Abstract
This review addresses how the combination of physiology, medicine and engineering principles contributed to the development and advancement of mechanical ventilation, emphasising the most urgent needs for improvement and the most promising directions of future development.
Several aspects of mechanical ventilation are introduced, highlighting on one side the importance of interdisciplinary research for further development and, on the other, the importance of training physicians sufficiently on the technological aspects of modern devices to exploit properly the great complexity and potentials of this treatment.
Educational aims
To learn how mechanical ventilation developed in recent decades and to provide a better understanding of the actual technology and practice.
To learn how and why interdisciplinary research and competences are necessary for providing the best ventilation treatment to patients.
To understand which are the most relevant technical limitations in modern mechanical ventilators that can affect their performance in delivery of the treatment.
To better understand and classify ventilation modes.
To learn the classification, benefits, drawbacks and future perspectives of automatic ventilation tailoring algorithms.
Short abstract
Physiology, medicine and engineering principles have contributed to the advancement of mechanical ventilation http://ow.ly/Fjlb30bFpLl
Introduction
Even though the concept of mechanical ventilation dates back to the 14th century with Vesalius [1], it is only in the last century that it has been widely introduced into routine clinical practice. Since their initial appearance, mechanical ventilators have become more sophisticated and expanded their application from the intensive care unit (ICU) to the respiratory medicine ward and even to patients’ homes for long-term treatments. This was the result of combining the advances in our understanding of respiratory physiology, pathophysiology and clinical management of patients together with technological progress in mechanical, electronic and biomedical engineering.
Nowadays, this evolution is still rapid, with new devices and an increased number of ventilation modes and strategies being introduced to improve outcomes, patient–ventilator interactions and patient care. Engineering has played and is still playing a relevant role in this process, not only in improving the technical performance of the ventilators but also in contributing to a better understanding of respiratory physiology and pathophysiology, and of how different ventilation strategies interact with the respiratory system.
This review addresses how the combination of physiology, medicine and engineering principles has contributed to the development and advancement of mechanical ventilation, highlighting the open issues and the most urgent needs for improvement with a view on the most promising directions of future development.
Evolution of mechanical ventilation
The use of mechanical tools to assist ventilation dates back to the late 19th century, when devices able to apply an alternating subatmospheric pressure around the body were used to restore ventilation by expanding the chest wall of patients [2]. However, it was only after the introduction of positive-pressure ventilation during the reappearance of poliomyelitis in the 1950s, when Bjorn Ibsen demonstrated a dramatic reduction of mortality in patients manually ventilated by tracheostomy, that mechanical ventilation started to be widespread [3].
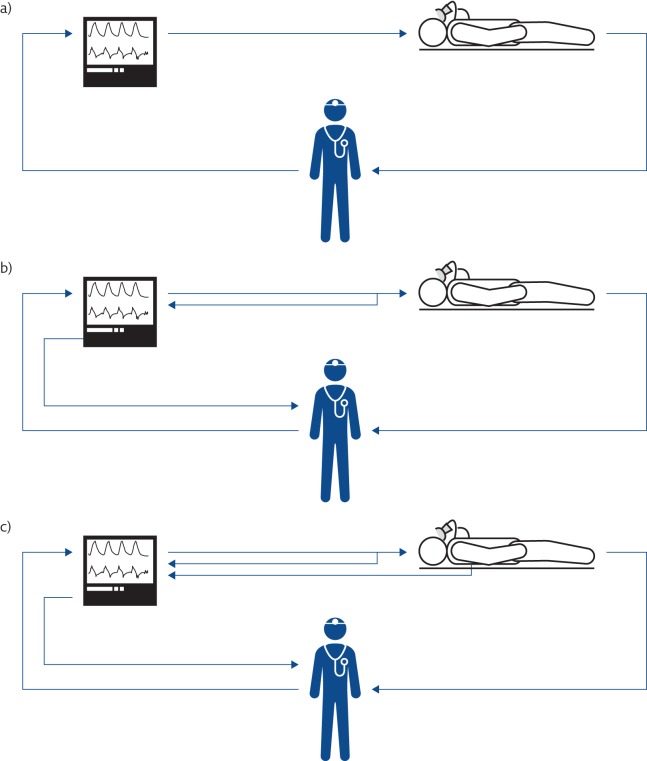
The first positive-pressure mechanical ventilators became available in 1940. Though characterised by a significant degree of sophistication, they were able to deliver only a pre-set tidal volume at a given respiratory rate (volume-control ventilation mode) with no or very limited capability to monitor ventilation variables (figure 1a).
Figure 1.
Evolution of the concept of mechanical ventilation. a) The first mechanical ventilators were not equipped with sensors. b) Mechanical ventilators monitor all the ventilation parameters, allowing both closed-loop control of the generated waveform and providing information to the clinicians. c) Mechanical ventilators monitor the condition of the patients and automatically adjust the ventilatory parameters on the basis of patients’ needs.
At that time, respiratory physiology had already established its foundations and was rapidly growing. The application of mathematical modelling to describe the relationships between flow and pressure (a wonderful example of what today would be considered a biomedical engineering approach), which marked the dawn of the mechanics of breathing, was introduced by Dixon and Brodie [4] in 1903, who modelled the lung as resistance and compliance. Rohrer [5] introduced a relevant simplification by considering only one pressure across the lung and one across the chest wall in 1915 (modelling confirmed by Mead et al. [6] in 1970), improving our capability to describe and understanding the mechanics of the respiratory system during both physiological and artificial breaths. Rahn et al. [7] introduced pressure–volume diagrams of the lung and thorax, and the concept of relaxation curves, in 1946, creating the background for the development of respiratory energetics. These and other studies constituted the physiological background that lead to the introduction of positive-pressure ventilation into clinical practice.
The main objective of mechanical ventilation was originally focused on restoring patients’ ventilation. This concept expanded as the other variables determining gas exchange were understood. Ashbaugh et al. [8] first described acute respiratory distress syndrome (ARDS) in 1967 and identified the positive end-expiratory pressure (PEEP) as “most helpful in combating atelectasis and hypoxæmia”. During the same period, animal studies showing oxygen toxicity when a high inspiratory oxygen fraction (FIO2) was used suggested increasing ventilation instead of FIO2 to treat hypoxaemia, leading to the (ab)use of increased tidal volume (VT).
From the early 1970s, ventilators benefited from the progresses in electronics, and started incorporating more advanced monitoring of flow and pressure variables (figure 1b). Improvements in monitoring also allowed the possibility of using real-time variables to control the action of the machine, with the intermittent mandatory ventilation mode opening the development of assisted mechanical ventilation as a way to manage the weaning of patients from periods of volume-controlled ventilation [9].
In the meantime, our understanding of the mechanics of breathing further improved thanks to the introduction of the concept of dynamic compression in airways during forced expiration by Fry, Hyatt and co-workers [10]; three-dimensional analysis of pressure, flow and volume curves [10, 11]; and a better understanding of chest wall mechanics from Campbell’s diagram for the partitioning of elastic and resistive work [12], and the two-compartment model of the chest wall by Konno and Mead [13].
In 1974, Webb and Tierney [14] demonstrated in animal models that ventilation with high distending pressures was leading per se to severe or even fatal pulmonary oedema, marking the beginning of a decades-long research effort aimed at understanding the adverse effects of mechanical ventilation and the mechanisms involved, and identifying appropriate strategies for preventing ventilation-induced lung injury (VILI), which despite the huge progress made so far, is still an open issue. Since then, major attention has been given to identifying the optimal ventilation strategy as a compromise between normalisation of blood gases and avoiding the development of VILI, resulting in the introduction of improved technologies for monitoring ventilation and lung conditions and the introduction of new advanced ventilation modes.
