Abstract
Extracellular polymeric substances (EPS) were quantified in flocculent and aerobic granular sludge developed in two sequencing batch reactors with the same shear force but different settling times. Several EPS extraction methods were compared to investigate how different methods affect EPS chemical characterization, and fluorescent stains were used to visualize EPS in intact samples and 20-μm cryosections. Reactor 1 (operated with a 10-min settle) enriched predominantly flocculent sludge with a sludge volume index (SVI) of 120 ± 12 ml g−1, and reactor 2 (2-min settle time) formed compact aerobic granules with an SVI of 50 ± 2 ml g−1. EPS extraction by using a cation-exchange resin showed that proteins were more dominant than polysaccharides in all samples, and the protein content was 50% more in granular EPS than flocculent EPS. NaOH and heat extraction produced a higher protein and polysaccharide content from cell lysis. In situ EPS staining of granules showed that cells and polysaccharides were localized to the outer edge of granules, whereas the center was comprised mostly of proteins. These observations confirm the chemical extraction data and indicate that granule formation and stability are dependent on a noncellular, protein core. The comparison of EPS methods explains how significant cell lysis and contamination by dead biomass leads to different and opposing conclusions.
The efficiency of biological wastewater treatment depends, first, upon the selection and growth of metabolically capable microorganisms and, second, upon the efficient separation of those organisms from the treated effluent. Bacteria usually aggregate to form suspended flocs, which can cause bulking and foaming problems if filamentous bacteria are present. Activated sludge flocs also settle relatively slowly, requiring large primary and secondary settling tanks before clear effluent can be released. Alternatively, aerobic granular sludge aggregates have been formed in sequencing batch reactors (SBRs) with short fill periods and various substrates (1a, 13, 15). As opposed to flocs, granules are dense and have high settling velocities. They can be described as a collection of self-immobilized cells into a somewhat spherical form and are considered to be a special case of biofilm growth (10).
Microbial aggregates form biofilms by creating a network of cells and extracellular polymeric substances (EPS), which include any substances of biological origin (9). The abbreviation “EPS” has often been expanded to extracellular polysaccharides or exopolysaccharides. However, EPS have been shown to be a rich matrix of polymers, including polysaccharides, proteins, glycoproteins, nucleic acids, phospholipids, and humic acids. EPS are typically reported to aid in the formation of a gel-like network that keeps bacteria together in biofilms, cause the adherence of biofilms to surfaces, and protect bacteria against noxious environmental conditions (24).
Because EPS are a major component of cell flocs and biofilms, they are hypothesized to play a central role in all types of biofilm formation, including flocculation and granulation. It is not fully understood what factors increase EPS formation, although several researchers hypothesize that hydraulic shear may contribute (21). Tay et al. (21) reported that increased aeration rates in a granular SBR resulted in an increased polysaccharide content and that granular sludge disintegrated when polysaccharides were lost from the system. Aerobic granules have also failed to form in systems with reduced aeration rates (6, 22). Researchers concluded that hydrodynamic shear force increases the production of cellular polysaccharides, which aid in the formation and stability of aerobic granules (11). However, several arguments exist against shear force being the necessary factor for granule formation. Most notably, granules were not stable in airlift reactors operated with longer settling times and high aeration rates (2). Therefore, the relationship between shear force, EPS formation, and granule stability is unclear.
In the present experiment, two SBRs were operated with the same aeration rate and superficial upflow gas velocity but with two different settling times. Long and short settling times were utilized to form flocculent and granular sludge, respectively, under the same shear conditions. The EPS were extracted at steady-state to determine whether the polysaccharide and protein contents varied between flocculent and granular sludge produced under the same aeration rate, and several extraction methods were compared. Intact, hydrated flocs and granules were also fluorescently stained to visualize the distribution of cells, polysaccharides, and proteins in situ.
MATERIALS AND METHODS
Reactor operation.
Two 5-liter column-type SBRs were operated for 6 months to develop flocculant and granular sludge, respectively. The reactors were constructed from Plexiglas shaped as a cylinder (height, 100 cm; diameter, 9 cm). They were aerated at a rate of 275 liters h−1 (superficial gas velocity of 1.2 cm s−1) with a 50% volumetric exchange ratio. The reactors were inoculated with 5 liters of activated sludge from a municipal wastewater treatment plant (initial mixed liquor suspended solid [MLSS] content of 2.5 g liter−1). The walls of the reactors were cleaned every 2 weeks, and the biofilm growth was discarded. Both reactors were fed from a common source of glucose and peptone with nutrients (similar to that used by (16)) at a volumetric loading rate of 2.4 kg of chemical oxygen demand (COD) m−3 day−1 (800 mg of COD liter−1 cycle−1). The total cycle time was 4 h with six cycles per day (90-min static fill, 120-min react, 2- or 10-min settle, 15-min draw, and 5- or 13-min idle). The only variation in operating strategy was the settling and idle times (10-min settle for reactor 1 and 2-min settle for reactor 2).
