Abstract
Background:
Recent studies suggest that traumatic brain injury (TBI) is a risk factor for subsequent ischemic stroke, even years after the initial insult. The mechanisms of the association remain unclear. The presence of traumatic subarachnoid hemorrhage (tSAH) may mediate the effect of TBI on long-term stroke risk, as it has previously been linked to short-term vasospasm and delayed cerebral ischemia.
Methods:
Using administrative claims data, we conducted a retrospective cohort study of acute care hospitalizations. Patients discharged with a first-recorded diagnosis of tSAH were followed for a primary diagnosis of stroke. They were matched to patients with TBI but not tSAH. Cox proportional hazards modeling was used to assess the association between tSAH and stroke while adjusting for covariates.
Results:
We identified 40 908 patients with TBI (20 454 patients with tSAH) who were followed for a mean of 4.3 + 1.8 years. A total of 531 had an ischemic stroke after discharge. There was no significant difference in stroke risk between those with tSAH (1.79%; 95% confidence interval [CI] 1.54%-2.08%) versus without tSAH (2.12%; 95% CI 1.83%-2.44%). The same pattern was found in adjusted analyses even when the group was stratified by age-group or by proxies of TBI severity.
Conclusions:
Our findings do not support a role of tSAH in mediating the association between TBI and protracted stroke risk. Further study is required to elucidate the mechanisms of long-term increased stroke risk after TBI.
Keywords: stroke, traumatic brain hemorrhage, cerebrovascular trauma, subarachnoid hemorrhage, intracranial vasospasm
Introduction
Recent studies have demonstrated that traumatic brain injury (TBI) is a strong risk factor for subsequent ischemic stroke; the risk period extends up to 5 years following the initial insult and is independent of traditional vascular risk factors.1,2 The mechanisms of the association remain unknown. Arterial dissection seems an unlikely candidate as patients with arterial dissection were excluded from the studied TBI population.2 One possibility is the protracted risk of vasospasm due to the presence of traumatic subarachnoid hemorrhage (tSAH) leading to delayed cerebral ischemia. Alternatively, tSAH may lead to long-term vascular remodeling that predisposes patients to ischemic stroke.
Traumatic brain injury represents an amalgam of heterogeneous pathologies. Traumatic SAH is present in 39% to 65% of cases3–6 and is associated with poor outcomes.3,7–9 The quantity of tSAH has been shown to predict incidence and severity of vasospasm, which occurs in 14% to 45% of all cases with TBI.10–12 Vasospasm due to tSAH leads to decreased cerebral blood flow,11,13,14 ischemia, and infarction in up to 17% of cases.15–19
While tSAH may lead to delayed cerebral ischemia, there is a paucity of data to guide physicians in managing the mildest cases of TBI with minimal tSAH. Current guidelines suggest monitoring such patients for at least 24 hours.20 A recent study of over 1000 patients with Glasgow Coma Scale scores of 14 or 15 and intracranial hemorrhage (47% of which had tSAH) suggested that safe discharge was possible given stable imaging and neurological examination after as little as 6 hours.21 Given that vasospasm most commonly begins 2 days after TBI,6,11 this practice
warrants further review, especially as a recent observational study from Taiwan found that post-TBI stroke peaked soon after discharge and tailed off thereafter.1 Therefore, we sought to determine whether or not the presence of tSAH in TBI was associated with stroke risk following discharge.
Methods
Study Design
We performed a retrospective cohort study using data on all inpatient discharges in California, Florida, and New York. These data were collected by the California Office of Statewide Health Planning and Development, the New York Statewide Planning and Research Cooperative System, and the Florida Agency for Health Care Administration. These 3 agencies provide these data to the Agency for Healthcare Research and Quality for its Healthcare Cost and Utilization Project.22 Each patient is assigned a personal linkage number that allows them to be followed anonymously through all subsequent hospitalizations. Up to 24 discharge diagnoses, labeled as present before admission or developed during hospitalization, are coded at each encounter using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) system.23 The institutional review board at Weill Cornell Medical College approved this study.
