Abstract
Iodide (I−)-accumulating bacteria were isolated from marine sediment by an autoradiographic method with radioactive 125I−. When they were grown in a liquid medium containing 0.1 μM iodide, 79 to 89% of the iodide was removed from the medium, and a corresponding amount of iodide was detected in the cells. Phylogenetic analysis based on 16S rRNA gene sequences indicated that iodide-accumulating bacteria were closely related to Flexibacter aggregans NBRC15975 and Arenibacter troitsensis, members of the family Flavobacteriaceae. When one of the strains, strain C-21, was cultured with 0.1 μM iodide, the maximum iodide content and the maximum concentration factor for iodide were 220 ± 3.6 (mean ± standard deviation) pmol of iodide per mg of dry cells and 5.5 × 103, respectively. In the presence of much higher concentrations of iodide (1 μM to 1 mM), increased iodide content but decreased concentration factor for iodide were observed. An iodide transport assay was carried out to monitor the uptake and accumulation of iodide in washed cell suspensions of iodide-accumulating bacteria. The uptake of iodide was observed only in the presence of glucose and showed substrate saturation kinetics, with an apparent affinity constant for transport and a maximum velocity of 0.073 μM and 0.55 pmol min−1 mg of dry cells−1, respectively. The other dominant species of iodine in terrestrial and marine environments, iodate (IO3−), was not transported.
Iodine is an essential trace element for humans and animals because of its important role as a constituent of thyroid hormones. In the thyroid gland in mammals, iodine is taken up by and accumulates in thyroid follicular cells as iodide ion (I−) (13). Iodine deficiency leads to endemic goiter and cretinism, and a 1993 World Health Organization report (39) estimated that 1.6 billion people, or 30% of the world's population, live in iodine-deficient areas. From a radioecological viewpoint, on the other hand, the long-lived radioisotope iodine-129 (129I; half-life, 1.6 × 107 years) is of great concern, since it is one of the most persistent radionuclides released from nuclear facilities into the environment (10, 24). Given its long half-life, 129I can participate in the biogeochemical cycling of iodine and potentially accumulate in the human thyroid gland (11). Therefore, it is necessary to obtain better information on the behavior of iodine in the environment for accurate safety assessments of nuclear facilities (27, 36).
When we consider the mobility and behavior of long-lived 129I, it is important to understand how iodine is retained in the environment. In the terrestrial environment, iodine is strongly adsorbed to soils, and high iodine concentrations in soils have been reported, e.g., 5 mg kg−1 as a worldwide average (9) and about 30 mg kg−1 as a representative value for Japanese soil (26). These values are much higher than those in their parental materials, such as rocks and plants (0.05 to 0.5 mg kg−1) (37), indicating that iodine is highly accumulated in soils. Although iodine sorption to soils is affected by various physicochemical parameters, including soil type, pH, Eh, salinity, and organic carbon content (16, 37), a number of studies have indicated that soil microorganisms are also involved in the process (5, 8, 20, 26). Muramatsu and Yoshida (26) found that autoclaving of soils significantly reduced the sorption of iodide. The decreased sorption was recovered by incubation of autoclaved soil with a smaller amount (1%) of fresh soil, suggesting an effect of increased microbial activities. Koch et al. (20) observed increased iodide sorption to soils treated with glucose and decreased sorption to soils treated with thymol (an antiseptic). Decreased iodine sorption to fumigated, air-dried, or gamma-irradiated soils has also been reported (8). Similarly, several studies with marine sediments (oxic sediments) showed the presence of very high concentrations of iodine (23, 30, 31). For instance, 96 to 1,990 mg of iodine kg−1 was observed in the surface sediments of the Southwest African Shelf (30). Since the total dissolved iodine concentration in seawater is only 60 μg liter−1 (38), it is apparent that these sediments are highly enriched in iodine. Malcolm and Price (23) speculated that iodine accumulation in sediments occurs as a result of bacterial activities. The importance of bacteria in iodide sorption was also suggested for Canadian Shield lake sediments (7).
