Abstract
Background:
Impaired awareness of hypoglycaemia (IAH) predisposes affected patients to severe hypoglycaemia. There are few data on prevalence of IAH in adults with insulin-treated type 2 diabetes in Asia. We aim to ascertain the prevalence of IAH among insulin-treated patients with type 2 diabetes in an outpatient clinic in a tertiary care centre in Singapore.
Methods:
A total of 374 patients with insulin-treated type 2 diabetes attending the outpatient diabetes clinic in a tertiary referral centre in Singapore were recruited over a 4-month period. Participants completed a questionnaire to document baseline characteristics and assess their hypoglycaemia awareness status, using a combination of the Clarke, Gold and Pedersen-Bjergaard methods.
Results:
Using the Clarke, Gold and Pedersen-Bjergaard methods, prevalence of IAH in our cohort was 9.6%, 13.4% and 33.2% respectively. Overall, 7.2% of participants suffered from severe hypoglycaemia in the preceding year. The IAH group had more episodes of severe hypoglycaemia across all three methods, compared with the normal awareness group (p < 0.01). There were no significant differences in mean HbA1c, duration of diabetes and insulin treatment between the IAH and normal awareness groups.
Conclusions:
IAH is prevalent in adults with insulin-treated type 2 diabetes in Asia, and is associated with significantly increased risk of severe hypoglycaemia.
Keywords: Diabetes mellitus type 2, hypoglycaemia, impaired awareness
Introduction
Strong emphasis has been placed on intensification of diabetes control since the publication of landmark trials such as the Diabetes Control and Complications Trial (DCCT) and the United Kingdom Prospective Diabetes Study.1,2 However, intensification of diabetes control is associated with a three-fold increase in the risk of severe hypoglycaemia (SH).3 SH, defined as an event requiring assistance of another person to correct hypoglycaemia, is associated with significant cardiovascular sequelae.4,5 A sharp rise in the incidence of SH has been reported in Asia, highlighting a need for further research in SH prevention.6,7
Early subjective recognition of the symptoms of hypoglycaemia allows affected individuals to take action to restore the blood glucose level. Impaired awareness of hypoglycaemia (IAH) is a phenomenon whereby patients have no symptoms or vague symptoms of hypoglycaemia and may not be able to take actions in time to stop the blood glucose from falling to a critical level. IAH is widely recognized as a significant risk factor for SH.8,9
IAH has been studied extensively in people with type 1 diabetes. The Gold, Clarke and Pedersen-Bjergaard methods are established methods to evaluate IAH in type 1 diabetes.8–10 Depending on the methodology used, prevalence of IAH in people with type 1 diabetes has been shown to range between 24–62.5%.11 Little is known about the prevalence of IAH in type 2 diabetes, with only one study estimating it to be 9.8% in a Scottish clinic population of patients with insulin-treated type 2 diabetes.12,13 Singapore is an example of an Asian country that has experienced a marked rise in the prevalence of diabetes over the past decade, from 9.0% in 1998 to 11.3% in 2010.14 IAH and the association with SH have not been evaluated in the Asian healthcare setting. Hence, it is timely to evaluate IAH and SH among patients with insulin-treated type 2 diabetes.
By utilizing the Gold, Clarke and Pedersen-Bjergaard methods, we hope to explore and assess the prevalence of IAH in patients with insulin-treated type 2 diabetes in an outpatient setting in Singapore.
Patients and methods
Participants
Insulin-treated adults with type 2 diabetes attending the Diabetes and Metabolism Centre at Singapore General Hospital between August and December 2015 were recruited. Based on data extrapolated from existing studies, a sample size of 318 would be required for an estimated prevalence of IAH of 9.8%.12 Inclusion criteria were patients older than 21 years with type 2 diabetes who have been treated with insulin therapy for more than 6 months. The age cutoff of 21 years was used as the Singapore General Hospital is an adult tertiary care centre. Exclusion criteria were pregnancy and end stage renal disease.