Study Participants
We defined TBI as the presence of ICD-9-CM codes 800.0-801.9, 803.0-804.9, 850.0-854.1, or 959.01 in any hospital discharge diagnosis field, per definitions from the Centers for Disease Control and Prevention.24,25 We identified adults age 18 years or older who were discharged alive after a first-recorded hospitalization with TBI from 2005 to 2010 in California, 2006 to 2013 in New York, and 2005 to 2013 in Florida. These dates were selected to allow at least 1 year of follow-up for all patients. These states were chosen as they provide a demographically heterogeneous population that represents nearly 20% of the US population.
Measurements
All patients were followed for a primary outcome of ischemic stroke defined as ICD-9-CM codes 433.x1, 434.x1, or 436 in the absence of a primary discharge code for rehabilitation (V57) or any diagnosis code for intracerebral hemorrhage (431) or SAH (430). This combination of diagnosis codes has been found to have high sensitivity and specificity for ischemic stroke.26
Patients with TBI were divided into 2 groups on the basis of presence of tSAH (ICD-9-CM codes 852.0 and 852.1). We then matched patients with and without tSAH on the basis of age, sex, race, insurance status, income, admission year, length of stay, discharge disposition, number of Elixhauser comorbidities, need for mechanical ventilation, coronary heart disease, hypertension, diabetes, congestive heart failure, peripheral vascular disease, chronic kidney disease, chronic obstructive pulmonary disease, atrial fibrillation, alcohol abuse, and tobacco use.
Patients with multiple visits for TBI were entered into the cohort at their index visit. We excluded patients with a history of ischemic stroke prior to or during the index hospitalization for TBI or trauma. We excluded nonresidents of California, New York, and Florida in order to maximize the probability of follow-up. A sensitivity analysis excluding patients with cervical artery dissection was performed in order to help rule out dissection as an etiology of stroke. In a subgroup analysis of mild TBI, we included only patients who were discharged from the hospital within 72 hours of admission. We also assessed whether or not tSAH is associated with venous thromboembolism (VTE) following discharge in order to assess the role of tSAH in long-term hypercoagulability.
Statistical Analysis
Baseline characteristics of the TBI group with tSAH were compared to the TBI group without tSAH using descriptive statistics. Kaplan-Meier survival statistics were used to calculate the cumulative rates for ischemic stroke. The log-rank test was used to compare the cumulative rates of ischemic stroke between those with tSAH and those without. Multivariable Cox proportional hazards models accounting for the matched design were used to assess the associations between tSAH and ischemic stroke. Inspection of log-log plots verified the proportional hazards assumption. All statistical analyses were performed using Stata/MP (version 13; StataCorps, College Station, Texas). The threshold of statistical significance was set at α = 0.05.
Results
We identified 20 454 patients with TBI and tSAH and matched them with 10 454 patients without tSAH. Patients were followed for a mean of 4.3 ± 1.8 years. The mean age was 48.9 (± 21.6) years. The majority of the patients were men (72.8%) and white (65.3%). Patients with tSAH were slightly older, were more likely to have hypertension, were less likely to carry a diagnosis of alcohol abuse, and had longer lengths of stay than those without tSAH. The groups had similar mean number of Elixhauser comorbidities (1.60 ± 1.56 for total cohort). See Table 1 for full characteristics of the cohort.
Table 1.
Characteristics of Patients With Traumatic Brain Injury Stratified by the Presence of Traumatic Subarachnoid Hemorrhage.