Analytical procedures.
MLSS and volatile suspended solid (VSS) content, effluent and volatile suspended solid (ESS and EVSS, respectively) content, and the sludge volume index (SVI) were measured one to three times per week, all according to APHA standard engineering methods (4). Substrate removal was measured weekly by the COD during the cycle and in the effluent by using Dr.Lange COD kits (according to the colorimetric COD standard method) (Dr.Lange GmbH, Düsseldorf, Germany). The specific oxygen uptake rate (SOUR) during a cycle was measured weekly during startup, and the endogenous SOUR was measured twice a week (4, 12). Endogenous oxygen uptake rate (OUR) samples were collected from the end of the react cycle and aerated for at least 2 h before the OUR measurement, whereas beginning and end of the react OUR samples were measured immediately after sampling. The development of flocs and granules was observed by using a stereomicroscope (Leica Wild MPS 46/52; Leica, Vienna, Austria), and images were obtained with an attached Kodak digital camera.
EPS extraction and chemical analysis.
EPS was characterized from nonhomogenized and homogenized samples by two extraction methods: the use of cation-exchange resin (Dowex) and alkaline treatment with heat (NaOH).
Sample pretreatment.
Approximately 0.5 g volatile solids (VS) were taken from each reactor at the end of the SBR cycle. The reactor volume taken for the 0.5 g of VS was estimated from previous VSS measurements. The actual mass of VS sampled for EPS extraction was determined from the MLSS and VSS contents measured at the time of sampling. The sample was centrifuged at 4°C and 10,000 rpm for 15 min. The supernatant was collected to determine chemical composition of reactor wash and loosely bound EPS. The samples were resuspended in Milli-Q water and centrifuged again. For nonhomogenized samples, the remaining pellet was resuspended in phosphate buffer (7) to a total volume of 100 ml. For homogenized samples, the pellet was resuspended in 40 ml of phosphate buffer and divided into two aliquots. Each aliquot was homogenized for 10 min in a homogenizer (RW 20 DZM; Janke & Kunkel, Staufen, Germany) at a maximum rpm of 980, and the two homogenized parts were combined with phosphate buffer to a 100-ml total volume.
Cation-exchange resin extraction.
EPS extraction using a Dowex 50x8, Na+ Form, cation-exchange resin (Fluka) was performed with a 0.5 g of VS-to-35 g of cation exchange resin ratio according to the method of Frølund et al. (7). The samples were stirred at 750 rpm for 4 h in the dark at 4°C. A blank sample with cation exchange resin and phosphate buffer (pH 7) was also tested.
NaOH and heat extraction.
Harvested samples (suspended in 100 ml of buffer) were adjusted to pH 11 by using 1 M NaOH and placed in plastic bottles before heating to 80°C for 30 min (modified from Tay et al. [21]). A blank with phosphate buffer adjusted to pH 11 with NaOH was also measured.
EPS harvesting and characterization.
After the Dowex and NaOH extraction, respective samples were centrifuged at 10,000 rpm for 1 min. The supernatant was transferred to clean centrifuge tubes and again centrifuged 10 min. Afterward, cell lysis was measured with a glucose-6-phosphate dehydrogenase kit (Fisher Scientific 345-A), and the remaining EPS supernatant was stored at −20°C in aliquots until chemical analyses were performed. Total organic carbon (TOC) was measured by using an Elementar High TOC II (Elementar, Hanau, Germany). Proteins and polysaccharides were measured according to the method of Frølund et al. (7) by using bovine albumin serum and glucose standards, respectively. Cell lysis was measured directly after Dowex EPS extraction by using a glucose-6-phosphate dehydrogenase kit and was negligible. Cell lysis from NaOH EPS extraction could not be measured with the kit, since the kit measures the presence of the glucose-6-phosphate dehydrogenase enzyme. Enzymes are typically active only within a pH range of 5 to 9, and controlled trials with the kit and NaOH extracts (pH 11) were not successful (23).
Fluorescence staining and CLSM.