Details of microbial effects on iodine accumulation in the environment remain to be solved. One possible explanation is that iodine is bound to a bacterial cell envelope or is actively transported into cells (8, 23, 31). To date, however, there have been no reports on bacteria capable of accumulating iodine. The predominant chemical forms of environmental iodine are iodide and iodate (IO3−) ions (16, 38), and previous studies of iodine uptake by brown algae and thyroid cells showed that the bioavailable iodine form is not iodate but iodide (14, 21). In this study, we attempted to isolate iodide-accumulating bacteria (IAB) from the environment and to characterize the mechanisms of their iodide accumulation. Such bacteria may contribute to the fixation of both stable iodine and 129I in soils and marine sediments.
MATERIALS AND METHODS
Bacterial strains.
Flexibacter aggregans NBRC15975 and Zobellia uliginosa NBRC14962T were purchased from the NITE Biological Research Center, Chiba, Japan. Arenibacter troitsensis JCM11736T was purchased from the Japan Collection of Microorganisms, Saitama, Japan.
Isolation of IAB.
Ninety-one bacterial strains which had been isolated from marine surface sediment, surface soil, surface seawater, and seaweed were used in a first screening. They were grown in PTYG liquid medium (1) (for soil isolates) or marine broth 2216 (Difco) (for marine isolates) and were inoculated onto agar plates. The agar plates contained 0.1 μM nonradioactive iodide (KI) and 370 kBq of 125I− ml−1 (Na125I at 80.5 Mbq nmol−1; Amersham Bioscience). This iodide concentration was similar to concentrations in seawater (38) and soil pore water (40). After incubation at 30°C, bacterial colonies were transferred to Hybond-XL membrane filters (Amersham Bioscience) by using sterile toothpicks. Finally, the membranes were exposed for 2 days in the dark, and the results were visualized with a Molecular Imager FX system (Bio-Rad). Bacterial strains showing dense and black spots were chosen as candidates for IAB.
In a second screening, the strains were cultured aerobically in liquid media containing nonradioactive iodide (0.1 μM) and 125I− (74 kBq ml−1). After cultivation for 24 h, 700 μl of each culture was placed on a silicone oil layer (500 μl; 35:65 mixture of SH556 and SH550; Toray Dow Corning Silicone) that had been placed over 100 μl of distilled water in a microcentrifuge tube. After centrifugation (13,000 × g at 4°C for 3 min), 50 μl of the supernatant sample was removed and placed in a scintillation vial, and the contents of the microcentrifuge tube were frozen at −80°C. The distilled water layer containing the cell pellet then was cut away by using a pair of dog nail clippers and was transferred to another scintillation vial. The activity of 125I was measured with an NaI scintillation counter (Aloka ARC-370 M) for 10 min. At zero time (before cultivation), 50 μl of the medium was removed for the measurement of 125I, and the total 125I concentration in the medium was calculated. To assess iodide-accumulating ability, the efficiency of accumulation by each strain was calculated with the following equation: percent accumulation = (total amount of 125I in cell pellets at 24 h/total amount of 125I in the medium at zero time) × 100. The efficiency of accumulation was also calculated with another equation: percent accumulation = 100 − (total amount of 125I in the supernatant at 24 h/total amount of 125I in the medium at zero time) × 100. In most instances, the values obtained with these two equations were similar.
Sequencing of 16S rRNA genes.
Genomic DNA was isolated by the method of Hiraishi (17). 16S rRNA gene sequences were determined by PCR amplification and direct sequencing with previously described conditions and reagents (19). The obtained 16S rRNA gene sequences were subjected to BLAST searches (http://www.ncbi.nlm.nih.gov/BLAST/) to determine 16S rRNA gene similarities. The retrieved sequences were aligned by using the CLUSTAL W program, version 1.6 (33).
Iodide uptake assay.
Cells grown in marine broth 2216 were harvested by centrifugation (10,000 × g at 4°C for 10 min) and washed twice with washing buffer, containing 330 mM NaCl, 30 mM MgCl2 · 6H2O, 6.8 mM CaCl2 · 2H2O, 2.2 mM K2HPO4, and 2.9 mM KH2PO4 (pH 6.0). The cell pellet was resuspended in the same buffer to achieve an optical density at 600 nm of 1.0 (equivalent to approximately 0.5 mg [dry weight] per ml). The transport assay was carried out with a 100-ml Erlenmeyer flask containing 10 ml of washed cell suspension. The reaction was started by the addition of a mixture of nonradioactive iodide and 125I− to yield final concentrations of 0.1 μM and 74 kBq ml−1, respectively. Glucose was added as an exogenous source of energy at a concentration of 28 mM. When iodate uptake was determined, nonradioactive iodate (KIO3) and 125IO3− were added at the same concentration as iodide. An iodate tracer was prepared from 125I− as described previously (25). The reaction mixture was incubated at 30°C on a shaker rotating at 180 rpm, and the activity of 125I in the cell pellet was measured at various times as described above. The radioactivity at zero time was subtracted from activities at subsequent times to calculate the net uptake by the cells.
