Abstract
MicroRNAs (miRNAs) may regulate a number of genes, each of which may have a variety of functions. We utilized an endoarterial biopsy catheter to assess the dysregulation of miRNAs in a porcine shunt model of pulmonary hypertension (PH). Two Yucatan micropigs underwent surgical anastomosis of the left pulmonary artery to the descending aorta. Endoarterial biopsy samples were obtained at baseline, and at regular intervals during the progression of PH. RNA, isolated from biopsy samples, was analyzed by Illumina miRNA expression microarrays (containing ∼1200 human miRNAs), Affymetrix Porcine GeneChips, Bioconductor, and GeneSpring. We examined a total of 925 genes in a PH whole genome microarray. Biopsy samples showed that 39 miRNAs were downregulated and 34 miRNAs were upregulated compared to baseline. The number of PH-associated genes reported to be controlled by each of the dysregulated miRNAs was in the range of 1–113. The five miRNAs that had the largest number of PH-associated genes were: miR-548c-3p, miR-520d-3p, miR-130a-5p, miR-30a-3p, and miR-let-7g-3p. Several of the dysregulated miRNAs have been associated with molecular pathways and biologic processes involved in PH. Among 29 miRNAs, which were predicted to be dysregulated by a systems biology approach, we found four that were dysregulated in our porcine shunt model. An endoarterial biopsy technique was successful in showing that a large number of miRNAs are dysregulated in a porcine shunt model of PH. Many of these miRNAs control multiple PH-associated genes, molecular pathways, and biologic processes. Endoarterial biopsy offers potential experimental and clinical diagnostic value.
Keywords: pulmonary hypertension, endoarterial biopsy, microRNA
Pulmonary hypertension (PH) often leads to right heart failure and death. Despite therapy, survival is about 60% at five years.1,2 Pulmonary arterial hypertension’s (PAH) molecular and genetic associated vascular remodeling mechanisms are incompletely understood. In both animal models and patients with PH, endothelin-1 and thromboxane A2 increase, while nitric oxide and prostacyclin decrease.3 Further, in a previous animal model study of PH,4 we demonstrated a change in myriad mRNAs levels as PH developed.
MicroRNAs (miRNAs) are small, non-coding RNAs, 21–23 nucleotides in length, which participate in a variety of biological processes, including regulation of gene expression, differentiation, developmental timing, proliferation, metabolism, and cell death.5,6 Several investigators have demonstrated a role for miRNAs in PH: MiR-21 affected hypoxia-induced pulmonary arterial smooth muscle cell proliferation and migration.7 MiR-17/92 caused dysregulated expression of bone morphogenic receptor type II,8 which is strongly associated with the development of PH. MiR-204 diminished proliferation, vascular remodeling, and pulmonary arterial pressure (PAP) in PH.9 Let-7d-3p was reduced in pulmonary arterial smooth muscle cells of patients with thromboembolic PH,10 which could cause proliferation of pulmonary arterial smooth muscle cells. Several miRNAs were dysregulated in rat models of PH caused by hypoxia and monocrotaline.11
The purpose and unique aspects of this study were the use of a pulmonary endoarterial biopsy catheter to detect stage-dependent miRNA changes in pulmonary endovascular samples obtained in a large animal model of PH, correlate these changes to the expression of miRNA-targeted genes, molecular pathways and biologic processes that have been associated with PH, and compare our experimental miRNA results to those predicted using a systems biology approach12 and experimental data from the literature. We posited the hypothesis that several miRNAs would be upregulated and several miRNAs downregulated as PH developed, that the changes would be dependent on the stage of PH and that multiple PH molecular pathways and biologic processes would potentially be affected.
Methods
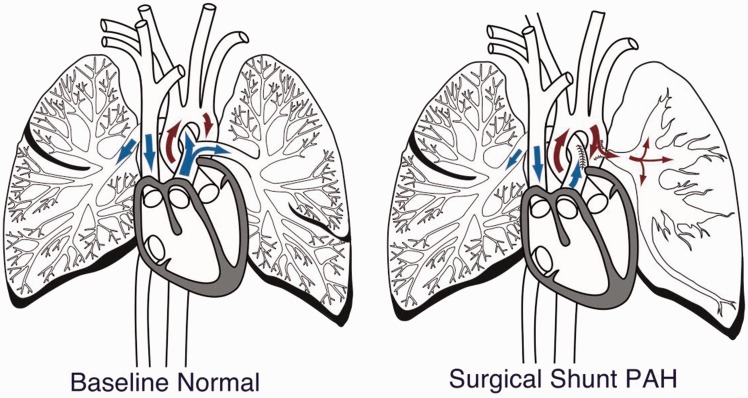
Two Yucatan micropigs underwent surgical anastomosis of the left pulmonary artery to the descending aorta (Fig. 1). Within a few weeks, the left PAP increased from normal to systemic level. Endoarterial biopsy samples were obtained percutaneously from 2–3 mm branch pulmonary arteries at baseline and at regular intervals (approximately days 7, 21, 60, and 180) during the progression of PH. Biopsy samples were procured with a 7.9 French endoarterial biopsy catheter (Figs. S1–S3) which allowed a correlation of the changes in miRNA expression to disease progression (Fig. 2). RNA, isolated from biopsy samples, was loaded into Illumina miRNA expression microarrays containing ∼1200 human miRNAs. Porcine and homo sapiens miRNA sequences are highly conserved. Raw data were normalized using a reference group to re-scale intensities in Illumina Beadstudio, and the data were then analyzed using Bioconductor and GeneSpring. Three groups were defined: (1) baseline (normal); (2) high flow low pressure (HFLP) (the surgical anastomosis was created and there was high flow to the left pulmonary artery but no significant elevation of PAP); and (3) pulmonary arterial hypertension (PH). Groups 2 and 3 miRNA expression intensities were compared with baseline. Only statistically significant (P value < 0.05, by Welsh’s T-test test) miRNA expression fluorescent intensity unit differences of HFLP and PH compared to baseline were included in the tables.
