancer is well established (11,12). An inflammatory microenvironment influences every hallmark of cancer e.g. cell proliferation, invasion, angiogenesis and metastasis (9). Macrophages are innate immune cells originating from peripheral blood monocytes with important roles in normal tissue homeostasis such as primary response to pathogens, resolution of inflammation and wound healing. They also constitute the most abundant immune cell present in the tumor stroma and display extensive diversity and plasticity (13–15). Within the tumor microenvironment monocytes differentiate into tumor-associated macrophages (TAMs), mainly due to tumor-derived chemo-tactic factors. TAMs are active in the progression of tumors and may, in response to various signals, exhibit dual roles in the microenvironment such as facilitate tumor growth, or in contrast, contribute to destruction of tumors (16,17). Simplified, human macrophages can be classified into two phenotypically extremes, the classically activated M1 phenotype considered to exhibit tumoricidal activities arising from stimulation of macrophages with Th1-cytokines such as interferon-γ (IFNγ) and/or microbial products like lipopolysaccharide (LPS). This phenotype is characterized by high levels of pro-inflammatory cytokines such as interleukin (IL)12, IL23, IL6, tumor necrosis factor-α (TNF-α) and reactive oxygen/nitrogen intermediates (ROI/RNI) (10,15).
On the contrary, the alternatively activated M2 macrophage is polarized by Th2-cytokines such as IL4 and IL13, and releases high levels of anti-inflammatory cytokines such as IL10 and transforming growth factor-β. In most tumors the infiltrating macrophages are polarized towards the M2 phenotype and show a pro-tumoral role (10,15,16). CD163 is a scavenger receptor that is regarded as highly specific for M2 macrophages, while CD68 is a pan-macrophage marker and stains both M1 and M2 phenotypes (18,19). Furthermore, TAMs produce growth promoting and sustaining cytokines, including epithelial growth factor, vascular endothelial growth factor and matrix metalloproteinases (MMPs) (20). Activation of (cell-signaling) urokinase receptor (uPAR) through binding of urokinase-type plasminogen activator (uPA) triggers the conversion of plasminogen to plasmin, which in turn, initiates a cascade of extracellular proteases, e.g. MMPs. MMPs degrade components of extracellular matrix leading to/promoting tumor cell invasion and metastatic progression (21,22).
In human breast cancer, the inflammatory cells, mainly lymphocytes and macrophages, can constitute as much as half of the tumor mass and several studies suggest that high density of TAMs is associated with high vascularity, high tumor grade, increased tumor size, nodal metastasis and reduced overall survival (23–25). Previously published studies have also associated high infiltration of CD68+ and/or CD163+ macrophages with ERα and PR-negative tumors and high Ki67 proliferative index, whereas there are inconsistent results of infiltration of macrophages, and HER-2 positivity (25–29).
The aim of the current study, undertaken with human breast cancer tissue, as well as with the human breast cancer cell line T47D was to examine, in human breast cancer, the relationship between infiltrating macrophages and their phenotype(s), hormone receptor status comprising PR, ERα and ERβ1, the expression of HER-2, MMP-9, uPAR and the proliferation marker Ki67. Furthermore, we investigated how conditioned media (CM) from macrophages of the M1 and M2 phenotypes, respectively, may influence the proliferation and the expression of the markers mentioned above.
Materials and methods
Immunohistochemistry
Breast cancer specimens being analyzed in this study were archival material stored in paraffin blocks, having been taken for diagnostic purpose prior to any treatment at the Department of Clinical Pathology and Cytology, Karlstad Central Hospital (Karlstad, Sweden). The study included tumor specimen from all patients (n=19) selected for neoadjuvant therapy at Karlstad Central Hospital between 2009 and 2012, two samples were excluded because of too little materials left. All samples were de-identified prior to analysis. Serial sections of 4 µm were cut from each sample and were mounted on IHC microscope glass slides (Dako, Glostrup, Denmark). The sections were de-paraffinized followed by antigen retrieval using PT-link at 97°C for 20 min in EnVision FLEX Target Retrieval Solution (Dako). The sections were incubated for 30 min with either of the following primary antibodies: Monoclonal rabbit anti-human estrogen receptor (ER)α (clone EP1, ready-to-use), monoclonal mouse anti-human progesterone receptor (PR; clone PgR 636, ready-to-use), monoclonal mouse anti-human-CD68 antibody (clone Kp1, ready-to-use), HercepTest™ polyclonal rabbit anti-human HER2, monoclonal mouse anti-human estrogen receptor β1 (clone PPG5/10, dilution 1:40), monoclonal mouse anti-human Ki-67 (clone MIB1, ready-to-use), polyclonal rabbit anti-human MMP-9 (1:50), monoclonal mouse anti-human uPAR (clone R4, 1:50) (all from Dako) and monoclonal mouse anti-human-CD163 antibody (clone 10D6, 1:200, Novocastra, Leica Microsystems, Newcastle, UK). The monoclonal mouse anti-human estrogen receptor β1 (clone PPG5/10, 1:40) (Dako) demanded an additional step of incubating sections with EnVision FLEX/mouse linker (Dako) for 15 min prior to addition of the secondary antibody. Immunohistochemical EnVision visualization system was performed with the standard method of horseradish peroxidase and 3, 3′-diamino-benzidine incubating the sections with secondary anti-mouse/anti-rabbit (ready-to-use) for 20 min and substrate working solution FLEX DAB sub-chromophore 5 min in Autostainer Link 48 according to the manufacturer (Dako).
Benign human cervix tissue was used as control for ERα and PR antibodies, benign cervix and breast carcinoma for ERβ1 and human tonsil for CD68, CD163, uPAR, MMP-9 and Ki67 antibodies. After immunostaining, slides were counter-stained with Mayer's haematoxylin, dehydrated, cleared and mounted using Tissue-Tek coverslipping film (Sakura Finetek, Torrence, CA, USA). Assessments of all immunostainings were done by a senior pathologist (A.S.). Positive immunoreactivity (IR) for ERα, ERβ1, PR and Ki67 were denoted as percentage of positive breast carcinoma cells while positive IR for HER-2 was scored from 0 to 3+ according to current clinical guidelines in Sweden. Staining for CD68 and CD163 were scored as 1–3 where 1 (1–10%, 'low'), 2 (10–30%, 'moderate') and 3 (>30%, 'high') indicating percentage of positive cells in the intratumoral and stromal area. The MMP-9 or uPAR immunoreactivity was determined by counting the total number of positive tumor (T) cells, and macrophage like stroma (S) cells, respectively. Cells were counted in five randomly selected 320×250 µm areas and ranged from 0 (negative), score 1 (1–10 positive cells/five areas), score 2 (10–30 positive cells/five areas) and score 3 (>30 positive cells/five areas). All scoring were performed at ×400 magnification and a resolution of 6.24 pixels/µm. Images at ×400 magnification were captured using a Leica DMD108 light microscope with an integrated camera.
