Abstract
Background
Breathlessness is the most common symptom reported by patients with pulmonary arterial hypertension (PAH). The Modified Borg Dyspnea Scale (MBS) is routinely obtained during the six-minute walk test in the assessment of PAH patients, but it is not known whether the MBS predicts clinical outcomes such as hospitalizations in PAH.
Methods
We performed a retrospective study of World Health Organization (WHO) Group 1 PAH patients followed at our center. The dates of the first three MBS and hospitalizations that occurred within three months of a documented MBS were collected. Marginal Cox hazard regression modeling was used to assess for a relationship between MBS and all-cause as well as PAH-related hospitalization.
Results
A total of 50 patients were included; most (92%) were functional class III/IV, 44% and 65% were treatment-naïve prior to their first MBS and hospitalization, respectively. The first recorded MBS was inversely correlated with the first recorded six-minute walk distance (6MWD) (r = –0.41, P < 0.01) but did not track with WHO functional class (r = 0.07, P = 0.63). MBS did not predict all-cause (hazard ratio [HR], 0.91; 95% confidence interval [CI], 0.76–1.08; P = 0.28) or PAH-related hospitalization (HR, 1.04; 95% CI, 0.89–1.23; P = 0.61), though there was a strong relationship between 6MWD and PAH-related hospitalization (P = 0.01). These findings persisted after multivariable adjustment.
Conclusions
Breathlessness as assessed by MBS does not predict all-cause or PAH-related hospitalization. Robust and validated patient-reported outcomes are needed in pulmonary vascular disease.
Keywords: Borg Scale, pulmonary hypertension, patient-reported outcomes, breathlessness, six-minute walk distance
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease of the pulmonary vasculature associated with significant morbidity, functional limitation, and a limited life expectancy.1 The decision to initiate or escalate treatment in PAH is multifaceted but World Health Organization (WHO) functional class, which is predominantly determined by the severity of patient-reported dyspnea with activity,2 is a major factor in risk assessment.2,3 Breathlessness is the most commonly reported symptom in PAH and as it can be perceived, interpreted, and rated by the patient, it is by definition a patient-reported outcome (PRO).4,5 PROs provide important information about health status and disease management not adequately captured by traditional endpoints. While several tools to assess health-related quality of life (HRQoL) have shown promise in PAH, these have not been well-validated or lack pragmatism.6,7
The pathophysiologic mechanisms underlying dyspnea in PAH are not well understood, but in a given patient may represent diminished right-sided cardiac output, abnormal oxygen uptake, ventilatory inefficiency during exercise, or cardiovascular deconditioning.8–10 Signals from the central nervous system and receptors in the right atrium, pulmonary vasculature, lung parenchyma, and chest wall may also contribute to the sensation of shortness of breath. The degree of dyspnea during exercise may therefore reflect a comprehensive “read-out” of multisystem interactions in PAH.
The Modified Borg Dyspnea Scale (MBS) is a 0 to 10 rated numerical score used to measure dyspnea as reported by the patient during submaximal exercise and is routinely administered during six-minute walk testing (6MWT), one of the most common and frequently used measures to assess disease severity in PAH. The MBS is reproducible within a single testing period and tracks with objective indices of exercise intensity in healthy controls and thus has been extrapolated for use in chronic lung disease.11,12 The MBS may provide a minimally cumbersome method of predicting clinical deterioration in PAH, especially since the 6MWT is used widely in PAH care. The MBS was shown to be a univariate predictor of mortality in PAH patients in a single study,13 but additional evidence tying the MBS to clinical events in pulmonary vascular disease is lacking. We sought to examine the relationship between MBS and hospitalization in patients with WHO Group 1 PAH. Hospitalization for PAH is a potentially clinically meaningful outcome and was the primary driver of composite endpoints in recently completed randomized clinical trials of medical therapies for PAH.14–16 We hypothesized that higher MBS would be associated with an increased rate of all-cause as well as PAH-related hospitalization.
Methods
Study sample
We performed a retrospective study of patients with WHO Group 1 PAH identified from the Rhode Island Hospital Pulmonary Hypertension Center Registry during 1999–2014. We included participants with a diagnosis of PAH confirmed by right heart catheterization and meeting traditional diagnostic criteria2 who were followed for at least six months and had at least one MBS recorded within the first six months after diagnosis. We excluded participants aged < 18 years. This study was approved by the Institutional Review Board of Rhode Island Hospital (IRB Registration #023914). Written consent was deemed not necessary for this retrospective review.