Fig. 1.
Surgical shunt model. The LPA has been disconnected from the main pulmonary artery and is now attached to the proximal descending aorta (surgical shunt PAH). The arrows depict the direction of circulatory flow. The left lung demonstrates hypertensive changes.
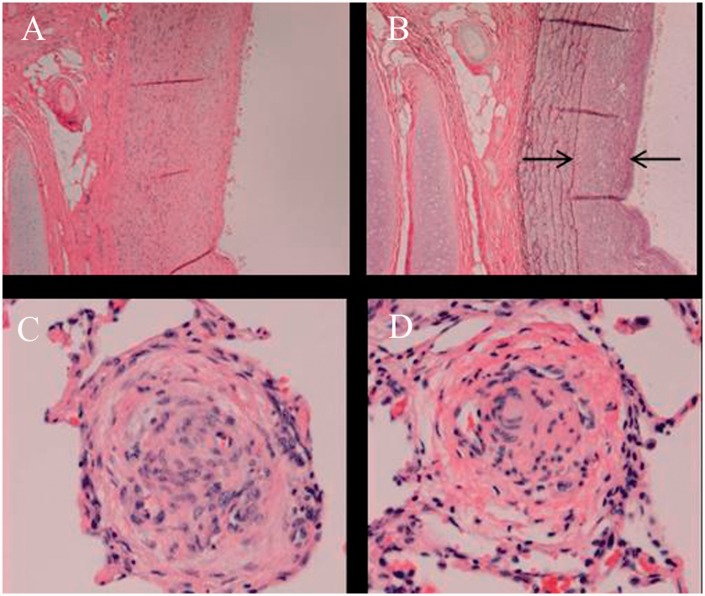
Fig. 2.
Histology at the time of necropsy. Specimens of hypertensive left lung obtained at the time of necropsy, showing significant thickening of the neointima (arrows: (a) hematoxylin and eosin; (b) elastin staining) and vascular occlusive and plexiform-like lesions (c, d). Magnification × 40.
We correlated the miRNA data to gene expression data obtained by us previously in the same experimental model4, data reported by White et al.12 and other publications. We examined the 100 most upregulated and 100 most downregulated genes 7 and 21 days after shunt creation for HFLP, and 60 and 180 days after shunt creation for PH. A total of 925 genes were examined. GeneCards (www.genecards.org) was used to correlate which miRNAs have been associated with these genes. These miRNAs were then compared to the miRNAs that were dysregulated in our model. Tables were generated listing the genes controlled by specific miRNAs, the function of the genes, and the direction of gene dysregulation. We also analyzed miRNAs that were dysregulated in different molecular pathways and biologic processes known to be involved in PH.
In addition, we compared the miRNAs that were dysregulated in our surgical PH model with miRNAs predicted to be dysregulated in PH by a systems biology approach.12 We analyzed key genes identified as being involved in PH by White et al.12 and examined which miRNAs have been associated with these genes using GeneCards. Dysregulated miRNAs in our model were then listed in tables with PH genes that have been reported to be associated with them. We also analyzed genes that were dysregulated in our model,4 identified which miRNAs control them and generated similar tables. The gene functions were obtained from GeneCards (www.genecards.org) or Google Scholar. Finally, we prepared a table listing the expression of 50 miRNAs previously reported to be associated with various models of PH and compared them to our experimental data.
Results
Tables 1 and 2 summarize the PAP in two animals that underwent endoarterial biopsies for miRNA analysis, and how the samples were segregated for the study of pulmonary arterial vascular miRNA expression at baseline, HFLP, and PH. Multiple miRNAs were dysregulated during progression from baseline to HFLP and then PH; 39 miRNAs were downregulated and 34 miRNAs were upregulated in PH compared to baseline. Tables 3, 4, and S1–S4 summarize the significant changes in miRNA expression, comparing baseline with HFLP (Tables S1 and S2), baseline to PH (Tables 3 and 4), and HFLP to PH (Tables S3 and S4).
Table 1.
Animal, days post-shunt surgery, and pulmonary artery pressure (PAP).
| Pig 1 |
Pig 2 |
||||
|---|---|---|---|---|---|
| Day | PAP | mPAP | Day | PAP | mPAP |
| 7 | 18/10 | 16 | 7 | 20/11 | 16 |
| 10 | 22/17 | 19 | 6 | 19/15 | 16 |
| 24 | 85/62 | 72 | 21 | 20/15 | 17 |
| 59 | 89/50 | 58 | 55 | 23/15 | 19 |
| 94 | 100/81 | 82 | 83 | 93/68 | 80 |
| 104 | 91/69 | 80 | |||
| 140 | 92/70 | 81 | |||
Table 2.
Animal, days post-shunt surgery, and pulmonary artery pressure (PAP).
| Baseline |
HFLP |
PH |
||||||
|---|---|---|---|---|---|---|---|---|
| Animal no., day | PAP | mPAP | Animal no., day | PAP | mPAP | Animal no., day | PAP | mPAP |
| 1, 7 | 18/10 | 16 | 1, 10 | 22/17 | 19 | 1, 24 | 85/62 | 72 |
| 2, 7 | 20/11 | 16 | 2, 6 | 19/15 | 16 | 1, 59 | 89/50 | 58 |
| 2, 21 | 20/15 | 17 | 1, 94 | 100/81 | 82 | |||
| 2, 55 | 23/15 | 19 | 2, 83 | 93/68 | 80 | |||
| 2, 104 | 91/69 | 80 | ||||||
| 2, 140 | 92/70 | 81 | ||||||
Table 3.
