Abstract
Background
Hispanics/Latinos are purportedly at increased risk for neurocognitive decline and dementias. Without dementia cures, low-cost, well-tolerated public health means for mitigating neurocognitive decline are needed.
Objective
We examined associations between neurocognition and cardiovascular health (CVH) metrics (Life’s Simple 7; LS7) among diverse Hispanics/Latinos. We hypothesized that higher LS7 would be associated with healthier brain function (neurocognitive performance).
Methods
We used baseline (2008–2011) Hispanic Community Health Study/Study of Latinos (HCHS/SOL; N = 9,623; ages 45–74 years) to examine neurocognition in relation to CVH LS7 scores.
Results
In age and sex adjusted models, a one unit LS7 score increase (range = 0–14) was associated with higher neurocognitive function on the B-SEVLT sum (0.23 [p <0.01]; range = 3–42), B-SEVLT recall (0.12 [p <0.01]; range = 0–15), Word Fluency (phonemic; 0.46 (p < 0.01); range = 0–49), and Digit Symbol Substitution (0.49 (p < 0.01); range = 0–83) tests, respectively. Stated differently, a change from the minimum LS7 (0) to maximum LS7 (14) score corresponded to higher scores on verbal learning (4.62) and memory (2.24), verbal fluency (7.0), and psychomotor processing speed (12). In fully adjusted models the associations were attenuated, but remained statistically significant. Incremental adjustments indicated that Latino background and, to a lesser extent, education were primary contributors to the evinced attenuations.
Conclusions
We found that higher neurocognitive function was associated with better LS7 CVH metrics among middle-aged and older Hispanics/Latinos. Associations between neurocognitive function and LS7 were strongest among two at-risk groups for neurocognitive decline and dementia, women and Hispanics/Latinos with lower education. Public health efforts to reduce cardiovascular disease morbidity and mortality may have additional neurocognitive benefits among at-risk Hispanics/Latinos.
Keywords: Cardiovascular system, cognition, epidemiology, Hispanic Americans
INTRODUCTION
“Staying sharp” into older adulthood is a major concern of most Americans. Driven by fear of Alzheimer’s disease and other dementias, loss of independence and self, many ask “what can I do to remain cognitively healthy into older adulthood?” In response, the Institute of Medicine (IOM) recently released a timely review with public health opportunities and recommendations for maintaining a cognitively “sharp” mind as the nation and global populations age [1]. In the context of this IOM report and state of science, [1, 2] we note that the brain consumes up to 20% of all cardiac output, about 20% of the body’s oxygen, and nearly 25% of glucose. Acute cerebral blood flow disruptions can have devastating and nearly immediate effects on tissue and function; while chronic insufficiencies have more insidious sequelae [3]. Thus, maintaining healthy brain vascular perfusion to demanding brain tissue is vital for its immediate survival and short- and longterm functional integrity. In this study, we examine neurocognitive function in relation to cardiovascular health (CVH) among middle-age and older Hispanics/Latinos.
Hispanics/Latinos are reportedly at increased risk for neurocognitive decline and dementias compared to Whites [4]. Without cures for dementias on the near horizon, public health means for reducing risk for neurocognitive decline and dementias are being sought. Healthy cardiovascular lifestyles are increasingly recognized as neurocognitively beneficial and may provide neuroprotection and resilience to impairment [5, 6]. If so, improving population-level cardiovascular health through modifiable lifestyles has the potential to mitigate the looming public health burden of Alzheimer’s disease and other dementias, especially among groups at high risk for these disorders [7].
In 2010, the American Heart Association (AHA) established national goals to improve Americans’ CVH by 20% while reducing cardiovascular diseases, deaths, and stroke by 20% by year 2020 [8]. Also known as Life’s Simple 7™ (LS7), the CVH goals include three behavioral (i.e., healthy diet, non-smoking, and physical activity) and four biomarker measures (i.e., blood pressure, body mass index, total cholesterol, and fasting blood glucose), each with quantified metrics [8]. Each of the seven components can be easily summarized into ranges indicative of “Ideal, Intermediate and Poor” CVH status. While few (<1%) Americans meet all seven “Ideal” CVH goals, equally few (about 2%) meet the “Poor” criteria [9–11]. Nevertheless, having more LS7s in the Ideal range is associated with better CVH, lowered cardiovascular disease incidence, and other adverse health outcomes [9, 12, 13]. Higher LS7 that are indicative of better CVH have been associated with more favorable neurocognitive function and lower incident neurocognitive impairment in African American and non-Latino White populations [5, 6].
The purpose of this study was to examine associations between neurocognitive function and LS7 CVH metrics among middle-aged and older Hispanics/Latinos. We hypothesized that higher LS7 would be associated with healthier brain function (neurocognitive performance). To achieve our study aims, we use baseline (2008–2011) data from the Hispanic Community Health Study/Study of Latinos (HCHS/SOL).
METHODS
Study sample
HCHS/SOL is a multiethnic, multisite, prospective cohort study of 16,415 community-dwelling Hispanic/Latino adults (18- to 74-years old). We focused on the middle-aged and older cohort (45- to 74-years old; N = 9,623) with baseline neurocognitive assessment data. HCHS/SOL design was formulated to estimate representative baseline risk factors for overall Hispanic/Latinos as well as for specific backgrounds, including Central Americans, Cubans, Dominicans, Mexicans, Puerto Ricans, and South Americans. Data were collected from field centers in four U.S. cities with substantial Hispanic/Latino population concentrations (Bronx, NY; Chicago, IL; Miami, FL; and San Diego, CA). Each field center recruited about 4,000 eligible, self-identified Hispanic/Latino adults, and detailed HCHS/SOL sampling methods are available elsewhere [14, 15].