Nucleotide sequence accession numbers.
The 16S rRNA gene sequences determined in this study have been deposited in the DDBJ database under the accession numbers shown in Table 1.
TABLE 1.
Iodide accumulation by IAB, moderate IAB, and non-IAB
| Strain | 16S rRNA gene accession no. | % Accumulation of iodide after 24 ha |
|---|---|---|
| IAB | ||
| C-7 | AB118679 | 86 ± 0.0 |
| C-12 | AB118674 | 82 ± 4.2 |
| C-21 | AB182553 | 83 ± 5.1 |
| U-2 | AB182554 | 79 ± 2.1 |
| U-3 | AB118680 | 89 ± 9.6 |
| U-5 | AB118681 | 83 ± 1.9 |
| Moderate IAB | ||
| C-4 | AB182555 | 17 ± 5.1 |
| C-8 | AB182556 | 15 ± 6.9 |
| Non-IAB | ||
| 4-5 | NDb | 2.8 ± 0.46 |
| C-1 | AB118672 | 0.70 ± 0.0 |
| C-3 | AB118678 | 4.7 ± 0.0 |
| C-6 | AB182557 | 0.33 ± 0.31 |
| Hj-3 | AB182558 | 0.35 ± 0.25 |
Means ± standard deviations of triplicate analyses.
ND, not determined.
RESULTS
Isolation of IAB.
In the first autoradiographic screening, most bacterial colonies exhibited black spots to some extent. Among these, 13 bacterial strains showing dense black spots were chosen as candidates for IAB. These strains were cultured in liquid medium for 24 h, and their capacities for accumulating iodide were quantified. In 6 of the 13 strains, 79 to 89% of total iodide was removed from the medium, and the corresponding amount of iodide was detected in the cells (Table 1). All of these strains originated from marine surface sediment and were used as IAB in further experiments. We also isolated from marine sediment two bacterial strains with relatively weak abilities to accumulate iodide. In these strains, designated “moderate IAB,” 15 to 17% of total iodide was accumulated in the cells (Table 1). The remaining five strains did not show any significant abilities to accumulate iodide (Table 1). When one of the IAB, strain C-21, was grown on solid medium containing 125I−, strong radioactivity was found to be incorporated into the cells, indicating that iodide actually accumulated in the IAB cells (Fig. 1).
FIG. 1.
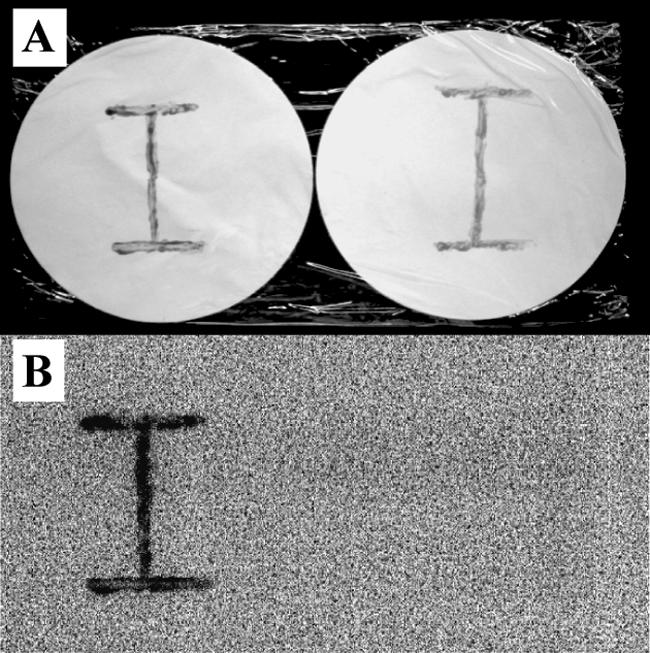
Iodide accumulation by IAB grown on solid medium. Strains C-21 (left) and C-6 (a control non-IAB) (right) were grown on marine agar 2216 containing 0.1 μM KI and 370 kBq of 125I− ml−1. After incubation for 3 days, the cells were removed from the medium and streaked on Hybond-XL membrane filters in the shape of an “I” (A). The membranes were exposed for 2 days in the dark, and the results were visualized with a Molecular Imager FX system (B).