Significantly downregulated microRNAs PH vs. baseline (P < 0.05).
| miRNA symbol | Illumina ID | Baseline | PH |
|---|---|---|---|
| HS_134 | ILMN_3168054 | 3916.21 | 79.38 |
| HS_150 | ILMN_3167952 | 8724.37 | 2076.18 |
| HS_169 | ILMN_3168235 | 58.82 | 6.34 |
| HS_221 | ILMN_3168335 | 15.54 | 0.00 |
| HS_56 | ILMN_3167249 | 229.25 | 18.22 |
| hsa-let-7f-2-3p | ILMN_3168709 | 1281.24 | 218.10 |
| hsa-miR-1201 | ILMN_3168604 | 31.83 | 0.00 |
| hsa-miR-1205 | ILMN_3168827 | 569.48 | 117.37 |
| hsa-miR-1229 | ILMN_3167337 | 177.30 | 43.83 |
| hsa-miR-124a:9.1 | ILMN_3168039 | 9629.83 | 3219.90 |
| hsa-miR-128b:9.1 | ILMN_3167491 | 3060.68 | 925.46 |
| hsa-miR-1304 | ILMN_3168882 | 12023.57 | 6677.06 |
| hsa-miR-1321 | ILMN_3168663 | 31.00 | 3.29 |
| hsa-miR-135a-3p | ILMN_3168798 | 376.77 | 151.44 |
| hsa-miR-212 | ILMN_3167761 | 37.30 | 16.59 |
| hsa-miR-219-2-3p | ILMN_3168597 | 99.25 | 14.28 |
| hsa-miR-29b-1-5p | ILMN_3168755 | 233.36 | 82.45 |
| hsa-miR-33a-3p | ILMN_3167691 | 127.02 | 1.76 |
| hsa-miR-33a-3p | ILMN_3168654 | 740.78 | 96.90 |
| hsa-miR-377 | ILMN_3168481 | 8687.78 | 4563.60 |
| hsa-miR-483-5p | ILMN_3168558 | 211.48 | 68.62 |
| hsa-miR-494 | ILMN_3168446 | 8875.62 | 343.70 |
| hsa-miR-495 | ILMN_3167052 | 370.00 | 45.81 |
| hsa-miR-496 | ILMN_3167393 | 90.38 | 5.18 |
| hsa-miR-519a | ILMN_3168168 | 32.58 | 1.72 |
| hsa-miR-520d-3p | ILMN_3167753 | 114.97 | 14.32 |
| hsa-miR-521 | ILMN_3168215 | 37.30 | 8.98 |
| hsa-miR-524-3p | ILMN_3167328 | 6444.52 | 2162.76 |
| hsa-miR-542-5p | ILMN_3167175 | 8796.46 | 1274.47 |
| hsa-miR-548c-5p | ILMN_3168540 | 493.90 | 161.33 |
| hsa-miR-551a | ILMN_3168265 | 11306.19 | 5572.32 |
| hsa-miR-586 | ILMN_3167515 | 4183.74 | 42.62 |
| hsa-miR-935 | ILMN_3168678 | 837.14 | 14.23 |
| hsa-miR-95 | ILMN_3166971 | 1310.30 | 311.18 |
| hsa-miR-99a-3p | ILMN_3168648 | 593.72 | 46.29 |
| solexa-5620-151 | ILMN_3168905 | 92.30 | 5.07 |
| solexa-603-1846 | ILMN_3167128 | 32.58 | 0.00 |
Table 4.
Significantly upregulated microRNAs PH vs. baseline (P < 0.05).
| miRNA symbol | Illumina ID | Baseline | PH |
|---|---|---|---|
| HS_135 | ILMN_3167512 | 0.00 | 11.79 |
| HS_151.1 | ILMN_3167470 | 0.00 | 12.39 |
| HS_157 | ILMN_3167993 | 0.00 | 192.76 |
| HS_170 | ILMN_3167684 | 11.78 | 80.14 |
| HS_206 | ILMN_3168217 | 0.00 | 304.34 |
| HS_251.1 | ILMN_3167879 | 0.00 | 111.71 |
| HS_262.1 | ILMN_3167361 | 21.39 | 259.94 |
| hsa-let-7d-3p | ILMN_3168710 | 0.00 | 1499.40 |
| hsa-let-7g-3p | ILMN_3168732 | 0.00 | 216.74 |
| hsa-miR-1185 | ILMN_3168241 | 0.00 | 18.57 |
| hsa-miR-1203 | ILMN_3168680 | 2.62 | 148.59 |
| hsa-miR-1237 | ILMN_3168818 | 0.00 | 20.75 |
| hsa-miR-1273 | ILMN_3168760 | 11.78 | 80.09 |
| hsa-miR-127-5p | ILMN_3168719 | 0.00 | 380.69 |
| hsa-miR-130a-5p | ILMN_3168870 | 0.00 | 912.52 |
| hsa-miR-16-2-3p | ILMN_3168672 | 0.00 | 941.07 |
| hsa-miR-187 | ILMN_3168167 | 0.00 | 1144.02 |
| hsa-miR-192-3p | ILMN_3168722 | 1.26 | 529.41 |
| hsa-miR-28-5p | ILMN_3167223 | 381.67 | 5756.85 |
| hsa-miR-29a | ILMN_3168589 | 597.66 | 1739.42 |
| hsa-miR-30a-3p | ILMN_3167158 | 0.00 | 3.36 |
| hsa-miR-331-5p | ILMN_3168706 | 0.00 | 1700.55 |
| hsa-miR-374a | ILMN_3168240 | 1613.60 | 5725.95 |
| hsa-miR-410 | ILMN_3167244 | 0.00 | 1534.57 |
| hsa-miR-519e-5p | ILMN_3168031 | 0.00 | 52.02 |
| hsa-miR-520g | ILMN_3168350 | 0.00 | 2772.39 |
| hsa-miR-548n | ILMN_3168639 | 0.00 | 728.32 |
| hsa-miR-568 | ILMN_3167039 | 17.25 | 1036.96 |
| hsa-miR-602 | ILMN_3167275 | 39.19 | 146.84 |
| hsa-miR-610 | ILMN_3167193 | 0.00 | 151.36 |
| hsa-miR-663 | ILMN_3167088 | 0.00 | 303.57 |
| solexa-2580-353 | ILMN_3168890 | 0.00 | 343.04 |
| solexa-3126-285 | ILMN_3168895 | 0.00 | 7.81 |
| solexa-7534-111 | ILMN_3168911 | 0.00 | 235.62 |
Table 5 lists the number of PH-associated genes controlled by each of the miRNAs that were found to be dysregulated in our model. The number was in the range of 1–113 (from a total of 925 genes). The five miRNAs with the most regulated genes were: miR-548c-3p (113), miR-520d-3p (106), miR-130a-5p (103), miR-30a-3p (84), and miR-let-7g-3p (83).