Intact, living, and hydrated granules and flocs were stained with fluorescently labeled probes with different excitation and emission spectra in order to visualize the distribution of cells, polysaccharides, and proteins in samples. After being stained, whole samples were either visualized directly by confocal laser scanning microscopy (CLSM; LSM510 META; Zeiss, Jeve, Germany) or embedded in cryosectioning compound (Microm, Walldorf, Germany) and cryosectioned into 20-μm sections (CM 3050S Kryostat; Leica, Bensheim, Germany) for direct visualization.
Staining.
Samples were stained in 1.5-ml Eppendorf tubes, covered with aluminum foil, and placed on a shaker table (100 rpm) for 15 min each. Fluorescein-isothiocyanate (FITC) (0.01%) is an amine reactive dye and stains all proteins and amino-sugars of cells and EPS (19). Concanavalin A (ConA) lectin conjugated with Texas Red (100 μg ml−1) was used in the present study to bind to α-mannopyranosyl and α-glucopyranosyl sugar residues. Syto 63 is a cell-permeative nucleic acid stain and was used to visualize all cells. All probes were purchased from Molecular Probes, and samples were washed with phosphate-buffered saline after each staining step. Granules were stained before and after cutting them in half in order to rule out any diffusion limitation of stains inside the granule structure. Based on these observations and reported diffusion coefficients of similar stains (5), there should have been no biases due to diffusion differences.
CLSM.
The probes were visualized on three channels with corresponding excitations and emissions: FITC (488 nm, BP 505 to 530 nm), ConA (543 nm, LP 560 nm), and Syto 63 (633 nm, LP 650 nm). Z-sectioning was performed on whole granules and rendered three-dimensionally by using the LSM 510 Viewer software (Zeiss).
RESULTS
Reactor operation and performance.
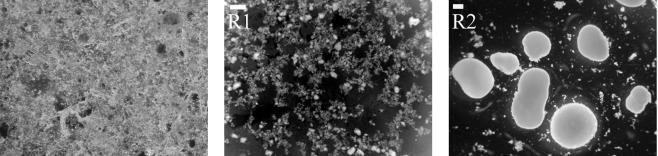
A detailed report of granule development and performance in these reactors is published separately (14). Due to the difference in settling time, the washout of sludge during the first 2 weeks of operation was much greater for reactor 2 (2 min settling) (the MLSS dropped to 0.7 g liter−1) than for reactor 1 (10 min settling) (the MLSS began to increase immediately after startup). After 1 week of operation, granules were observed in both reactors. On day 56 of operation, the SVIs of reactors 1 and 2 were 60 and 63 ml g−1, respectively, and the settling time seemed to have no effect on granule formation. However, after 80 days of operation, the reactors began to diverge in terms of sludge characteristics, MLSS, and SVI. For reactor 1, the granules always coexisted with flocculent sludge, whereas the flocs in reactor 2 were continuously washed out with the short settling time, leaving predominantly granular sludge. Images of reactor inoculum and steady-state sludge are presented in Fig. 1. In parallel studies (not presented here), three separate reactors were operated with the same strategy as reactor 2, and all formed granules with similar properties.
FIG. 1.
Reactor inoculum and steady-state sludge (day 200) from reactors 1 and 2 (R1 and R2; scale = 1 mm).
The steady-state values for MLSS, VSS, effluent SS, SVI, OUR, and COD removal are summarized in Table 1. It is clear that both reactors performed well in terms of COD removal and OUR. The reactors differed in the properties of the sludge, MLSS content, and settling characteristics. Most significantly, the granular reactor 2 developed an average MLSS of 8.8 g liter−1 and an SVI of 50 ml g−1 compared to an MLSS of 3.0 g liter−1 and an SVI of 120 ml g−1 for the flocculent reactor 1.
TABLE 1.
Steady-state values for standard measurements (days 120 to 220 of operation)
| Property or performance parameter | Steady-state value ± SDa
|
|
|---|---|---|
| Reactor 1 (10-min settle) | Reactor 2 (2-min settle) | |
| Sludge properties | ||
| MLSS (g liter−1) | 3.0 ± 0.2 | 8.8 ± 0.5 |
| VSS (g liter−1) | 2.7 ± 0.2 | 8.0 ± 0.5 |
| SVI (ml g−1) | 120 ± 12 | 50 ± 2 |
| ESS (mg liter−1) | 280 ± 90 | 170 ± 50 |
| Reactor performance | ||
| Endogenous SOURb | 12 ± 1 | 8 ± 2 |
| COD removal (%) | 96 ± 1 | 96 ± 1 |
Values are reported with 95% confidence (as determined by analysis of variance).
SOUR is reported as follows: mg of O2/(g of VSS h).
EPS characterization.