Phylogenetic positions of IAB.
In a preliminary phylogenetic analysis, approximately 450-bp 16S rRNA gene fragments of IAB (strains C-7, C-12, C-21, U-2, U-3, and U-5) were sequenced and aligned. All of these strains were located within the Cytophaga-Flavobacterium-Bacteroides phylum and were closely related to each other, with sequence similarities of 95.7 to 99.8%. One of the strains, strain C-21, was chosen as a representative strain, and approximately 1,400 bp of its 16S rRNA gene was sequenced. Strain C-21 was most closely related to F. aggregans NBRC15975 (28) and A. troitsensis (29), members of the family Flavobacteriaceae. Sequence similarities between strain C-21 and these two bacteria were 99.8 and 99.4%, respectively. Other related organisms were Arenibacter latericius (18), Z. uliginosa (4), and Zobellia galactanovorans (4), with sequence similarities of 94.7, 92.0, and 91.8%, respectively. Approximately 450-bp 16S rRNA gene fragments of two moderate IAB (strains C-4 and C-8) were also sequenced and aligned. These strains were closely related to Pseudorhodobacter ferrugineus (formerly Agrobacterium ferrugineum) (35) within the α subclass of the Proteobacteria.
In order to confirm the distribution of iodide-accumulating abilities among the related microorganisms, F. aggregans NBRC15975, A. troitsensis JCM11736T, and Z. uliginosa NBRC14962T were cultured in marine broth 2216 containing iodide (0.1 μM). These three bacterial strains accumulated 85.6, 89.3, and 40.5% of total iodide during cultivation for 24 h, respectively.
Iodide accumulation by IAB.
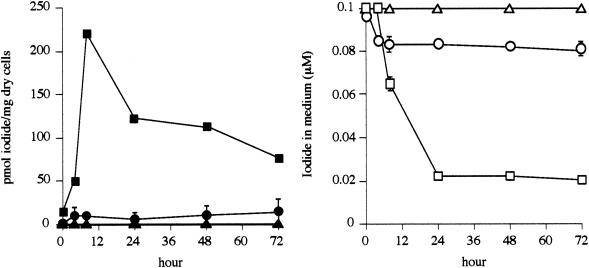
The time course of iodide accumulation by strain C-21 was observed. In addition, iodide accumulation by moderate IAB (strain C-4) and non-IAB (strain C-6) was also determined. As shown in Fig. 2, the maximum iodide content of 220 ± 3.6 (mean ± standard deviation) pmol per mg of dry cells was observed at 8 h in strain C-21. At 24 h, the iodide content had decreased to approximately half of that at 8 h, but iodide accumulated by the cells was not released into the medium. At the end of the cultivation (72 h), the iodide concentration in the medium had decreased to 0.02 μM, indicating that 80% of total iodide was removed from the medium (Fig. 2). Strain C-4, a moderate IAB, showed a maximum iodide content of 14 ± 0.66 pmol per mg of dry cells at 72 h, and approximately 20% of total iodide was removed from the medium. Strain C-6, a non-IAB, however, did not remove iodide from the medium, and the maximum iodide content of this strain was only 0.45 ± 0.012 pmol per mg of dry cells at 24 h. The iodide concentration factors for these three strains were calculated based on the ratio of the iodide concentration in cells (on a dry-weight basis) to that in the medium. The maximum iodide concentration factors for strains C-21, C-4, and C-6 were 5.5 × 103 (at 24 h), 1.7 × 102 (at 72 h), and 4.5 (at 24 h), respectively.
FIG. 2.
Time course of iodide accumulation by strains C-21 (squares), C-4 (circles), and C-6 (triangles) grown in marine broth 2216 containing iodide. The results are expressed as the means ± standard deviations of triplicate analyses. The absence of error bars indicates that the error was smaller than the symbol. Closed symbols represent the iodide content in the cells; open symbols represent the iodide concentration in the medium.
Effect of iodide concentration on iodide accumulation.