Table 5.
Number of PH-associated genes regulated by each miRNA.
| miR | Number of genes regulated | miR | Number of genes regulated | miR | Number of genes regulated |
|---|---|---|---|---|---|
| miR-548c-3p | 113 | miR-520g | 35 | miR-1304 | 15 |
| miR-520d-3p | 106 | miR-410 | 32 | miR-219-2-3p | 14 |
| miR-130a-5p | 103 | miR-586 | 31 | miR-154-3p | 13 |
| miR-30a-3p | 84 | miR-29b-1-5p | 31 | miR-1321 | 12 |
| let-7g-3p | 83 | miR-16-2-3p | 31 | miR-127-5p | 12 |
| let-7f-2-3p | 78 | miR-494 | 29 | miR-331-5p | 11 |
| miR-363-5p | 49 | miR-33a-3p | 29 | miR-619 | 11 |
| miR-495 | 48 | miR-548i | 29 | miR-643 | 9 |
| miR-519a | 48 | miR-192-3p | 29 | miR-1287 | 8 |
| let-7d-3p | 48 | miR-185-3p | 27 | miR-208b | 8 |
| miR-548n | 46 | miR-1205 | 25 | miR-99a-3p | 7 |
| miR-519e-5p | 46 | miR-212 | 24 | miR-28-5p | 7 |
| miR-33a-3p | 45 | miR-187 | 23 | miR-556 | 7 |
| miR-135a-3p | 43 | miR-10b-3p | 23 | miR-610 | 5 |
| miR-133b | 40 | miR-371-5p | 22 | miR-524-3p | 4 |
| miR-548c-5p | 39 | miR-218-1-3p | 21 | miR-525-3p | 3 |
| miR-377 | 38 | miR-1237 | 18 | miR-497 | 2 |
| miR-548a-5p | 37 | miR-935 | 17 | miR-664 | 1 |
Tables S5–S10 list selected miRNAs that were dysregulated in our model, their target genes (with their function) and the fold-change in the level (compared to baseline) of the targeted gene expression at 7, 21, 60 and 180 days after shunt creation. Tables S5 and S6 compare baseline with HFLP, Tables S7 and S8 compare baseline with PH, and Tables S9 and S10 compare HFLP with PH.
Table S11 lists molecular pathways involved in PH, the dysregulated miRNAs (with the direction of regulation), and target genes (with the direction of regulation). Among the signaling pathways are four that have been targeted by approved drugs: nitric oxide, endothelin, prostacyclin, and calcium channels. In addition, the TGF-β pathway is known to be genetically linked to familial and non-familial PH. The PDGF pathway has been targeted with Imatinib. Other pathways associated with PH include: NF-Kappa-β, PPAR-γ, protein kinase, MAP kinase, and oncogenes. Several miRNAs dysregulated in our model appear to have an effect on multiple signaling pathways. For example, miR-130a-5p was dysregulated in our PH model and it appears to have an effect on at least one intermediate of the following signaling pathways: nitric oxide, endothelin, prostacyclin, calcium channels, PDGF, protein kinase, MAP kinase, and oncogenes.
Table S12 summarizes prominent biological processes involved in PH, the dysregulated miRNAs (with the direction of regulation), and target genes (with the direction of regulation). Among these biological processes are: proliferation, inflammation, apoptosis, cellular adhesion and motility, ubiquitination, microtubule assembly, extracellular matrix effects, mitochondrial metabolism, and tumor suppression. These have been shown to be important in endothelial, smooth muscle, and adventitial cells during the development of PH. Several miRNAs dysregulated in our model had effects on multiple biological processes associated with PH. For example, let-7d-3p was dysregulated and appeared to have an effect on at least one intermediate involved in cellular proliferation, cell adhesion, inflammation, mitochondrial metabolism, apoptosis, and tumor suppression.
Several dysregulated miRNAs in our model and their known effects on signaling pathways and biologic process associated with PH are summarized in Tables S13 and S14. The list of miRNAs, pathways, and biologic processes is not exhaustive, but it provides a glimpse of the myriad relationships of miRNAs and cellular functions.
Table 6 lists the miRNAs that were dysregulated in our experimental model compared with the miRNAs that were predicted to be dysregulated by a systems biology approach.12 Among 29 miRNAs, which were predicted by the systems biology approach to be dysregulated in PH, we found only four that were dysregulated in our model. Table S15 lists 50 miRNAs from the published literature that were strongly associated with PH in several experimental models, the direction of change and the references. Among these 50, six were found to be dysregulated in our model.
Table 6.
Comparison of miRNA dysregulation in the current study with a systems biology approach.