Modified Borg Dyspnea Scale and clinical variables
All 6MWT performed at our institution prior to 2002 were performed according to published studies.17,18 Thereafter, 6MWT were conducted as per the 2002 American Thoracic Society standards.19 The MBS recorded at walk test conclusion was collected along with dates of administration. The first MBS performed at our institution was defined as the first test. Up to four serial scores were recorded; the fourth walk date was used for right censoring purposes. Additional clinical data, including demographics, PAH sub-type as designated by the PAH clinician, functional class, serial six-minute walk distance (6MWD), and PAH therapies, were extracted from the registry database.
Hospitalization
Dates and details of hospital admissions were collected from the medical record. A hospitalization event was classified as PAH-related if the admission was due to disease progression (e.g., worsening exercise tolerance, dyspnea, syncope), right heart failure, or initiation or transition of prostacyclin analogue therapies. Hospitalizations related to complications of parenteral prostacyclin analogue administration (e.g., subcutaneous injection site pain or soft-tissue infection, Hickman catheter fracture or infection) but not progression of disease requiring addition of or change in prostacyclin analogue were categorized as all-cause hospitalizations. An additional analysis was performed in which hospitalizations related to complications of parenteral prostacyclin analogue therapy were excluded entirely from all-cause hospitalizations. Dates of MBS were paired with the corresponding sequential hospitalization date if this occurred within a three-month period (the recommended interval for repeating 6MWT in PAH).3 A sensitivity analysis was performed which included MBS collected within one month of hospitalization.
Statistical analysis
Descriptive statistics were reported as medians and quartiles and categorical data were expressed as counts and percentages. Differences between those with and without one or more PAH-related hospitalization were assessed using Wilcoxon or Fisher’s exact tests (when applicable). Pearson’s correlation was used to evaluate the relationship between MBS and markers of disease severity (functional class and 6MWD). Differences in the first recorded MBS among PAH sub-groups were assessed using a generalized mixed model assuming a binomial distribution with the GLIMMIX procedure. Marginal repeated hazard regression analysis was used to model the relationship between MBS and all-cause and PAH-related hospitalizations adjusted for age and body mass index using the PHREG procedure. Significance was established at the 0.05 level and all interval estimates were calculated for 95% confidence.
Results
A total of 54 patients with WHO Group 1 PAH were eligible, of whom two (4%) were excluded for missing MBS and two (4%) were excluded because follow-up was less than six months. The final study sample included 50 patients, 21 (42%) of whom had one or more PAH-related hospitalizations (Table 1). The median age was 62 years (age range, 49–71 years), 84% were women and 38% had idiopathic PAH. Most (92%) were functional class III/IV, 44% were treatment-naïve, and 14% were on combination therapy at the time of their first MBS; 65% were treatment-naïve and 8% were on combination therapy at the time of their first hospitalization. The median MBS was 4 (interquartile range [IQR], 3–7) among those who had a PAH-related hospitalization and 3.5 (IQR, 3–5) among those who did not. Six (12%) participants died during the study period.
Table 1.
Characteristics of the study sample.
| Total cohort | PAH-related hospitalization | No PAH-related hospitalization | P value | |
|---|---|---|---|---|
| n | 50 | 21 | 29 | |
| Age, years | 62 (49–71) | 62 (47–66) | 62 (52–72) | 0.99 |
| Sex, female | 42 (84) | 18 (86) | 24 (83) | 0.99 |
| Race/ethnicity | 0.63 | |||
| White | 43 (86) | 18 (86) | 25 (86) | |
| Black | 5 (10) | 3 (14) | 2 (7) | |
| Hispanic | 6 (12) | 3 (14) | 3 (10) | |
| Body mass index, kg/m2 | 30 (26–37) | 32 (27–38) | 28 (25–35) | 0.21 |
| PAH diagnosis | ||||
| Idiopathic | 19 (38) | 7 (33) | 12 (41) | |
| Connective tissue disease | 20 (40) | 8 (38) | 12 (41) | |
| Congenital heart disease | 3 (6) | 2 (10) | 1 (3) | |
| HIV | 6 (12) | 3 (14) | 3 (10) | |
| Portopulmonary | 1 (2) | 0 | 1 (3) | |
| Sickle cell* | 1(2) | 1 (5) | 0 | |
| Smoking status | 0.55 | |||
| Current | 4 (8) | 1 (5) | 3 (10) | |
| Former | 29 (58) | 11 (52) | 18 (62) | |
| Never | 17 (34) | 9 (43) | 8 (28) | |
| Functional class | ||||
| I | 1 (2) | 0 | 1 (3) | |
| II | 3 (6) | 0 | 3 (10) | |
| III | 42 (84) | 18 (86) | 24 (83) | |
| IV | 4 (8) | 3 (14) | 1 (3) | |
| Six-minute walk distance, m | 321 (168–366) | 248 (134–338) | 341 (213–387) | 0.07 |
| Modified Borg Scale | 4 (3–7) | 4 (3–7) | 3.5 (3–5) | 0.37 |
Reported as n (%) or median (interquartile range).