Strain C-21 was cultured for 24 h with iodide concentrations of 0.1 μM to 1 mM. The growth of this strain was not affected significantly even with 1 mM iodide (data not shown). Although the iodide content of this strain increased with increasing iodide concentrations, the percent accumulation of iodide decreased with much higher levels of iodide (Table 2). Similarly, the iodide concentration factor also decreased from 5.8 × 103 (at 0.1 μM) to 2.4 (at 1 mM). These results suggested that iodide accumulation by IAB was a saturable process.
TABLE 2.
Effect of iodide concentration on iodide accumulation by strain C-21a
| Iodide concn (μM) | % Accumulation of iodide | pmol of iodide/mg of dry cellsb |
|---|---|---|
| 0.1 | 82.7 | 101 ± 7.6 |
| 1 | 25.8 | 310 ± 0.72 |
| 10 | 3.5 | 430 ± 1.3 |
| 100 | 0.5 | 610 ± 13 |
| 1,000 | 0.2 | 2,400 ± 320 |
All data were obtained after cultivation for 24 h.
Means ± standard deviations of triplicate analyses.
Kinetics of iodide uptake.
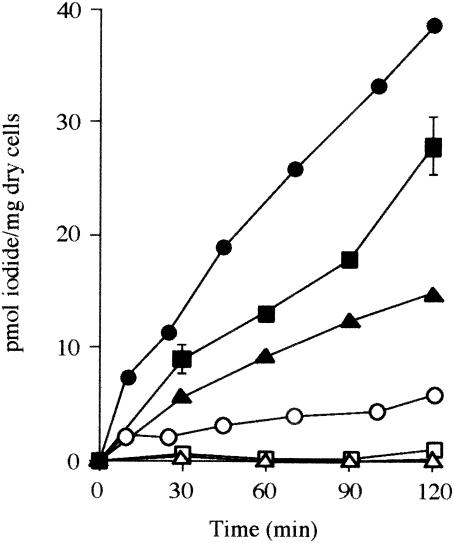
Iodide transport by IAB was assayed with washed cell suspensions of IAB. As shown in Fig. 3, cells were unable to take up iodide without glucose. However, the cumulative uptake of iodide was observed when the cell suspensions were incubated with glucose. Autoclaved cell suspensions did not take up iodide (data not shown). Initial rates of uptake of iodide with various nonradioactive iodide concentrations (0.02 to 0.5 μM) were determined with strain C-21. Iodide transport by this strain showed Michaelis-Menten kinetics, with an apparent affinity constant and a maximum velocity of 0.073 μM and 0.55 pmol min−1 mg of dry cells−1, respectively. Finally, initial rates of uptake of iodide and iodate by strain C-21 were compared. The values for iodide and iodate were 0.32 ± 0.0036 and 0.012 ± 0.0022 pmol min−1 mg of dry cells−1, respectively.
FIG. 3.
Iodide uptake by washed cell suspensions of strains C-7 (circles), C-21 (squares), and U-3 (triangles). The uptake experiments were conducted as described in Materials and Methods. Results for cells incubated in the presence (solid symbols) or absence (open symbols) of glucose are shown and are expressed as the means ± standard deviations of duplicate analyses. The absence of error bars indicates that the error was smaller than the symbol. Similar results were obtained for other IAB (C-12, U-2, and U-5).
DISCUSSION
The chemical behavior of iodine in the environment is affected by microorganisms. Tsunogai and Sase (34) reported that nitrate-reducing bacteria have the potential to reduce iodate to iodide. Iodate reduction by iron- and sulfate-reducing bacteria has also been reported (12, 15). In previous studies, we found that a wide variety of terrestrial and marine bacteria have capacities for methylating iodide to form methyl iodide (CH3I), a significant volatile organic iodine compound in the atmosphere (1-3). In addition, we recently found that certain marine bacteria (Roseovarius spp. and unidentified bacteria) oxidize iodide to form molecular iodine (I2) (3a). Together with these processes, a number of studies have shown that microorganisms participate in the fixation of iodine in the environment (5, 7, 8, 20, 23, 26, 30, 31). Considering the fact that several species of bacteria possess the abilities to accumulate certain elements (6, 22), it would not be surprising if they directly take up iodine and play significant roles in the fixation of iodine. In the present study, we found that certain marine bacteria actually possess capacities for accumulating iodide and that the uptake of iodide is a saturable process which requires an exogenous source of energy.