Neurocognitive tests
Neurocognitive tests were administered in the participants’ preferred language during face-to-face interviews by trained, bilingual research assistants. Four neurocognitive tests were used in this study: 1) Six-Item Screener (SIS); [16] 2) Brief-Spanish English Verbal Learning Test (B-SEVLT); [17, 18] 3) phonemic Word Fluency (henceforth, WF) test of the Multilingual Aphasia Examination; [19, 20] and (3) Digit Symbol Subtest test (DSS; also referred to as DST) [21]. These neurocognitive tests and scoring procedures have been previously described [22]. Briefly, the SIS (range 0–6) is a mental status test that was scored dichotomously with a value of 4 or lower representing “cognitive impairment”. The cutpoint reflects previous validation work in patients with dementia [16]. The B-SEVLT is an episodic learning and memory test with two scores: 1) the summed total correctly learned items across three learning trials (B-SEVLT-sum; range 0–45) and 2) total correctly recalled items (B-SEVLT recall; range 0–15) following an interference trial. WF is a verbal fluency test (continuous) of summed correctly generated words (beginning with letters F and A) within 1 minute. DSS is a mental processing speed exam that was continuous total of correct responses (range 0–90). Detailed descriptives of the distributions of the neurocognitive tests including means, standard deviations, medians, and percentiles (5th, 25th, 75th, and 95th) are provided in Supplementary Table 1. All four continuous measures were standardized (i.e., z-score transformed) to the population of interest to facilitate test scores comparisons and inferences.
Life’s Simple 7
CVH indicators including diet, physical activity, smoking, body mass index, blood pressure, cholesterol, and glucose were measured per AHA criteria and coded categorically as Ideal (2 points), Intermediate (1 point), or Poor (0) [8]. We then calculated the sum of the seven LS7 criterion scores (range 0–14) for each participant as previously published. Individual LS7 indicator attributes are presented in Supplementary Table 2. A detailed presentation of the distribution of the LS7 indicators in HCHS/SOL target population has been previously published [23].
Covariates
Covariates included six demographic and socioeconomic indicators including: 1) age in years, and age squared (to accommodate the possibility of a curvilinear relationship between age and cognition), 2) sex, 3) Hispanic/Latino background (Dominican, Central American, Cuban, Mexican, Puerto-Rican, and South American), 4) education (<12-years; high school or equivalent; some college; and college or more), 5) household income (< = $20,000; $20,001–$50,000; >$50,000; and unreported; note that respondents with unreported income were included as an analytical category to avoid excluding observations from our analytical sample), and 5) language preference (Spanish/English). In additional analyses, we accounted for study site in the model to examine whether variations in locales can explain any reported associations between our primary predictor and outcomes.
Analytic procedures
Data analytic procedures designed for complex survey sample designs in the Stata software package (12.1) were used to perform all study analyses, and appropriate methods for subpopulations analyses of complex survey data were applied to generate our estimates [24]. Specifically we used a Taylor Series Linearization approach to variance estimation to obtain correct standard errors [25]. We excluded respondents who did not report a specific Latino background from the analyses (n = 227). We also excluded respondents reporting prevalent stroke or transient ischemic attack diagnoses (n = 349). Finally, we excluded (n = 192) respondents with missing values on the model covariates. Our analytic sample consisted on n = 8,855 Hispanic/Latino participants. The study protocol was reviewed and approved by the institutional review boards at Michigan State University and all other participating sites.
Our data analyses were conducted in three steps. First, we generated analytic sample descriptive statistics for the variables of interest (Table 1). Additionally we characterized the distribution of our AHA LS7 predictor variable and grouped respondents in three categories: the lowest (i.e., 0–25th percentiles), Inter Quartile Range (IQR = >25th and <75th), and those in the highest (i.e., 75th to 100). Subsequently, we calculated descriptive statistics across the three generated categories, and tested for significant associations between our covariates and the above-mentioned categorical LS7 variable using survey adjusted chi-squared tests (Table 1).
Table 1.
Descriptive statistics for Hispanic Community Health Study/Study of Latinos (transient ischemic attack/stroke free/coronary heart disease) participants 45–74 years of age
| AHA LS7
|
|||||
|---|---|---|---|---|---|
| Overall Sample | <25th % | IQR | >75th % | χ2-Test | |
| Sex | |||||
| Male | 43.9 | 41.2 | 44.8 | 45.3 | p = 0.0666 |
| Female | 56.1 | 58.8 | 55.2 | 54.8 | |
| Age | |||||
| 45–54 | 49.4 | 40.8 | 48.7 | 64.4 | p = 0.0000 |
| 55–64 | 31.3 | 35.5 | 31.6 | 24.0 | |
| 65–74 | 19.3 | 23.8 | 19.7 | 11.6 | |
| Background | |||||
| Dominican | 9.3 | 6.5 | 10.6 | 10.1 | p = 0.0000 |
| Central American | 6.8 | 6.2 | 6.6 | 8.3 | |
| Cuban | 27.5 | 38.4 | 25.0 | 17.9 | |
| Mexican | 33.4 | 24.7 | 34.8 | 42.9 | |
| Puerto Rican | 17.0 | 19.7 | 17.0 | 12.9 | |
| South American | 5.9 | 4.5 | 6.0 | 7.9 | |
| Education | |||||
| Less than HS | 39.6 | 43.2 | 40.5 | 31.3 | p = 0.0000 |
| HS or equivalent | 21.7 | 22.4 | 21.1 | 22.5 | |
| More than HS | 38.7 | 34.4 | 38.5 | 46.2 | |
| Income | |||||
| < = $20,000 | 44.5 | 48.7 | 44.1 | 39.2 | p = 0.0000 |
| $20,001–$50,000 | 34.6 | 32.4 | 34.1 | 39.2 | |
| > = $50,001 | 11.4 | 6.8 | 12.3 | 15.5 | |
| Not Reported | 9.6 | 12.1 | 9.5 | 6.1 | |
| Language preference | |||||
| Spanish | 86.4 | 87.0 | 86.5 | 85.5 | p = 0.7149 |
| English | 13.6 | 13.0 | 13.6 | 14.5 | |
| Center | |||||
| Bronx | 25.1 | 23.4 | 26.5 | 23.6 | p = 0.0000 |
| Chicago | 12.9 | 9.8 | 13.3 | 16.5 | |
| Miami | 36.4 | 47.1 | 33.7 | 27.7 | |
| San Diego | 25.6 | 19.6 | 26.5 | 32.2 | |
Note 1: Life’s Simple 7 (LS7) CVH indicators were measured per AHA criteria and coded categorically as Ideal (2 points), Intermediate (1 point) or Poor (0). We then calculated the sum of the seven LS7 criterion scores (range 0–14) for each participant. Note 2: IQR stands for Inter Quartile Range.