Initially, EPS extraction was performed by using only the Dowex cation-exchange method combined with homogenization (14), and the results were opposite those reported by using the NaOH heating method (21). To understand the influence of method on granular sludge characterization, the EPS from steady-state samples was chemically extracted and characterized by different methods. Nonhomogenized and homogenized samples were extracted by using (i) alkaline treatment with NaOH at 80°C and (ii) stirring with cation-exchange resin. Table 2 presents an overview of all results.
TABLE 2.
EPS component analysis for each extraction method
| Sample and reactor | Pretreatment | Mean value (mg g−1 VSS−1) ± SEa
|
|||
|---|---|---|---|---|---|
| Protein | Carbohydrates | TOC | PN/PSb | ||
| NaOH extraction | |||||
| Reactor 1 | Nonhomogenized | 199 ± 28 | 26 ± 9 | 227 ± 44 | 7.8 |
| Reactor 1 | Homogenized | 190 ± 28 | 30 ± 9 | 234 ± 44 | 6.4 |
| Reactor 2 | Nonhomogenized | 185 ± 28 | 18 ± 9 | 86 ± 44 | 10.9 |
| Reactor 2 | Homogenized | 210 ± 28 | 26 ± 9 | 203 ± 44 | 7.9 |
| Dowex extraction | |||||
| Reactor 1 | Nonhomogenized | 39 ± 5 | 5 ± 2 | 33 ± 4 | 7.5 |
| Reactor 1 | Homogenized | 50 ± 5 | 8 ± 2 | 44 ± 4 | 6.6 |
| Reactor 2 | Nonhomogenized | 23 ± 5 | 3 ± 2 | 15 ± 4 | 7.6 |
| Reactor 2 | Homogenized | 73 ± 5 | 11 ± 2 | 69 ± 4 | 6.7 |
Standard errors were calculated based on separate extractions of triplicate samples.
PN/PS, protein/polysaccharide ratio.
The total TOC measurement indicates the amount of EPS extracted, and the values for each reactor are presented in Fig. 2 for both nonhomogenized and homogenized sludge samples. In general, the NaOH extraction yielded much more total TOC for each sample than the corresponding Dowex extraction. Triplicate NaOH extractions also had a greater variability than triplicate Dowex extractions, which is represented by the error bars in Fig. 2. For the predominantly flocculent reactor 1, homogenization had little effect on the total TOC extracted by using either the NaOH or Dowex method. In contrast, homogenization of granular reactor 2 samples increased TOC yields by >200% for both extraction methods.
FIG. 2.
Total TOC of EPS extracted from reactor 1 (flocculent) and 2 (granular) by using each extraction methods. Gray bars represent homogenized samples. Error bars indicate the standard deviations of triplicate extractions.
The protein content was greater than the polysaccharide content of all EPS extracts (data presented in Table 2 for homogenized samples). The alkaline treatment yielded >200% more proteins and carbohydrates than the corresponding Dowex treatment, which corresponded to the increase in TOC with alkaline extraction (Fig. 2). Between reactors 1 and 2, the EPS of granules (R2) had higher protein levels than EPS from flocs and granules (R1), whereas the carbohydrate content increased only slightly.
In situ EPS staining and fluorescent microscopy.
Fluorescently labeled lectins such as ConA have been used to stain glycoconjugates in heterotrophic, multispecies biofilms, and FITC is an amine reactive dye that stains proteins and other amine-containing compounds (3, 17, 18, 20). These stains may also bind with protein and glycoconjugate groups associated with cell walls, so a counterstain with Syto 63 was used to distinguish EPS from cells. Figure 3 shows the resultant staining from a 20-μm cryosection taken from the middle of a granule (ca. 260 μm below the surface). A freshly sampled granule was stained before sectioning, and fluorescence was viewed on three separate channels for each stain. Figure 3 shows that cells and polysaccharides were restricted to the outer edge of the granule structure, with the cells clustered within 100 μm of the surface. Figure 3C shows that FITC stained proteins throughout the entire granule, with no overlapping of FITC with other stains in the center. At least 10 cryosections were viewed from five different granules, all with similar observations.
FIG. 3.
Fluorescent staining of 20-μm cryosections from a reactor 2 granule. Cells stained with Syto 63 (A), polysaccharides stained with ConA (B), and proteins stained with FITC (C) are shown. Scale bar, 100 μm. Images were obtained with a ×10 objective lens.