To date, biochemical mechanisms of iodine accumulation have been characterized only for two types of organisms, mammals (thyroid gland) and brown algae. The normal thyroid maintains a concentration of iodide 20 to 40 times higher than that in plasma (32). On the other hand, an iodide concentration factor of 1.5 × 105 has been reported for one brown alga (Laminaria digitata) (21). Our results showed that IAB accumulate iodide from the medium at a concentration factor of approximately 6 × 103. Thus, their iodide-accumulating ability is lower than that of the brown alga but much higher than that of thyroid cells. Our study of the kinetics of iodide uptake by IAB revealed that the apparent affinity constant and the maximum velocity are 0.073 μM and 0.55 pmol min−1 mg of dry cells−1, respectively. Since L. digitata has an apparent affinity constant for iodide of 420 μM and a maximum velocity of 65 pmol min−1 mg of tissue−1 (21), the decreased ability of IAB may be attributed to their high affinity and low velocity for iodide transport.
In the thyroid gland, iodide is taken up by a sodium-iodide symporter (13, 14, 32). This transmembrane protein cotransports sodium ion with iodide ion into the thyroid against the concentration gradient, and the driving force for the process is the electrochemical gradient of sodium ion across the membrane. On the other hand, the uptake of iodide by brown algae is dependent on oxidative power. Küpper et al. (21) showed that iodide in seawater is first oxidized to hypoiodous acid or molecular iodine by cell wall haloperoxidase, and these oxidized iodine species then freely penetrate algal cells by means of facilitated diffusion. The present study indicated that iodide transport by IAB is a saturable process and that glucose is required for iodide uptake (Fig. 3). Therefore, it is reasonable to consider that the process is mediated by an active transport system (not simple diffusion or sorption). More detailed investigations are needed for a full understanding of this uptake system. Determinations of the energy source and the specificity of iodide uptake by IAB are under way in our laboratory.
In conclusion, the present study provides evidence that IAB inhabit the natural environment. IAB may play a significant role in the fixation of stable iodine as well as long-lived 129I in the environment. In addition, it will be interesting to examine the physiological function of iodine accumulated by IAB cells.
Acknowledgments
We thank H. Suzuki (Radioisotope Research Center, Chiba University) for technical support.
REFERENCES
- 1.Amachi, S., Y. Kamagata, T. Kanagawa, and Y. Muramatsu. 2001. Bacteria mediate methylation of iodine in marine and terrestrial environments. Appl. Environ. Microbiol. 67:2718-2722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amachi, S., M. Kasahara, S. Hanada, Y. Kamagata, H. Shinoyama, T. Fujii, and Y. Muramatsu. 2003. Microbial participation in iodine volatilization from soils. Environ. Sci. Technol. 37:3885-3890. [DOI] [PubMed] [Google Scholar]
- 3.Amachi, S., M. Kasahara, T. Fujii, H. Shinoyama, S. Hanada, Y. Kamagata, T. Ban-Nai, and Y. Muramatsu. 2004.. Radiotracer experiments on biological volatilization of organic iodine from coastal seawaters. Geomicrobiol. J. 21:481-488. [Google Scholar]
- 3a.Amachi, S., Y. Muramatsu, Y. Akiyama, K. Miyazaki, S. Yoshiki, S. Hanada, Y. Kamagata, T. Ban-Nai, H. Shinoyama, and T. Fujii. Isolation of iodide-oxidizing bacteria from iodide-rich natural gas brines and seawaters. Microbial Ecol., in press. [DOI] [PubMed]
- 4.Barbeyron, T., S. L'Haridon, E. Corre, B. Kloareg, and P. Potin. 2001. Zobellia galactanovorans gen. nov., sp. nov., a marine species of Flavobacteriaceae isolated from a red alga, and classification of [Cytophaga] uliginosa (ZoBell and Upham 1944) Reichenbach 1989 as Zobellia uliginosa gen. nov., comb. nov. Int. J. Syst. Evol. Microbiol. 51:985-997. [DOI] [PubMed] [Google Scholar]
- 5.Behrens, H. 1982. New insights into the chemical behavior of radioiodine in aquatic environments, p. 27-40. Environmental migration of long-lived radionuclides. IAEA Proceedings Series STI/PUB/597. International Atomic Energy Agency, Vienna, Austria.