Isolation of human monocytes and their differentiation to M1 or M2 macro phage phenotype, and collection of macrophage conditioned media
The generation of human monocyte-derived macrophages was conducted as previously described (30). Briefly, 45 ml of buffy coat obtained from healthy blood donors at Clinical Immunology and Transfusion Medicine, Akademiska University Hospital (Uppsala, Sweden) was mixed with an equal volume of PBS containing 3 mM EDTA (Sigma-Aldrich, St. Louis, MO, USA) and was then gradient centrifuged with Ficoll Paques PLUS (GE Healthcare, Little Chalfont, UK). The separated band of peripheral blood mononuclear cells (PBMC) was collected, and pelleted cells were washed by repeated centrifugation steps. Monocytes were purified by adherence to the cell culture dishes and macrophages were generated by culturing monocytes for 6 days in RPMI-1640 (RPMI) (Life Technologies, Carlsbad, CA, USA) with 20% heat-inactivated fetal calf serum (FCS) (Thermo Scientific, Waltham, MA, USA) and 20 ng/ml macrophage colony-stimulating factor (M-CSF; R&D Systems, Minneapolis, MN, USA).
For further differentiation of the macrophages 100 ng/ml LPS (Sigma-Aldrich) and 20 ng/ml IFNγ (R&D Systems) were added to generate the M1 phenotype. Conversely, 20 ng/ml IL4 and 20 ng/ml IL13 (both from R&D Systems) were added to generate the M2 phenotype. M0-macrophages were cultured in RPMI +5% FCS without any additions. After 48 h, the differentiated macrophages were washed twice with PBS and were, furthermore, cultured in RPMI with 5% FCS for another 48 h. Thereafter the conditioned media (CM) from M1 and M2 macrophages, containing neither LPS plus IFNγ nor IL4 plus IL13, was collected, centrifuged to remove cellular debris and then stored in aliquots at −20°C. Macrophages of either M0, M1 or M2 phenotype were also lysed for RNA isolation and reverse transcriptase quantitative PCR (RT-qPCR) at two different time points, first directly after washing and removal of the prior addition of LPS, IFNγ, IL4, IL13 and second, after the 48 h incubation in RPMI with 5% FCS.
Cell culture and cell cycle analysis
The human ductal breast epithelial tumor cell line T47D was purchased from American Type Culture Collection (Manassas, VA, USA). The cells were cultured at 37°C with 5% CO2 in RPMI medium supplemented with 10% FCS, 2 mM L-glutamine, 100 U/ml of penicillin, 100 µg/ml of streptomycin (Life Technologies). For treatment with macrophage CM, cells were seeded at 25,000 cells/cm2 onto cell culture plates (Greiner Bio-One, Frickenhausen, Germany) and were allowed to adhere for 48 h before treatment with M1 and M2 CM for another 48 h. Cells that were exposed to RPMI +5% FCS only, served as untreated controls. After the respective treatment, RNA extraction was undertaken with fractions of the cells. Alternatively, in order to investigate possible epigenetic effect of M1 and M2 CM, respectively, on the hormone receptor expression of T47D cell line, cells were re-seeded and cultured for another 72 and 140 h prior to RNA extraction.
To investigate the effect of CM from M1 and M2 phenotypes on cell growth of the T47D cell line, cells were cultured and treated for 48 h as described above. Next, they were detached by trypsinization and counted in a hemocytometer. Approximately 250,000 cells from each treatment (including untreated controls) were collected for cell cycle analysis. These cells were washed with PBS containing 1% bovine serum albumin (BSA), centrifuged 10 min at 200 × g and resuspended in 450 µl ice-cold PBS/BSA prior to the addition of 5 ml ice-cold 70% ethanol. Samples were stored at −20°C until analysis, prior to which Triton X-100 (Sigma-Aldrich) was added to a final concentration of 0.1% and samples were incubated for 5 min at 6°C. Thereafter, the cells were centrifuged 10 min at 200 × g and resuspended in 1 ml PBS/BSA and this procedure was repeated once. The cells were then resuspended in PBS/BSA and 0.1% Triton X-100, 200 µg/ml RNaseA and 50 µg/ml propidium iodide (the latter two items were obtained from Sigma-Aldrich) were added followed by incubation in the dark at room temperature for 45 min. Cell cycle analysis was performed on FACSCalibur flow cytometer (BD Biosciences, Franklin Lakes, NJ, USA) and the results was calculated using ModFit LT v3.1 (Verity Software House, Inc., Topsham, ME, USA).
RNA extraction, cDNA synthesis and quantification of mRNA (RT-qPCR)
Total RNA was extracted from M0, M1 and M2 macrophages as well as from cultured T47D cells treated as described above using RNeasy Plus Mini kit (Qiagen, Hilden, Germany) in accordance with the manufacturer's instructions. For purity and quantification of the extracted RNA absorbance was measured at wavelengths 260 and 280 nm using a NanoQuant plate with the M200 Pro plate reader (Tecan, Männedorf, Switzerland). Synthesis of cDNA was performed from 0.2 µg of total RNA using High Capacity cDNA Reverse Transcription kit (Applied Biosystems, Foster City, CA, USA) with a total reaction volume of 20 µl in accordance with the manufacturer's instructions. Expression of CCL2, CCL17, CCL18, CXCL9, IL6, IL8, IL10, IL12, NFκB and TNFα mRNA in M0, M1 and M2 macrophages and ERα, ERβ1, PR, HER-2, p21 and p27 mRNA in T47D cells was evaluated by RT-qPCR, using StepOnePlus real-time PCR with Power SYBR-Green Master Mix (both from Applied Biosystems) in a total volume of 25 µl containing 4 µl of cDNA (diluted 5×) and 200 nM of each primer. All primer sequences are listed in Table I. Samples were run in duplicates with appropriate negative controls and gene expression was normalized to the housekeeping genes POLR2F and GAPDH. The efficiency of the primers was calculated using LinRegPCR software (31) and the size of the amplified PCR products were validated using agarose gel-electrophoresis. Fold changes were calculated using the ΔΔCq method.
Table I.