| miRNAs predicted from White et al.12 | miRNAs experimentally found by White et al.12 | miRNAs found in our data | miRNAs predicted by White et al.12 that we found | miRNAs found by us but not predicted by White et al.12 | miRNAs predicted by White et al.12 and not found by us | miRNAs experimentally predicted by White et al.12 and not found by us |
|---|---|---|---|---|---|---|
| miR-200bc | let-7a,f | hsa-miR-548n | miR-130 | hsa-miR-548n | miR-200bc | let-7a,f |
| miR-216 | miR-126 | let-7d-3p | miR-135/a | let-7d-3p | miR-216 | miR-126 |
| miR- 153 | miR-138 | let-7f-2-3p | miR-219/2-3p | let-7f-2-3p | miR- 153 | miR-138 |
| miR-1 | miR-143/145 | let-7g-3p | miR-410 | let-7g-3p | miR-1 | miR-143/145 |
| miR-130 | miR-144 | miR-10b-3p | miR-10b-3p | miR-145 | miR-144 | |
| miR-135 | miR-204 | miR-1205 | miR-1205 | miR-148 | miR-204 | |
| miR-145 | miR-21 | miR-1237 | miR-1237 | miR-149 | miR-21 | |
| miR-148 | miR-210 | miR-127-5p | miR-127-5p | miR-17-92 | miR-210 | |
| miR-149 | miR-22 | miR-1287 | miR-1287 | miR-182 | miR-22 | |
| miR-17-92 | miR-276 | miR-130* | miR-1304 | miR-190 | miR-276 | |
| miR-182 | miR-302b | miR-1304 | miR-130a-5p | miR-204 | miR-302b | |
| miR-190 | miR-30c | miR-130a-5p | miR-1321 | miR-205 | miR-30c | |
| miR-204 | miR-322 | miR-1321 | miR-133b | miR-21 | miR-322 | |
| miR-205 | miR-367 | miR-133b | miR-154-3p | miR-221/222 | miR-367 | |
| miR-21 | miR-450 | miR-135a-3p | miR-16-2-3p | miR-224 | miR-450 | |
| miR-219 | miR-451 | miR-154-3p | miR-185-3p | miR-24 | miR-451 | |
| miR-221/222 | miR-16-2-3p | miR-187 | miR-27a/b | |||
| miR-224 | miR-185-3p | miR-192-3p | miR-290 | |||
| miR-24 | miR-187 | miR-208b | miR-340 | |||
| miR-27a/b | miR-192-3p | miR-212 | miR-361 | |||
| miR-290 | miR-208b | miR-218-1-3p | miR-375 | |||
| miR-340 | miR-212 | miR-28-5p | miR-383 | |||
| miR-361 | miR-218-1-3p | miR-29b-1-5p | miR-455 | |||
| miR-375 | miR-219-2-3p | miR-30a-3p | miR-873 | |||
| miR-383 | miR-28-5p | miR-331-5p | miR-96 | |||
| miR-410 | miR-29b-1-5p | miR-33a | ||||
| miR-455 | miR-30a-3p | miR-33a-3p | ||||
| miR-873 | miR-331-5p | miR-363-5p | ||||
| miR-96 | miR-33a | miR-371-5p | ||||
| miR-33a-3p | miR-377 | |||||
| miR-363-5p | miR-494 | |||||
| miR-371-5p | miR-495 | |||||
| miR-377 | miR-495 | |||||
| miR-410 | miR-495 | |||||
| miR-494 | miR-496 | |||||
| miR-495 | miR-519a | |||||
| miR-495 | miR-519e-5p | |||||
| miR-495 | miR-520d-3p | |||||
| miR-496 | miR-520g | |||||
| miR-519a | miR-524-3p | |||||
| miR-519e-5p | miR-525-3p | |||||
| miR-520d-3p | miR-548a-5p | |||||
| miR-520g | miR-548c-3p | |||||
| miR-524-3p | miR-548c-5p | |||||
| miR-525-3p | miR-548i | |||||
| miR-548a-5p | miR-548n | |||||
| miR-548c-3p | miR-556 | |||||
| miR-548c-5p | miR-586 | |||||
| miR-548i | miR-610 | |||||
| miR-548n | miR-619 | |||||
| miR-556 | miR-643 | |||||
| miR-586 | miR-663 | |||||
| miR-610 | miR-935 | |||||
| miR-619 | miR-99a-3p | |||||
| miR-643 | ||||||
| miR-663 | ||||||
| miR-935 | ||||||
| miR-99a-3p |
Discussion
The pathophysiologic changes in the pulmonary arterial wall observed in PH include endothelial cell, smooth muscle cell and fibroblast dysfunction, abnormal vasoconstricion, proliferation, hypertrophy, fibrosis, plexiform and angiomatoid lesions, and necrotizing arteritis.13 The molecular and genetic changes responsible for these changes remain to be elucidated. There has been growing interest in the study of genes, mRNAs and more recently, miRNAs, associated with PH.
Boucherat et al.14 provided examples of miRNAs that have been shown to be dysregulated in different cell types of the pulmonary vascular wall in PH. In endothelial cells, miR-130/301, miR-17/92, miR-125, miR-21, and miR-210 were upregulated and miR 424/503 was downregulated. In smooth muscle cells, miR-130/301, miR-145, miR-21, miR-190, miR-29b, and miR17/92 were upregulated and miR-204, miR-193, miR-30c, miR206, miR-328, and miR-17/92 were downregulated. In adventitital fibroblasts miR-124 was downregulated.
There have been an increasing number of reports examining the association of miRNAs with PH. Chen et al.15 showed upregulation of miR-29 in estrogen-associated heritable PH. Deng et al.16 reported that inhibition of miR-143-3p blocked hypoxia-induced PH in mice. Meloche et al.17 demonstrated that bromodomain-containing protein 4 played a key role in PH and that its expression was miR-204 dependent. Liu et al.18 reported that defective miRNA processing affects the risk of developing PH. Meloche et al.19 showed that restoring the expression of miR-223 in lungs of rats with monocrotaline-induced PH reversed established PH. McLendon et al.20 demonstrated improvement in rat sugen/hypoxia PH by inhibition of miR-145. Wallace et al.21 reported reduced development of hypoxia-induced PH in mice by restoration of miR-96. Bi et al.22 showed that monocrotaline-induced PH could be ameliorated by miR-27b inhibition in rats. Bertero et al.23 demonstrated a global regulatory role for miR-130/301 in endothelial and arterial smooth muscle cell function in PH. Recently, Chun et al. described a high level of complexity of miRNA regulation in PH and offered computational techniques to aid in organizing and prioritizing pathways and molecules with respect to miRNA biology.24
With the growing evidence for miRNAs dysregulation in PH, the dilemma arises of how to detect these changes clinically. Several investigators have measured circulatory miRNAs in patients with PH. Rhodes et al.25 showed that reduced circulatory miR-150 was associated with poor survival in PH. Courboulin et al.9 reported that downregulation of miR-204 in buffy coat cells correlated with PH severity. Bertero et al.26 demonstrated increased plasma miR-130/301 levels in patients with increasing severity of PH. Sarrion et al.27 reported that 61 circulating miRNAs were dysregulated in 12 patients with idiopathic PH compared with ten controls. Wei et al.28 showed that several miRNAs, detected in buffy coat samples, were dysregulated in human subjects with PH. Therefore, it appears that many miRNA changes can be detected in the circulation, but the origin of the miRNAs, which could include white cells, macrophages, or different vascular wall cells, remains to be elucidated.