- 6.Belliveau, B. H., M. E. Starodub, C. Cotter, and J. T. Trevors. 1987. Metal resistance and accumulation in bacteria. Biotechnol. Adv. 5:101-127. [DOI] [PubMed] [Google Scholar]
- 7.Bird, G. A., and W. Schwartz. 1996. Distribution coefficients, Kds, for iodide in Canadian Shield lake sediments under oxic and anoxic conditions. J. Environ. Radioact. 35:261-279. [Google Scholar]
- 8.Bors, J., and R. Martens. 1992. The contribution of microbial biomass to the adsorption of radioiodide in soils. J. Environ. Radioact. 15:35-49. [Google Scholar]
- 9.Bowen, H. J. M. 1979. Environmental chemistry of the elements, p. 60-61. Academic Press Ltd., London, United Kingdom.
- 10.Buraglio, N., A. Aldahan, G. Possnert, and I. Vintersved. 2001. 129I from the nuclear reprocessing facilities traced in precipitation and runoff in northern Europe. Environ. Sci. Technol. 35:1579-1586. [DOI] [PubMed] [Google Scholar]
- 11.Cohen, B. L. 1985. The origin of I in soil and the 129I problem. Health Phys. 49:279-285. [DOI] [PubMed] [Google Scholar]
- 12.Councell, T. B., E. R. Landa, and D. R. Lovley. 1997. Microbial reduction of iodate. Water Air Soil Pollut. 100:99-106. [Google Scholar]
- 13.De La Vieja, A., O. Dohan, O. Levy, and N. Carrasco. 2000. Molecular analysis of the sodium/iodide symporter: impact on thyroid and extrathyroid pathophysiology. Physiol. Rev. 80:1083-1105. [DOI] [PubMed] [Google Scholar]
- 14.Eskandari, S., D. D. F. Loo, G. Dai, O. Levy, E. M. Wright, and N. Carrasco. 1997. Thyroid Na+/I− symporter: mechanism, stoichiometry, and specificity. J. Biol. Chem. 272:27230-27238. [DOI] [PubMed] [Google Scholar]
- 15.Farrenkopf, A. M., M. E. Dollhopf, S. N. Chadhain, G. W. Luther III, and K. H. Nealson. 1997. Reduction of iodate in seawater by bacterium Shewanella putrefaciens strain MR-4. Mar. Chem. 57:347-354. [Google Scholar]
- 16.Fuge, R., and C. C. Johnson. 1986. The geochemistry of iodine. Environ. Geochem. Health 8:31-54. [DOI] [PubMed] [Google Scholar]
- 17.Hiraishi, A. 1992. Direct automated sequencing of 16S rDNA amplified by polymerase chain reaction from bacterial cultures without DNA purification. Lett. Appl. Microbiol. 15:210-213. [DOI] [PubMed] [Google Scholar]
- 18.Iwanova, E. P., O. I. Nedashkovskaya, J. Chun, A. M. Lysenko, G. M. Frolova, V. I. Svetashev, M. V. Vysotskii, V. V. Mikhailov, A. Huq, and R. R. Colwell. 2001. Arenibacter gen. nov., new genus of the family Flavobacteriaceae, and description of a new species, Arenibater latericius sp. nov. Int. J. Syst. Evol. Microbiol. 51:1987-1995. [DOI] [PubMed] [Google Scholar]
- 19.Kanagawa, T., Y. Kamagata, S. Aruga, T. Kohno, M. Horn, and M. Wagner. 2000. Phylogenetic analysis of and oligonucleotide probe development for Eikelboom type 021N filamentous bacteria isolated from bulking activated sludge. Appl. Environ. Microbiol. 66:5043-5052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Koch, J. T., D. B. Rachar, and B. D. Kay. 1989. Microbial participation in iodide removal from solution by organic soils. Can. J. Soil Sci. 69:127-135. [Google Scholar]
- 21.Küpper, F. C., N. Schweigert, E. Ar Gall, J.-M. Legendre, H. Vilter, and B. Kloareg. 1998. Iodine uptake in Laminariales involves extracellular, haloperoxidase-mediated oxidation of iodide. Planta 207:163-171. [Google Scholar]
- 22.Ledin, M. 2000. Accumulation of metals by microorganisms—processes and importance for soil systems. Earth Sci. Rev. 51:1-31. [Google Scholar]