Primer sequences used for qPCR.
| Gene name | Forward primer sequence 5′-3′ | Reverse primer sequence 5′-3′ | Genebank accession no. |
|---|---|---|---|
| CCL2 | CAGCCAGATGCAATCAATGCC | TGGAATCCTGAACCCACTTCT | NM_002982.3 |
| CCL17 | CTTCTCTGCAGCACATCCAC | AGTACTCCAGGCAGCACTCC | NM_002987.2 |
| CCL18 | CTCCTTGTCCTCGTCTGCAC | TCAGGCATTCAGCTTCAGGT | NM_002988.3 |
| CXCL9 | CCAGTAGTGAGAAAGGGTCGC | AGGGCTTGGGGCAAATTGTT | NM_002416.2 |
| ERα | GGGAAGTATGGCTATGGAATCTG | TGGCTGGACACATATAGTCGTT | NM_000125.3 |
| ERβ1 | TCCATCGCCAGTTATCACATCT | CTGGACCAGTAACAGGGCTG | NM_001437.2 |
| GAPDH | CAACAGCGACACCCACTCCT | CACCCTGTTGCTGTAGCCAAA | NM_002046.4 |
| HER-2 | TGTGACTGCCTGTCCCTACAA | CCAGACCATAGCACACTCGG | NM_001005862.2 |
| IL6 | GATCCAAAAACCACCCCTGACCC | CAATCTGAGGTGCCCATGCTAC | NM_000600.3 |
| IL8 | CATGACTTCCAAGCTGGCCGTG | CCACTCTCAATCACTCTCAGTTC | NM_000584.3 |
| IL10 | CTGGGGGAGAACCTGAAGA | GGCCTTGCTCTTGTTTTCAC | NM_000572.2 |
| IL12 | CAGCCTGGGAAACATAACAAGAC | CTCCTGCCTCATCCTCCTGAA | NM_002187.2 |
| MMP-9 | GGGACGCAGACATCGTCATC | TCGTCATCGTCGAAATGGGC | NM_004994.2 |
| NFκB | CCAACAGATGGCCCATACCT | AACCTTTGCTGGTCCCACAT | NM_001165412.1 |
| p21 | TTAGCAGCGGAACAAGGAGT | AGCCGAGAGAAAACAGTCCA | NM_000389.4 |
| P27 | TAATTGGGGCTCCGGCTAACT | TGCAGGTCGCTTCCTTATTCC | NM_004064.3 |
| POLR2F | ATGTCAGACAACGAGGACAATTT | TTCGGCATTCTCCAAGTCATC | NM_001301129.1 |
| PR | ACCCGCCCTATCTCAACTACC | AGGACACCATAATGACAGCCT | NM_000926.4 |
| TNF-α | CCTCTCTCTAATCAGCCCTCTG | GAGGACCTGGGAGTAGATGAG | NM_000594.3 |
| uPAR | GAGCTATCGGACTGGCTTGAA | CGGCTTCGGGAATAGGTGAC | NM_002659.3 |
qPCR, quantitative PCR; CCL, chemokine (C-C motif) ligand; CXCL, chemokine (C-X-C motif) ligand; ERα, estrogen receptor α; ERβ1, estrogen receptor β; GAPDH, glyceraldehyde 3 phosphate dehydrogenase; HER-2, human epidermal growth factor receptor 2; IL, interleukin; MMP, matrix metalloproteinase; NF, nuclear factor; POLR2F, RNA polymerase II subunit F; PR, progesterone receptor; TNF, tumor necrosis factor; uPAR, urokinase plasminogen activator receptor.
Immunocytochemistry
Approximately 200,000 cells of T47D treated as described in the cell culture section were detached by trypsinization and centrifuged for 10 min at 300 × g. Cells were resuspended in PBS and spun onto positively charged microscopic glass slides (Thermo Scientific). Slides were allowed to dry and fixed in 4% formaldehyde solution for 10 min prior to immunostaining using monoclonal rabbit anti-human estrogen receptor α (clone EP1, ready-to-use), monoclonal mouse anti-human progesterone receptor (clone PgR 636, ready-to-use,) mouse anti-human estrogen receptor β1 (clone PPG5/10, 1:40) monoclonal mouse anti-human Ki-67 (clone MIB1, ready-to-use) and monoclonal mouse anti-human uPAR (clone R4, 1:50) (all from Dako) as previously described.
Ethics
The study has been approved by the Uppsala Ethics Committee (license 2014/498).
Statistics
Data are presented as mean ± SEM. A paired Student's t-test was used for all cell counting experiments comparing treated samples vs. untreated controls and also for the RT-qPCR mRNA expression data comparing ΔCt values for treated samples vs. untreated controls. Basal mRNA expression levels and cell cycle distributions were compared using an unpaired Student's t-test. A Jonckheere-Terpstra test was used to analyze significant associations between increasing CD68+ mononuclear cell infiltration in the biopsies with increasing tumor cell expression of Ki67, uPAR, HER2 or MMP-9, or decreasing tumor cell expression of ERα, ERβ or PR.
Results
The number of macrophages in human breast cancer tissue have a positive association with the expression of uPAR or Ki67 as well as an inverse association with ERα or PR
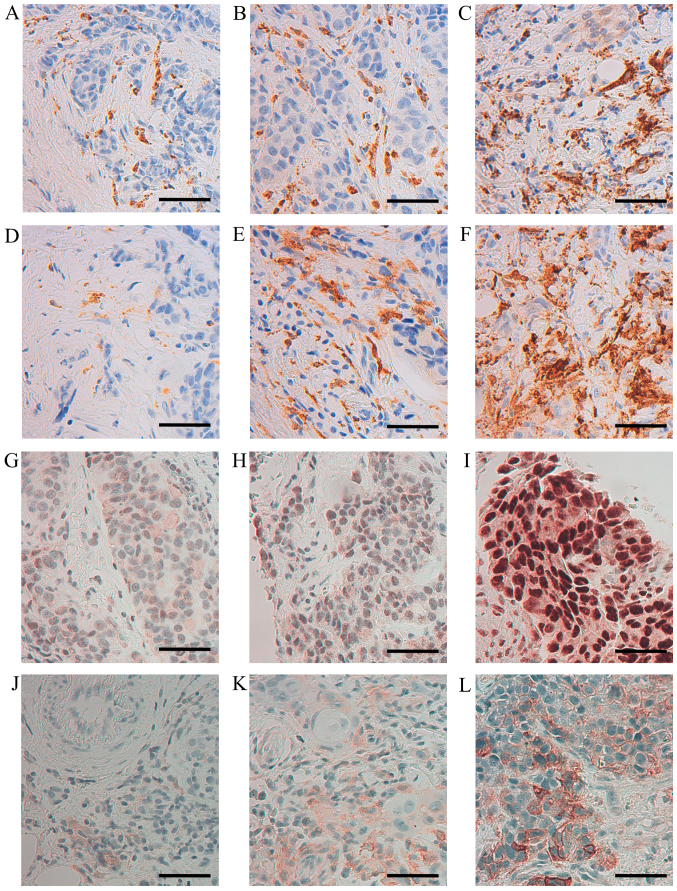
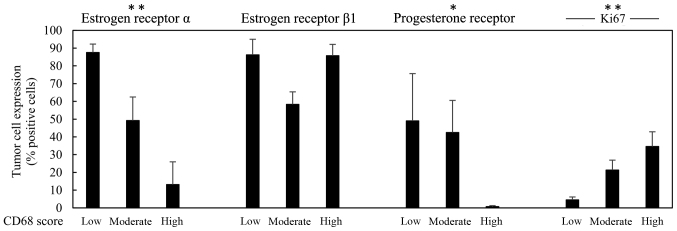
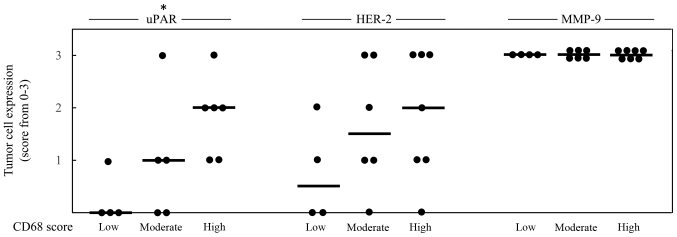
To investigate association between infiltration of macrophages and selected markers in human breast cancer tissue, 17 breast cancer biopsies were immunohistochemically stained for CD68, CD163, ERα, PR, HER-2, ERβ1, Ki67, MMP-9 and uPAR. The immunoreactivity (IR) of the selected antigens was evaluated and is presented in Table II. Representative images of the IR obtained by staining of CD68, CD163, ERβ1 and uPAR are shown in Fig. 1. The score for CD68 and CD163 were equal in 15/17 cases strongly indicating that the M2 macrophage phenotype is the dominant one being present in the currently investigated biopsies. In Figs. 2 and 3, the score 1–3 of CD68 is positively associated with higher expression of Ki67 or uPAR, respectively. No statistical significant association could be seen between CD68 and the expression of HER-2, ERβ1 or MMP-9, while an inverse association between CD68 and ERα as well as between CD68 and PR, could be noted (Fig. 2).