In this experimental study, we showed that miRNA changes can be detected directly in the tissue that is affected pathologically by PH. We utilized an endoarterial catheter, inserted percutaneously in a minimally invasive manner, to obtain pulmonary endovascular samples safely and of adequate size and quality to allow identification of miRNA changes sequentially during the development of PH. Measuring miRNA (and other molecular) changes directly in individual patients at specific stages of their disease process offers great promise for advancing knowledge of the molecular mechanisms and designing specific personalized therapy.
We showed that multiple miRNAs were dysregulated in a porcine model of PH created surgically by connecting the left pulmonary artery to the descending aorta. We have previously reported the reliable development of PH after several weeks in this model.4,29 We performed an analysis of miRNA changes comparing the endovascular biopsy samples in three groups: (1) baseline (normal pressure and flow); (2) increased flow but low pressure (the first weeks after the surgical anastomosis); and (3) systemic level PH. We then compiled tables relating the dysregulated miRNAs with genes that have been associated with experimental or clinical PH. Several miRNAs regulate multiple PH genes, including: miR-548c-3p (113); miR-520d-3p (106); miR-130a-5p (103); miR-30a-3p (84); and miR-let-7g-3p (83). Among these, the miR-130 and miR-30 families of miRNAs have been shown by others to be implicated in the etiology of PH.14 In our model of PH, miR-520d-3p expression levels decreased eightfold from 115 to 14. MiR-520d-3p targets over 1400 genes including phosphodiesterase 5A, which has been shown to be dysregulated and is a target of therapy in PH. Also in our model, two miRNAs of the let-7 family were upregulated: let-7d-3p from 0 to 1500 and let-7g-3p from 0 to 217. Let-7d-3p targets 819 genes including endothelin-1, which is known to be dysregulated and its receptor is a target of therapy in PH.
Several signaling pathways have been implicated in PH. Those involving nitric oxide, endothelin, prostacyclin, and calcium channels have been the targets of drug therapies. Many molecular intermediates in these pathways have been shown to be the targets of miRNAs that were dysregulated in our model (Tables S11 and S13). Further work may elucidate miRNAs with effects on multiple PH implicated pathways and could become the targets of therapy. For example, miR-130a-5p was dysregulated in our model and it has been shown to affect at least one intermediate of the following signaling pathways: nitric oxide, endothelin, prostacyclin, calcium channels, PDGF, protein kinase, MAP kinase, and oncogenes. Interestingly, miR-130 has been shown by others to be significantly upregulated in pulmonary vascular cells14 and to be a global key regulator in PH.30 In our data, there was a significant change in the level of miR-130a-5p, but not miR-130 or miR-130a. The nucleotide sequences of miR-130, miR-130a and miR-130a-5p are very different. This exclusive dysregulation of miR-130a-5p, but not miR-130 or miR-130a in PH has not been reported previously and its significance remains to be determined.
Multiple biological processes have been reported to be involved in PH, including cellular proliferation, apoptosis, inflammation, cell migration, and disordered mitochondrial metabolism. Tables S12 and S14 provide selective miRNA and target gene data from our model relating to these biological processes. Analysis of these and other molecular changes may identify key miRNAs, which may have effects on multiple biologic processes and become potential treatment targets for PH. For example, let-7d-3p was dysregulated in our model and appeared to have an effect on at least one intermediate involved in cellular proliferation, cell adhesion, inflammation, mitochondrial metabolism, apoptosis, and tumor suppression. While it is tempting to speculate that the miRNA that was shown to affect the largest number of cellular functions in PH would be among the most important to target therapeutically, it is also possible that the key miRNA or miRNAs involved in PH still have not been discovered or that targeting a miRNA with critical effects on a single or only a few biological pathways may result in a more important biologic effect.
Among 29 miRNAs, which were predicted by a theoretical approach to be dysregulated in PH,12 we found only four that were dysregulated in our model (Table 6). Among 50 miRNAs, which were shown to be strongly associated in other experimental models of PH, we found only six that were dysregulated in our model (Table S15). These discrepancies may be related to the specific surgical model, the animal species or the stages of the PH disease process that were studied. Alternatively, direct measurement of miRNA levels may be more accurate in a specific experimental or human subject at a particular stage of the disease than the theoretical approach.
In further support of the importance of direct examination of pulmonary endovascular tissue to study miRNA levels is the work of Schlosser et al.31 These investigators measured a set of miRNAs (miR-17, -21, 130b, -145, -204, -424, and 503) that have been causally implicated in PH in 3 rat models (monocrotaline, SU5416 + hypoxia, and chronic hypoxia), a cohort of patients with PH and healthy subjects. They found PH model dependent perturbations in plasma and tissue miRNA levels; however, none of these were conserved across all three experimental models. The miRNA changes were context dependent and were not consistent across the rodent models and human PH.
In light of the findings of Schlosser et al.,31 the specific miRNA changes detected in our porcine shunt model of chronic PH may or may not have direct relevance to human PH. Perhaps more important than the specific miRNA changes observed in our model is the methodology described in our study. The ability to obtain pulmonary vascular biopsy samples safely and of adequate size and quality to perform studies in a patient with PH of any etiology or at any particular stage of their disease would be revealing and potentially useful in designing individualized medical therapy.