This progress was also possible thanks to the introduction of microprocessors in mechanical ventilators, starting in the early 1980s. Microprocessors are silicon electronic circuits able to execute programs; therefore, they can provide very complex functions that can be easily changed by selecting different programs, allowing a modern mechanical ventilator to rapidly modify its behaviour (e.g. changing from a volume-control to a pressure-control or a pressure-assist ventilation mode simply by selecting different control algorithms executed by the device). In addition, microprocessors allow the implementation of sophisticated signal processing of the measurements provided by sensors, leading not only to better measurement accuracy and better rejection of noise and artefacts, but also to the generation of new information by appropriately computing and integrating several variables. Moreover, this expanded information can be used by the ventilator itself to optimise the ventilation parameters on the basis of patient needs, creating the so-called “closed-loop” ventilation strategies or intelligent or smart ventilation modes (figure 1c).
The mechanical ventilator: the challenge of delivering accurate ventilation
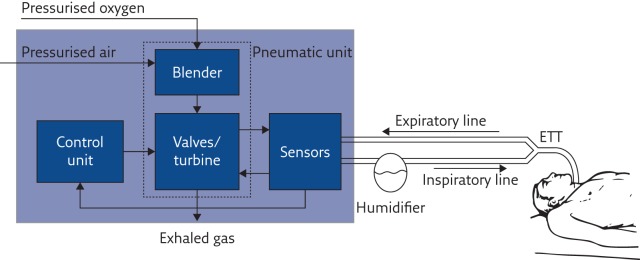
Once a ventilation strategy is defined, the ventilator should deliver it to the patient in the most accurate way. To achieve this, the machine must sense all variables that define the breathing pattern with high accuracy and adjust its action in real time. Modern ventilators achieve this by combining cutting-edge technology of actuators, sensors and digital electronics together with sophisticated data processing algorithms. The basic components of a typical mechanical ventilator are shown in figure 2.
Figure 2.
Basic structure and main functional components of a mechanical ventilator. ETT: endotracheal tube.
Pneumatic unit
The pressure source provides the energy required to overcome the elastic and resistive load imposed by the patient’s respiratory system, and it is used to reduce their work of breathing. The pressurised air is mixed with the appropriate amount of oxygen by the blender, and delivered to the patient by fast valves that modulate the amount of gas flowing to and from the patient. Thanks to the recent progress in electronics and electric motors, instead of using valves, in some modern ventilators a fast-response, brushless-driven turbine acts as a variable pressurised air source, making the device independent of centralised medical compressed air distribution but still providing good performance [15].
Sensors
In modern mechanical ventilators, all relevant ventilation variables (pressure, flow and FIO2) are measured by appropriate sensors that provide information to the control unit in order to adjust, in real time, the valves/turbines for delivering the desired ventilation mode.
Modern pressure transducers produce stable, precise and fast measurement of pressure but, despite this, pressure variables provided by ventilators are at times still outside the tolerance range, even in absence of leaks [16]. Aimed at measuring an extensive variable, flow sensors (that also provide volume data by computing the time integral of flow) must be placed in series with the patient in the breathing circuit and, therefore, are exposed to humidity, condensation and the patient’s exhalation, making them potential sites of cross-contamination. Moreover, flow meters must guarantee a wide operating range of measurements, high accuracy and stability, adequate dynamic response, robustness to changes in gas composition, temperature and vapour condensation, and at the same time, impose a low pneumatic resistance and dead space on the patient.
The need to improve flow measurement technology is highlighted by the several comparative publications on performance of mechanical ventilators that quite often identify the delivered volume as the variable showing the lowest accuracy and repeatability [16–18].
The most common approaches to measuring flow include variable-orifice sensors, pneumotachographs or hot-wire anemometers. Promising technologies for the future are micromachined and fibreoptic-based flow meters [19]. In particular, thermal flow sensors micromachined on a single silicon chip are already available and used in some mechanical ventilators. They have the advantages of presenting high sensitivity and accuracy, a wide measurement range, fast response, low temperature and gas composition sensitivity, low dead space and resistance [20], and the production cost could lead, in the future, to the availability of pre-calibrated disposable devices.
Ventilator circuit
Few studies have addressed the impact of ventilator circuits on overall performance in adult ventilation [21–23], while greater attention has been paid to this in paediatric and neonatal ventilation. The small VT of neonatal ventilation makes it crucial to consider the shunt compliance of the circuits when measuring volumes, either by excluding it by measuring flow after the circuit at the airways opening or by estimating and digitally compensating for it [24, 25]. Also, specific design of the ventilation circuits improves the transmission of ventilation waveforms to the patient with possible consequences on the overall treatment [26].
Digital signal processing algorithms
Compared to previous generations of mechanical ventilators, the use of microprocessors allowed the development of sophisticated signal processing algorithms to compensate for intrinsic flaws in sensor technologies, consequently improving performance. As a drawback, these algorithms are mostly unknown to the users, who thus cannot understand or predict the behaviour of the ventilator in situations and conditions different from these considered during the development of the algorithms.
As volume measurements are obtained by digital integration of the flow signals, the signal processing also significantly contributes to volume accuracy by compensating for drift and leaks, especially during noninvasive ventilation, where leak estimation and compensation algorithms play a major role. Recent studies have shown that, even if compensation algorithms can markedly reduce measurement errors, overall performance are not yet ideal [16, 27] and improvements are still needed, especially during variable leaks [28].
Maintenance
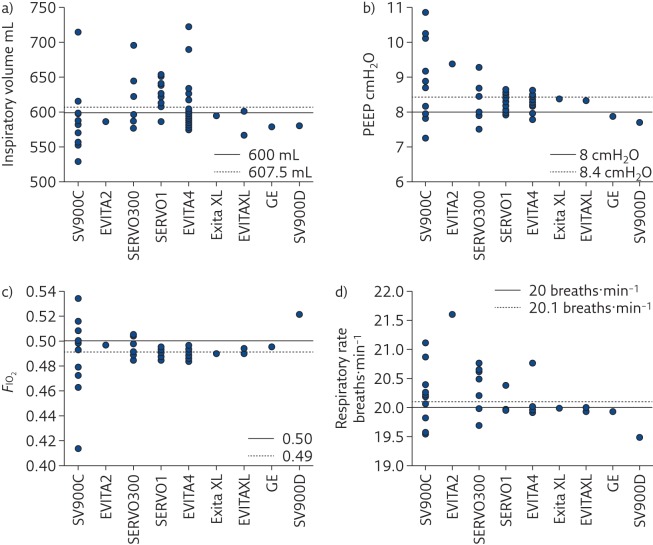
Even if each mechanical ventilator undergoes accurate calibration and testing before being set in operation, preserving performance over time is the responsibility of maintenance and servicing procedures, which have not always shown to be appropriate [29]. Even when the same model of mechanical ventilator is considered, wide variability in performance between devices has been observed (figures 3 and 4), both in the high-end machines used in the ICU [17] and in simpler units for home ventilation [30], highlighting the urgency of improving lifetime quality control of such devices, especially for flow sensors.
Figure 3.
Measured values of a) inspiratory volume, b) PEEP, c) FIO2 and d) respiratory rate delivered to a test lung by several devices in four intensive care units grouped by ventilator model. EVITA4: Draeger Evita 4 (25 machines); SERVOI: Siemens/Maquet Servo I (16 machines); SVC900C: Siemens SV900C (12 machines); SERVO300: Siemens/Maquet Servo 300 (seven machines); EVITAXL: Draeger Evita XL (three machines); SV900D: Siemens SV900D (one machine); EVITA2: Draeger Evita 2 (one machine) and ENGSTROM: GE Engstrom (one machine). Reproduced and modified from [17] with permission from the publisher.
Figure 4.