Table II.
Immunohistochemical staining of 17 breast cancer biopsies.
| Sample | CD68 | CD163 | ERα (%) | ERβ1 (%) | PR (%) | HER-2 | Ki-67 (%) | MMP-9 (T/S) | uPAR (T/S) | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 3 | 3 | 90 | 100 | 0 | 3+ | 15 | 3 | 3 | 2 | 0 |
| 2 | 1 | 1 | 100 | 95 | 100 | 2+ | 0 | 3 | 3 | 0 | 0 |
| 3 | 2 | 2 | 75 | 30 | 75 | 0 | 25 | 3 | 2 | 1 | 0 |
| 4 | 3 | 3 | 0 | 95 | 0 | 3+ | 50 | 3 | 3 | 1 | 0 |
| 5 | 2 | 2 | 0 | 70 | 0 | 3+ | 25 | 3 | 3 | 0 | 0 |
| 6 | 1 | 1 | 80 | 95 | 5 | 1+ | 5 | 3 | 3 | 0 | 0 |
| 7 | 2 | 2 | 30 | 60 | 10 | 3+ | 8 | 3 | 2 | 0 | 0 |
| 8 | 1 | 1 | 80 | 60 | 90 | 0 | 5 | 3 | 2 | 0 | 0 |
| 9 | 1 | 2 | 90 | 95 | 1 | 0 | 8 | 3 | 3 | 1 | 0 |
| 10 | 3 | 3 | 0 | 90 | 0 | 0 | 70 | 3 | 3 | 3 | 3 |
| 11 | 3 | 3 | 1 | 90 | 1 | 2+ | 50 | 3 | 2 | 2 | 0 |
| 12 | 3 | 3 | 0 | 95 | 0 | 3+ | 25 | 3 | NA | 1 | NA |
| 13 | 2 | 1 | 40 | 60 | 0 | 2+ | 10 | 3 | 2 | NA | NA |
| 14 | 2 | 2 | 60 | 80 | 70 | 1+ | 45 | 3 | 2 | 3 | 2 |
| 15 | 3 | 3 | 1 | 80 | 4 | 1+ | 15 | 3 | 3 | 2 | 0 |
| 16 | 3 | 3 | 0 | 50 | 0 | 2+ | 17 | 3 | 3 | NA | NA |
| 17 | 2 | 2 | 90 | 50 | 100 | 0 | 15 | 3 | 1 | 1 | 0 |
HER-2, human epidermal growth factor 2; MMP-9, matrix metalloproteinase-9; uPAR, urokinase-type plasminogen activator receptor; ERα, estrogen receptor α; ERβ1, estrogen receptor β1; PR, progesterone receptor. T, positive tumor cells; S, positive macrophage-like stroma cells; NA, not available.
Figure 1.
Immunohistochemical staining of CD68, CD163, ERβ1 and uPAR in human, breast cancer biopsies. Representative images of CD68 (A) score 1, (B) score 2, (C) score 3; CD163 (D) score 1, (E) score 2, (F) score 3; ERβ1 (G) 30% positive tumor cells, (H) 50% positive tumor cells (I) 100% positive tumor cells; uPAR (J) score 1, (K) score 2, (L) score 3 (×400 magnification, calibration bar is 50 µm in all micrographs).
Figure 2.
Infiltration density of CD68+ mononuclear cells in a total of 17 cases ranged from score 1–3; n=4 cases CD68+ score 1 (low), n=6 cases CD68+ score 2 (moderate) and n=7 cases CD68+ score 3 (high) and the expression of estrogen receptor α, estrogen receptor β1, progesterone receptor and Ki67 all denoted as percentage (%) of positive breast carcinoma cells. Results are presented as mean values ± SEM. The Jonckheere-Terpstra test was used to determine significant associations between increasing levels of CD68+ cells and decreasing amount of tumor cells expressing ERα, ERβ1 or PR, or increasing amount of tumor cells expressing Ki67 (*p<0.05, **p<0.01, ***p<0.001).
Figure 3.
Infiltration density of CD68+ mononuclear cells in a total of 17 cases ranged from score 1–3; n=4 cases CD68+ score 1 (low), n=6 cases CD68+ score 2 (moderate) (for uPAR one case with CD68 score 2 was not available) and n=7 cases CD68+ score 3 (high) (for uPAR one case with CD68 score 3 was not available). The infiltration density of CD68+ mononuclear cells and the expression of uPAR, HER-2 and MMP-9 in tumor cells all denoted as score 0–3 of positive breast carcinoma cells; 0 = negative, 1 = low, 2 = medium and 3 = high. Each case is represented by a dot and the median value of each group is indicated by a line. The Jonckheere-Terpstra test was used to determine significant associations between increasing levels of CD68+ cells and increasing tumor cell expression of uPAR, HER-2 or MMP9 (*p<0.05, **p<0.01, ***p<0.001).
Conditioned media from cultured human macrophages decrease cell proliferation, reduce protein expression of Ki-67, and mRNA expression of ERα and PR, and increase mRNA expression of ERβ1 in T47D
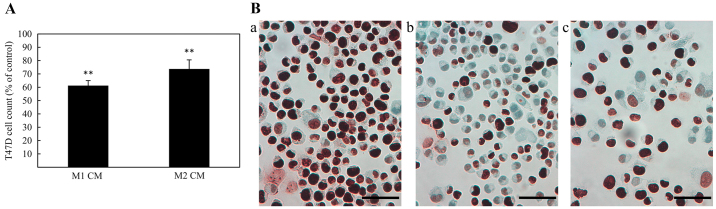
For investigation of the effect of CM (48 h challenge) from M1 or M2 macrophages on the proliferation of T47D, cells were counted in a hemocytometer and immunocytochemically stained for the proliferation marker, Ki67. As shown in Fig. 4A and B, both M1 and M2 macrophage CM caused a decrease in cell number as well as a reduced Ki67 protein expression, when compared with untreated controls. In addition, cell cycle distribution analysis for T47D cells treated with macrophage CM indicated that treatment with M1 CM caused an accumulation of cells in G0/G1 phase (Table III).