Second, we used survey linear regression models to test the associations between the continuous AHA LS7 indicator (range 0–14) and each of the standardized neurocognitive tests (Table 2). All analyses were re-executed using the original metrics of the neurocognitive tests and are available in Supplementary Table 3. We fit survey logistic regression models to test the association between LS7 and the dichotomous SIS outcome (i.e., low mental status score). In both the linear and logistic regression models, we started by testing unadjusted (bivariate) associations. Subsequently, we assessed how controlling for our covariates affected these associations by first adjusting for age and sex, and then controlling for Latino background, education, income, and language preference. The crude, age and sex, and fully adjusted models are presented in Table 2. Results of models using the original neurocognitive metrics are presented in Supplementary Table 3. Results of incremental adjustments to these covariates to examine the attenuation in associations between LS7 scores and each of the neurocognitive outcomes are included in Supplementary Table 4. In additional models (available from authors) we also accounted for Field Center site. Accounting for Field Center site did not notably affect the reported associations between the primary predictor and outcomes of interest and the Field Center covariate was excluded to maintain model parsimony. To facilitate the interpretation of our results, we calculated and plotted the marginal means of our standardized continuous outcomes, and marginal probabilities for the dichotomous SIS, across the continuum of LS7 index values for the unadjusted, age-sex adjusted, and fully adjusted regression models (Fig. 1). Additionally, we calculated and presented the estimated marginal means of the continuous neurocognitive outcomes in their original metric in Supplementary Figure 1.
Table 2.
Associations between AHA LS7 CVH indicator (range 0–14) and neurocognitive scores from the Hispanic Community Health Study/Study of Latinos
| Model 1 | Model 2 | Model 3 | |
|---|---|---|---|
| SEVLT Sum | b/se | b/se | b/se |
| LS7 (0–14) | 0.05*** | 0.04*** | 0.01* |
| 0.01 | 0.01 | 0.01 | |
| SEVLT Recall | b/se | b/se | b/se |
| LS7 (0–14) | 0.04*** | 0.03*** | 0.01* |
| 0.01 | 0.01 | 0.01 | |
| Word Fluency | b/se | b/se | b/se |
| LS7 (0–14) | 0.06*** | 0.05*** | 0.03*** |
| 0.01 | 0.01 | 0.01 | |
| Digit Symbol Test | b/se | b/se | b/se |
| LS7 (0–14) | 0.05*** | 0.04*** | 0.01** |
| 0.01 | 0.01 | 0.00 | |
| Cognitive Impairment (SIS< = 4) |
OR/95%CI | OR/95%CI | OR/95%CI |
| LS7 (0–14) | 0.90*** | 0.92*** | 0.93** |
| 0.87,0.93 | 0.88,0.96 | 0.89,0.97 |
Note 1: Survey linear regression models were used to test the association between LS7 and each of the four continuous standardized neurocognitive tests (SEVLT Sum, SEVLT Recall, Word Fluency, and Digit Symbol Test). Survey logistic regression models to test the association between LS7 and the dichotomous SIS outcome. Note 2: Model 1 reports unadjusted (bivariate) associations. Model 2 controls for age and sex. Model 3 includes additional controls for Latino background, education, income, and language preference. Note 3: b is the unstandardized beta coefficient and se stands for standard error. OR indicates odds ratio, and CI stands for confidence interval. Note 4: Life’s Simple 7 (LS7) CVH indicators were measured per AHA criteria and coded categorically as Ideal (2 points), Intermediate (1 point) or Poor (0). We then calculated the sum of the seven LS7 criterion scores (range 0–14) for each participant. Note 5: SEVLT stands for Spanish English Verbal Learning Test. SIS indicates Six-Item Screener.
p <0.05;
p < 0.01;
p < 0.001.
Fig. 1.

Estimated marginal means of standardized continuous neurocognitive outcomes and marginal probabilities of neurocognitive impairment (SIS< = 4) from the Hispanic Community Health Study/Study of Latinos. Note 1: The marginal means are based on survey linear regression models used to test the association between LS7 and each of the four continuous standardized neurocognitive tests (SEVLT Sum, SEVLT Recall, Word Fluency, and Digit Symbol Test). The marginal probabilities are based on survey logistic regression models used totest the association between LS7 and dichotomous SIS (< = 4). Note 2: M1 reports unadjusted (bivariate) associations. M2 controls for age and sex. M3 includes additional controls for Latino background, education, income, and language preference.