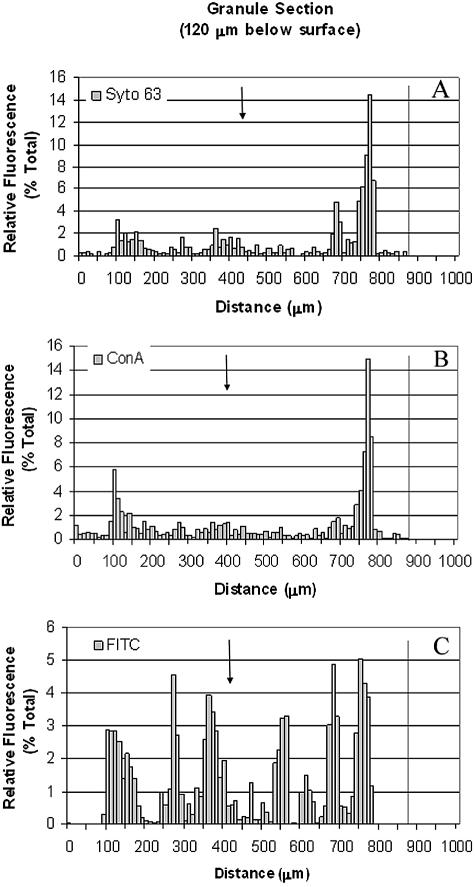
For several sections, the fluorescence intensity of each stain along one diameter of the granule was quantified by image analysis. The relative intensities, or percent total, are displayed in Fig. 4 for a section extracted 120 μm below the granule surface. The main distribution of cells and polysaccharides were at the edge of the section, whereas the protein stain was unevenly distributed across the entire diameter. This quantification confirms the microscopic observations shown in Fig. 3. It also shows that the Syto and ConA stains were detected at the center of the granules albeit at low levels, indicating that the stains were able to diffuse into the entire sample.
FIG. 4.
Relative fluorescence of each stain through a 20-μm cryosection, cut 120 μm below the granule surface. The granule edge is marked by a dotted line, and an arrow marks the granule center. Cells (Syto 63) (A) and polysaccharides (ConA) (B) are clustered within the first 200 mm of the granule edge, and proteins (FITC) are distributed unevenly throughout the granule (C).
Whole flocs and granules were also observed under the microscope. One floc is displayed in Fig. 5. EPS (both polysaccharide and protein staining) was concentrated in a flocculent center, whereas some polysaccharides were present around the network of filamentous fungi. To view the EPS distribution on the outer edge of a granule, a stack of 10-μm optical sections were taken into a depth of 130 μm within the granule. A projection of this stack of images is shown in Fig. 5 without the FITC channel, since it overlapped with both Syto 63 and ConA staining at low magnification. The projection clearly shows that cells are embedded in a large network of polysaccharide material on the outer edge of the granule. This observation correlates with the cryosectioning data presented in Fig. 3 and 4. Under a higher magnification, specific cell clusters were observed in association with polysaccharides and proteins.
FIG. 5.
(A) Floc stained with Syto 63 (red), ConA (blue), and FITC (green) and viewed with a ×10 objective lens. Scale, 100 μm. The majority of EPS is localized at a flocculent center, whereas some polysaccharides are present around filamentous fungi. (B) Three-dimensional projection of a 130-μm stack of optical sections from the outer edge of a granule. Cells (red) and polysaccharides (blue) are evident. Scale, 100 μm. Cells are embedded in a large polysaccharide matrix.
DISCUSSION
Reactor operation and sludge properties.
The effect of settling time on floc and granule formation followed previously reported research (1). Reactor 1, with a minimal settling velocity (νmin) of 2.4 m h−1, formed predominantly flocculent sludge, and reactor 2 (νmin of 12 m h−1) formed predominantly granular sludge. However, granules were observed in both reactors, indicating that slow settling times do not prevent granule formation but rather prevent granular sludge from becoming dominant in the reactor. The short settling times cause continuous biomass washout, thus affecting species selection over time (14).
EPS extraction and characterization.
The results for EPS content differ strikingly with those reported by Tay et al. (21). For an SBR operated with a superficial gas velocity of 1.2 cm s−1, the same as used in this experiment, Tay et al. reported a polysaccharide/protein ratio (PS/PN ratio) of 15. In that experiment, the polysaccharide content was hypothesized to contribute more to the granule structure and stability than the protein content (21). However, in the current experiment, the protein content was much higher than the polysaccharide content. Using a similar extraction method of NaOH and heat for nonhomogenized granules, the protein/polysaccharide ratio (PN/PS ratio) was calculated to be ca. 11 for reactor 2 (see Table 2). This trend was confirmed by methods of homogenization and Dowex cation-exchange extraction for both reactor 1 and 2 samples, and the data were correlated with a short EPS literature review shown in Table 3. For a variety of biofilm types and extraction methods, proteins have been reported more abundant than polysaccharides in EPS.