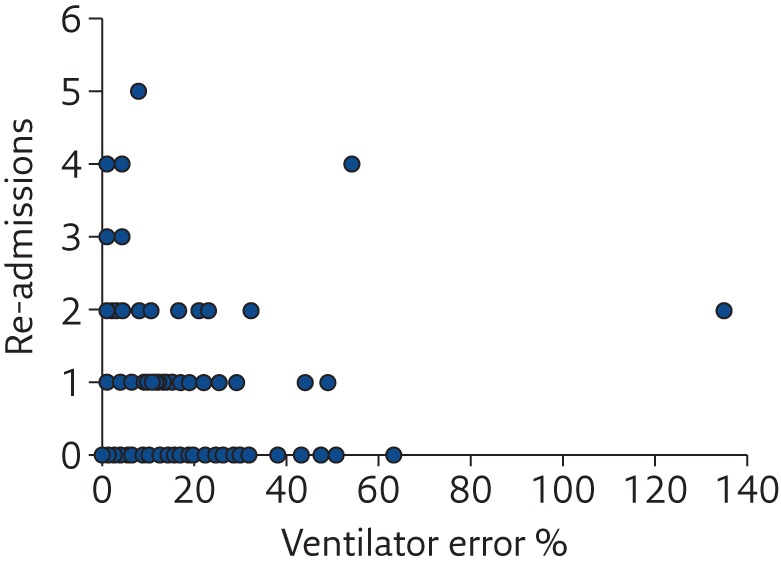
Relationship between the number of unplanned hospital re-admissions of home-ventilated patients in the previous year and the index of error of the home ventilator. Reproduced and modified from [30] with permission from the publisher.
Ventilation modes
Even if the concept of mechanical ventilation is apparently simple, there are virtually infinite possible patterns of interaction between the ventilator and the patient, namely the modes of ventilation. Originally, the classification of these modes was relatively simple, with “controlled ventilation modes” being used when the ventilator was imposing its action to the patient and “assisted ventilation modes” used when the ventilator was supporting the breathing of the patient. However, since the introduction of microprocessors, the number of modes of ventilation has grown exponentially. Moreover, in addition to the implicit complexity of this matter, many manufacturers have introduced different (and often trademarked) names for similar modes, mostly for pursuing marketing and branding strategies, making it even more difficult for the user to understand the principles and ideal use of each ventilation mode available on a given ventilator.
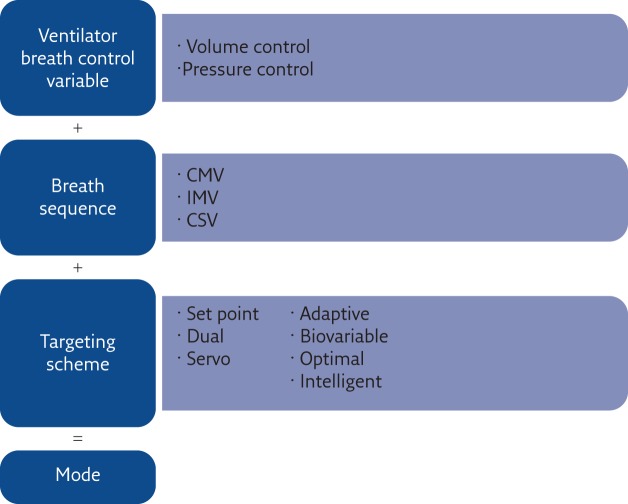
This is a relevant issue that probably deserves more attention in order to improve our ability to provide the best ventilation to our patients, whichever ventilator is used. Starting in 1992, Robert Chatburn developed a structured approach that lead to a well-defined and structured taxonomy to name ventilation modes depending on their essential features, making it possible to understand the features of a ventilation modality from its name [31].
Chatburn’s approach (figure 5) is based on the definition of a mode of mechanical ventilation as the combination of three elements:
1) the ventilator breath control variable
2) the breath sequence
3) the targeting scheme
They used 10 maxims to progressively describe how to classify a given ventilation mode by understanding what it does (table 1). Flow charts are also proposed to suggest a procedure for classifying a given ventilation mode by applying the maxims. Unfortunately, the introduction of such a structured taxonomy into clinical practice requires relevant and simultaneous efforts both in the educational programmes of medical schools and in the implementation of this naming approach in the ventilators by the manufacturers. This is unlikely to occur without a firm and combined effort from all medical societies of anaesthesia, intensive care and respiratory medicine, which is still lacking.
Figure 5.
Construction of the ventilation mode taxonomy suggested by Chatburn. The name of a ventilation mode results from three elements. CMV: continuous mandatory ventilation; IMV: intermittent mandatory ventilation; CSV: continuous spontaneous ventilation. Reproduced and modified from [31] with permission from the publisher.
Table 1.
Chatburns’ maxims for understanding ventilator operation
| A breath is one cycle of positive flow (inspiration) and negative flow (expiration) defined in terms of the flow versus time curve |
| A breath is assisted if the ventilator provides some or all of the work of breathing |
| A ventilator assists breathing using either pressure control or volume control based on the equation of motion for the respiratory system |
| Breaths are classified according to the criteria that trigger (start) and cycle (stop) inspiration |
| Trigger and cycle events can be either patient initiated or ventilator initiated |
| Breaths are classified as spontaneous or mandatory based on both the trigger and cycle events |
| Ventilators deliver three basic breath sequences: CMV, IMV and CSV |
| Ventilators deliver five basic ventilatory patterns: VC-CMV, VC-IMV, PC-CMV, PC-IMV and PC-CSV |
| Within each ventilatory pattern, there are several types that can be distinguished by their targeting schemes (set-point, dual, biovariable, servo, adaptive, optimal and intelligent) |
| A mode of ventilation is classified according to its control variable, breath sequence and targeting schemes |
CMV: continuous mandatory ventilation; IMV: intermittent mandatory ventilation; CSV: continuous spontaneous ventilation; VC: volume control; PC: pressure control. Reproduced and modified from [31] with permission from the publisher.
Since in-depth description of different ventilation modes is outside the goal of this review, the reader can find detailed information elsewhere [32]. As most of the recent innovation in mechanical ventilation is focused in developing new approaches for the implementation of a targeting scheme, the following paragraphs are focused on this topic, using Chatburn’s classification as reference (table 2).
Table 2.
Classification of different targeting schemes
| Scheme | Description | Advantage | Disadvantage |
| 1) Set-point (s) | The operator sets all parameters of the pressure waveform (pressure control modes) or volume and flow waveforms (volume control modes) | Simplicity | Changing patient conditions may make settings inappropriate |
| 2) Dual (d) | The ventilator can automatically switch between volume control and pressure control during a single inspiration | It can adjust to changing patient conditions and ensure either a pre-set VT or peak inspiratory pressure, whichever is deemed most important | It may be complicated to set correctly and may need constant readjustment if not automatically controlled by the ventilator |
| 3) Servo (r) | The output of the ventilator (pressure/volume/flow) automatically follows a varying input | Support by the ventilator is proportional to inspiratory effort | It requires estimates of artificial airway and/or respiratory system mechanical properties |
| 4) Adaptive (a) | The ventilator automatically sets target(s) between breaths in response to varying patient conditions | It can maintain stable VT delivery with pressure control for changing lung mechanics or patient inspiratory effort | Automatic adjustment may be inappropriate if algorithm assumptions are violated or if they do not match physiology |
| 5) Biovariable (b) | The ventilator automatically adjusts the inspiratory pressure or VT randomly | It simulates the variability observed during normal breathing and may improve oxygenation or mechanics | Manually set range of variability may be inappropriate to achieve goals |
| 6) Optimal (o) | The ventilator automatically adjusts the targets of the ventilatory pattern to either minimise or maximise some overall performance characteristic (e.g. work rate of breathing) | It can adjust to changing lung mechanics or patient inspiratory effort | Automatic adjustment may be inappropriate if algorithm assumptions are violated or if they do not match physiology |
| 7) Intelligent (i) | This is a targeting scheme that uses artificial intelligence programmes such as fuzzy logic, rule-based expert systems and artificial neural networks | It can adjust to changing lung mechanics or patient inspiratory effort | Automatic adjustment may be inappropriate if algorithm assumptions are violated or if they do not match physiology |
Reproduced and modified from [31] with permission from the publisher.