Third, we separately tested for the differential nonadditive effects of age, sex, and education on the association between each of the neurocognitive tests and LS7 scores. We did so by refitting the fully adjusted models (detailed in step 2 above) while accounting for 1) LS7 by age (measured using 3 groups; 1=45–54, 2 = 55–64, and 3 = 65+), 2) LS7 by sex, and 3) LS7 by education interactions. The interactions from model estimates are included in Supplementary Table 5. The interactions from model estimates models based on the original test metrics are included in Supplementary Table 6. Interaction effects are best understood when visualized [26]. Consequently, we calculated the marginal means (for the standardized neurocognitive tests) and probabilities (for the dichotomous SIS) resulting from the continuous by categorical interactions of LS7 with 1) sex, 2) age, and 3) education, and plotted the estimates and their 95% confidence interval bounds (Figs. 2, 3, and 4). For further clarity, we explicitly tested and plotted contrasts for the linear predictions resulting from these interactions (Supplementary Figures 5, 6, and 7). As with above, we recalculated and presented the estimated marginal means of the continuous neurocognitive outcomes in their original metric in Supplementary Figures 2–4.
Fig. 2.
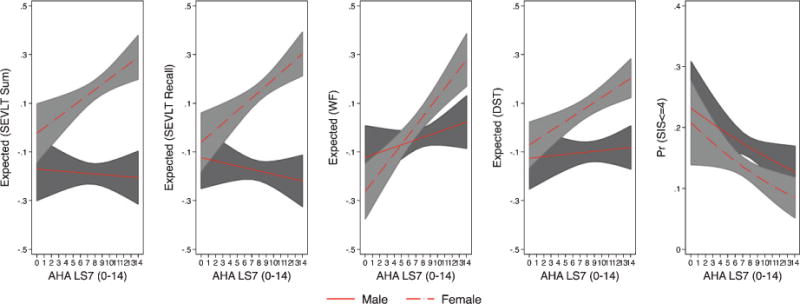
Estimated marginal means of standardized continuous neurocognitive outcomes and marginal probabilities of neurocognitive impairment (SIS< = 4) by sex from the Hispanic Community Health Study/Study of Latinos. Note 1: The marginal means are based on survey linear regression models used to test the association between LS7 and the standardized continuous neurocognitive outcomes. The marginal probabilities are based on survey logistic regression model used to test the association between LS7 and dichotomous SIS (< = 4). Note 2: The estimated marginal means of neurocognitive function and probabilities of impairment result from fully adjusted models (controlling for age, Latino background, education, income, and language preference) with an interaction between LS7 and sex.
Fig. 3.
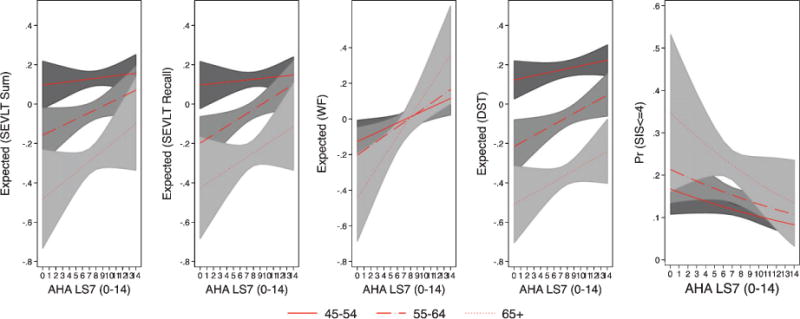
Estimated marginal means of standardized continuous neurocognitive outcomes and marginal probabilities of neurocognitive impairment (SIS< = 4) by age groups from the Hispanic Community Health Study/Study of Latinos. Note 1: The marginal means (and 95% CI) are based on survey linear regression models used to test the association between LS7 and the standardized continuous neurocognitive outcomes. The marginal probabilities (and 95% CI) are based on survey logistic regression models used to test the association between LS7 and dichotomous SIS (< = 4). Note 2: The estimated marginal means of neurocognitive function and probabilities of impairment result from fully adjusted models (controlling for sex, Latino background, education, income, and language preference) with an interaction between LS7 and age.
Fig. 4.
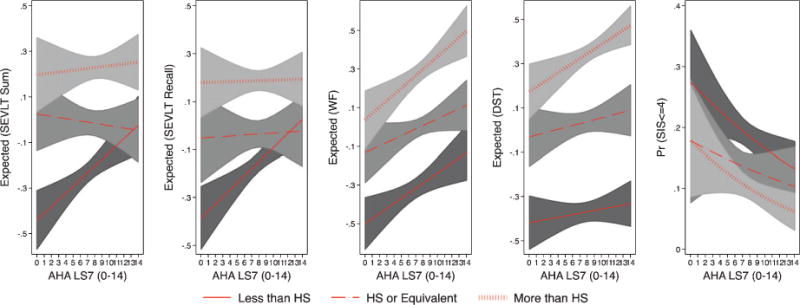
Estimated marginal means of standardized continuous neurocognitive outcomes and marginal probabilities of neurocognitive impairment (SIS< = 4) by education groups in the Hispanic Community Health Study/Study of Latinos. Note 1: The marginal means (and 95% CI) are based on survey linear regression models used to test the association between LS7 and the standardized continuous neurocognitive outcomes. The marginal probabilities (and 95% CI) are based on survey logistic regression models used to test the association between LS7 and dichotomous SIS (< = 4). Note 2: The estimated marginal means of neurocognitive function and probabilities of impairment result from fully adjusted models (controlling for age, sex, Latino background, income, and language preference) with an interaction between LS7 and education.
RESULTS
Descriptive statistics
Survey weighted descriptive statistics for the overall analytic sample and by LS7 quartiles are presented in Table 1. Slightly more than half of our sample was female (55%), and the mean age was 56.3 years with close to half of respondents (47.3%) reporting ages between 45–54 years. Mexican-origin was the largest Latino group (32.2%), followed by Cubans (27.8%), and Puerto Ricans (18%). Two-in-five respondents reported less than a high-school education (40.4%), and as many (45.7%) reported a household income of less than $20,000. Finally, the overwhelming majority of respondents (86.6%) chose Spanish as their preferred language.