Hemodynamically defined as PAH. P values not calculated when cells contained zero.
HIV, human immunodeficiency virus; PAH, pulmonary arterial hypertension.
The first recorded MBS was inversely correlated with the first recorded 6MWD (r = –0.41, P < 0.01) but did not track with WHO functional class (r = 0.07, P = 0.63). There was a negative correlation between functional class and the first recorded 6MWD (r = –0.43, P < 0.01). No differences were found between PAH subgroups and MBS (P = 0.15). As such, participants with all PAH diagnoses were analyzed as one cohort.
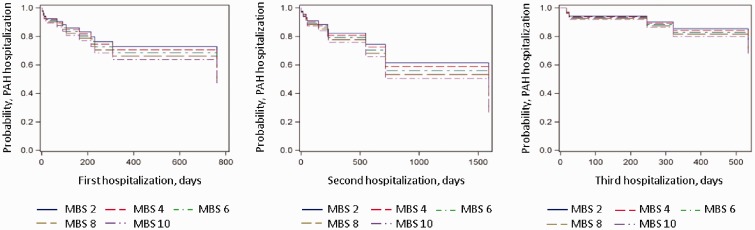
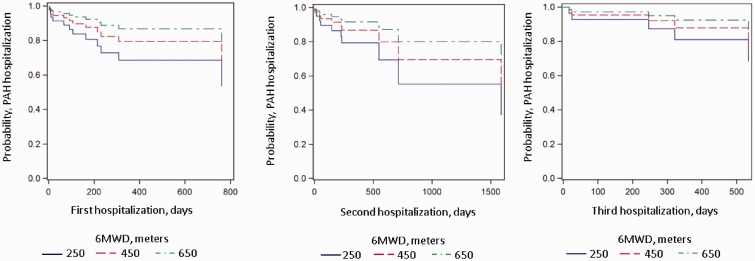
There was no relationship between MBS and all-cause (P = 0.28) or PAH-related hospitalizations (P = 0.61) (Table 2 and Fig. 1). Results were unchanged after adjustment for age and body mass index (both main effect and interaction effect). Maximum recorded MBS also had no relationship with all-cause or PAH-related hospitalizations (data not shown). A subgroup analysis including only those who had a MBS performed within one month before hospitalization (n = 33) yielded identical results (HR, 1.01; 95% CI, 0.82–1.23; P = 0.95). Results were also unchanged when hospitalizations related to parenteral PAH therapy (n = 4) were excluded from the model for MBS and all-cause hospitalizations (HR, 0.92; 95% CI, 0.77–1.11; P = 0.39). There was a significant relationship between 6MWD and PAH-related hospitalization such that for every 1-m increase in 6MWD, the rate of PAH-related hospitalization decreased 10% (HR, 0.10; 95% CI, 0.99–1.00; P = 0.01) (Fig. 2). There was no evidence to place the proportionality assumption into question (P = 0.59) for this result. No significant relationship was found between all-cause hospitalizations and 6MWD (P = 0.36).
Table 2.
Relationship between Modified Borg Scale and hospitalizations.
| Model | Hazard ratio | 95% CI | P value | |
|---|---|---|---|---|
| All-cause hospitalization | Basic | 0.91 | 0.76–1.08 | 0.28 |
| Adjusted* | 0.91 | 0.76–1.10 | 0.33 | |
| PAH-related hospitalization† | Basic | 1.04 | 0.89–1.23 | 0.61 |
| Adjusted* | 1.12 | 0.92–1.35 | 0.25 |
Age and body mass index.