Targeting schemes 1 and 2: set-point and dual schemes
The set-point and dual schemes are both manual targeting schemes in which the operator directly adjusts the controlled output [33]. The majority of ventilation modalities is included in this category. These modes can be divided into synchronised or non-synchronised ventilation modes.
In synchronised modes, the advances of technologies resulted in improved trigger performance [34–36]. While in adults, the use of either the patient’s flow or pressure triggering results in non-clinically significant difference in performance [37], in neonatal ventilation, where VT is extremely small and respiratory rates high, flow triggering improves synchrony [38]. However, the patient’s triggering is still an open issue, especially in presence of leaks and during noninvasive ventilation [39, 40].
Targeting scheme 3: servo targeting schemes
Servo targeting ventilation modes are characterised by the ventilator generating an output that follows a varying input in real time. Automatic tube compensation (ATC), neurally adjusted ventilator assist (NAVA), proportional assist ventilation (PAV) and PAV-plus are examples of this category. They allow high degree of synchrony with patients and support the work of breathing, assigning to the patient’s neural respiratory control the definition of the ventilation waveform.
ATC mathematically estimates the pressure drop due to the endotracheal tube (ETT) from the patient flow and continuously adjusts the pressure at the airway opening in order to maintain the pressure at the tip of the ETT at the set value, compensating (only) for the resistive workload added to the patient by the high resistance of the tube. NAVA measures the electrical activity of the diaphragm by transoesophageal electromyography and applies an airway opening pressure proportional to it to the patient. It allows better synchronisation with the patient, the delivery of physiological waveforms and it is robust in case of leaks and auto-PEEP [41, 42]. PAV and PAV-plus support both the elastic and resistive work of the patient, and are based on the application of the equation of motion of the respiratory system, therefore requiring the knowledge of the patient’s respiratory resistance (Rdyn) and compliance (Cdyn). PAV-plus continuously monitors lung mechanics in order to adjust Rdyn and Cdyn to follow changes in patient’s conditions and resulting in better performance [33].
Targeting scheme 4: adaptive targeting
Adaptive targeting allows the ventilator to automatically set one ventilation variable to maintain another ventilation variable at a predefined value. In this way, the ventilator can cope with changes in patient conditions. Examples of ventilation modes using this scheme are: mandatory rate ventilation, in which the inspiratory pressure is modified to maintain the set respiratory frequency; adaptive flow/adaptive inspiratory time, in which the inspiratory time and inspiratory flow are automatically adjusted to maintain an inspiratory/expiratory ratio of 1/2; adaptive pressure control, in which inspiratory pressure is adapted to deliver a target VT regardless of any change in lung mechanics or patient effort; and mandatory minute ventilation, in which minute ventilation is maintained close to a target value by increasing/decreasing mandatory breaths or by an automatic adjustment of the inspiratory pressure. It is worthy to underline that these ventilation modes cannot be considered forms of automatic weaning [33].
Targeting scheme 5: biovariable targeting
Variable ventilation is a ventilation modality that provides a variable VT or peak pressure by adding random fluctuations to the set value in order to mimic the natural variability of the respiration. The rationale is based on the noise-enhanced amplification of a useful signal in a nonlinear system by stochastic resonance. Indeed, including appropriately designed noise in mechanical ventilators improves gas exchange [43]. Moreover, variability in the respiratory system has been found to be important for cell activity, as it positively modulates surfactant secretion [44, 45]; it is also related to the maturation of breathing control in infants [46, 47] and correlates with weaning success [48–50]. Animal studies have shown that variability improves ventilation efficiency and lung compliance in the preterm lung without increasing lung inflammation or lung injury [51, 52].
Adjusting mechanical ventilation for patients’ condition and needs: optimal and intelligent targeting schemes
When, in 1998, Dreyfuss and Saumon demonstrated that the key ventilation variable responsible for the development of VILI is not the absolute pressure applied to the lung but the VT, it became clear that, in order to avoid VILI, the settings of ventilator parameters must be accurately tailored to the individual patient. However, even if the VT was physiologically corrected by adjusting it for body weight, many patients were injured anyway. A significant advancement in our understanding of VILI was provided by the use of computed tomography (CT) to study ARDS. It was thanks to CT scans that we learned that ARDS is not a homogeneous, but a highly heterogeneous, disease [53, 54]. Three-dimensional lung images showed that in a diseased lung, there are nonaerated regions due to either a complete filling of the alveolar spaces with liquid and cells, and/or to the collapse of potentially recruitable pulmonary units (atelectasis) [55]. This has major consequences in tailoring mechanical ventilation: a VT estimated from the body size of a given patient might be excessive as the actual aerated lung can be much smaller than its anatomical dimensions (a concept called “baby lung”). The translation of this concept to clinical practice was demonstrated by the ARDSNet trial in 2000, in which a significant reduction of mortality was shown when a VT of 6 mL⋅kg−1 was used instead of 12 mL⋅kg−1 [56]. This and other studies underlined the importance of applying a lung-protective ventilation strategy in which the minimisation of side-effects of ventilation gains priority when applying this treatment. However, even if we now understand much more of the pathophysiology of ventilation, we are still not able to fully implement these concepts in the clinical practice. For example, we learned that ventilation parameters should be adjusted on the amount of the aerated lung of the patient and not simply on his/her body weight. However, the size of the ventilated lung changes from patient to patient and in the same patient with time. Moreover, as part of the nonaerated regions can be due to atelectasis, the application of different PEEP, ventilatory patterns and lung volume history can markedly impact the dimension of the baby lung by recruiting or de-recruiting atelectatic regions, thus making the baby lung size a highly dynamic variable and not a static one [57, 58], thus requiring continuous adjustment of ventilator parameters. Therefore, PEEP should be optimised aiming to maximise the fraction of recruited lung [59, 60], thus reducing inspiratory pressures as much as possible to minimise over-distention of lung tissues [56]. Unfortunately, the only clinically available validated technology for assessing lung volume recruitment is CT, which is obviously unsuitable for long-term bedside monitoring.
This highlights the importance of both a proper understanding of the pathophysiological mechanisms and quantification of related relevant variables in optimising ventilation. Similar reasoning applies to other specific conditions, such as the presence of intrinsic PEEP in obstructed patients and the controlled unloading of the respiratory muscles during weaning.
Even if these physiopathology concepts are now well understood, the key variables needed to tailor ventilation are not yet measurable at the bedside, leading to a significant gap between what we know about an ideal ventilation strategy and what can be applied in the clinic. For this reason, research efforts have been devoted in recent years to improving technologies for assessing physiological variables at the bedside and to incorporate into mechanical ventilators these enhanced monitoring capabilities for providing the clinicians with the information needed to properly tailor mechanical ventilation. Moreover, availability of measurements of specific physiological variables enables the implementation of automatic tailoring algorithms.
Monitoring physiological conditions
Pressure at the airway opening, ventilation volumes and FIO2 are monitored by all modern mechanical ventilators. Beside oxygenation, which can be easily measured at bedside by pulse oximetry, distribution of lung ventilation and mechanics are among the most relevant variables for optimising ventilation. Therefore, recently developed noninvasive techniques for lung function monitoring are now becoming available in mechanical ventilators, allowing bedside monitoring of new relevant variable for optimising ventilation.
Monitoring distribution of ventilation: electrical impedance tomography
After decades of research, electrical imperdance tomography (EIT) is now commercially available to monitor the pattern of regional ventilation of the lungs during mechanical ventilation, and to show how it changes by modifying ventilation mode and parameters [61]. Recent studies showed that EIT can be an effective tool to titrate optimal PEEP [62–65].