The estimated means for the neurocognitive tests were 22.3 (standard deviation (SD) = 7.0), 8.0 (SD = 3.6), 18.3 (SD = 8.9), and 33.8 (SD = 16.4) for the SEVLT Sum, SEVLT Recall, Word Fluency, and Digit Symbol Substitution, respectively. The estimated mean for the LS7 index was 7.6 (SD = 2.5). Detailed descriptives for the outcomes and primary exposure variable are presented in Supplementary Table 1.
Crude associations between neurocognitive measures and LS7
Unadjusted linear regression models showed consistent significant positive associations between the neurocognitive outcomes and higher LS7 scores (Table 2, and Fig. 1). A one-unit improvement in the LS7 index was associated with 0.05 (p < 0.001), 0.04 (p < 0.001), 0.06 (p < 0.01), and 0.05 (p < 0.001) higher B-SEVLT Sum, B-SEVLT Recall, Word Fluency, and Digit Symbol Substitution standardized scores, respectively (the equivalent of 0.33, 0.16, 0.5, and 0.86 units change under the original metric; Supplementary Table 3). Stated differently, these associations correspond to 4.62 B-SEVLT Sum, 2.24 B-SEVLT Recall, 7.0 Word Fluency, and 12.0 Digit Symbol Substitution higher points on the four tests, respectively, concomitant with a change from the minimum (score 0) to the maximum (score 14) on the LS7 index (Supplementary Figure 1). Similarly, the unadjusted logistic regression model indicated that higher LS7 scores indicative of better CVH were associated (OR = 0.90; 95% CI = 0.87–0.93) with decreased odds of low mental status scores (SIS< = 4). A difference in the probability of low mental status equivalent to 0.20, concomitant with a change from the minimum (score 0) to the maximum (score 14) on the LS7 index (Supplementary Figure 1).
Adjusted associations
Age and sex adjustments led to expected attenuations in the reported associations between neurocognitive function and LS7 scores (Table 2, and Fig. 1). These attenuations equaled 30.1%, 29.4%, 8.5%, and 43% in the magnitude of the reported beta coefficients for the B-SEVLT Sum, B-SEVLT Recall, Word Fluency, and Digit Symbol Substitution, respectively (Supplementary Table 4). However, all reported associations remained statistically significant (p <0.001), and this was consistent across all five neurocognitive domains considered. More specifically, after age and sex adjustments, a one-unit improvement in the LS7 index was associated with 0.03 (p < 0.001), 0.03 (p < 0.001), 0.05 (p < 0.01), and 0.03 (p < 0.001) higher B-SEVLT Sum, B-SEVLT Recall, Word Fluency, and Digit Symbol Substitution standardized scores (Table 2), respectively (the equivalent of 0.23, 0.12, 0.46, and 0.49 units change under the original metric; Supplementary Table 3). These associations correspond to 3.22, 1.68, 6.44, and 6.9 higher points on the four tests, respectively, concomitant with a change from the minimum (score 0) to the maximum (score 14) on the LS7 index (Supplementary Figure 1). Additional adjustment for demographic and socioeconomic confounders further reduced the magnitudes of the reported relationships but did not completely attenuate their statistical significance (Table 2). Incremental adjustments indicated that Latino heritage and, to a lesser extent, education were primary contributors to the evidenced attenuations (Supplementary Table 4). Specifically, controlling for education led to additional attenuations that were equivalent to 16.4%, 14.2%, 17.3%, and 22.2% for the B-SEVLT Sum, B-SEVLT Recall, Word Fluency, and Digit Symbol Substitution, respectively. Controlling for Latino heritage further attenuated the associations by 23.2%, 24.3%, 19.4% and 9.5%, respectively. After accounting for all covariates (Latino background, education, income, and linguistic preference) a one-unit improvement in the LS7 score was associated with 0.01 (p < 0.05), 0.01 (p < 0.05), 0.03 (p < 0.01), and 0.01 (p < 0.01) standard deviations increase in B-SEVLT Sum, B-SEVLT Recall, Word Fluency, and Digit Symbol Substitution tests, respectively (the equivalent of 0.08, 0.04, 0.25, and 0.18 units change under the original metric; Supplementary Table 3). The attenuation in the odds ratios of the low mental status models were much smaller (Table 2). That is, the significant associations between low mental status and cardiovascular health (i.e., higher LS7 scores) were not markedly affected by the factors that we considered in this study. The fully adjusted logistic regression model indicated that LS7 scores remained negatively associated (OR = 0.93; 95% CI = 0.89–0.97) with low mental status (SIS< = 4). Figure 1 presents the expected means (in SD units) neurocognitive scores and probability of low mental status per unit change in the LS7 score resulting from the unadjusted and adjusted models detailed above. Supplementary Figure 1 presents the expected means (in tests metrics) neurocognitive scores per unit change in the LS7 score resulting from the unadjusted and adjusted models detailed above.
Interaction effects
There was a significant multiplicative effect of sex on the association of LS7 with B-SEVLT recall (p = 0.004), and Word Fluency (p = 0.011). Specifically, neurocognitive function for female participants was more likely to significantly improve with higher LS7 scores compared to males, as evidenced by different slopes of the regression lines for females and males in Fig. 2. The interaction between age and LS7 scores were consistently statistically insignificant for all considered neurocognitive measures (similar regression lines slope in Fig. 3). We did not find consistent multiplicative effects for education (Fig. 4) on all neurocognitive outcomes. However, better LS7 scores were associated with improved SEVLT-Sum scores (p = 0.027) among respondents reporting less than high school education compared to those reporting high school or equivalent level of schooling and with higher SEVLT-recall scores (p = 0.028) among respondents reporting high school or equivalent level of schooling compared to those reporting college or more education. The predicted means for the interaction models using the cognitive tests metrics are presented in Supplementary Figures 3, 4, and 5 for the sex, age, and education interactions, respectively. To further facilitate the interpretation of the tested interactions and show specific regions of significance in differences, we plotted the contrasts in linear predictions resulting from these interactions across the LS7 score continuum for the age, sex, and education groups in Supplementary Figures 5, 6, and 7, respectively.