Progression of cardiopulmonary symptoms, right heart failure, and/or change in pulmonary arterial hypertension therapy.
CI, confidence interval.
Fig. 1.
Marginal repeat hazards modeling for PAH-related hospitalization by MBS. X axis is the time to PAH-related hospitalization in days; Y axis is the likelihood of event. The legend corresponds to range of MBS representing each line.
Fig. 2.
Marginal repeat hazards modeling for PAH-related hospitalization by 6MWD. X axis is the time to PAH-related hospitalization in days; Y axis is the likelihood of event. The legend corresponds to range of 6MWD representing each line.
Discussion
We have shown that routine MBS collection at the end of the 6MWT does not predict all-cause or PAH-related hospitalization in PAH patients from a single center. This is despite a modest inverse correlation between MBS and 6MWD and a strong relationship between longer 6MWD and decreased rates of PAH-related hospitalization. The lack of association between the MBS (mean and maximum values) and a morbidity measure suggests it is an inadequate PRO in pulmonary vascular disease.
A straightforward and validated tool that would allow for patient-characterized shortness of breath is appealing in PAH, since such a measure could represent the sum total of complex cardiopulmonary, nervous system, and musculoskeletal interactions. The MBS is reproducible and tracks with objective indices of exercise intensity such as heart rate, minute ventilation, oxygen consumption, and workload in patients with chronic obstructive pulmonary disease (COPD) and advanced lung disease.12,20 Developed to capture both the rate of perceived exertion and the intensity of the sensation of breathlessness during exercise as a one-dimensional scale,18 the MBS would be a pragmatic tool in PAH since it is easy to measure, inexpensive, and can be repeated on successive clinical encounters to assess response to treatment, especially since the 6MWD is so widely incorporated into routine PAH care.2
Patient-reported dyspnea independently predicts mortality in both pulmonary and non-pulmonary diseases.21,22 In COPD patients, subjective dyspnea is superior to pulmonary function tests in predicting clinical outcomes including survival.23 Dyspnea has also been linked to hospitalizations in COPD, and importantly, quantified dyspnea has a greater impact on HRQoL than objective spirometric measurements.24,25 The MBS has been linked to survival in pulmonary fibrosis and when administered to asthmatic patients after bronchodilator treatment predicts hospitalization or relapse more so than forced expiratory volume in 1 second.23,26,27 While functional class designation has been repeatedly linked to outcomes in PAH and was inversely correlated with 6MWD in our study, only a single study in PAH has demonstrated a relationship between Borg score, which also captures breathlessness, and survival, and this relationship dissipated after multivariable adjustment.13 Khair et al. recently reported the minimal clinically important difference (MID) for MBS (approximately 1 unit) anchored against 6MWD in a PAH cohort.28 We also demonstrated a significant inverse correlation between MBS and 6MWD in our study, but the relationship between MBS and clinical events in PAH has not been established.
Over ten HRQoL tools have been studied in PAH.29 The Cambridge Pulmonary Hypertension Outcome Review (CAMPHOR), which includes breathlessness as a scale, is the most established but multi-dimensional and proprietary in nature.6,30–32 The Short Form-36 (validated in other chronic conditions) correlates with 6MWD and functional class and tracks with PAH therapy; however, the MID with PAH-therapy is large, particularly in the domain of physical activity limitation, suggesting it may be too blunt an instrument for the disease state.32,33 Other metrics (e.g., the Minnesota Living with Heart Failure Questionnaire and emPHasis-10) have either been adapted or designed to be PAH-specific and appear promising but require further validation and are not currently routinely in use, as is the MBS.7,13,34
In our cohort, the median MBS was 3 (IQR, 2.5–5.0) and the maximum was 4.8 (IQR, 3.6–7.0) despite the majority of patients being of advanced functional class before their first hospitalization. This suggests that the MBS in the context of 6MWT may lack the sensitivity needed to adequately discriminate patients’ sense of dyspnea across their disease trajectories, complex treatment regimens (which may impact HRQoL), or may represent a regression to the mean phenomena as patients are subjected to repeated tests (although there was also no relationship between maximum MBS and hospitalizations). Given that the reported MID is small, it is possible that slow incremental changes over time are not perceptible on any given test, leading to a lack of granularity.28 The change in MBS before and after 6MWT may have added value, but this is not routinely collected at our institution. Interestingly, 6MWD did have an inverse relationship with PAH-related hospitalizations, lending face validity to the assessment of the clinical encounter and its relationship to events, since both 6MWT and MBS are performed concurrently.