Figure 4.
(A) Effects of conditioned media (CM) from macrophages of M1 and M2 phenotype on the proliferation of T47D breast cancer cells. Results are expressed as percentage of mean value ± standard deviation. Asterisks indicate significant differences compared to the untreated control (100%) (*p<0.05, **p<0.01, ***p<0.001). (B) Immunoreactivity for the proliferation marker Ki67 in T47D breast cancer cell line. Representative examples of the effect from treatment with conditioned media (CM) from macrophages (b) of M1 or (c) M2 phenotype demonstrating a reduced Ki67 protein immunoreactivity compared with (a) untreated T47D cells (×400 magnification, calibration bar is 50 µm in all micrographs).
Table III.
Cell cycle analysis of T47D cells treated with macrophage CM.
| Treatment | Cells in G1/G0-phase (%) | Cells in S-phase (%) | Cells in G2/M-phase (%) |
|---|---|---|---|
| RPMI 5% FCS | 70 | 20 | 10 |
| M1 CM | 85 | 5 | 10 |
| M2 CM | 75 | 15 | 10 |
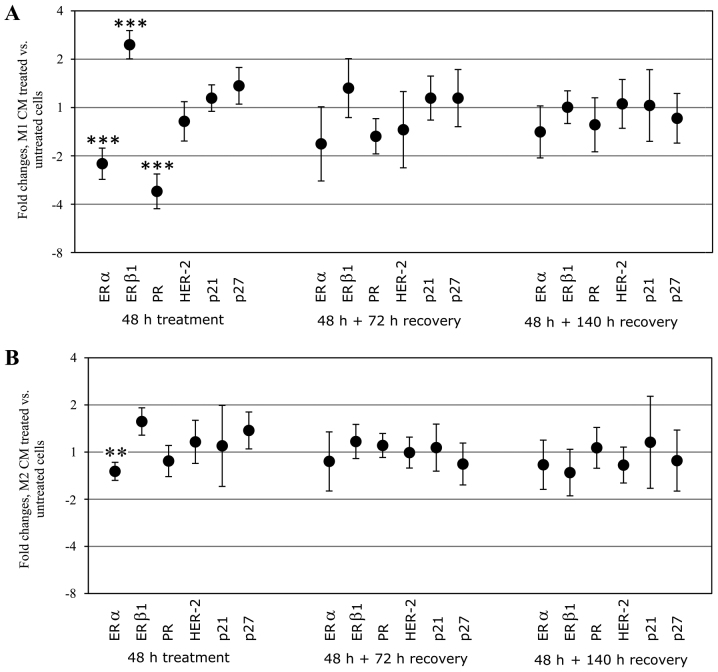
The mRNA expression of ERα, ERβ1, PR, HER-2, p21, p27, MMP-9 and uPAR, respectively, was analyzed in treated T47D cells and was compared with untreated controls. In cells treated with M1 CM there was a significant downregulation of ERα and PR mRNA. In contrast, the expression of ERβ1 was significantly upregulated with M1 CM. Treatment with CM from M2 macrophages also significantly downregulated the mRNA expression of ERα, however, to a lesser extent than treatment with M1 CM. When the CM was removed, normal expression of hormone receptors in T47D was restored after 140 h. No significant change in HER-2 or the cell cycle regulatory genes p21 and p27 expression in mRNA could be observed in response to either M1 or M2 CM treatment (Fig. 5). There was no detectable level of MMP-9 mRNA in T47D cells (data not shown). Gene expression of uPAR indicated downregulation at the mRNA level, however, no immunoreactivity for the uPAR protein could be confirmed in the T47D cells making this mRNA data less relevant (data not shown).
Figure 5.
Relative mRNA expression of estrogen receptor α (ERα), estrogen receptor β1 (ERβ1), progesterone receptor (PR), human epidermal growth factor receptor 2 (HER-2), p21 and p27 in T47D breast cancer cell line treated with CM from M1 (A) and M2 (B) macrophages. Fold changes were calculated using the ΔΔCq method and results are compared with untreated control cells. Results are mean values ± SEM using CM obtained from at least four different macrophage batches from different donors. Asterisks indicate significant differences of ΔCT values between treatment and control (*p<0.05, **p<0.01, ***p<0.001).
Conditioned media from cultured human macrophages reduce ERα protein expression in T47D
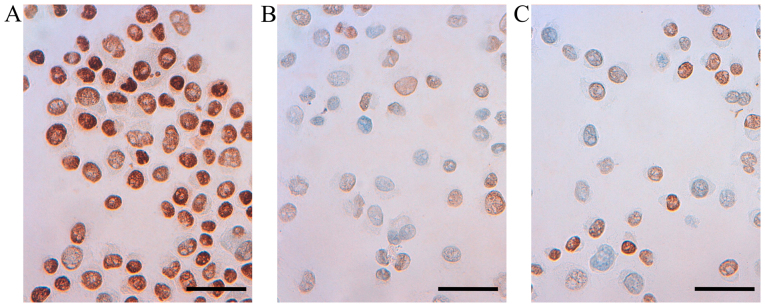
Immunocytochemical staining for ERα, ERβ1, PR and uPAR in T47D cells treated with CM from M1 or M2 macrophages was performed, and were compared with untreated control cells. In accordance with the downregulation of ERα mRNA in cells treated with M1 CM (above), reduced immunoreactivity for ERα protein could be observed in T47D treated with conditioned media from either M1 or M2 macrophages (Fig. 6).
Figure 6.
Representative immunoreactivity for estrogen receptor α (ERα) in T47D breast cancer cell line (A) untreated T47D control cells (B) T47D cells treated with CM from M1 macrophage phenotype for 48 h (C) T47D cells treated with CM from M2 macrophage phenotype for 48 h (×400 magnification, calibration bar is 50 µm in all micrographs).
Immunocytochemical staining for PR in untreated and treated T47D cells demonstrated a very strong nuclear staining with only a slightly weaker positivity in a few of the T47D cells treated with M1 CM. Staining for ERβ1 revealed a very strong nuclear staining of both untreated T47D and T47D cells treated with M1 or M2 CM. No immunoreactivity for the uPAR protein could be confirmed in the T47D cells (Table IV).
Table IV.