Methods
Each participant completed a questionnaire which incorporated the Minimally Modified Clarke’s questionnaire, Gold single-item scale and Pedersen-Bjergaard single-item scale to assess IAH. The Clarke method comprises eight questions. Responses are graded as reduced awareness (R) or aware (A). A sum of four R responses and above indicates IAH.9 The Gold method comprises a single question ‘Do you know when your hypoglycaemia is starting?’. The response is in the form of a seven-point Likert scale ranging from 1 (always aware) to 7 (never aware). A score of 4 and above suggests IAH.8 The Pedersen-Bjergaard method comprises a single question ‘Can you feel when your blood sugar is low?’. Responses were in the form of ordered categories from ‘never’ to ‘always’. Any response that is not ‘always’ indicates IAH.10 In our questionnaire, the frequency of SH in the preceding year was obtained through participants’ response to the number of time in which they required third party assistance to recognize and treat their hypoglycaemia.4 Baseline characteristics and diabetes treatment regimens were also recorded. To facilitate the participation of non-English speaking individuals, the questionnaire was translated into Chinese and made available when required. We followed the principles and process recommended by the International Society for Pharmacoeconomics and Outcomes Research for translation of the questionnaire.15 The participant could seek clarification of the content of the questionnaire from a member of the investigator team if required. HbA1c was measured using the Tina-quant® HbA1c Gen. 3 assay (Roche Diagnostics Ltd, Switzerland). The results were DCCT aligned.1
This study was approved by the SingHealth Centralised Institutional Review Board (Singapore). Informed consent was obtained prior to the study.
Statistical analysis
All results are expressed as mean ± standard deviation (SD) unless otherwise stated. To compare the differences between groups, a two-sample t-test, Mann–Whitney U test or Chi-square test was used, as appropriate. A p value of <0.05 was considered statistically significant. A Spearman’s correlation coefficient was used to assess the relationship between the two variables to assess the relationship between Clarke, Gold and Pedersen-Bjergaard methods. All statistical analyses were performed using IBM SPSS Statistics for Windows, Version 23.0 (Armonk, NY: IBM Corp).
Results
Participant characteristics
A total of 374 participants completed the study. A total of 50.8% of participants were female and 49.2% were male. The mean age of the cohort was 58 ± 13.1 years, with mean duration of diabetes of 17.4 ± 8.7 years, median insulin duration of 6 years [interquartile range (IQR): 3–10 years], and mean HbA1c of 72 ± 20 mmol/mol (8.7 ± 1.8%). The majority of the participants were Chinese (58%), followed by Indian (23.5%), Malay (13.4%) and others (5.1%). An English version of the questionnaire was administered to 77.5% of the participants; 22.5% completed the Chinese version.
Prevalence of IAH
The prevalence of IAH ranged from 9.6–33.2% depending on the scoring method used (Figure 1). There was a fall in prevalence of IAH from 33.2% to 18.2% when the criteria for IAH in Pedersen-Bjergaard method was revised such that both ‘always’ as well as ‘usually’ were classified as normal awareness, while ‘sometimes’ and ‘never’ were classified as IAH.
Figure 1.
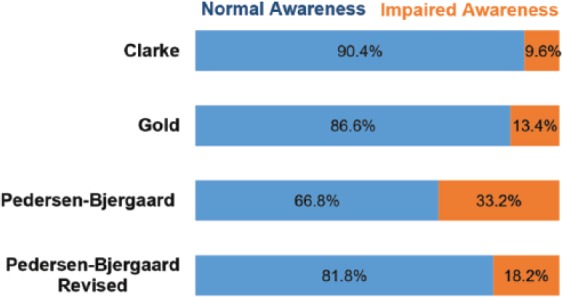
Prevalence of normal awareness of hypoglycaemia (blue bar) vs impaired awareness of hypoglycaemia (orange bar) as measured by Clarke, Gold, Pedersen-Bjergaard and revised Pedersen-Bjergaard methods.
There was moderate correlation between the Clarke and Gold methods (rs = 0.56, p < 0.001), whereas the Pedersen-Bjergaard method correlated weakly with Clarke and Gold methods (rs = 0.46 and 0.49, ps < 0.001 respectively). With the revised version of Pedersen-Bjergaard method, the Spearman’s correlation coefficients increased to 0.60 with Clarke method and 0.61 with Gold method (ps < 0.001).
There were no significant differences in the IAH prevalence detected by the English version compared with that of the Chinese version, with the IAH prevalence being 9.7% versus 9.5%, p = 0.97 according to Clarke method; 33.4% versus 32.1%, p = 0.82 according to Pedersen-Bjergaard method; and 13.1% versus 14.3%, p = 0.78 according to Gold method.