Hospitalization as an outcome is an important determinant of survival, cost, HRQoL for the patient and burden on the caretaker in PAH.35,36 Though hospitalization has not been a primary outcome for PAH clinical trials, it has been recognized as a key component of a composite endpoint in several studies.37–39 Despite provider- and geography-specific thresholds for hospitalization, hospital admission is one of the few accepted objective measures of clinical worsening in pulmonary vascular disease.40,41 We found no relationship between hospitalizations (both all-cause and PAH-related) and MBS suggesting MBS may not be a relevant measure of breathlessness or a robust predictor of clinical events in patients with PAH. These results are somewhat surprising considering that increased dyspnea is a common complaint in patients admitted for worsening PAH and given that functional class, a somewhat subjective dyspnea metric, is a powerful predictor of outcome in PAH.42–44 Our study suggests that the degree of dyspnea at the end of 6MWT is not a predictor of later decompensation.
The study was limited by its retrospective nature, small sample size, and single-center design. While we controlled for variation in time between MBS assessment and hospitalization with mixed modeling, residual confounding could exist due to the introduction of PAH therapy or cardiopulmonary conditioning programs, for example. While there was no relationship between maximum MBS and hospitalization, with repeat testing there may be a learning phenomenon and a dilution of an underlying relationship between mean scores and outcomes; low mean MBS in our cohort may also represent suboptimal effort or modification of exertion by patients so as not to elicit breathlessness during 6MWT. We do not have detailed data about the exact timing of the initiation of PAH therapy and a given MBS and the interval between therapeutic changes or additions and MBS was not strictly protocolized. Still, our study sample included only patients with MBS performed three months prior to a hospitalization, the recommended interval for serial reassessments in PAH3 (and one month in a smaller group, with identical results), in order to increase sensitivity and the likelihood that a change in MBS would signal a clinical decline with subsequent hospitalization. While the majority of patients were treatment-naïve at the time that MBS was recorded prior to their first hospitalization, MBS was also a poor predictor of a second or third hospitalization when nearly all patients were on therapy at the time MBS was measured. Although MBS were similar in PAH subtypes in our cohort, subgroup sample sizes were small and this may not be generalizable to the PAH population-at-large (e.g., for all connective tissue disease versus idiopathic patients).
In this retrospective single-center study, we found no association between patient-reported dyspnea as measured by the MBS at the end of 6MWT and all-cause or PAH-related hospitalization. Despite the lack of an association between MBS and hospitalization, the 6MWD recorded at the same time as the MBS tracked inversely with hospitalization rates. These findings suggest that dyspnea as measured by MBS is an insensitive predictor of disease severity in PAH and that other PROs are needed to assess the risk of disease progression in PAH.
Acknowledgements
The authors thank the staff and participants of the Rhode Island Hospital Pulmonary Hypertension Center. This work has previously been presented in abstract form (A7384) at the 2016 American Thoracic Society International Conference, San Francisco, California, May 18, 2016.
Conflict of interest
CEV has received past consulting fees from Bayer, United Therapeutics, and Actelion, as well as grants to her institution from Actelion. JRK serves on the steering committees for Bayer and serves as a site investigator for clinical studies sponsored by Actelion, Bayer, Pulmonary Hypertension Association, NIH, and United Therapeutics. DB has received a grant funded in part by Actelion.
Funding
This work was supported by the National Institutes of Health P20GM103652.