Immunoreactivity of T47D cells treated with CM from M1 or M2 macrophages.
| Treatment | ERα | ERβ1 | PR | Ki-67 | uPAR |
|---|---|---|---|---|---|
| RPMI 5% FCS | +++ | +++ | +++ | +++ | − |
| M1 CM | + | +++ | ++ | + | − |
| M2 CM | + | +++ | +++ | ++ | − |
ERα, estrogen receptor α; ERβ1, estrogen receptor β1; PR, progesterone receptor; uPAR, urokinase-type plasminogen activator receptor. +++, very strong immunoreactivity; ++, strong immunoreactivity; +, weak immunoreactivity; −, negative immunoreactivity.
Characterization of cultured macrophages of M0, M1 and M2 phenotypes using quantitative PCR
CM generated from the cultured human M1 and M2 macrophages exhibited different effects on the breast carcinoma cell line T47D. To study possible differences in the CM used, RT-qPCR was performed on cultured macrophages of M0, M1 and M2 phenotype. Macrophages were terminated for RNA extraction at two different time points, first directly after the washing and removal of the additives used for differentiation of the M1 and M2 macrophages (LPS, IFNγ, IL4 and IL13), i.e. at the start of collection of CM (0 h) and second at the end of collection of CM (48 h incubation). The M1 macrophage phenotype was found to express significantly lower mRNA level of IL10 and significantly higher mRNA levels of IL6, IL8, IL12, CXCL9, TNFα and NFκB, these upregulated genes had a higher significant upregulation at the start of media collection (0 h) than at the end (48 h) (Table V). Expression of uPAR and MMP-9 mRNA in M0, M1 and M2 macrophages has been previously investigated (32) showing in that study no difference between either phenotype.
Table V.
mRNA expression levels in macrophages of M1 and M2 phenotypes in comparison to M0.
| Target mRNA | M0 0 h | M1 0 h | M2 0 h | M0 48 h | M1 48 h | M2 48 h |
|---|---|---|---|---|---|---|
| IL6 | 1 | U 190-foldb | U 10-fold | ND | ND | ND |
| IL8 | 1 | U 1700-foldc | D 2-folda | 1 | U 26-foldc | U 2-folda |
| IL10 | 1 | D 5-folda | 1 | 1 | U 5-fold | 1 |
| IL12 | 1 | U 5-foldc | 1 | D 2-fold | 1 | D 3-fold |
| CCL2 | 1 | D 2-fold | 1 | D 2-fold | 1 | D 8-fold |
| CCL17 | 1 | D 10-fold | U 4-fold | D 5-fold | ND | U 3-fold |
| CCL18 | 1 | U 26-fold | U 115-fold | D 2-fold | U 30-folda | U 30-fold |
| CXCL9 | 1 | U 14,000-foldc | 1 | D 5-fold | U 190-folda | D 3-fold |
| TNF-α | 1 | U 95-foldc | 1 | D 2-fold | U 7-folda | D 5-folda |
| NFκB | 1 | U 9-foldb | 1 | 1 | U 4-folda | 1 |
p<0.05,
p<0.01,
p<0.001. ND, not detected; U, upregulation; D, downregulation. 0 h indicate differentiated macrophages; 48 h indicate macrophages 48 h post-differentiation.
Discussion
Breast cancer, being the most common malignancy in women, constitute a heterogeneous disease in which the status of hormone receptors, HER-2 and Ki67 are routinely used to categorize tumors and to provide predictive information of response to hormonal therapy or targeted treatments. Adenocarcinoma of the breast are frequently infiltrated with TAMs and the aim of the current study was to investigate a possible relationship between such infiltration and the expression of receptors for various hormones as well as for HER-2, Ki67, MMP-9 and uPAR in 17 breast tumor biopsies chosen for neoadjuvant therapy, and in addition in a human breast cancer cell line.
It has been demonstrated that high number of M2 macrophages correlate with poor outcome in breast cancer (27) and that all histological locations of TAM have prognostic value (29). In our current study we noted both intratumoral and stromal dominance of M2 macrophages indicated by a large proportion of CD68+/CD163+ macrophages in all 17 breast tumor biopsies. Effects of macrophages on the markers analyzed in the current study could contribute to the understanding of the underlying mechanisms of TAMs in tumor progression. With regard to uPAR, there are indications that tumor cells which express this receptor may stimulate macrophage polarization towards a more tumor permissive M2 macrophage phenotype with the ability to promote tumor invasion and metastasis (22). uPAR is of major interest in breast cancer, ligation of uPAR has been shown to elicit an activation of uPA a validated biomarker for a worse outcome in breast cancer and also to activate MMP-9 that correlate with poor prognosis in breast cancer (21,33,34).
In the current study, we could demonstrate a significant positive association between the level of uPAR expressed on the surface of the tumor cell and the macrophage score. The number of MMP-9-positive tumor cells was high in all the 17 tumor biopsies selected for neoadjuvant therapy and therefore no association with the level of macrophages or uPAR could be demonstrated. Moreover, a significant positive association between the extent of macrophage infiltration and the expression of Ki67 was found, Ki67 is a proliferation marker and high expression is associated with a more aggressive tumor growth and higher risk of developing recurrent disease (35). Numerous studies have revealed that the expression of the growth factor HER-2 is associated with poor prognosis in breast cancer, however the link between macrophage infiltrates and HER-2 status is inconsistent (25–29). We could not demonstrate a significant positive association between the extent of macrophage infiltration and the expression of HER-2. Loss of ERα or PR expression in breast cancer gives a worse prognosis and exclude the possibility to treat these patients with hormone blocking therapy (36). We found that the amount of M2 macrophage infiltration is inversely associated with the expression of ERα as well as of PR. These findings support previous reports which suggest that high infiltration of macrophages expressing either CD68 or CD163 is associated with ERα and PR-negative tumors (25–29), and demonstrate another possible route for TAMs in breast cancer to act in an unfavorable manner.
Moreover, we demonstrate that CM from macrophages of M1 and M2 phenotype could decrease the amount of ERα at the mRNA levels in vitro in the breast carcinoma cell line T47D. The downregulation of ERα mRNA was accompanied by an apparent decrease in ERα immunoreactivity, as demonstrated by immunocytochemistry.
Our current findings are in concert with those by Stossi et al (37). Thus, these authors also reported that conditioned media from THP-1 macrophages induced a loss of expression of ERα mRNA and protein in MCF-7 breast cancer cell line via the involvement of MAPK and c-Jun. There are previous reports of high contents of inflammatory cytokines and of infiltrating leukocytes in ERα-negative tumors (38) and several macrophage-derived cytokines have been implicated in the downregulation of ERα (39). We observed that IL6, IL8, IL12, CXCL9, TNFα and NFκB were upregulated in the M1 macrophages in comparison with the M2 macrophages, although expressed also by M2. TNF-α as well as NFκB have been associated with suppression of ERα in vitro (39–41). Moreover, IL8 was overexpressed in ER-negative breast cancer cells (42). A previous observation that IL6 elicited a loss of ERα mRNA expression and caused methylation of the promoter for ERα in MCF-7 cells (43), could not be confirmed in our study. Thus, when the CM was removed and cells were re-cultured in RPMI the expression of hormone receptors was restored after 140 h.