Lung mechanics: forced oscillation technique
With the forced oscillation technique (FOT), a small-amplitude, high-frequency oscillatory pressure is applied to the airway opening, and the study of the relationship between the applied pressure and the resulting flow at different frequencies (the so-called respiratory impedance) allows the characterisation of specific features of the lung mechanical properties, even in presence of spontaneous breathing and without requiring oesophageal manometry [66]. In particular, recent studies have demonstrated potential usefulness of FOT for monitoring lung mechanics during mechanical ventilation [67–70], proved that it is sensitive and specific to changes in peripheral lung mechanics [71–73], and showed that FOT can be used as bedside tool for monitoring recruitment/derecruitment [74]. Specifically, monitoring lung mechanics by FOT during a decremental PEEP trial allows the identification of the open-lung PEEP, defined as the minimum PEEP level required to prevent lung derecruitment after a recruitment manoeuvre, with high sensitivity and specificity when compared with CT [75, 76]. FOT may also be a useful tool for optimising PEEP in obstructed patients as it allows the monitoring of the presence of tidal expiratory flow limitation [71, 72] and, in general, can be used to monitor the status and evolution of respiratory mechanics in the ventilated patient.
These are only examples of how cutting-edge technologies have resulted in new monitoring tools for optimising ventilation. Other physiological variables and measurement technologies have been studied for monitoring blood gases, lung mechanics, proinflammatory mediators, etc. [77].
Computational tools
The idea of automating mechanical ventilation was suggested in 1957 [78]. The rationale of “intelligent” ventilators is to improve patient management by analysing and integrating information coming from large number of sources, and guaranteeing continuous adjustment of the ventilation even when expert personnel are not constantly available, improving patients’ treatment and minimising clinical errors. Moreover, these tools can be also used for educational purposes.
However, ventilation management is a complex process involving the analysis of multiple parameters, subjective strategies and multiple goals (gas exchange, lung protection and weaning). Physiology, as well as the peculiarity of the pathology, must be taken into account, several clinical data must be gathered from many sources and the condition of the patient must be monitored continuously. Finally, priorities of goals, clinical preferences and local clinical protocols must be also considered.
Until now, different variables have been considered as input for optimising ventilation strategies. They include variables describing the general condition of the patients, haemodynamic variables, gas exchange, lung mechanics and the work of breathing [79]. When implementing these strategies in mechanical ventilators, there is the need to identify a trade-off between the complete description of the patient condition and the complexity of managing many variables, availability of the data, invasiveness of measurements and acquisition of data from different equipment.
The same can be said for the output variables: the simplest system controls only one variable. This is the case for algorithms that adjust FIO2 to maintain target saturation. Ideally, on the basis of the condition of the patient, algorithms should be able to control not only a single variable but all the ventilator settings and even suggest specific manoeuvres or pharmacological treatments to the clinician.
Engineering science has developed several computational tools that start from a set of input (measured) variables and can determine the desired outputs (settings) [79]. They include mathematical models and classical controllers (e.g. proportional, integrative and derivative controllers), rule-based expert systems (e.g. systems that use coded clinical protocols), and other artificial intelligence techniques (fuzzy logic, neural networks and genetic algorithms) or hybrid systems (combinations of the aforementioned systems). All these engineering technologies can be used to model the cardiorespiratory system and/or the decisional process of the clinicians to automatically identify an optimal ventilation strategy.
Mathematical models have the advantage of describing pathophysiological processes. However, in order to be patient-specific, the identification of the parameters of the model is required. This is often difficult as these parameters are usually not measurable with noninvasive techniques and estimation errors, being unavoidable, must be considered. Classical control science controllers are commonly used to modify one or few ventilator parameters to maintain one or few physiological variables within a predefined range.
Rule-based expert systems are built using the available ventilation protocols and clinician expertise. They have the disadvantages of being related to protocols that may be subjective and specific for single centres.
Artificial intelligence techniques do not require modelling the system on a mechanistic basis. They are able to handle large number of variables and they can be trained on large existing data sets. As a limitation, their outputs are difficult to predict as they are determined by the complex interaction of many rules.
All these tools have been and are currently applied to implement both open-loop (decision support) systems and closed-loop systems. Closed-loop systems have the major advantage of allowing a continuous adaptation of ventilation to the patient without requiring the intervention of clinicians. However, as they apply changes to a patient’s therapy without involving, or at least requiring, the acknowledgment of a clinician, they open serious issues of safety and accountability [79] that will require, in some countries, compliance with current legislation.
Targeting scheme 6: optimal targeting schemes
These schemes allow automatic adjustment of ventilator settings to either minimise or maximise some overall performance characteristic. Different optimal targeting schemes have been proposed in the literature [79]. They include:
schemes involving a simple controller used for the automatic adjustment of FIO2 to maintain patient saturation within an optimal range, or to adjust inspiratory pressure or minute ventilation on the basis of carbon dioxide exchange
more complex procedures including mathematical models and explicit objective functions for optimising more than one variable (e.g. optimising respiratory rate, VT, inspiratory/expiratory time and PEEP for minimising the elastance of the respiratory system and regulating end-tidal carbon dioxide)
A ventilation modality available in commercial mechanical ventilators that follows this scheme is adaptive support ventilation (ASV). ASV is designed to minimise the work of breathing. Its inputs are body weight, FIO2, PEEP and %MV to be supported by the machine, and the target is to automatically adjust respiratory rate and VT, considering resistance, compliance and auto-PEEP. Some expert rules are used to maintain frequency and VT in safe ranges and reduce the risk of auto-PEEP. Because of these rules, ASV can be better classified as a form of hybrid scheme that combines mathematical modelling and artificial intelligence. Studies have shown that ASV can select specific VT and respiratory rate settings [80] to achieve the same arterial partial pressure of carbon dioxide as the clinicians do and allowing weaning automation [81].
The new modality Intellivent-ASV adds to the ASV controller (ventilation controller) an oxygenation controller that adjusts PEEP and FIO2, depending on the ARDS network tables to maintain oxygen saturation measured by pulse oximetry (SpO2) within a pre-set range. Moreover, it includes a test for probing weaning readiness of patients [82].
Targeting scheme 7: intelligent targeting schemes
Several systems were described in literature for setting ventilation parameters based on some form of artificial intelligence. Examples of this targeting scheme are a fuzzy-logic system used for FIO2 control, or for optimising respiratory rate and VT on the basis of lung mechanics, SpO2, carbon dioxide, body temperature, end-tidal carbon dioxide, and the patient’s body weight and height or to implement the “open-lung approach”; and artificial neural networks, genetic algorithms, fuzzy-logic systems and their combination for the support of ventilation management specific to some common lung pathologies [79].
Studies applying rule-based expert systems include those by Laubscher et al. [83], which was included in the development of ASV, and Dojat et al. [84], which was integrated in a commercial ventilator under the name of Smartcare. Smartcare is a rule-based expert system for automatic control of pressure support ventilation. It processes respiratory rate, end-tidal carbon dioxide, pressure support and VT. It aims to maintain the patient in a “comfort zone” defined by a combination of VT, respiratory frequency and end-tidal carbon dioxide, and to progressively decrease the inspiratory pressure. Studies assessing the impact of using this ventilation mode found that it has better or similar performance to weaning managed by experienced personnel [85–89]. However, it is not well suited for patients with low spontaneous breathing activity or during worsening of airway obstruction [82].
Educational perspective
This review has mainly focused on describing how the combination of physiology, medicine and engineering concepts and tools are being used to improve mechanical ventilation. To go further, two kinds of advancements are required. The first is the development of cost-effective, accurate, miniaturised and reliable sensors to measure patient variables (e.g. pressure, flow and oxygenation) and actuators to build machines reliably providing the desired pressure/flow to patients (e.g. blowers and valves). The second is the application of mathematical models, control systems and artificial intelligence to tailor the action of the mechanical ventilator to each patient’s needs. While progress in sensors and actuators is mostly a technological issue (relatively independent of respiratory pathophysiology), application of control/intelligence tools to the specific case of mechanical ventilation is something that goes beyond the pure discipline of engineering, since it requires a deep knowledge of the pathophysiology of the respiratory system. This is a challenging task that must be carried out by a truly interdisciplinary approach, joining the knowledge and expertise of bioengineers and physicians. Although this kind of approach has already been followed “informally” since the beginning of mechanical ventilation, interdisciplinary efforts are more and more necessary as the complexity of modern mechanical ventilators increases as these machines are among the most complex medical devices in terms of integrating and controlling a variety of input and output variables.