References
- 1.Peacock AJ, Murphy NF, McMurray JJ, et al. An epidemiological study of pulmonary arterial hypertension. Eur Respir J 2007; 30: 104–109. [DOI] [PubMed] [Google Scholar]
- 2.Galie N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D60–72. [DOI] [PubMed] [Google Scholar]
- 3.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension. The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiography (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46: 903–975. [DOI] [PubMed] [Google Scholar]
- 4.Benza RL, Miller DP, Barst RJ, et al. An evaluation of long-term survival from time of diagnosis in pulmonary arterial hypertension from the REVEAL Registry. Chest 2012; 142: 448–456. [DOI] [PubMed] [Google Scholar]
- 5.Parshall MB, Schwartzstein RM, Adams L, et al. An official American Thoracic Society statement: update on the mechanisms, assessment, and management of dyspnea. Am J Respir Crit Care Med 2012; 185: 435–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.McCabe C, Bennett M, Doughty N, et al. Patient-reported outcomes assessed by the CAMPHOR questionnaire predict clinical deterioration in idiopathic pulmonary arterial hypertension and chronic thromboembolic pulmonary hypertension. Chest 2013; 144: 522–530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yorke J, Corris P, Gaine S, et al. emPHasis-10: development of a health-related quality of life measure in pulmonary hypertension. Eur Respir J 2014; 43: 1106–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyamoto S, Nagaya N, Satoh T, et al. Clinical correlates and prognostic significance of six-minute walk test in patients with primary pulmonary hypertension. Comparison with cardiopulmonary exercise testing. Am J Respir Crit Care Med 2000; 161: 487–492. [DOI] [PubMed] [Google Scholar]
- 9.Tolle JJ, Waxman AB, Van Horn TL, et al. Exercise-induced pulmonary arterial hypertension. Circulation 2008; 118: 2183–2189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.de Jesus Perez VA. Pumping it up! Angiogenesis and muscle deconditioning in pulmonary hypertension. Am J Respir Crit Care Med 2014; 190: 250–251. [DOI] [PubMed] [Google Scholar]
- 11.Borg E, Borg G, Larsson K, et al. An index for breathlessness and leg fatigue. Scand J Med Sci Sports 2010; 20: 644–650. [DOI] [PubMed] [Google Scholar]
- 12.Bausewein C, Farquhar M, Booth S, et al. Measurement of breathlessness in advanced disease: a systematic review. Respir Med 2007; 101: 399–410. [DOI] [PubMed] [Google Scholar]
- 13.Cenedese E, Speich R, Dorschner L, et al. Measurement of quality of life in pulmonary hypertension and its significance. Eur Respir J 2006; 28: 808–815. [DOI] [PubMed] [Google Scholar]
- 14.Channick RN, Delcroix M, Ghofrani HA, et al. Effect of macitentan on hospitalizations: results from the SERAPHIN trial. JACC Heart Fail 2015; 3: 1–8. [DOI] [PubMed] [Google Scholar]
- 15.Galie N, Barbera JA, Frost AE, et al. Initial Use of ambrisentan plus tadalafil in pulmonary arterial hypertension. N Engl J Med 2015; 373: 834–844. [DOI] [PubMed] [Google Scholar]
- 16.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373: 2522–2533. [DOI] [PubMed] [Google Scholar]
- 17.Holden DA, Rice TW, Stelmach K, et al. Exercise testing, 6-min walk, and stair climb in the evaluation of patients at high risk for pulmonary resection. Chest 1992; 102: 1774–1779. [DOI] [PubMed] [Google Scholar]
- 18.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc 1982; 14: 377–381. [PubMed] [Google Scholar]
- 19.ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166: 111–117. [DOI] [PubMed] [Google Scholar]
- 20.Mador MJ, Rodis A, Magalang UJ. Reproducibility of Borg scale measurements of dyspnea during exercise in patients with COPD. Chest 1995; 107: 1590–1597. [DOI] [PubMed] [Google Scholar]
- 21.Pesola GR, Ahsan H. Dyspnea as an independent predictor of mortality. Clin Respir J 2016; 10: 142–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maltoni M, Pirovano M, Scarpi E, et al. Prediction of survival of patients terminally ill with cancer. Results of an Italian prospective multicentric study. Cancer 1995; 75: 2613–2622. [DOI] [PubMed] [Google Scholar]
- 23.Nishimura K, Izumi T, Tsukino M, et al. Dyspnea is a better predictor of 5-year survival than airway obstruction in patients with COPD. Chest 2002; 121: 1434–1440. [DOI] [PubMed] [Google Scholar]