DISCUSSION
More than ever, middle-aged and older adults are interested and invested in maintaining their physical and neurocognitive health. Herein, we found that higher neurocognitive function was associated with better cardiovascular health among middle-aged and older Latinos of diverse backgrounds. The cross-sectional associations between neurocognitive function and higher or Ideal LS7s were substantial, and our findings are consistent with mounting evidence that cardiovascular health is important to neurocognitive function [27, 28]. Our study extends existing evidence to diverse and understudied Hispanic/Latino populations. The American Heart Association 2020 goals for reducing cardiovascular morbidity and mortality that were used in this study were associated with higher neurocognitive function. With the one exception of lifelong learning, AHA’s LS7 2020 goals overlap with brain health findings and initiatives of the Alzheimer’s Association [27, 29]. In principle, concerted heart and brain health efforts could yield high public health dividends for an increasingly diverse and older US population.
As US and global populations age, stroke and neurocognitive disorders (e.g., Alzheimer’s disease) will continue their ascension as leading causes of global and national disease burden unless effective public health prevention measures are implemented [30]. In the late 19th and early 20th centuries, dementia was largely considered a consequence of brain vascular pathology, which was later overshadowed by intensified focus on neuronal etiologies [31, 32]. Now in the early 21st century, the boundaries between Alzheimer’s disease and related dementia etiologies are beginning to dissolve, [33] and there is renewed interest in brain vascular pathology that have important implications for brain disease prevention. By extension, public health education and promotion campaigns to improve population-level CVH would likely have concomitant brain health effects.
Reis et al. reported that more favorable LS7s among African American and White youths in the Coronary Artery Risk Development in Young Adults (CARDIA) study followed over 25-years were associated with better neurocognitive function in midlife [5]. Additionally, Thacker and colleagues demonstrated in the REasons for Geographic And Racial Differences in Stroke (REGARDS) study of African Americans and Whites that Intermediate and Ideal LS7 levels were associated with lower incident neurocognitive “impairment.” [6] Our HCHS/SOL study extends extant work to middle-aged and older, community-dwelling Hispanic/Latino adults. Our cross-sectional study is unique in that we demonstrated contemporaneous associations between neurocognition and LS7s, which is consistent with non-human work, [34] and further supports extant findings and assertions that higher levels of CVH (i.e., LS7) may have additional and more immediate neurocognitive functional benefits. Furthermore, these benefits appear to be consistent across multiple domains of neurocognitive health including mental status/orientation, episodic learning and memory, verbal fluency, and psychomotor speed.
We observed notably stronger associations between better neurocognitive function and LS7 scores among women compared to men. This observation is noteworthy in that women are at higher risk for Alzheimer’s disease and related dementias than men, [35] and improved CVH health may have the added benefit of improving and maintaining neurocognitive function in women. Secondly, low education is an important dementia risk, and we observed that the strongest associations between neurocognitive measures of verbal episodic learning and memory and LS7s were among those at the lowest education levels compared to the other more educated groups. Indeed, our findings showed that low education participants with the highest LS7s levels statistically “caught up” with the neurocognitive learning and memory performance of Hispanics/Latinos with post-secondary education. This is important since Hispanics/Latinos have among the lowest high school completion rates in the US [36]. Thus, our HCHS/SOL findings indicated that among Hispanic/Latino adults increasing LS7s had the strongest neurocognitive effects among two groups at increased risk for decline and dementia, namely women and those with the lowest educational levels. Nevertheless, these neurocognitive associations with LS7 and education among Hispanics/Latinos warrant further investigation. Thirdly, the association between higher LS7 scores and better neurocognitive performance was not age differentiated. This suggests that improved cardiovascular health plays an equally important role in neurocognitive health of middle-aged and older Latino adults. Middle-age is recognized as a vulnerable period for developing cardiovascular diseases and neurocognitive decline. Our findings indicate that better CVH was associated with higher neurocognitive function among midlife and older Hispanics/Latinos age that could help foster healthy neurocognitive aging.
A limitation of our findings was that they were cross-sectional and that we did not ascertain neurocognitive decline or disorders (e.g., dementias). Thus, we are unable to determine if LS7s leads to better neurocognitive function or visa versa. Regardless of the associations’ true directionality, better understanding CVH and neurocognitive function relationships is warranted given the global health priority status of Alzheimer’s disease and related dementias [7]. Secondly, the sex differences in neurocognitive learning and memory in relation to LS7 that we observed in this study are intriguing and merit additional and focused exploration. Overall Hispanic/Latino CVH compared favorably to Whites and other ethnic/racial groups with the exception of higher cohort BMI [8, 10, 23]. As the prospective HCHS/SOL cohort advances from midlife to older age, we will monitor associations between CVH, neurocognitive function.
CONCLUSION
In this study, we found that higher neurocognitive function was associated with better cardiovascular health among middle-aged and older Hispanic/Latino HCHS/SOL participants. The newly established LS7 cardiovascular health goals were established in order to reduce cardiovascular morbidity and mortality by 20% by year 2020, but we observed immediate associations in neurocognitive health in this study that we anticipate would have long-term benefits for neurocognitive health and dementia prevention. We will follow the HCHS/SOL cohort to determine what effect higher LS7s have on CVH and neurocognitive health as the cohort advances in age into the coming decade. Given the rapid expansion of US Hispanics/Latinos, the population will continue to exert major influences on US economics, society and policies in coming decades. For the US to maintain its global position, it is vital for the nation to find affordable public health methods to maintain and improve Hispanic/Latino neurocognitive and cardiovascular health.