| Characteristica | No tSAH (N = 20 454) | tSAH (N = 20 454) |
|---|---|---|
| Age, mean (SD), y | 48.6 (21.6) | 49.2 (21.4) |
| Female | 5504 (26.9) | 5628 (27.5) |
| Raceb | ||
| White | 13 138 (64.2) | 13 559 (66.3) |
| Black | 2286 (11.2) | 1555 (7.6) |
| Hispanic | 3414 (16.7) | 3467 (17.0) |
| Asian | 648 (3.2) | 846 (4.1) |
| Other | 968 (4.7) | 1027 (5.0) |
| Payment sourcec | ||
| Medicare | 5000 (24.5) | 5114 (25.0) |
| Medicaid | 2516 (12.3) | 2687 (13.1) |
| Private | 7652 (37.4) | 7635 (37.3) |
| Self-pay | 2530 (12.4) | 2374 (11.6) |
| Other | 2756 (13.5) | 2644 (12.9) |
| Disposition | ||
| Home | 11 976 (58.6) | 10 491 (51.3) |
| Transfer to acute care facility | 802 (3.9) | 1116 (5.5) |
| Transfer to rehab/nursing home | 5426 (26.5) | 6938 (33.9) |
| Home health care | 1580 (7.7) | 1373 (6.7) |
| Against medical advice | 539 (2.6) | 424 (2.1) |
| Other | 131 (0.6) | 112 (0.6) |
| Length of stay, mean (SD), d | 10.6 (19.7) | 11.8 (17.2) |
| Hypertension | 6061 (29.6) | 6303 (30.8) |
| Diabetes | 2533 (12.4) | 2688 (13.1) |
| Coronary heart disease | 1574 (7.7) | 1665 (8.1) |
| Congestive heart failure | 629 (3.1) | 625 (3.1) |
| Peripheral vascular disease | 378 (1.9) | 361 (1.8) |
| Chronic obstructive pulmonary disease | 840 (4.1) | 830 (4.1) |
| Atrial fibrillation | 1072 (5.2) | 1127 (5.5) |
| Chronic kidney disease | 423 (2.1) | 446 (2.2) |
| Alcohol abuse | 6562 (32.1) | 6277 (30.7) |
| Tobacco use | 523 (2.6) | 531 (2.6) |
| Number of Elixhauser comorbidities, mean (SD) | 1.6 (1.6) | 1.7 (1.6) |
| Mechanical ventilation | 6193 (30.3) | 6155 (30.1) |
Abbreviations: SD, standard deviation; tSAH, traumatic subarachnoid hemorrhage.
aData are presented as number (%) unless otherwise specified.
bSelf-reported by patients or their surrogates. Numbers do not sum to group totals because of missing race/ethnicity data.
cNumbers do not sum to group totals because of missing payment-source data.
Overall, 531 of 40 908 patients with TBI experienced an ischemic stroke after discharge. There was no significant difference in the cumulative stroke rate between those patients with tSAH (1.79%; 95% confidence interval [CI] 1.54%-2.08%) versus without tSAH (2.12%; 95% CI 1.83%-2.4%), resulting in a hazard ratio (HR) of 0.77 (95% CI 0.63-0.94). The risk in both groups was protracted (see Figure 1). When stratified by age (less than 45 years old, 45-65 years old, 65-85 years old, greater than 85 years old), there was likewise no difference in stroke rate between tSAH and non-tSAH TBI. Similarly, risks were similar when stratified by proxies of TBI severity including discharge disposition and mechanical ventilation. In a sensitivity analysis limiting the cohort to those patients without dissection, our results were essentially unchanged.
Figure 1.
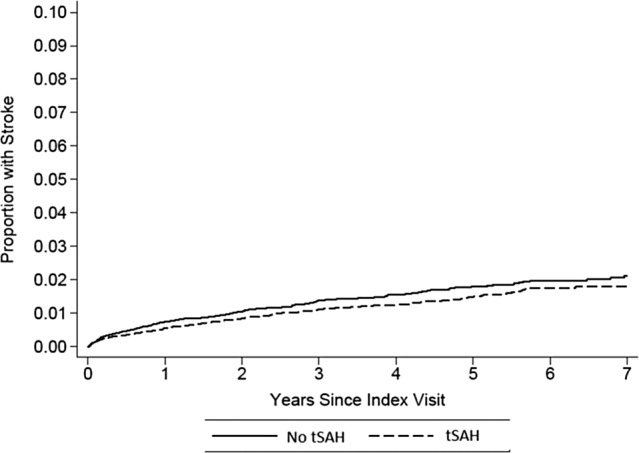
Cumulative stroke risk after TBI stratified by the presence of tSAH. TBI indicates traumatic brain injury; tSAH, traumatic subarachnoid hemorrhage.
When the outcome was changed to from ischemic stroke to VTE, there was no association between tSAH and VTE (HR 1.12, 95% CI 0.90-1.33).
When limiting the cohort to those patients who were hospitalized for less than 72 hours with TBI, 79 (1.17%) of 6738 patients had a stroke after discharge. The presence of tSAH was not associated with a greater risk of stroke (HR 1.11, 95% CI 0.66-1.87).