Moreover, the effect of CM from M1 or M2 macrophage phenotypes on cell proliferation, and expression of HER-2, Ki67 and hormone receptors, was investigated in the T47D cell line. After 48 h of treatment, both M1 and M2 macrophage CM caused a decrease in the cell number of T47D compared with untreated controls, with the highest effect elicited by the M1 macrophage. This was accompanied by a reduced protein expression of Ki67 in T47D. However, this finding could not be confirmed in the tumor biopsies where a strong infiltration of macrophages was associated with high Ki67 proliferation index. It is most likely that TAM contribute to tumor growth in vivo by means not taken into account in our in vitro experiments, for instance by contributing to sustained angiogenesis (23,24).
The prognostic role of the hormone receptor ERβ1 in breast cancer is less clear (7,8) and whether an association between macrophages and expression of ERβ1 in breast cancer exists is not known. The expression of ERβ1 was high in the 17 breast tumor biopsies analyzed and we could not demonstrate any association between the amount of macrophage and the tumor cell expression of ERβ1. However, the mRNA level of ERβ1 in T47D cells was upregulated when treated with macrophage CM. Unlike ERα, ERβ has a putative anti-proliferative effect when binding to its ligand (7). M1 CM caused an accumulation of T47D cells in G0/G1 phase and a decrease of cells in S-phase, indicating a cell cycle arrest in G0/G1. This inhibitory effect of M1 CM on T47D cells is in agreement with previous studies on the colon cancer cell line HT-29 and the lung cancer cell line H520 (30,32,44) and suggests that macrophages of the M1 phenotype in breast cancer tumor stroma might have an inhibitory effect and reduce the growth of breast cancer cells. However, it was also stated that CM from M1 macrophage phenotype attenuated the effect of chemotherapy for cells which responds with a G0/G1 cell cycle arrest (44). In the current study, we could not observe any changes on the cell cycle inhibitory gene p21 and this has been observed previously in the small cell lung cancer cell line H69 (32).
In conclusion, our in vivo and in vitro results confirm the potential of the macrophages alone to influence the expression of PR and ERα. We also demonstrated in vivo and in vitro differences in the influence of Ki67, HER-2 and ERβ1, though; our in vivo results demonstrated a significant positive association of macrophages and the tumor cell expression of uPAR and Ki67. Our result support previous studies suggesting that macrophages are involved in impairing the prognosis for breast cancer patients and that there could be a reason to control the level of macrophages in some breast carcinoma patients.
Acknowledgments
The present study was supported financially by the County Council of Värmland, Karlstad University and Örebro University.
References
- 1.Siegel RL, Miller KD, Jemal A. Cancer Statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 2.Goldhirsch A, Wood WC, Coates AS, Gelber RD, Thürlimann B, Senn HJ. Panel members: Strategies for subtypes - dealing with the diversity of breast cancer: Highlights of the St. Gallen International Expert Consensus on the Primary Therapy of Early Breast Cancer 2011. Ann Oncol. 2011;22:1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferretti G, Felici A, Papaldo P, Fabi A, Cognetti F. HER2/neu role in breast cancer: From a prognostic foe to a predictive friend. Curr Opin Obstet Gynecol. 2007;19:56–62. doi: 10.1097/GCO.0b013e328012980a. [DOI] [PubMed] [Google Scholar]
- 4.Thomas C, Gustafsson JA. The different roles of ER subtypes in cancer biology and therapy. Nat Rev Cancer. 2011;11:597–608. doi: 10.1038/nrc3093. [DOI] [PubMed] [Google Scholar]
- 5.Diep CH, Daniel AR, Mauro LJ, Knutson TP, Lange CA. Progesterone action in breast, uterine, and ovarian cancers. J Mol Endocrinol. 2015;54:R31–R53. doi: 10.1530/JME-14-0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci USA. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Haldosén LA, Zhao C, Dahlman-Wright K. Estrogen receptor beta in breast cancer. Mol Cell Endocrinol. 2014;382:665–672. doi: 10.1016/j.mce.2013.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Leung YK, Lee MT, Lam HM, Tarapore P, Ho SM. Estrogen receptor-beta and breast cancer: Translating biology into clinical practice. Steroids. 2012;77:727–737. doi: 10.1016/j.steroids.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 10.Biswas SK, Allavena P, Mantovani A. Tumor-associated macrophages: Functional diversity, clinical significance, and open questions. Semin Immunopathol. 2013;35:585–600. doi: 10.1007/s00281-013-0367-7. [DOI] [PubMed] [Google Scholar]
- 11.Balkwill F, Mantovani A. Inflammation and cancer: Back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 12.Coussens LM, Werb Z. Inflammation and cancer. Nature. 2002;420:860–867. doi: 10.1038/nature01322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sica A, Mantovani A. Macrophage plasticity and polarization: In vivo veritas. J Clin Invest. 2012;122:787–795. doi: 10.1172/JCI59643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hao NB, Lü MH, Fan YH, Cao YL, Zhang ZR, Yang SM. Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol. 2012;2012:948098. doi: 10.1155/2012/948098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Allavena P, Sica A, Solinas G, Porta C, Mantovani A. The inflammatory micro-environment in tumor progression: The role of tumor-associated macrophages. Crit Rev Oncol Hematol. 2008;66:1–9. doi: 10.1016/j.critrevonc.2007.07.004. [DOI] [PubMed] [Google Scholar]
- 16.Sica A, Schioppa T, Mantovani A, Allavena P. Tumour-associated macrophages are a distinct M2 polarised population promoting tumour progression: Potential targets of anti-cancer therapy. Eur J Cancer. 2006;42:717–727. doi: 10.1016/j.ejca.2006.01.003. [DOI] [PubMed] [Google Scholar]
- 17.Sica A, Allavena P, Mantovani A. Cancer related inflammation: The macrophage connection. Cancer Lett. 2008;267:204–215. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 18.Lau SK, Chu PG, Weiss LM. CD163: A specific marker of macrophages in paraffin-embedded tissue samples. Am J Clin Pathol. 2004;122:794–801. doi: 10.1309/QHD6YFN81KQXUUH6. [DOI] [PubMed] [Google Scholar]
- 19.Holness CL, Simmons DL. Molecular cloning of CD68, a human macrophage marker related to lysosomal glycoproteins. Blood. 1993;81:1607–1613. [PubMed] [Google Scholar]
- 20.Matsumoto H, Koo SL, Dent R, Tan PH, Iqbal J. Role of inflammatory infiltrates in triple negative breast cancer. J Clin Pathol. 2015;68:506–510. doi: 10.1136/jclinpath-2015-202944. [DOI] [PubMed] [Google Scholar]