It goes without saying that bioengineers focused on designing and building mechanical ventilators must be deeply educated on respiratory pathophysiology. However, it is nowadays not so obvious that physicians dealing with mechanical ventilation have been sufficiently educated on the technological aspects of these complex medical devices. Physicians attending patients under acute or chronic mechanical ventilation are faced with considerable difficulties to properly understand how these devices work. As mentioned before in this review, a major difficulty is the intrinsic complexity of modern control/intelligence algorithms implemented in current devices. This difficulty is increased by the lack of standardisation and the fact that, for marketing and commercial strategies, companies building ventilators tend to add new definitions of ventilation modes and variants without disclosing the algorithms with sufficient detail for the user to really understand how the machine is working. The result is that in most cases the physician is using a sort of “black box” as therapeutic device. Accordingly, it seems necessary that, when being specifically trained to use mechanical ventilation, physicians should be also provided with a sufficient education on the technological issues involved in these modern medical devices. In that way, they will be in optimal professional conditions to select the best therapeutic option for each individual patient.
Conclusions
Patients’ needs are changing with time because of the evolution of both pathology severity and treatments. Therefore, an optimal ventilation strategy is an adaptive process aiming to treat the acute condition and either support the gradual weaning from the ventilator or adjust to the natural fluctuations of chronic disorders. Such a tailoring could improve outcomes and comfort, reduce adverse events, and avoid prolonging ventilation, with its associated risks and costs. To reach these goals, advancement of our understanding in pathophysiology, medicine and engineering must be combined with an interdisciplinary approach to fully address the complexity of mechanical ventilation for further improving patients’ treatment.
Educational questions
- Ventilation variables displayed by mechanical ventilators are always within the accuracy limits declared by manufacturers
- a) True
- b) False
- c) Only at the beginning of their life cycle
- Ventilation mode names indicated by modern mechanical ventilators uniquely identify the characteristics of the mode
- a) Yes, all ventilators use only standardised names
- b) No, there might be different names for the same mode as manufacturers are not obliged to use a standardised taxonomy
- c) Only intensive care unit ventilators are required to use a standardised taxonomy
- The technology of a sensor fully characterises its performance
- a) Yes
- b) Only in modern mechanical ventilators
- c) No, in modern mechanical ventilators the use of different data processing algorithms can lead to very different overall performance of the same sensor technology
- d) Only for flow sensors
- The difference between an optimal and an intelligent targeting scheme is
- a) The optimal targeting scheme identifies the best ventilation setting while the intelligent scheme, the best ventilation mode
- b) The optimal targeting scheme adjust ventilation parameters in order to maximise or minimise a comprehensive physiological feature such as work of breathing, while the intelligent scheme uses artificial intelligence tools to optimise the ventilation settings on the basis of coded knowledge
- c) The two terms refer to the same approach
- In mechanical ventilators, the difficulty to provide accurate measurements of the ventilation variables is usually:
- a) Similar for all variables
- b) Higher for volumes compared to pressure, FIO2 and time
- c) Lower for pressure compared to volume
- d) Higher for pressure compared to FIO2 and time
Suggested answers
c.
b.
c.
b.
b.
Disclosures
R. Dellaca EDU-0078-2017_Dellaca (1.2MB, pdf)
C. Veneroni EDU-0078-2017_Veneroni (1.2MB, pdf)
Footnotes
Conflict of interest Disclosures can be found alongside this article at breathe.ersjournals.com
References
- 1.Vesalius A. De humani corporis fabrica. 1543.
- 2.Dalziel J. On sleep and apparatus for promoting artificial respiration. Br Assoc Adv Sci 1838; 2: 127–134. [Google Scholar]
- 3.Lassen HCA. A preliminary report on the 1952 epidemic of poliomyelitis in Copenhagen with special reference to the treatment of acute respiratory insufficiency. Lancet 1953; 1: 37–41. [DOI] [PubMed] [Google Scholar]
- 4.Dixon WE, Brodie TG. Contributions to the physiology of the lungs: Part I. The bronchial muscles, their innervation, and the action of drugs upon them. J Physiol 1903; 29: 97–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rohrer F. Der Zusammenhang der Atemkräfte und ihre Abhängigkeit vom Dehnungszustand der Atmungsorgane. Pflügers Arch Eur J Physiol 1916; 165: 419–444. [Google Scholar]
- 6.Mead J, Takishima T, Leith D. Stress distribution in lungs: a model of pulmonary elasticity. J Appl Physiol 1970; 28: 596–608. [DOI] [PubMed] [Google Scholar]
- 7.Rahn H, Otis AB, Chadwick LE, et al. . The pressure-volume diagram of the thorax and lung. Am J Physiol 1946; 146: 167–178. [DOI] [PubMed] [Google Scholar]
- 8.Ashbaugh DG, Bigelow DB, Petty TL, et al. . Acute respiratory distress in adults. Lancet 1967; 2: 319–323. [DOI] [PubMed] [Google Scholar]
- 9.Downs JB, Klein EF, Desautels D, et al. . Intermittent mandatory ventilation: a new approach to weaning patients from mechanical ventilators. Chest 1973; 64: 331–335. [DOI] [PubMed] [Google Scholar]
- 10.Fry DL, Hyatt RE. Pulmonary mechanics. A unified analysis of the relationship between pressure, volume and gasflow in the lungs of normal and diseased human subjects. Am J Med 1960; 29: 672–689. [DOI] [PubMed] [Google Scholar]
- 11.Hyatt RE, Schilder DP, Fry DL. Relationship between maximum expiratory flow and degree of lung inflation. J Appl Physiol 1958; 13: 331–336. [DOI] [PubMed] [Google Scholar]
- 12.Campbell E. The Respiratory Muscles and the Mechanics of Breathing. London, Lloyd-Luke, 1958. [Google Scholar]
- 13.Konno K, Mead J. Measurement of the separate volume changes of rib cage and abdomen during breathing. J Appl Physiol 1967; 22: 407–422. [DOI] [PubMed] [Google Scholar]
- 14.Webb HH, Tierney DF. Experimental pulmonary edema due to intermittent positive pressure ventilation with high inflation pressures. Protection by positive end-expiratory pressure. Am Rev Respir Dis 1974; 110: 556–565. [DOI] [PubMed] [Google Scholar]
- 15.Thille AW, Lyazidi A, Richard J-CM, et al. . A bench study of intensive-care-unit ventilators : New versus old and turbine-based versus compressed gas-based ventilators ventilators. Intensive Care Med 2009; 35: 1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Garnier M, Quesnel C, Fulgencio J-P, et al. . Multifaceted bench comparative evaluation of latest intensive care unit ventilators. Thompson JP, editor. Br J Anaesth 2015; 115: 89–98. [DOI] [PubMed] [Google Scholar]