Frequency of SH in IAH and normal awareness groups
The overall prevalence of self-reported SH in the preceding 1 year was 7.2%. The IAH group had a statistically significant higher proportion of patients with SH, as well as greater number of episodes of SH per patient per year compared with the normal awareness group. This difference was consistent regardless of the hypoglycaemia awareness assessment method used (Table 1). There were no significant differences in mean HbA1c, duration of diabetes and insulin treatment regimens between the IAH and normal awareness groups.
Table 1.
Characteristics of patients in normal awareness versus IAH groups. (n = 374). Values are expressed as mean ± SD, median (IQR), n (%).
| Hypoglycaemia awareness | Clarke |
Gold |
Pedersen-Bjergaard |
Pedersen-Bjergaard Revised |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normal | Impaired | p | Normal | Impaired | p | Normal | Impaired | p | Normal | Impaired | p | |
| Number | 338 | 36 | − | 324 | 50 | − | 250 | 124 | − | 306 | 68 | − |
| Age (years) | 57.7 ± 12.7 | 61.4 ± 15.8 | 0.18 | 57.9 ± 12.5 | 59.0 ± 16.3 | 0.66 | 58.4 ± 11.6 | 57.2 ± 15.6 | 0.45 | 58.1 ± 12.5 | 57.7 ± 15.6 | 0.84 |
| Duration of DM (years) | 17.2 ± 8.7 | 19.1 ± 8.6 | 0.22 | 17.4 ± 8.5 | 17.4 ± 9.7 | 0.97 | 17.4 ± 8.3 | 17.6 ± 9.4 | 0.83 | 17.5 ± 8.5 | 17.1 ± 9.3 | 0.77 |
| Duration of insulin treatment (years) | 6 (3–10) | 5 (3–9.75) | 0.57 | 6 (3–10) | 5 (2–10) | 0.31 | 6 (3–10) | 6 (3–10) | 0.38 | 6 (3–11) | 5 (2–8.75) | 0.06 |
| HbA1c (%) | 8.7 ± 1.9 | 8.6 ± 1.6 | 0.78 | 8.7 ± 1.9 | 8.5 ± 1.6 | 0.52 | 8.7 ± 1.8 | 8.7 ± 1.9 | 0.90 | 8.7 ± 1.9 | 8.8 ± 1.8 | 0.80 |
| HbA1c (mmol/mol) | 72 ± 21 | 70 ± 17 | 0.78 | 72 ± 21 | 69 ± 17 | 0.52 | 72 ± 20 | 72 ± 21 | 0.90 | 72 ± 21 | 73 ± 20 | 0.80 |
| Mean no. of SH episodes per person per year | 0.04 ± 0.22 | 0.67 ± 0.93 | 0.001 | 0.06 ± 0.36 | 0.30 ± 0.54 | 0.005 | 0.04 ± 0.21 | 0.22 ± 0.61 | 0.001 | 0.05 ± 0.24 | 0.32 ± 0.74 | 0.003 |
| No. of patients with SH in preceding year (%) | 10 (3.0) | 17 (47.2) | <0.001 | 14 (4.3) | 13 (26.0) | <0.001 | 8 (3.2) | 19 (15.3) | <0.001 | 12 (3.9) | 15 (22.1) | <0.001 |
DM, diabetes mellitus; IAH, Impaired awareness of hypoglycaemia; IQR, interquartile range; SD, standard deviation; SH, severe hypoglycaemia.
Discussion
Hypoglycaemia forms a challenging aspect of diabetes management. Very little is known about the prevalence of IAH in adults with type 2 diabetes, particularly in Asia. To our knowledge, this is the first study which uses three methods of assessment of IAH to assess prevalence of IAH among the insulin-treated type 2 diabetes patient attending a tertiary care centre. This study is also the first study to address the issue of IAH and SH in an Asian healthcare setting. Moreover, this study incorporates both English and Chinese translated versions of questionnaire to assess IAH in adults with type 2 diabetes outside of Europe and North America. Remarkably, the Chinese translated version of questionnaire correlated well with the English version and detected a similar rate of IAH.