- 21.Noh H, Hong S, Huang S. Role of urokinase receptor in tumor progression and development. Theranostics. 2013;3:487–495. doi: 10.7150/thno.4218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hu J, Jo M, Eastman BM, Gilder AS, Bui JD, Gonias SL. uPAR induces expression of transforming growth factor β and interleukin-4 in cancer cells to promote tumor-permissive conditioning of macrophages. Am J Pathol. 2014;184:3384–3393. doi: 10.1016/j.ajpath.2014.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis CE, Leek R, Harris A, McGee JO. Cytokine regulation of angiogenesis in breast cancer: The role of tumor-associated macrophages. J Leukoc Biol. 1995;57:747–751. doi: 10.1002/jlb.57.5.747. [DOI] [PubMed] [Google Scholar]
- 24.Leek RD, Hunt NC, Landers RJ, Lewis CE, Royds JA, Harris AL. Macrophage infiltration is associated with VEGF and EGFR expression in breast cancer. J Pathol. 2000;190:430–436. doi: 10.1002/(SICI)1096-9896(200003)190:4<430::AID-PATH538>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 25.Mahmoud SM, Lee AH, Paish EC, Macmillan RD, Ellis IO, Green AR. Tumour-infiltrating macrophages and clinical outcome in breast cancer. J Clin Pathol. 2012;65:159–163. doi: 10.1136/jclinpath-2011-200355. [DOI] [PubMed] [Google Scholar]
- 26.Mohammed ZM, Going JJ, Edwards J, Elsberger B, Doughty JC, McMillan DC. The relationship between components of tumour inflammatory cell infiltrate and clinicopathological factors and survival in patients with primary operable invasive ductal breast cancer. Br J Cancer. 2012;107:864–873. doi: 10.1038/bjc.2012.347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tiainen S, Tumelius R, Rilla K, Hämäläinen K, Tammi M, Tammi R, Kosma VM, Oikari S, Auvinen P. High numbers of macrophages, especially M2-like (CD163-positive), correlate with hyaluronan accumulation and poor outcome in breast cancer. Histopathology. 2015;66:873–883. doi: 10.1111/his.12607. [DOI] [PubMed] [Google Scholar]
- 28.Medrek C, Pontén F, Jirström K, Leandersson K. The presence of tumor associated macrophages in tumor stroma as a prognostic marker for breast cancer patients. BMC Cancer. 2012;12:306. doi: 10.1186/1471-2407-12-306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gwak JM, Jang MH, Kim DI, Seo AN, Park SY. Prognostic value of tumor-associated macrophages according to histologic locations and hormone receptor status in breast cancer. PLoS One. 2015;10:e0125728. doi: 10.1371/journal.pone.0125728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Engström A, Erlandsson A, Delbro D, Wijkander J. Conditioned media from macrophages of M1, but not M2 phenotype, inhibit the proliferation of the colon cancer cell lines HT-29 and CACO-2. Int J Oncol. 2014;44:385–392. doi: 10.3892/ijo.2013.2203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ruijter JM, Ramakers C, Hoogaars WM, Karlen Y, Bakker O, van den Hoff MJ, Moorman AF. Amplification efficiency: Linking baseline and bias in the analysis of quantitative PCR data. Nucleic Acids Res. 2009;37:e45. doi: 10.1093/nar/gkp045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hedbrant A, Wijkander J, Seidal T, Delbro D, Erlandsson A. Macrophages of M1 phenotype have properties that influence lung cancer cell progression. Tumour Biol. 2015;36:8715–8725. doi: 10.1007/s13277-015-3630-9. [DOI] [PubMed] [Google Scholar]
- 33.Duffy MJ, O'Donovan N, McDermott E, Crown J. Validated biomarkers: The key to precision treatment in patients with breast cancer. Breast. 2016;29:192–201. doi: 10.1016/j.breast.2016.07.009. [DOI] [PubMed] [Google Scholar]
- 34.Zhao S, Ma W, Zhang M, Tang D, Shi Q, Xu S, Zhang X, Liu Y, Song Y, Liu L, et al. High expression of CD147 and MMP-9 is correlated with poor prognosis of triple-negative breast cancer (TNBC) patients. Med Oncol. 2013;30:335. doi: 10.1007/s12032-012-0335-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dai X, Xiang L, Li T, Bai Z. Cancer hallmarks, biomarkers and breast cancer molecular subtypes. J Cancer. 2016;7:1281–1294. doi: 10.7150/jca.13141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dunnwald LK, Rossing MA, Li CI. Hormone receptor status, tumor characteristics, and prognosis: A prospective cohort of breast cancer patients. Breast Cancer Res. 2007;9:R6. doi: 10.1186/bcr1639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Stossi F, Madak-Erdoğan Z, Katzenellenbogen BS. Macrophage-elicited loss of estrogen receptor-α in breast cancer cells via involvement of MAPK and c-Jun at the ESR1 genomic locus. Oncogene. 2012;31:1825–1834. doi: 10.1038/onc.2011.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chavey C, Bibeau F, Gourgou-Bourgade S, Burlinchon S, Boissière F, Laune D, Roques S, Lazennec G. Oestrogen receptor negative breast cancers exhibit high cytokine content. Breast Cancer Res. 2007;9:R15. doi: 10.1186/bcr1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee SH, Nam HS. TNF alpha-induced down-regulation of estrogen receptor alpha in MCF-7 breast cancer cells. Mol Cells. 2008;26:285–290. [PubMed] [Google Scholar]
- 40.Bhat-Nakshatri P, Campbell RA, Patel NM, Newton TR, King AJ, Marshall MS, Ali S, Nakshatri H. Tumour necrosis factor and PI3-kinase control oestrogen receptor alpha protein level and its transrepression function. Br J Cancer. 2004;90:853–859. doi: 10.1038/sj.bjc.6601541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Oida K, Matsuda A, Jung K, Xia Y, Jang H, Amagai Y, Ahn G, Nishikawa S, Ishizaka S, Jensen-Jarolim E, et al. Nuclear factor-ĸB plays a critical role in both intrinsic and acquired resistance against endocrine therapy in human breast cancer cells. Sci Rep. 2014;4:4057. doi: 10.1038/srep04057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Freund A, Chauveau C, Brouillet JP, Lucas A, Lacroix M, Licznar A, Vignon F, Lazennec G. IL-8 expression and its possible relationship with estrogen-receptor-negative status of breast cancer cells. Oncogene. 2003;22:256–265. doi: 10.1038/sj.onc.1206113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.D'Anello L, Sansone P, Storci G, Mitrugno V, D'Uva G, Chieco P, Bonafé M. Epigenetic control of the basal-like gene expression profile via Interleukin-6 in breast cancer cells. Mol Cancer. 2010;9:300. doi: 10.1186/1476-4598-9-300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hedbrant A, Erlandsson A, Delbro D, Wijkander J. Conditioned media from human macrophages of M1 phenotype attenuate the cytotoxic effect of 5-fluorouracil on the HT-29 colon cancer cell line. Int J Oncol. 2015;46:37–46. doi: 10.3892/ijo.2014.2696. [DOI] [PMC free article] [PubMed] [Google Scholar]