- 17.Govoni L, Dellaca RL, Penuelas O, et al. . Actual performance of mechanical ventilators in ICU: A multicentric quality control study. Med Devices Evid Res 2012; 5: 111–119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leipälä JA, Iwasaki S, Milner A, et al. . Accuracy of the volume and pressure displays of high frequency oscillators. Arch Dis Child Fetal Neonatal Ed 2004; 89: F174–F176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schena E, Massaroni C, Saccomandi P, et al. . Flow measurement in mechanical ventilation: a review. Med Eng Phys 2015; 37: 257–264. [DOI] [PubMed] [Google Scholar]
- 20.Silvestri S, Schena E. Micromachined flow sensors in biomedical applications. 2012; 3: 225–243. [Google Scholar]
- 21.Hess D, McCurdy S, Simmons M. Compression volume in adult ventilator circuits: a comparison of five disposable circuits and a nondisposable circuit. Respir Care 1991; 36: 1113–1118. [PubMed] [Google Scholar]
- 22.Disposable adult breathing circuits for use with critical care ventilators. Health Devices 1994; 23: 104–129. [PubMed] [Google Scholar]
- 23.Wang JS, Hung WT, Lin CY. Leakage of disposable breathing circuits. J Clin Anesth 1992; 4: 111–115. [DOI] [PubMed] [Google Scholar]
- 24.Castle RA, Dunne CJ, Mok Q, et al. . Accuracy of displayed values of tidal volume in the pediatric intensive care unit. Crit Care Med 2002; 30: 2566–2574. [DOI] [PubMed] [Google Scholar]
- 25.Cannon ML, Cornell J, Tripp-Hamel DS, et al. . Tidal volumes for ventilated infants should be determined with a pneumotachometer placed at the endotracheal tube. Am J Respir Crit Care Med 2000; 162: 2109–2112. [DOI] [PubMed] [Google Scholar]
- 26.Wheeler KI, Moore GP, Morley CJ, et al. . Comparison of two ventilator circuits for Drager Babylog high-frequency ventilation. J Paediatr Child Health 2011; 47: 211–216. [DOI] [PubMed] [Google Scholar]
- 27.Itagaki T, Bennett DJ, Chenelle CT, et al. . Performance of leak compensation in all-age ICU ventilators during volume-targeted neonatal ventilation: a lung model study. Respir Care 2017; 62: 10–21. [DOI] [PubMed] [Google Scholar]
- 28.Lujan M, Sogo A, Grimau C, et al. . Influence of dynamic leaks in volume-targeted pressure support noninvasive ventilation: a bench study. Respir Care 2015; 60: 191–200. [DOI] [PubMed] [Google Scholar]
- 29.Beydon L, Liu N, Hassapopoulos J, et al. . Test of 20 similar intensive care ventilators in daily use conditions--evaluation of accuracy and performances. Intensive Care Med 1992; 18: 32–37. [DOI] [PubMed] [Google Scholar]
- 30.Farré R, Navajas D, Prats E, et al. . Performance of mechanical ventilators at the patient’s home: a multicentre quality control study. Thorax 2006; 61: 400–404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chatburn RL, El-Khatib M, Mireles-Cabodevila E. A taxonomy for mechanical ventilation: 10 fundamental maxims. Respir Care 2014; 59: 1747–1763. [DOI] [PubMed] [Google Scholar]
- 32.Cairo JM. Mosby’s Respiratory Care Equipment. Amsterdam, Elsevier Health Sciences, 2013. [Google Scholar]
- 33.Chatburn RL, Mireles-Cabodevila E. Closed-loop control of mechanical ventilation: description and classification of targeting schemes. Respir Care 2011; 56: 85–102. [DOI] [PubMed] [Google Scholar]
- 34.Richard J-C, Carlucci A, Breton L, et al. . Bench testing of pressure support ventilation with three different generations of ventilators. Intensive Care Med 2002; 28: 1049–1057. [DOI] [PubMed] [Google Scholar]
- 35.Thille AW, Lyazidi A, Richard J-CM, et al. . A bench study of intensive-care-unit ventilators: new versus old and turbine-based versus compressed gas-based ventilators. Intensive Care Med 2009; 35: 1368–1376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Takeuchi M, Williams P, Hess D, et al. . Continuous positive airway pressure in new-generation mechanical ventilators: a lung model study. Anesthesiology 2002; 96: 162–172. [DOI] [PubMed] [Google Scholar]
- 37.Kondili E. Patient-ventilator interaction. Br J Anaesth 2003; 91: 106–119. [DOI] [PubMed] [Google Scholar]
- 38.Keszler M. State of the art in conventional mechanical ventilation. J Perinatol 2009; 29: 262–275. [DOI] [PubMed] [Google Scholar]
- 39.Oto J, Chenelle CT, Marchese AD, et al. . A comparison of leak compensation in acute care ventilators during noninvasive and invasive ventilation: a lung model study. Respir Care 2013; 58: 2027–2037. [DOI] [PubMed] [Google Scholar]
- 40.Stell IANM, Paul G, Lee KC, et al. . Noninvasive ventilator triggering in chronic a test lung comparison. Am J Respir Crit Care Med 2001; 164: 2092–2097. [DOI] [PubMed] [Google Scholar]
- 41.Lellouche F, Brochard L. Advanced closed loops during mechanical ventilation (PAV, NAVA, ASV, SmartCare). Best Pract Res Clin Anaesthesiol 2009; 23: 81–93. [DOI] [PubMed] [Google Scholar]
- 42.Branson RD, Johannigman JA. Innovations in mechanical ventilation. Respir Care 2009; 54: 933–947. [DOI] [PubMed] [Google Scholar]
- 43.Suki B, Alencar AM, Sujeer MK, et al. . Life-support system benefits from noise. Nature 1998; 393: 127–128. [DOI] [PubMed] [Google Scholar]
- 44.Arold SP, Suki B, Alencar AM, et al. . Variable ventilation induces endogenous surfactant release in normal guinea pigs. Am J Physiol Lung Cell Mol Physiol 2003; 285: L370–L375. [DOI] [PubMed] [Google Scholar]
- 45.Arold SP, Bartolák-Suki E, Suki B. Variable stretch pattern enhances surfactant secretion in alveolar type II cells in culture. Am J Physiol Lung Cell Mol Physiol 2009; 296: L574–L581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Frey U, Silverman M, Barabási AL, et al. . Irregularities and power law distributions in the breathing pattern in preterm and term infants. J Appl Physiol 1998; 85: 789–797. [DOI] [PubMed] [Google Scholar]
- 47.Engoren M, Courtney SE, Habib RH. Effect of weight and age on respiratory complexity in premature neonates. J Appl Physiol 2009; 106: 766–773. [DOI] [PubMed] [Google Scholar]
- 48.Wysocki M, Cracco C, Teixeira A, et al. . Reduced breathing variability as a predictor of unsuccessful patient separation from mechanical ventilation. Crit Care Med 2006; 34: 2076–2083. [DOI] [PubMed] [Google Scholar]
- 49.Kaczmarek J, Kamlin COF, Morley CJ, et al. . Variability of respiratory parameters and extubation readiness in ventilated neonates. Arch Dis Child Fetal Neonatal Ed 2013; 98: F70–F73. [DOI] [PubMed] [Google Scholar]
- 50.Engoren M. Approximate entropy of respiratory rate and tidal volume during weaning from mechanical ventilation. Crit Care Med 1998; 26: 1817–1823. [DOI] [PubMed] [Google Scholar]
- 51.Berry CA, Suki B, Polglase GR, et al. . Variable ventilation enhances ventilation without exacerbating injury in preterm lambs with respiratory distress syndrome. Pediatr Res 2012; 72: 384–392. [DOI] [PubMed] [Google Scholar]
- 52.Pillow JJ, Musk GC, McLean CM, et al. . Variable ventilation improves ventilation and lung compliance in preterm lambs. Intensive Care Med 2011; 37: 1352–1359. [DOI] [PubMed] [Google Scholar]
- 53.Gattinoni L, Mascheroni D, Torresin A, et al. . Morphological response to positive end expiratory pressure in acute respiratory failure. Computerized tomography study. Intensive Care Med 1986; 12: 137–142. [DOI] [PubMed] [Google Scholar]
- 54.Maunder RJ, Shuman WP, McHugh JW, et al. . Preservation of normal lung regions in the adult respiratory distress syndrome. Analysis by computed tomography. JAMA 1986; 255: 2463–2465. [PubMed] [Google Scholar]
- 55.Remy-Jardin M, Remy J, Giraud F, et al. . Computed tomography assessment of ground-glass opacity: semiology and significance. J Thorac Imaging 1993; 8: 249–264. [DOI] [PubMed] [Google Scholar]