Discussion
In a large, heterogeneous population of patients with TBI we found a subsequent protracted risk of ischemic stroke, consistent with prior reports.1,2 While those prior reports failed to find a relation to TBI subtype, including skull fracture, concussion, and intracranial bleeding, they did not specifically evaluate the role of tSAH. Contrary to our hypothesis, the presence of tSAH was not associated with subsequent stroke, even in analyses stratified by age and proxies of TBI severity such as discharge disposition and requirement for mechanical ventilation.
While vasospasm from tSAH may lead to delayed cerebral ischemia, it is transient and usually resolves within 2 to 3 weeks.6,15,27 Our study, like a recent retrospective cohort study of patients from California, failed to find a preponderance of early ischemic stroke risk, but rather a continued elevated risk of ischemic stroke throughout the follow-up period, even among patients discharged within 72 hours of TBI.2 As vasospasm usually resolves within weeks, it is unlikely to mediate the protracted risk of stroke following TBI.
If TBI truly increases the long-term risk of ischemic stroke, the mechanisms by which it does so remain unclear. Given the extended period of increased stroke risk that we and others found following TBI, vascular dissection seems unlikely to be the mechanism as it is relatively rare (complicating approximately 3.2% blunt head and neck injuries), has a low rate of subsequent ischemic stroke, and tends to heal rather quickly.28–30 One prior study documented a continued risk of ischemic stroke after TBI even after excluding patients with arterial dissection.2 We similarly found no difference in our results after excluding all patients with dissections.
Hypercoagulability occurs during post-TBI hospitalization and leads to a 3- to 4-fold increase in VTE regardless of the presence or timing of anticoagulation31,32; nonetheless, the duration of the hypercoagulability and relationship to arterial clot and ischemic stroke remain unknown. As with ischemic stroke, we found no significant relationship between tSAH and VTE. Further study is necessary to explore the role of hypercoagulability in long-term stroke risk after TBI.
It is also possible that TBI may lead to cognitive–behavioral changes that could increase the use of substances such as tobacco and cocaine, increasing in turn the risk of ischemic stroke; conversely, patients who use more substances linked with ischemic stroke make be at greater risk for TBI.33 Patients with TBI may accrue traditional vascular risk factors at higher rates. While prior studies have adjusted for the presence of traditional vascular risk factor diagnoses such as hypertension and diabetes,1,2 they were unable to adjust for the severity of those risk factors. In addition, patients with TBI may be less adherent to medications that mitigate ischemic stroke risk. Alternatively, the administration of certain medications following TBI may increase the incidence of ischemic stroke. Antipsychotic medications, for instance, are commonly prescribed for neuropsychological sequelae of TBI and may contribute to higher stroke risk.34,35 Finally the inflammatory milieu following TBI, regardless of the presence of tSAH, may initiate an as (of) yet uncharacterized vascular remodeling that predisposes to ischemic stroke. Future research is required to further understand the association between TBI and long-term ischemic stroke risk.
There are several important limitations to our study. First, we cannot rule out that there may have been a significant number of patients without ICD-9-CM codes for tSAH who actually did have tSAH, thus increasing the risk of a type II error. Second, we cannot be sure that there were not unmeasured confounders that either increased the ischemic stroke risk among the non-tSAH patients or lowered the ischemic stroke risk among the patients with tSAH. Third, we lacked data regarding finer points of assessment and management such as radiographic features of tSAH (extent, location, etc), medication lists, and so on.
Conclusion
We found no evidence that the presence of tSAH is associated with increased long-term stroke risk among patients with TBI. Further studies will be needed to elucidate the mechanisms of increased long-term stroke risk after TBI.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by grant K23NS082367 (Dr Kamel) from the National Institute of Neurological Disorders and Stroke (http://www.ninds.nih.gov/) and a grant from the Michael Goldberg Stroke Research Fund (Dr Kamel).