Supplementary Material
Acknowledgments
Drs. Gonzalez and Tarraf receive support for this work from R01-AG48642 and previously received support from NHLBI HC-65233. The Hispanic Community Health Study/Study of Hispanic/Latinos was carried out as a collaborative study supported by contracts from the National Heart, Lung, and Blood Institute (NHLBI) to the University of North Carolina (N01-HC65233), University of Miami (N01-HC65234), Albert Einstein College of Medicine (N01-HC65235), Northwestern University (N01-HC65236), and San Diego State University (N01-HC65237). The following Institutes/Centers/Offices contribute to the HCHS/SOL through a transfer of funds to the NHLBI: National Institute on Minority Health and Health Disparities, National Institute on Deafness and Other Communication Disorders, National Institute of Dental and Craniofacial Research, National Institute of Diabetes and Digestive and Kidney Diseases, National Institute of Neurological Disorders and Stroke, NIH Institution-Office of Dietary Supplements.
This work was supported by the National Institute on Aging, National Institute of Neurologic Disorders and Stroke, and National Heart Lung Blood Institute. Its contents are solely the responsibility of the authors and do not necessarily represent the official views of the NIH.
The authors thank the staff and participants of HCHS/SOL for their important contributions. Investigators website - http://www.cscc.unc.edu/hchs/. In addition, we wish to thank Alan S. Conceicao for his assistance in preparing this manuscript for publication.
Footnotes
Handling Associate Editor: David Libon
Authors’ disclosures available online (http://jalz.com/manuscript-disclosures/15-1125r2).
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JAD-151125.
References
- 1.Institute of Medicine. Cognitive Aging: Progress in Understanding and Opportunities for Action. National Academies Press; Washington DC: 2015. [PubMed] [Google Scholar]
- 2.Snyder HM, Corriveau RA, Craft S, Faber JE, Greenberg SM, Knopman D, Lamb BT, Montine TJ, Nedergaard M, Schaffer CB, Schneider JA, Wellington C, Wilcock DM, Zipfel GJ, Zlokovic B, Bain LJ, Bosetti F, Galis ZS, Koroshetz W, Carrillo MC. Vascular contributions to cognitive impairment and dementia including Alzheimer’s disease. Alzheimers Dement. 2015;11:710–717. doi: 10.1016/j.jalz.2014.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zlokovic BV. Neurovascular pathways to neurodegeneration in Alzheimer’s disease and other disorders. Nat Rev Neurosci. 2011;12:723–738. doi: 10.1038/nrn3114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gurland BJ, Wilder DE, Lantigua R, Stern Y, Chen J, Killeffer EH, Mayeux R. Rates of dementia in three ethnoracial groups. Int J Geriatr Psychiatry. 1999;14:481–493. [PubMed] [Google Scholar]
- 5.Reis JP, Loria CM, Launer LJ, Sidney S, Liu K, Jacobs DR, Zhu N, Lloyd-Jones DM, He K, Yaffe K. Cardiovascular health through young adulthood and cognitive functioning in midlife. Ann Neurol. 2013;73:170–179. doi: 10.1002/ana.23836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Thacker EL, Gillett SR, Wadley VG, Unverzagt FW, Judd SE, McClure LA, Howard VJ, Cushman M. The American Heart Association Life’s Simple 7 and Incident Cognitive Impairment: The REasons for Geographic And Racial Differences in Stroke (REGARDS) Study. J Am Heart Assoc. 2014;3:e000635. doi: 10.1161/JAHA.113.000635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Health Organization. First WHO Ministerial Conference on Global Action Against Dementia.2015. [Google Scholar]
- 8.Lloyd-Jones DM, Hong Y, Labarthe D, Mozaffarian D, Appel LJ, Van Horn L, Greenlund K, Daniels S, Nichol G, Tomaselli GF, Arnett DK, Fonarow GC, Ho PM, Lauer MS, Masoudi FA, Robertson RM, Roger VL, Schwamm LH, Sorlie P, Yancy CW, Rosamond WD, on behalf of the American Heart Association Strategic Planning Task F, Statistics C Defining and setting national goals for cardiovascular health promotion and disease reduction. Circulation. 2010;121:586–613. doi: 10.1161/CIRCULATIONAHA.109.192703. [DOI] [PubMed] [Google Scholar]
- 9.Folsom AR, Yatsuya H, Nettleton JA, Lutsey PL, Cushman M, Rosamond WD. Community prevalence of ideal cardiovascular health, by the American Heart Association definition, and relationship with cardiovascular disease incidence. J Am Coll Cardiol. 2011;57:1690–1696. doi: 10.1016/j.jacc.2010.11.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shay CM, Ning H, Allen NB, Carnethon MR, Chiuve SE, Greenlund KJ, Daviglus ML, Lloyd-Jones DM. Status of cardiovascular health in US adults: Prevalence estimates from the National Health and Nutrition Examination Surveys (NHANES) 2003–2008. Circulation. 2011;125:45–56. doi: 10.1161/CIRCULATIONAHA.111.035733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ford ES, Greenlund KJ, Hong Y. Ideal cardiovascular health and mortality from all causes and diseases of the circulatory system among adults in the United States / clinical perspective. Circulation. 2012;125:987–995. doi: 10.1161/CIRCULATIONAHA.111.049122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dong C, Rundek T, Wright CB, Anwar Z, Elkind MSV, Sacco RL. Ideal Cardiovascular health predicts lower risks of myocardial infarction, stroke, and vascular death across Whites, Blacks, and Hispanics: The Northern Manhattan Study. Circulation. 