TABLE 3.
EPS composition of different biofilm samples as determined by different methods
| EPS extraction method | Sludge type | Protein (mg/g of VS) | Carbohydrate (mg/g of SS) | Reference |
|---|---|---|---|---|
| Heating (70°C) | UASBa | 80 | 13 | Schmidt and Ahring (19a) |
| Heating (80°C) | Activated sludge | 121 | 8 | Frølund et al. (7) |
| NaOH (pH 11) | Activated sludge | 96 | 22 | Frølund et al. (7) |
| Dowex and mixing | Activated sludge | 243 | 48 | Frølund et al. (7) |
| Dowex and mixing | Sewer biofilm | 154 | 12 | Jahn and Nielsen (10a) |
| Dowex and mixing | Anaerobic granules | 140 | 41 | Batstone and Keller (1) |
UASB, upflow anaerobic sludge blanket.
The comparison of EPS extraction methods showed two trends with respect to homogenization and alkaline treatment. Homogenization had little effect on the amount of EPS extracted from flocculent sludge, but it greatly aided the EPS extraction from granular sludge samples. Because granules can be very large (>1 mm), they have a surface-to-volume limitation for both NaOH and cation-exchange resin. The resin exchanges monovalent cations (in this case Na+) with divalent cations (mainly Ca2+ and Mg2+) that are responsible for the cross-linking of charged compounds in the EPS matrix. By homogenizing the sample beforehand, the cation-exchange resin interacts with more total EPS, removing more total divalent cations, and increasing the repulsion of EPS components and their water solubility. Alkaline treatment causes charged groups, such as carboxylic groups in proteins and polysaccharides, to be ionized since their isoelectric points are typically below pH 4 to 6. This also causes a repulsion between EPS components and increases their water solubility (18). Therefore, homogenization should be used when samples with vastly different shapes and sizes are compared. Homogenization eliminates a method bias for more efficient extraction from samples with a greater surface area/volume ratio, as was shown in Fig. 2 with the effect of homogenization for flocs versus granules. Only the homogenized Dowex extraction showed granules with more total EPS than flocs, although this observation was clearly made with the microscopic staining. When samples with a low surface area/volume ratio such as flocs are compared, homogenization is not essential.
EPS extraction with alkaline and heat treatment produced much more TOC, proteins, and polysaccharides than Dowex extraction. This increase was most likely due to severe cell lysis caused by the high pH and heat treatment. The extent of cell lysis during extraction is difficult to measure in undefined samples, and the glucose-6-phosphate dehydrogenase kit was not applicable for alkaline samples, since high pH and heat are known to disrupt macromolecules such as enzymes and proteins. Previous studies reported that boiling and addition of NaOH cause severe cell lysis in activated sludge, whereas a few hours of mixing with Dowex does not cause strong lysis (18). The NaOH extraction also produced more total TOC from flocculent EPS (R1) than granular EPS (R2) as shown in Fig. 2, but the Dowex extraction or microscopic staining of polysaccharides and proteins did not confirm this observation. Therefore, extraction with NaOH and heat should be avoided.
Fluorescence staining of EPS and microscopy.
Flocs were comprised of a small center of EPS and cells surrounded by a network of filamentous bacteria and fungi. In contrast, the center of granules was labeled mostly with the protein stain, and cells and polysaccharides were isolated to the outer layer of granules, as shown with the cryosectioning data. This result correlates with another study by DeBeer et al., in which anaerobic flocs and granules were stained for EPS polysaccharide distribution. In loose flocs, the highest concentration of EPS was found in the center, whereas 50% of the EPS in granules was concentrated in a 40-μm layer at the surface (5). This reflects the polysaccharide distribution stained by ConA in aerobic granules, with the outer layer being ca. 100 μm thick.
The center of the granules was mostly stained by FITC, which stains cells or free amino groups. A subsequent counterstaining with Syto 63 resulted in few signals from the granule center, suggesting that the majority of the granule volume was comprised of noncellular material. The origin of this material can be inferred by the microscopic staining of flocs and smaller granules, in which the flocculent center is comprised of cells and EPS together. The bacteria in these aggregates continue to grow, creating an ever-larger granule. As the particle size increases, so does the mass transfer limitation of oxygen within the outer layer of active biomass (8). Mass transport limitations eventually create various layers of aerobic, anaerobic, and dead biomass within granules. The aerobic layer of biomass has been reported to be 800 μm in diameter, which is much longer than observed in the present study (20). Therefore, the exact structure of aerobic granules is probably dependent on reactor operation, species selection, and biofilm growth morphology. The general observations suggest that granule centers are comprised of old aggregates of dead or dormant biomass and EPS, thus explaining the uneven distribution of FITC throughout the granule structure.