- 23.Malcolm, S. J., and N. B. Price. 1984. The behaviour of iodine and bromine in estuarine surface sediments. Mar. Chem. 15:263-271. [Google Scholar]
- 24.Moran, J. E., S. Oktay, P. H. Santschi, and D. R. Schink. 1999. Atmospheric dispersal of 129iodine from nuclear fuel reprocessing facilities. Environ. Sci. Technol. 33:2536-2542. [Google Scholar]
- 25.Muramatsu, Y., S. Uchida, and Y. Ohmomo. 1990. Determination of I-129 and I-127 in soil and tracer experiments on the adsorption of iodine in soil. J. Radioanal. Nucl. Chem. 138:377-384. [Google Scholar]
- 26.Muramatsu, Y., and S. Yoshida. 1999. Effects of microorganisms on the fate of iodine in the soil environment. Geomicrobiol. J. 16:85-93. [Google Scholar]
- 27.Muramatsu, Y., S. Yoshida, U. Fehn, S. Amachi, and Y. Ohmomo. 2004. Studies with natural and anthropogenic iodine isotopes: iodine distribution and cycling in the global environment. J. Environ. Radioact. 74:221-232. [DOI] [PubMed] [Google Scholar]
- 28.Nakagawa, Y., T. Sakane, M. Suzuki, and K. Hatano. 2002. Phylogenetic structure of the genera Flexibacter, Flexithrix, and Microscilla deduced from 16S rRNA sequence analysis. J. Gen. Appl. Microbiol. 48:155-165. [DOI] [PubMed] [Google Scholar]
- 29.Nedashkovskaya, O. I., M. Suzuki, M. V. Vysotskii, and V. V. Mikhailov. 2003. Arenibacter troitsensis sp. nov., isolated from marine bottom sediment. Int. J. Syst. Evol. Microbiol. 53:1287-1290. [DOI] [PubMed] [Google Scholar]
- 30.Price, N. B., and S. E. Calvert. 1973. The geochemistry of iodine in oxidized and reduced recent marine sediments. Geochim. Cosmochim. Acta 37:2149-2158. [Google Scholar]
- 31.Price, N. B., and S. E. Calvert. 1977. The contrasting geochemical behaviours of iodine and bromine in recent sediments from the Namibian shelf. Geochim. Cosmochim. Acta 41:1769-1775. [Google Scholar]
- 32.Smyth, P. P. A., and R. M. Dwyer. 2002. The sodium iodide symporter and throid disease. Clin. Endocrinol. 56:427-429. [DOI] [PubMed] [Google Scholar]
- 33.Thompson, J. D., D. G. Higgins, and T. J. Gibson. 1994. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 22:4673-4680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tsunogai, S., and T. Sase. 1969. Formation of iodide-iodine in the ocean. Deep Sea Res. 16:489-496. [Google Scholar]
- 35.Uchino, Y., T. Hamada, and A. Yokota. 2002. Proposal of Pseudorhodobacter ferrugineus gen nov, comb nov, for a non-photosynthetic marine bacterium, Agrobacterium ferrugineum, related to the genus Rhodobacter. J. Gen. Appl. Microbiol. 48:309-319. [DOI] [PubMed] [Google Scholar]
- 36.Vandecasteele, C. M., M. Van Hees, F. Hardeman, G. Voigt, and B. J. Howard. 2000. The true absorption of 131I, and its transfer to milk in cows given different stable iodine diets. J. Environ. Radioact. 47:301-317. [Google Scholar]
- 37.Whitehead, D. C. 1984. The distribution and transformations of iodine in the environment. Environ. Int. 10:321-339. [Google Scholar]
- 38.Wong, G. T. F. 1991. The marine geochemistry of iodine. Rev. Aquat. Sci. 4:45-73. [Google Scholar]
- 39.World Health Organization. 1993. Global prevalence of iodine deficiency disorders. MDIS working paper no. 1. World Health Organization, Geneva, Switzerland.
- 40.Yuita, K., T. Tanaka, C. Abe, and S. Aso. 1991. Dynamics of iodine, bromine, and chlorine in soil: 1. Effect of moisture, temperature, and pH on the dissolution of the triad from soil. Soil Sci. Plant Nutr. 37:61-73. [Google Scholar]