2012;125:2975–2984. doi: 10.1161/CIRCULATIONAHA.111.081083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ogagarue ER, Lutsey PL, Klein R, Klein BE, Folsom AR. Association of ideal cardiovascular health metrics and retinal microvascular findings: The Atherosclerosis Risk in Communities Study. J Am Heart Assoc. 2013;2:e000430. doi: 10.1161/JAHA.113.000430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.LaVange LM, Kalsbeek WD, Sorlie PD, Avilés-Santa LM, Kaplan RC, Barnhart J, Liu K, Giachello A, Lee DJ, Ryan J, Criqui MH, Elder JP. Sample design and cohort selection in the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:642–649. doi: 10.1016/j.annepidem.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sorlie PD, Avilés-Santa LM, Wassertheil-Smoller S, Kaplan RC, Daviglus ML, Giachello AL, Schneiderman N, Raij L, Talavera G, Allison M, LaVange L, Chambless LE, Heiss G. Design and implementation of the Hispanic Community Health Study/Study of Latinos. Ann Epidemiol. 2010;20:629–641. doi: 10.1016/j.annepidem.2010.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 17.Gonzélez HM, Mungas DM, Reed BR, Marshall S, Haan M. A new verbal learning and memory test for English- and Spanish-speaking older people. J Int Neuropsychol Soc. 2001;7:544–555. doi: 10.1017/s1355617701755026. [DOI] [PubMed] [Google Scholar]
- 18.González HM, Mungas D, Haan MN. A verbal learning and memory test for English- and Spanish-speaking older Mexican-American adults. Clin Neuropsychol. 2002;16:439–451. doi: 10.1076/clin.16.4.439.13908. [DOI] [PubMed] [Google Scholar]
- 19.Lezak M, Howieson DB, Loring DW. Neuropsychological Assessment. Oxford University Press; New York: 2004. [Google Scholar]
- 20.Benton AL, Hamsher K. Multilingual Aphasia Examination, Ed 2. AJA Associates; Iowa City: 1989. [Google Scholar]
- 21.Wechsler D. WAIS-R Manual. Psychological Corporation; San Antonio, TX: 1981. [Google Scholar]
- 22.González HM, Tarraf W, Gouskova N, Gallo LC, Penedo FJ, Davis SM, Lipton RB, Argüelles W, Choca JP, Catellier DJ, Mosley TH. Neurocognitive function among middle-aged and older Hispanic/Latinos: Results from the Hispanic Community Health Study/Study of Latinos. Arch Clin Neuropsychol. 2015;30:68–77. doi: 10.1093/arclin/acu066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.González HM, Tarraf W, Rodríguez CJ, Gallo LC, Sacco RL, Talavera GA, Heiss G, Kizer JR, Hernandez R, Davis S, Schneiderman N, Daviglus ML, Kaplan RC. Cardiovascular health among diverse Hispanics/Latinos: Hispanic Community Health Study/Study of Latinos (HCHS/SOL) results. Am Heart J. 2016;176:134–144. doi: 10.1016/j.ahj.2016.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Heeringa SG, West BT, Berglund PA. Appliedsurvey data analysis. CRC Press; 2010. [Google Scholar]
- 25.Rust K. Variance estimation for complex estimators in sample surveys. J Off Stat. 1985;1:381–397. [Google Scholar]
- 26.Mitchell MN. Interpreting and visualizing regression models using Stata. Stata Press books; 2012. [Google Scholar]
- 27.Baumgart M, Snyder HM, Carrillo MC, Fazio S, Kim H, Johns H. Summary of the evidence on modifiable risk factors for cognitive decline and dementia: A population-based perspective. Alzheimers Dement. 2015;11:718–726. doi: 10.1016/j.jalz.2015.05.016. [DOI] [PubMed] [Google Scholar]
- 28.Daviglus ML, Plassman BL, Pirzada A, Bell CC, Bowen PE, Burke JR, Connolly ES, Jr, Dunbar-Jacob JM, Granieri EC, McGarry K, Patel D, Trevisan M, Williams JW., Jr Risk factors and preventive interventions for Alzheimer disease: State of the science. Arch Neurol. 2011;68:1185–1190. doi: 10.1001/archneurol.2011.100. [DOI] [PubMed] [Google Scholar]
- 29.Ngandu T, Lehtisalo J, Solomon A, Levälahti E, Ahtiluoto S, Antikainen R, Bäckman L, Hänninen T, Jula A, Laatikainen T, Lindström J, Mangialasche F, Paajanen T, Pajala S, Peltonen M, Rauramaa R, Stigsdotter-Neely A, Strandberg T, Tuomilehto J, Soininen H, Kivipelto M. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
- 30.Mathers CD, Loncar D. Projections of global mortality and burden of disease from 2002 to 2030. PLoS Med. 2006;3:e442. doi: 10.1371/journal.pmed.0030442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Alzheimer A, Frankfurt AM. Neuere Arbeiten liber die Dementia senilis und die auf atheromatoser Gefässerkrankung basierenden Gehirnkrankheiten. Mschr Psychiat Neurol. 1898;3:101–115. [Google Scholar]
- 32.Alzheimer A. Die arteriosklerotische Atrophie des Gehirns. Allg Zschr Psychiat Psych gerichtl Med. 1895;51:809–812. [Google Scholar]
- 33.Iadecola C. The pathobiology of vascular dementia. Neuron. 2013;80:844–866. doi: 10.1016/j.neuron.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.van Praag H, Shubert T, Zhao C, Gage FH. Exercise enhances learning and hippocampal neurogenesis in aged mice. J Neurosci. 2005;25:8680–8685. doi: 10.1523/JNEUROSCI.1731-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mielke MM, Vemuri P, Rocca WA. Clinical epidemiology of Alzheimer’s disease: Assessing sex and gender differences. Clin Epidemiol. 2014;6:37–48. doi: 10.2147/CLEP.S37929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.United States Census Bureau. American Community Survey (ACS) 2014 https://www.census.gov/programs-surveys/acs/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