References
- 1. Chen YH, Kang JH, Lin HC. Patients with traumatic brain injury: population-based study suggests increased risk of stroke. Stroke. 2011;42(10):2733–2739. [DOI] [PubMed] [Google Scholar]
- 2. Burke JF, Stulc JL, Sklarus LE, Sears ED, Zahuranec DB, Morgenstern LB. Traumatic brain injury may be an independent risk factor for stroke. Neurology. 2013;81(1):33–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eisenberg HM, Gary HE, Jr, Aldrich EF, et al. Initial CT findings in 753 patients with severe head injury. A report from the NIH Traumatic Coma Data Bank. J Neurosurg. 1990;73(5):688–698. [DOI] [PubMed] [Google Scholar]
- 4. Mattioli C, Beretta L, Gerevini S, et al. Traumatic subarachnoid hemorrhage on the computerized tomography scan obtained at admission: a multicenter assessment of the accuracy of diagnosis and the potential impact on patient outcome. J Neurosurg. 2003;98(1):37–42. [DOI] [PubMed] [Google Scholar]
- 5. Servadei F, Murray GD, Teasdale GM, et al. Traumatic subarachnoid hemorrhage: demographic and clinical study of 750 patients from the European brain injury consortium survey of head injuries. Neurosurgery. 2002;50(2):261–267 [DOI] [PubMed] [Google Scholar]
- 6. Zubkov AY, Lewis AI, Raila FA, Zhang J, Parent AD. Risk factors for the development of post-traumatic cerebral vasospasm. Surg Neurol. 2000;53(2):126–130. [DOI] [PubMed] [Google Scholar]
- 7. Gaetani P, Tancioni F, Tartara F, et al. Prognostic value of the amount of post-traumatic subarachnoid haemorrhage in a six month follow up period. J Neurol Neurosurg Psychiatry. 1995;59(6):635–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Abraszko R, Zurynski Y, Dorsch N. The importance of traumatic subarachnoid hemorrhage. J Clin Neurosci. 1996;3(1):21–25. [DOI] [PubMed] [Google Scholar]
- 9. Kakarienka A, Braakman R, Schakel EH. Clinical significance of the finding of subarachnoid blood on CT scan after head injury. Acta Neurochir (Wien). 1994;129(1-2):1–5. [DOI] [PubMed] [Google Scholar]
- 10. Lin TK, Tsai HC, Hsieh TC. The impact of traumatic subarachnoid hemorrhage on outcome: a study with grouping of traumatic subarachnoid hemorrhage and transcranial Doppler sonography. J Trauma Acute Care Surg. 2012;73(1):131–136. [DOI] [PubMed] [Google Scholar]
- 11. Oertel M, Boscardin WJ, Obrist WD, et al. Posttraumatic vasospasm: the epidemiology, severity, and time course of an underestimated phenomenon: a prospective study performed in 299 patients. J Neurosurg. 2005;103(5):812–824. [DOI] [PubMed] [Google Scholar]
- 12. Sander D, Klingelhofer J. Cerebral vasospasm following post-traumatic subarachnoid hemorrhage evaluated by transcranial Doppler ultrasonography. J Neurol Sci. 1993;119(1):1–7. [DOI] [PubMed] [Google Scholar]
- 13. Martin NA, Doberstein C, Zane C, Caron MJ, Thomas K, Becker DP. Posttraumatic cerebral arterial spasm: transcranial Doppler ultrasound, cerebral blood flow, and angiographic findings. J Neurosurg. 1992;77(4):575–583. [DOI] [PubMed] [Google Scholar]
- 14. Lee JH, Martin NA, Alsina G, McArthur DL, Zuacha K, Hovda AD, et al. Hemodynamically significant cerebral vasospasm and outcome after head injury: a prospective study. J Neurosurg. 1997;87(2):221–233. [DOI] [PubMed] [Google Scholar]
- 15. Weber M, Grolimund P, Seiler RW. Evaluation of posttraumatic cerebral blood flow velocities by transcranial Doppler ultrasonography. Neurosurgery. 1990;27(1):106–112. [DOI] [PubMed] [Google Scholar]
- 16. Macpherson P, Graham DI. Correlation between angiographic findings and the ischaemia of head injury. J Neurol Neurosurg Psychiatry. 1978;41(2):122–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Pasqualin A, Vivenza C, Rosta L, Licata C, Cavazzani P, Da Pian R. Cerebral vasospasm after head injury. Neurosurgery. 1984;15(6):855–858. [PubMed] [Google Scholar]
- 18. Taneda M, Kataoka K, Akai F, Asai T, Sakata I. Traumatic subarachnoid hemorrhage as a predictable indicator of delayed ischemic symptoms. J Neurosurg. 1996;84(5):762–768. [DOI] [PubMed] [Google Scholar]
- 19. Chan KH, Dearden NM, Miller JD. The significance of posttraumatic increase in cerebral blood flow velocity: a transcranial Doppler ultrasound study. Neurosurgery. 1992;30(5):697–700. [PubMed] [Google Scholar]
- 20. Unden J, Ingebrigtsten T, Romner B; for the Scandinavian Neurotrauma Committee (SNC). Scandinavian guidelines for initial management of minimal, mild and moderate head injuries in adults: an evidence and consensus-based update. BMC Med. 2013;11:50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Kreitzer N, Lyons MS, Hart K, et al. Repeat neuroimaging of mild traumatic brain-injured patients with acute traumatic intracranial hemorrhage: clinical outcomes and radiographic features. Acad Emerg Med. 2014;21(10):1083–1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Agency for Healthcare Research and Quality. Healthcare Cost and Utilization Project. http://hcupnet.ahrq.gov. Accessed July 21, 2015.