- 56.Acute Respiratory Distress Syndrome Network,Brower RG, Matthay MA, et al. . Ventilation with lower tidal volumes as compared with traditional tidal volumes for acute lung injury and the acute respiratory distress syndrome. N Engl J Med 2000; 342: 1301–1308. [DOI] [PubMed] [Google Scholar]
- 57.Crotti S, Mascheroni D, Caironi P, et al. . Recruitment and derecruitment during acute respiratory failure: a clinical study. Am J Respir Crit Care Med 2001; 164: 131–140. [DOI] [PubMed] [Google Scholar]
- 58.Pelosi P, Goldner M, McKibben A, et al. . Recruitment and derecruitment during acute respiratory failure. Am J Respir Crit Care Med 2001; 164: 122–130. [DOI] [PubMed] [Google Scholar]
- 59.Ware LB, Matthay MA. The acute respiratory distress syndrome. N Engl J Med 2000; 342: 1334–1349. [DOI] [PubMed] [Google Scholar]
- 60.Brower RG, Lanken PN, MacIntyre N, et al. . Higher versus lower positive end-expiratory pressures in patients with the acute respiratory distress syndrome. N Engl J Med 2004; 351: 327–336. [DOI] [PubMed] [Google Scholar]
- 61.Gong B, Krueger-Ziolek S, Moeller K, et al. . Electrical impedance tomography : functional lung imaging on its way to clinical practice? Expert Rev Respir Med 2015; 9: 721–737. [DOI] [PubMed] [Google Scholar]
- 62.Wolf GK, Gómez-Laberge C, Rettig JS, et al. . Mechanical ventilation guided by electrical impedance tomography in experimental acute lung injury. Crit Care Med 2013; 41: 1296–1304. [DOI] [PubMed] [Google Scholar]
- 63.Liu S, Tan L, Möller K, et al. . Identification of regional overdistension, recruitment and cyclic alveolar collapse with electrical impedance tomography in an experimental ARDS model. Crit Care 2016; 20: 119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Muders T, Luepschen H, Zinserling J, et al. . Tidal recruitment assessed by electrical impedance tomography and computed tomography in a porcine model of lung injury. Crit Care Med 2012; 40: 903–911. [DOI] [PubMed] [Google Scholar]
- 65.Costa EL V, Borges JB, Melo A, et al. . Bedside estimation of recruitable alveolar collapse and hyperdistension by electrical impedance tomography. Intensive Care Med 2009; 35: 1132–1137. [DOI] [PubMed] [Google Scholar]
- 66.Kaczka DW, Dellacà RL. Oscillation mechanics of the respiratory system: applications to lung disease. Crit Rev Biomed Eng 2011; 39: 337–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Farre R, Ferrer M, Rotger M, et al. . Servocontrolled generator to measure respiratory impedance from 0.25 to 26 Hz in ventilated patients at different PEEP levels. Eur Respir J 1995; 8: 1222–1227. [DOI] [PubMed] [Google Scholar]
- 68.Bellardine CL, Ingenito EP, Hoffman A, et al. . Heterogeneous airway versus tissue mechanics and their relation to gas exchange function during mechanical ventilation. Ann Biomed Eng 2005; 33: 626–641. [DOI] [PubMed] [Google Scholar]
- 69.Kaczka DW, Hager DN, Hawley ML, et al. . Quantifying mechanical heterogeneity in canine acute lung injury: impact of mean airway pressure. Anesthesiology 2005; 103: 306–317. [DOI] [PubMed] [Google Scholar]
- 70.Kaczka DW, Brown RH, Mitzner W. Assessment of heterogeneous airway constriction in dogs: a structure-function analysis. J Appl Physiol 2008; 106: 520–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dellacà RL, Rotger M, Aliverti A, et al. . Noninvasive detection of expiratory flow limitation in COPD patients during nasal CPAP. Eur Respir J 2006; 27: 983–991. [DOI] [PubMed] [Google Scholar]
- 72.Dellaca RL, Santus P, Aliverti A, et al. . Detection of expiratory flow limitation in COPD using the forced oscillation technique. Eur Respir J 2004; 24: 332–333. [DOI] [PubMed] [Google Scholar]
- 73.Johnson MK, Birch M, Carter R, et al. . Use of reactance to estimate transpulmonary resistance. Eur Respir J 2005; 25: 1061–1069. [DOI] [PubMed] [Google Scholar]
- 74.Dellaca RL, Andersson Olerud M, Zannin E, et al. . Lung recruitment assessed by total respiratory system input reactance. Intensive Care Med 2009; 35: 2164–2172. [DOI] [PubMed] [Google Scholar]
- 75.Dellacà RL, Zannin E, Kostic P, et al. . Optimisation of positive end-expiratory pressure by forced oscillation technique in a lavage model of acute lung injury. Intensive Care Med 2011; 37: 1021–1030. [DOI] [PubMed] [Google Scholar]
- 76.Kostic P, Zannin E, Andersson Olerud M, et al. . Positive end-expiratory pressure optimization with forced oscillation technique reduces ventilator induced lung injury: a controlled experimental study in pigs with saline lavage lung injury. Crit Care 2011; 15: R126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nieman GF, Satalin J, Andrews P, et al. . Personalizing mechanical ventilation according to physiologic parameters to stabilize alveoli and minimize ventilator induced lung injury (VILI). Intensive Care Med Exp 2017; 5: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Saxton GA, Myers G. A servomechanism for automatic regulation of pulmonary ventilation. J Appl Physiol 1957; 11: 326–328. [DOI] [PubMed] [Google Scholar]
- 79.Tzavaras A, Weller PR. Classical approaches and intelligent systems in ventilation management: a survey. Crit Rev Biomed Eng 2010; 38: 157–188. [DOI] [PubMed] [Google Scholar]
- 80.Arnal J, Nafati C, Wysocki M, et al. . Utilization of adaptive support ventilation (ASV) in a polyvalent intensive care unit. Intensive Care Med 2004; 30: S84. [Google Scholar]
- 81.Dongelmans DA, Veelo DP, Paulus F, et al. . Weaning automation with adaptive support ventilation: a randomized controlled trial in cardiothoracic surgery patients. Anesth Analg 2009; 108: 565–571. [DOI] [PubMed] [Google Scholar]
- 82.Alam M, Jones G, Kahl W, et al. . Modeling the weaning of intensive care unit patients from mechanical ventilation: A review. Crit Rev Biomed Eng 2014; 42: 25–61. [DOI] [PubMed] [Google Scholar]
- 83.Laubscher TP, Heinrichs W, Weiler N, et al. . An adaptive lung ventilation controller. IEEE Trans Biomed Eng 1994; 41: 51–59. [DOI] [PubMed] [Google Scholar]
- 84.Dojat M, Pachet F, Guessoum Z, et al. . NéoGanesh: a working system for the automated control of assisted ventilation in ICUs. Artif Intell Med 1997; 11: 97–117. [DOI] [PubMed] [Google Scholar]
- 85.Dojat M, Harf A, Touchard D, et al. . Clinical evaluation of a computer-controlled pressure support mode. Am J Respir Crit Care Med 2000; 161: 1161–1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lellouche F, Mancebo J, Jolliet P, et al. . A multicenter randomized trial of computer-driven protocolized weaning from mechanical ventilation. Am J Respir Crit Care Med 2006; 174: 894–900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Rose L, Presneill JJ, Johnston L, et al. . A randomised, controlled trial of conventional versus automated weaning from mechanical ventilation using SmartCareTM/PS. Intensive Care Med 2008; 34: 1788–1795. [DOI] [PubMed] [Google Scholar]
- 88.Schädler D, Engel C, Elke G, et al. . Automatic control of pressure support for ventilator weaning in surgical intensive care patients. Am J Respir Crit Care Med 2012; 185: 637–644. [DOI] [PubMed] [Google Scholar]
- 89.Burns KEA, Meade MO, Lessard MR, et al. . Wean Earlier and Automatically with New Technology (the WEAN Study). A multicenter, pilot randomized controlled trial. Am J Respir Crit Care Med 2013; 187: 1203–1211. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
R. Dellaca EDU-0078-2017_Dellaca (1.2MB, pdf)
C. Veneroni EDU-0078-2017_Veneroni (1.2MB, pdf)