- 24.Yu TC, Zhou H, Suh K, et al. Assessing the importance of predictors in unplanned hospital readmissions for chronic obstructive pulmonary disease. Clinicoecon Outcomes Res 2015; 7: 37–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahler DA, Faryniarz K, Tomlinson D, et al. Impact of dyspnea and physiologic function on general health status in patients with chronic obstructive pulmonary disease. Chest 1992; 102: 395–401. [DOI] [PubMed] [Google Scholar]
- 26.Schneider JE, Lewis LM, Ferguson I, et al. Repeated dyspnea score and percent FEV1 are modest predictors of hospitalization/relapse in patients with acute asthma exacerbation. Respir Med 2014; 108: 1284–1291. [DOI] [PubMed] [Google Scholar]
- 27.Nishiyama O, Taniguchi H, Kondoh Y, et al. A simple assessment of dyspnoea as a prognostic indicator in idiopathic pulmonary fibrosis. Eur Respir J 2010; 36: 1067–1072. [DOI] [PubMed] [Google Scholar]
- 28.Khair RM, Nwaneri C, Damico RL, et al. The minimal important difference in Borg Dyspnea Score in pulmonary arterial hypertension. Ann Am Thorac Soc 2016; 13: 842–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Taichman DB, Doyle RL. Health-related quality of life and patient-reported outcomes in pulmonary arterial hypertension. Proc Am Thorac Soc 2008; 5: 623–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Twiss J, McKenna S, Ganderton L, et al. Psychometric performance of the CAMPHOR and SF-36 in pulmonary hypertension. BMC Pulm Med 2013; 13: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCollister DH, Beutz M, McLaughlin V, et al. Depressive symptoms in pulmonary arterial hypertension: prevalence and association with functional status. Psychosomatics 2010; 51: 339–339.e8. [DOI] [PubMed] [Google Scholar]
- 32.Chua R, Keogh AM, Byth K, et al. Comparison and validation of three measures of quality of life in patients with pulmonary hypertension. Intern Med J 2006; 36: 705–710. [DOI] [PubMed] [Google Scholar]
- 33.Keogh AM, McNeil KD, Wlodarczyk J, et al. Quality of life in pulmonary arterial hypertension: improvement and maintenance with bosentan. J Heart Lung Transplant 2007; 26: 181–187. [DOI] [PubMed] [Google Scholar]
- 34.Bonner N, Abetz L, Meunier J, et al. Development and validation of the living with pulmonary hypertension questionnaire in pulmonary arterial hypertension patients. Health Qual Life Outcomes 2013; 11: 161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kirson NY, Birnbaum HG, Ivanova JI, et al. Excess costs associated with patients with pulmonary arterial hypertension in a US privately insured population. Appl Health Econ Health Policy 2011; 9: 293–303. [DOI] [PubMed] [Google Scholar]
- 36.Burger CD, Long PK, Shah MR, et al. Characterization of first-time hospitalizations in patients with newly diagnosed pulmonary arterial hypertension in the REVEAL registry. Chest 2014; 146: 1263–1273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Barst RJ, Oudiz RJ, Beardsworth A, et al. Tadalafil monotherapy and as add-on to background bosentan in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2011; 30: 632–643. [DOI] [PubMed] [Google Scholar]
- 38.McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 2010; 55: 1915–1922. [DOI] [PubMed] [Google Scholar]
- 39.Galie N, Olschewski H, Oudiz RJ, et al. Ambrisentan for the treatment of pulmonary arterial hypertension: results of the ambrisentan in pulmonary arterial hypertension, randomized, double-blind, placebo-controlled, multicenter, efficacy (ARIES) study 1 and 2. Circulation 2008; 117: 3010–3019. [DOI] [PubMed] [Google Scholar]
- 40.McLaughlin VV, Badesch DB, Delcroix M, et al. End points and clinical trial design in pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54(1 Suppl): S97–107. [DOI] [PubMed] [Google Scholar]
- 41.Galie N, Simonneau G, Barst RJ, et al. Clinical worsening in trials of pulmonary arterial hypertension: results and implications. Curr Opin Pulm Med 2010; 16(Suppl. 1): S11–19. [DOI] [PubMed] [Google Scholar]
- 42.Taichman DB, McGoon MD, Harhay MO, et al. Wide variation in clinicians’ assessment of New York Heart Association/World Health Organization functional class in patients with pulmonary arterial hypertension. Mayo Clin Proc 2009; 84: 586–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002; 40: 780–788. [DOI] [PubMed] [Google Scholar]
- 44.Barst RJ, Chung L, Zamanian RT, et al. Functional class improvement and 3-year survival outcomes in patients with pulmonary arterial hypertension in the REVEAL Registry. Chest 2013; 144: 160–168. [DOI] [PubMed] [Google Scholar]