Our model mimics several congenital cardiac lesions, which have a systemic to pulmonary shunt, including patent ductus arteriosus, surgical aorto-pulmonary shunts (Waterston and Potts), hemitruncus, and perhaps even ventricular septal defects. The miRNA changes observed in our model may relate to the increase in flow, the increase in pressure or both. In our model, we observed different miRNA expression profiles comparing the experimental stage of increased flow–normal pressure with the stage of systemic level PH. Further analysis may help discover separate molecular pathways in vascular tissue for increased flow versus increased pressure. Ma et al.32 identified miRNAs implicated in PH in patients with congenital heart disease. They compared the miRNA profile in lung tissue specimens obtained at the time of surgery in six patients with ventricular septal defects and PH versus six patients with ventricular septal defects and no PH. Compared with the non-PH patients, the PH patients had 62 miRNAs that were upregulated and 12 miRNAs that were downregulated. The level of miRNA-27b correlated with the level of preoperative PH. They concluded that miRNAs might be important regulators of PH in congenital heart disease.
There are two potential strategies for miRNA-based therapies: miRNA mimetics and miRNA antagonists.32 Mimetics of miR-204, miR-424, miR-503, and let-7f have been reported to ameliorate or reverse PH in animal models.9,11,33 Anti-miR-17 was shown to provide beneficial therapy for experimental PH.34 There are currently no reports of miRNA based therapies in patients with PH.
There are several limitations to our study. MiRNA data were obtained from only two animals. However, multiple time points at high flow–low pressure and PH were compared, and the miRNA changes were statistically significant. Another limitation is that some miRNAs regulate thousands of genes and we have listed only a limited number of genes taken from our 7-, 21-, 60-, and 180-day sort list, key PH genes listed by White et al.12 and a drug-targeted gene list from our 2013 paper.4 The list of gene functions associated with each gene is not exhaustive. Also, we have not yet performed experiments altering miRNA levels to assess the effects on PH in our model. Furthermore, the biopsy catheter obtained samples from medium-sized arteries and it is not clear how their molecular findings are representative of changes that occur at arteriolar level or in the large pulmonary arteries. An additional limitation is that the exact cellular origin of the miRNAs was not determined. Endoarterial biopsy provides a tissue sample composed mostly of smooth muscle cells, with occasional endothelial and neo-intimal cells.35 To determine more precisely the pulmonary arterial wall cell type origin of the miRNA changes, selective culturing of cell from biopsy samples could be performed.36 Another limitation is that the dysregulated miRNAs may relate only to our specific shunt or porcine model of PH and may not apply to clinical PH. This provides further support to the paradigm of percutaneous pulmonary endoarterial biopsy, which may be performed in a sequential and minimally invasive manner.
In conclusion, a large number of miRNAs are dysregulated in a porcine shunt model of chronic PH. Each of these miRNAs may regulate multiple PH-associated genes, PH-associated signaling pathways, and biological processes. The roles of dysregulated miRNAs and their correspondingly regulated genes in PH await further study. MiRNA mimetic or antagonist therapy may have an application in human PH. The data presented in this study lend support to our hypothesis that regulation of miRNAs and genes is a very complex process with multiple miRNAs and genes upregulated and downregulated to different degrees and in different directions at various “stages” of PH. Endoarterial biopsy provides an innovative method to assess miRNA, gene, and other molecular regulation in normal and hypertensive pulmonary arteries at specific stages of the disease and opens a new window for precision therapy.
Acknowledgments
The authors thank Rene Mireles, Vascular BioSciences for the illustrations.
Conflict of interest
David Mann is a shareholder, patent holder and employee of Vascular BioSciences. Roy Williams is a warrant holder of Vascular BioSciences.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Archer S, Rich S. Primary pulmonary hypertension: A vascular biology and translational research “Work in progress”. Circulation 2000; 102: 2781–2791. [DOI] [PubMed] [Google Scholar]
- 2.Newman JH, Fanburg BL, Archer SL, et al. Pulmonary arterial hypertension: Future directions: Report of a National Heart, Lung and Blood Institute/Office of Rare Diseases Workshop. Circulation 2004; 109: 2947–2952. [DOI] [PubMed] [Google Scholar]
- 3.Morrell NW, Adnot S, Archer SL, et al. Cellular and molecular basis of pulmonary arterial hypertension. J Am Coll Cardiol 2009; 54: S20–S31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rothman A, Davidson S, Wiencek RG, et al. Vascular histomolecular analysis by sequential endoarterial biopsy in a shunt model of pulmonary hypertension. Pulm Circ 2013; 3: 50–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grosshans H, Filipowicz W. Molecular biology: the expanding world of small RNAs. Nature 2008; 451: 414–416. [DOI] [PubMed] [Google Scholar]
- 6.Selbach M, Schwanhäusser B, Thierfelder N, et al. Widespread changes in protein synthesis induced by microRNAs. Nature 2008; 455: 58–63. [DOI] [PubMed] [Google Scholar]
- 7.Sarkar J, Gou D, Turaka P, et al. MicroRNA-21plays a role in hypoxia-mediated pulmonary artery smooth muscle cell proliferation and migration. Am J Physiol Lung Cell Mol Physiol 2010; 299: L861–871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brock M, Trenkmann M, Gay RE, et al. Interleukin-6 modulates the expression of the bone morphogenic protein receptor type II through a novel STAT3-microRNA cluster 17/92 pathway. Circ Res 2009; 104: 1184–1191. [DOI] [PubMed] [Google Scholar]