In our study, the Pedersen-Bjergaard method estimated a much higher prevalence of IAH compared with the Clarke and Gold methods. This is in keeping with known data from studies evaluating IAH in type 1 diabetes.11 The Clarke and Gold methods estimated the prevalence of IAH to be between 9.6–13.4% which is close to the prevalence of 9.8% as reported by Schopman and colleagues12,13 There are differences in methodologies between our study and the Schopman and colleagues’ study; the latter used the Gold method as the only method of assessing IAH whereas we used a combination of Clarke, Gold and Pedersen-Bjergaard methods, and demonstrated the moderate correlation between Clarke and Gold methods. Each method of assessing IAH has its strengths and limitations. The Clarke method, with its composite scoring nature, gives further objective stratifications of different levels of IAH. The Gold and Pedersen-Bjergaard methods have the advantages of being simple and quick to administer but the Pedersen-Bjergaard method may overestimate the prevalence of IAH.11 Different methods may be deployed in different settings to evaluate IAH. It would be reasonable to use the Gold method in a busy outpatient clinic setting to screen for IAH among the insulin-treated type 2 diabetes patients. The positive findings from the screening Gold questionnaire may alert the clinicians to assess the problems of IAH in greater details during the brief outpatient visit. The Clarke method will be very useful in quantifying the improvement in hypoglycaemia awareness when interventions such as continuous subcutaneous insulin infusion or islet cell transplantation are used to tackle the issue of IAH and SH, as the data have greater granularity.16
Studies on patients with type 1 diabetes have identified association between age, disease duration and prevalence of IAH.17,18 We have not found significant differences in duration and treatment of diabetes between the IAH and normal hypoglycaemia awareness groups. This suggests that we cannot apply the usual risk factors for IAH to type 2 diabetes patients. With rising prevalence of type 2 diabetes globally, more work is needed in this area to identify patients at risk of IAH and SH.
Our study has a number of limitations. Firstly, due to the limited number of studies done on the prevalence of IAH in type 2 diabetes, these questionnaires have not been extensively validated for use in type 2 diabetes. Ideally, these assessment tools should be validated prior to being used to establish the prevalence of IAH in our context. However, rigorous validation studies using self-monitoring of blood glucose in a large cohort of participants is an extensive process. The intention of our study is to explore the use of these instruments in type 2 diabetes. To overcome the shortcomings of this study, we conducted cognitive interviews with 16 participants for each assessment tool, and concluded that they possess excellent face validity before we adopted them for this study. The striking similarity of the pattern of variation of the estimated IAH prevalence in our study sample and previously published data from a European centre suggest that the behaviour of these tools are rather robust across cultural contexts.11 Secondly, this questionnaire-based study used predominantly self-reported parameters and did not include blood glucose monitoring in the documentation of hypoglycaemia. We plan to test criteria validity in a subsequent study by incorporating diary of hypoglycaemic episodes. Thirdly, we would like to highlight that deterioration from normal awareness of hypoglycaemia to IAH is a continuous spectrum. Use of questionnaires with various cutoffs for detecting IAH artificially stratifies participants into discrete groups of either with intact hypoglycaemia awareness or with IAH. Therefore, thorough clinical assessment of hypoglycaemia awareness should still be an important component of diabetes consultation.
Conclusions
IAH is prevalent in the outpatient clinic in our institution and is associated with significantly increased risk of SH. There is a need to systematically evaluate IAH in type 2 diabetes patients as this has not been the focus of type 2 diabetes care in the past.
Acknowledgments
The authors would like to thank the staff at the Diabetes and Metabolism Centre, Singapore General Hospital for their assistance in facilitating the study.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Ling Zhu, Department of Endocrinology, Singapore General Hospital, 20 College Road, Singapore 169856, Singapore.
Li Chang Ang, Academic Clinical Program, Division of Medicine, Singapore General Hospital, Singapore.
Wee Boon Tan, Academic Clinical Program, Division of Medicine, Singapore General Hospital, Singapore.
Xiaohui Xin, Academic Clinical Program, Division of Medicine, Singapore General Hospital, Singapore.
Yong Mong Bee, Department of Endocrinology, Singapore General Hospital, Singapore.
Su-Yen Goh, Department of Endocrinology, Singapore General Hospital, Singapore.
Ming Ming Teh, Department of Endocrinology, Singapore General Hospital, Singapore.