- 23. Agency for Healthcare Research and Quality. HCUP methods series: methodological issues when studying readmissions and revisits using hospital administrative data. http://www.ncup-us.ahrq.gov/reports/methods/2011_01.pdf. Accessed July 21, 2015.
- 24. Thurman DJ, Sniezek JE, Johnson D, Greenspan A, Smith SM. Guidelines for Surveillance of Central Nervous System Injury. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC; 1995. [Google Scholar]
- 25. Marr A, Coronado VG. Annual Data Submission Standards. Central Nervous System Injury Surveillance. Atlanta, GA: US Department of Health and Human Services, Public Health Service, CDC; 2001. [Google Scholar]
- 26. Tirschwell DL, Longstreth WT., Jr Validating administrative data in stroke research. Stroke. 2002;33(10):2465–2470. [DOI] [PubMed] [Google Scholar]
- 27. Gomez CR, Backer RJ, Bucholz RD. Transcranial Doppler ultrasound following closed head injury: vasospasm or vasoparalysis? Surg Neurol. 1991;35(1):30–35. [DOI] [PubMed] [Google Scholar]
- 28. Hughes KM, Collier B, Greene KA, Kurek S. Traumatic carotid artery dissection: a significant incidental finding. Am Surg. 2000;66(11):1023–1027. [PubMed] [Google Scholar]
- 29. Touzé E, Gauvrit JY, Moulin T, Meder JF, Bracard S, Mas JL. Risk of stroke and recurrent dissection after a cervical artery dissection: a multicenter study. Neurology. 2003;61(10):1347–1351. [DOI] [PubMed] [Google Scholar]
- 30. Baracchini C, Tonello S, Meneghetti G, Ballotta E. Neurosonographic monitoring of 105 spontaneous cervical artery dissections: a prospective study. Neurology. 2010;75(21):1864–1870. [DOI] [PubMed] [Google Scholar]
- 31. Phelan HA. Pharmacologic venous thromboembolism prophylaxis after traumatic brain injury: a critical literature review. J Neurotrauma. 2012;29(10):1821–1828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Massaro AM, Doerfler S, Nawalinski K, et al. Thromboelastography defines late hypercoagulability after TBI: a pilot study. Neurocrit Care. 2015;22(1):45–51. [DOI] [PubMed] [Google Scholar]
- 33. Ilie G, Mann RE, Hamilton H, et al. Substance use and related harms among adolescents with and without traumatic brain injury. J Head Trauma Rehabil. 2015;30(5):293–301. [DOI] [PubMed] [Google Scholar]
- 34. Glenn MB. Sudden cardiac death and stroke with the use of antipsychotic medications: implications for clinicians treating individuals with traumatic brain injury. J Head Trauma Rehabil. 2010;25(1):68–70. [DOI] [PubMed] [Google Scholar]
- 35. Gill SS, Rochon PA, Herrmann N, et al. Atypical antipsychotic drugs and risk of ischaemic stroke: population based retrospective cohort study. BMJ. 2005;330(7489):445. [DOI] [PMC free article] [PubMed] [Google Scholar]