The cells on the outer edge of granules are embedded in a large network of polysaccharides stained by ConA (Fig. 5), which were all counterstained by FITC and Syto 63. The microscopic results suggest that the outer, active layer of cells excrete EPS with a large proportion of polysaccharides. The center of the granule was mostly stained by FITC but not by Syto 63. Therefore, the center could be composed of dead cells, which have leaked proteins and other amine-containing compounds into the granule center. The chemical extraction of EPS does not distinguish between proteins excreted by dead cells or live cells. Therefore, chemical extraction from granules is bound to contain some proteins and polysaccharides released from dead cells at the center, which may constitute a large percentage of the total structure for a large granule. Given this consideration, the comparison of chemical EPS extraction data from different biofilm structures (flocs and granules) is difficult. Microscopic staining can be used in conjunction to understand the distribution of EPS in situ, providing insight that the polysaccharides are significant components of EPS in the outer edges of granules, although they are but a small fraction of the total TOC extracted. Unfortunately, quantification of staining is difficult due to the specificity of lectins, which stain specific configurations of sugar residues, and the nonspecificity of FITC, which stain all amino groups.
Overall.
The method used for chemical extraction of EPS affects the total TOC, proteins, and polysaccharides extracted. Homogenization before extraction releases more total EPS from granule samples and has only a small effect on flocculent samples. Hot alkaline treatment with heat causes cell lysis that contaminates EPS with much higher levels of both proteins and polysaccharides than EPS extracted with cation-exchange resin. When comparing samples with different surface area/volume ratios are being compared, homogenization should be performed before chemical extraction in order to prevent method bias. Microscopic results showed that granular sludge has an outer layer (ca. 200 μm thick) of biomass and EPS containing large amounts of polysaccharides. The center of the granule contained proteins as the major component and intact cells and polysaccharides to a lesser extent.
Acknowledgments
This study was supported by the German Research Foundation and a U.S. Department of Education GAANN Fellowship.
We thank the MedTech Institute, Technical University of Munich, for the use of the Leica Kryostat.
REFERENCES
- 1. Batstone, D. J., and J. Keller. 2001. Variation of bulk properties of anaerobic granules with wastewater type. Water Res. 35:1723-1729. [DOI] [PubMed] [Google Scholar]
- 1a.Beun, J. J., A. Hendriks, M. C. M. van Loosdrecht, E. Morgenroth, P. A. Wilderer, and J. J. Heijnen. 1999. Aerobic granulation in a sequencing batch reactor. Water Res. 33:2283-2290. [DOI] [PubMed] [Google Scholar]
- 2.Beun, J. J., M. C. M. van Loosdrecht, and J. J. Heijnen. 2002. Aerobic granulation in a sequencing batch airlift reactor. Water Res. 36:702-712. [DOI] [PubMed] [Google Scholar]
- 3.Boessmann, M., C. Staudt, T. R. Neu, H. Horn, and D. C. Hempel. 2003. Investigation and modeling of growth, structure, and oxygen penetration in particle supported biofilms. Chem. Eng. Technol. 26:219-222. [PubMed] [Google Scholar]
- 4.Clesceri, L. S., A. E. Greenberg, and A. D. Eaton (ed.). 1998. Standard methods for the examination of water and wastewater. American Public Health Association/American Water Works Association/Water Environment Federation, Washington, D.C.