References
- 1. The Diabetes Control and Complications Trial Research Group. The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. N Engl J Med 1993; 329: 977–986. [DOI] [PubMed] [Google Scholar]
- 2. UK Prospective Diabetes Study (UKPDS) Group. Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet Lond Engl 1998; 352: 837–853. [PubMed] [Google Scholar]
- 3. The Diabetes Control and Complications Trial Research Group. Hypoglycemia in the Diabetes Control and Complications Trial. Diabetes 1997; 46: 271–286. [PubMed] [Google Scholar]
- 4. Seaquist ER, Anderson J, Childs B, et al. Hypoglycemia and diabetes: a report of a workgroup of the American Diabetes Association and the Endocrine Society. Diabetes Care 2013; 36: 1384–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zoungas S, Patel A, Chalmers J, et al. Severe hypoglycemia and risks of vascular events and death. N Engl J Med 2010; 363: 1410–1418. [DOI] [PubMed] [Google Scholar]
- 6. Kim JT, Oh TJ, Lee YA, et al. Increasing trend in the number of severe hypoglycemia patients in Korea. Diabetes Metab J 2011; 35: 166–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Chen Y-J, Yang C-C, Huang L-C, et al. Increasing trend in emergency department visits for hypoglycemia from patients with type 2 diabetes mellitus in Taiwan. Prim Care Diabetes 2015; 9: 490–496. [DOI] [PubMed] [Google Scholar]
- 8. Gold AE, MacLeod KM, Frier BM. Frequency of severe hypoglycemia in patients with type I diabetes with impaired awareness of hypoglycemia. Diabetes Care 1994; 17: 697–703. [DOI] [PubMed] [Google Scholar]
- 9. Clarke WL, Cox DJ, Gonder-Frederick LA, et al. Reduced awareness of hypoglycemia in adults with IDDM. A prospective study of hypoglycemic frequency and associated symptoms. Diabetes Care 1995; 18: 517–522. [DOI] [PubMed] [Google Scholar]
- 10. Pedersen-Bjergaard U, Agerholm-Larsen B, Pramming S, et al. Activity of angiotensin-converting enzyme and risk of severe hypoglycaemia in type 1 diabetes mellitus. Lancet Lond Engl 2001; 357: 1248–1253. [DOI] [PubMed] [Google Scholar]
- 11. Geddes J, Wright RJ, Zammitt NN, et al. An evaluation of methods of assessing impaired awareness of hypoglycemia in type 1 diabetes. Diabetes Care 2007; 30: 1868–1870. [DOI] [PubMed] [Google Scholar]
- 12. Schopman JE, Geddes J, Frier BM. Prevalence of impaired awareness of hypoglycaemia and frequency of hypoglycaemia in insulin-treated type 2 diabetes. Diabetes Res Clin Pract 2010; 87: 64–68. [DOI] [PubMed] [Google Scholar]
- 13. Schopman J. Hypoglycaemia in diabetes (academic thesis). University of Amsterdam, 2013. [Google Scholar]
- 14. Singapore Health Factors - Disease Burden, Ministry of Health, Singapore: https://www.moh.gov.sg/content/moh_web/home/statistics/Health_Facts_Singapore/Disease_Burden.html (2016, accessed November 2016). [Google Scholar]
- 15. Wild D, Grove A, Martin M, et al. Principles of good practice for the translation and cultural adaptation process for patient-reported outcomes (PRO) measures: report of the ISPOR Task Force for Translation and Cultural Adaptation. Value Health J Int Soc Pharmacoeconomics Outcomes Res 2005; 8: 94–104. [DOI] [PubMed] [Google Scholar]
- 16. Gehlaut RR, Dogbey GY, Schwartz FL, et al. Hypoglycemia in type 2 diabetes–more common than you think: a continuous glucose monitoring study. J Diabetes Sci Technol 2015; 9: 999–1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Geddes J, Schopman JE, Zammitt NN, et al. Prevalence of impaired awareness of hypoglycaemia in adults with type 1 diabetes. Diabet Med J Br Diabet Assoc 2008; 25: 501–504. [DOI] [PubMed] [Google Scholar]
- 18. Olsen SE, Asvold BO, Frier BM, et al. Hypoglycaemia symptoms and impaired awareness of hypoglycaemia in adults with type 1 diabetes: the association with diabetes duration. Diabet Med J Br Diabet Assoc 2014; 31: 1210–1217. [DOI] [PubMed] [Google Scholar]