- 9.Courboulin A, Paulin R, Giguère NJ, et al. Role for miR-204 in human pulmonary arterial hypertension. J Exp Med 2011; 208: 535–548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang L, Guo LJ, Liu J, et al. MicroRNA expression profile of pulmonary artery smooth muscle cells and the effect of let-7d in chronic thromboembolic pulmonary hypertension. Pulm Circ 2013; 3: 654–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Caruso P, MacLean MR, Khanin R, et al. Dynamic changes in lung microRNA profiles during the development of pulmonary hypertension due to chronic hypoxia and monocrotaline. Arterioscler Thromb Vasc Biol 2010; 30: 716–723. [DOI] [PubMed] [Google Scholar]
- 12.White K, Loscalzo J, Chan SY. Holding our breath: The emerging and anticipated roles of microRNA in pulmonary hypertension. Pulm Circ 2012; 2: 278–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heath D, Edwards JE. The pathology of hypertensive pulmonary vascular disease; a description of six grades of structural changes in the pulmonary arteries with special reference to congenital cardiac septal defects. Circulation 1958; 18: 533–547. [DOI] [PubMed] [Google Scholar]
- 14.Boucherat O, Potus F, Bonnet S. MicroRNA and pulmonary hypertension. Adv Exp Med Biol 2015; 888: 237–252. [DOI] [PubMed] [Google Scholar]
- 15.Chen X, Talati M, Fessel JP, et al. Estrogen metabolite 16α-hydroxyestrone exacerbates bone morphogenetic protein receptor type II-associated pulmonary arterial hypertension through microRNA-29-mediated modulation of cellular metabolism. Circulation 2016; 133: 82–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Deng L, Blanco FJ, Stevens H, et al. MicroRNA-143 activation regulates smooth muscle and endothelial cell crosstalk in pulmonary arterial hypertension. Circ Res 2015; 117: 870–883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meloche J, Potus F, Vaillancourt M, et al. Bromodomain-containing protein 4: the epigenetic origin of pulmonary arterial hypertension. Circ Res 2015; 117: 525–535. [DOI] [PubMed] [Google Scholar]
- 18.Lui S, Tang J, Huang L, et al. Cordyceps militaris alleviates severity of murine acute lung injury through mirnas-mediated cxcr2 inhibition. Cell Physiol Biochem 2015; 36: 2003–2011. [DOI] [PubMed] [Google Scholar]
- 19.Meloche J, Le Guen M, Potus F, et al. MiR-223 reverses experimental pulmonary arterial hypertension. Am J Physiol Cell Physiol 2015; 309: C363–C372. [DOI] [PubMed] [Google Scholar]
- 20.McLendon JM, Joshi SR, Sparks J, et al. Lipid nanoparticle delivery of a microRNA-145 inhibitor improves experimental pulmonary hypertension. J Control Release 2015; 210: 67–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wallace E, Morrell NW, Yang XD, et al. A sex-specific microRNA-96/5-hydroxytryptamine 1B axis influences development of pulmonary hypertension. Am J Respir Crit Care Med 2015; 191: 1432–1442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bi R, Bao C, Jiang L, et al. MicroRNA-27b plays a role in pulmonary hypertension by modulating peroxisome proliferator-activated receptor γ dependent Hsp90-eNOS signaling and nitric oxide production. Biochem Biophys Res Commun 2015; 460: 469–475. [DOI] [PubMed] [Google Scholar]
- 23.Bertero T, Cottrill K, Krauszman A, et al. The microRNA-130/301 family controls vasoconstriction in pulmonary hypertension. J Biol Chem 2015; 290: 2069–2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chun HJ, Bonnet S, Chan SY. Translating microRNA biology in pulmonary hypertension. It will take more than “miR” words. Am J Respir Crit Care Med 2017; 195: 167–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rhodes CJ, Wharton J, Boon RA, et al. Reduced microRNA-150 is associated with poor survival in pulmonary arterial hypertension. Am J Respir Crit Care Med 2013; 187: 294–302. [DOI] [PubMed] [Google Scholar]
- 26.Bertero T, Lu Y, Annis S, et al. Systems-level regulation of microRNA networks by miR-130/301promotes pulmonary hypertension. J Clin Invest 2014; 124: 3514–3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sarrion I, Milian L, Juan G, et al. Role of circulating miRNAs as biomarkers in idiopathic pulmonary arterial hypertension: possible relevance of miR-23a. Oxid Med Cell Longev 2015; 2015: 792–846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wei L, Li G, Zheng J, et al. Roles of microRNA in vascular diseases in cardiac and pulmonary systems. Pharmazie 2014; 69: 643–647. [PubMed] [Google Scholar]
- 29.Rothman A, Wiencek RG, Davidson S, et al. Hemodynamic and histologic characterization of a swine (Sus scrofa domestica) model of chronic pulmonary arterial hypertension. Comp Med 2001; 61: 258–262. [PMC free article] [PubMed] [Google Scholar]
- 30.Parikh VN, Jin C, Rabello S, et al. MicroRNA-21 integrates pathogenic signaling to control pulmonary hypertension: results of a network bioinformatics approach. Circulation 2012; 125: 1520–1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schlosser K, Taha M, Deng Y, et al. Discordant regulation of microRNA between multiple experimental models and human pulmonary hypertension. Chest 2015; 148: 481–490. [DOI] [PubMed] [Google Scholar]
- 32.Ma K, Zhao Q, Chen W, et al. Human lung microRNA profiling in pulmonary arterial hypertension secondary to congenital heart defect. Pediatr Pulmonol 2015; 50: 1214–1223. [DOI] [PubMed] [Google Scholar]
- 33.Kim KH, Jeong YT, Oh H, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat Med 2013; 19: 83–92. [DOI] [PubMed] [Google Scholar]
- 34.Pullamsetti SS, Doebele C, Fisher A, et al. Inhibition of microRNA-17 improves lung and heart function in experimental pulmonary hypertension. Am J Respir Crit Care Med 2012; 185: 409–419. [DOI] [PubMed] [Google Scholar]
- 35.Rothman A, Mann DM, Behling CA, et al. Percutaneous pulmonary endoarterial biopsy in an experimental model of pulmonary hypertension. Chest 1998; 114: 241–250. [DOI] [PubMed] [Google Scholar]
- 36.Rothman A, Mann DM, House MT, et al. Transvenous procurement of pulmonary artery smooth muscle and endothelial cells using a novel endoarterial biopsy catheter in a canine model. J Am Coll Cardiol 1996; 27: 218–224. [DOI] [PubMed] [Google Scholar]