- 5.DeBeer, D., V. O'Flaharty, J. Thaveesri, P. Lens, and W. Verstraete. 1996. Distribution of extracellular polysaccharides and flotation of anaerobic sludge. Appl. Microbiol. Biotechnol. 46:197-201. [Google Scholar]
- 6.de Kreuk, M. K., and M. C. M. van Loosdrecht. 2004. Selection of slow-growing organisms as a means for improving aerobic granular sludge stability. Water Sci. Technol. 49:9-17. [PubMed] [Google Scholar]
- 7.Frolund, B., R. Palmgren, K. Keiding, and P. H. Nielsen. 1996. Extraction of extracellular polymers from activated sludge using a cation exchange resin. Water Res. 30:1749-1758. [Google Scholar]
- 8.Gapes, D., B.-M. Wilén, and J. Keller. 2004.. Mass transport impacts in flocculent and granular biomass from SBR systems. Water Sci. Technol. 50:203-212. [PubMed]
- 9.Geesey, G. G. 1982. Microbial exopolymers: ecological and economic considerations. ASM News 48:9-14. [Google Scholar]
- 10.Grotenhuis, J. T. C., M. Smit, A. A. M. Van Lammeren, A. J. M. Stams, and A. J. B. Zehnder. 1991. Localization and quantification of extracellular polymers in methanogenic granular sludge. Appl. Microbiol. Biotechnol. 36:115-119. [Google Scholar]
- 10a.Jahn, A., and P. H. Nielsen. 1995. Extraction of extracellular polymeric substances (EPS) from biofilms using a cation exchange resin. Water Sci. Technol. 32:157-164. [Google Scholar]
- 11.Liu, Y., and J.-H. Tay. 2002. The essential role of hydrodynamic shear force in the formation of biofilm and granular sludge. Water Res. 36:1653-1665. [DOI] [PubMed] [Google Scholar]
- 12.Manning, J. F. 1986. The biological removal of phosphorous in a sequencing batch reactor. Ph.D. thesis. University of Notre Dame, Notre Dame, Ind.
- 13.McSwain, B. S., R. L. Irvine, and P. A. Wilderer. 2004. The effect of intermittent feeding on aerobic granule structure. Water Sci. Technol. 49:19-25. [PubMed] [Google Scholar]
- 14.McSwain, B. S., R. L. Irvine, and P. A. Wilderer. 2004. The influence of settling time on the formation of aerobic granules. Water Sci. Technol. 50:195-202. [PubMed]
- 15.Morgenroth, E., T. Sherden, M. C. M. van Loosdrecht, J. J. Heijnen, and P. A. Wilderer. 1997. Aerobic granular sludge in a sequencing batch reactor. Water Res. 31:3191-3194. [Google Scholar]
- 16.Moy, B. Y. P., J.-H. Tay, S.-K. Toh, Y. Liu, and S. T. L. Tay. 2002. High organic loading influences the physical characteristics of aerobic sludge granules. Lett. Appl. Microbiol. 34:407-412. [DOI] [PubMed] [Google Scholar]
- 17.Neu, T. R., G. D. W. Swerhone, and J. R. Lawrence. 2001. Assessment of lectin-binding analysis for in situ detection of glycoconjugates in biofilm systems. Microbiology 147:299-313. [DOI] [PubMed] [Google Scholar]
- 18.Nielsen, P. H., and A. Jahn. 1999. Extraction of EPS, p. 50-72. In J. Wingender, T. R. Neu, and H.-C. Flemming (ed.), Microbial extracellular polymeric substances: characterization, structure, and function. Springer-Verlag, Berlin, Germany.
- 19.Schmid, M., A. Thill, U. Purkhold, M. Walcher, J. Y. Bottero, P. Ginestet, P. H. Nielsen, S. Wuertz, and M. Wagner. 2003. Characterization of activated sludge flocs by confocal laser scanning microscopy and image analysis. Water Res. 37:2043-2052. [DOI] [PubMed] [Google Scholar]
- 19a.Schmidt, J. E., and B. K. Ahrins. 1994. Extracellular polymers in granular sludge from different upflow anaerobic sludge blanket (UASB) reactors. Appl. Microbiol. Biotechnol. 42:457-462. [Google Scholar]
- 20.Tay, J.-H., V. Ivanov, S. Pan, and S. T. L. Tay. 2002. Specific layers in aerobically grown microbial granules. Lett. Appl. Microbiol. 34:254-257. [DOI] [PubMed] [Google Scholar]
- 21.Tay, J.-H., Q.-S. Liu, and Y. Liu. 2001. The role of cellular polysaccharides in the formation and stability of aerobic granules. Lett. Appl. Microbiol. 33:222-226. [DOI] [PubMed] [Google Scholar]
- 22.Tsuneda, S., T. Nagano, T. Hoshino, Y. Ejiri, N. Noda, and A. Hirata. 2003. Characterization of nitrifying granules produced in an aerobic upflow fluidized bed reactor. Water Res. 37:4965-4973. [DOI] [PubMed] [Google Scholar]
- 23.Voet, D., and J. G. Voet. 1995. Rates of enzymatic reactions, p. 360-362. In D. Voet and J. G. Voet (ed.), Biochemistry. John Wiley & Sons, Inc., New York, N.Y.
- 24.Wingender, J., T. R. Neu, and H.-C. Flemming. 1999. What are bacterial extracellular polymeric substances?, p. 1-20. In J. Wingender, T. R. Neu, and H.-C. Flemming (ed.), Microbial extracellular polymeric substances: characterization, structure, and function. Springer, Berlin, Germany.