Abstract
In pulmonary hypertension (PH), right ventricular (RV) performance determines survival. Pulmonary artery (PA) stiffening is an important biomechanical event in PH and also predicts survival based on the PA relative area change (RAC) measured at rest using magnetic resonance imaging (MRI). In this exploratory study, we sought to generate novel hypotheses regarding the influence of stress RAC on PH prognosis and the interaction between PA stiffening, RV performance and survival. Fifteen PH patients underwent dobutamine stress-MRI (ds-MRI) and right heart catheterization. RACREST, RACSTRESS, and ΔRAC (RAC STRESS – RAC REST) were correlated against resting invasive hemodynamics and ds-MRI data regarding RV performance and RV-PA coupling efficiency (n’vv [RV stroke volume/RV end-systolic volume]). The impact of RAC, RV data, and n’vv on ten-year survival were determined using Kaplan–Meier analysis. PH patients with a low ΔRAC (<−2.6%) had a worse long-term survival (log-rank P = 0.045, HR for death = 4.46 [95% CI = 1.08–24.5]) than those with ΔRAC ≥ −2.6%. Given the small sample, these data should be interpreted with caution; however, low ΔRAC was associated with an increase in stress diastolic PA area indicating proximal PA stiffening. Associations of borderline significance were observed between low RACSTRESS and low n’vvSTRESS, Δη’VV, and ΔRVEF. Further studies are required to validate the potential prognostic impact of ΔRAC and the biomechanics potentially connecting low ΔRAC to shorter survival. Such studies may facilitate development of novel PH therapies targeted to the proximal PA.
Keywords: pulmonary hypertension, relative area change, dobutamine, magnetic resonance imaging, prognosis
Pulmonary arterial hypertension (PAH) results from obstructive or obliterative disease within the small resistance vessels of the lung. Chronic thomboembolic pulmonary hypertension (CTEPH) results from unresolved clot in the major pulmonary vessels. Various hemodynamic variables predict adverse clinical outcomes in both forms of pulmonary hypertension (PH). These most frequently include data which reflect RV performance, such as RV stroke volume,1 mixed venous oxygen saturation (MvO2),2 and serum N terminal-pro brain natriuretic peptide (NT-proBNP) concentration.3 The prognostic value of hemodynamic pressure measurements have proven less consistent in past studies, either as mean pulmonary artery pressure (mPAP),4–6 which reflects the constant component of RV afterload, or PA pulse pressure,7 which is more indicative of pulsatile load. This has led investigators to seek alternative mechanisms by which RV failure evolves in patients with PH, in the hope that compensatory treatments can be introduced to arrest this event.
PH is commonly associated with pathological stiffening of the proximal pulmonary arteries (PA), a process that is known to further impair RV performance.8 The basic mechanism(s) responsible for PA stiffening in PH are complex and will depend upon several biomechanical factors including the PA wall thickness and the non-linear dependence of the PA elastic modulus on blood pressure.4,9 PA pressure will in turn be determined by RV stroke volume and RV afterload. Given these complexities, interactions between PA stiffening, RV performance, and outcome should ideally be studied using invasive hemodynamic methods, combined with volumetric data from MRI. However, it is difficult to conduct these studies in patients with PH. Moreover, recent data demonstrate that MRI, used alone, can be used to accurately assess both PA stiffening, via the PA relative area change (RAC),10 and the efficiency of coupling between the RV and PA, using the ratio of RV stroke volume over RV end-systolic volume (SV/ESV, also termed η’VV),11 although the accuracy of this approximation is still the subject of some debate.
RAC is defined as the ratio of the change in PA area during the cardiac cycle (AMAX – AMIN) over the PA area at either diastole (AMIN) or systole (AMAX). Gan et al. recently demonstrated a negative curvilinear relationship between RAC and mean PAP measured at rest in PH patients.12 They also demonstrated that patients with a low resting RAC (≤16%) had a poorer prognosis, a finding that was later corroborated by Swift et al.13 This later study also demonstrated that abnormal RAC could be detected even in PH patients with early disease, as characterized by a mild elevation in PVR (<4 Woods Units [WU]) and normal RV function. This suggests a potential role for RAC as a risk stratification biomarker,13 perhaps facilitating treatment intensification in appropriate patients.
In the current study, we sought to generate novel hypotheses regarding the influence of stress RAC on PH prognosis and the interaction between PA stiffening, RV performance, and survival. A recent study by Sharma et al. illustrates that stress testing can reveal additional predictive information to studies performed at rest. Using dobutamine-stress echocardiography, this group was able to accurately detect sub-clinical reductions in RV contractile reserve using a stress protocol similar to the current study.8 Our approach was to measure RAC at rest and stress using MRI in a small cohort of PH patients. Long-term follow-up in a national PH center provided prognostic information. We chose MRI over echocardiography because of its superior tissue contrast, higher spatial resolution, and ability to image the PA at any angle, making it the recommended modality for this purpose.14
Methods
Study participants
Fifteen participants were recruited during elective inpatient assessment for suspected PH at the Western Infirmary (now Queen Elizabeth University Hospital), Glasgow, UK, between August 2004 and July 2005. All underwent a standard series of diagnostic tests, including right heart catheterization,15 in addition to dobutamine stress-MRI (ds-MRI). Five younger volunteers underwent ds-MRI alone. All participants gave informed written consent to a study protocol that had been approved by the West of Scotland Research and Ethics Committee. Exclusion criteria included contraindications to MRI, e.g. claustrophobia, metal implants or foreign bodies, and contraindications to dobutamine, e.g. aortic stenosis, hypokalemia, and dobutamine allergy. No participants were taking chronotropic medication at the time of MRI and no patients had received any disease-targeted therapy for PH.
Follow-up and survival
All cases were followed up at a national PH center. The frequency of clinic visits was dependent on clinical progress and varied between one and six months. Survival information was retrieved retrospectively from a prospectively populated database. This was calculated, in days, from the date of right heart catheterization to death or the date of database review in surviving patients. Patients were excluded from survival analyses if an explicitly unrelated cause of death was recorded.
MRI examination
All studies were performed on a 1.5 T MRI scanner (Sonata Magnetom, Siemens, Germany) using an identical protocol in patients and younger volunteers. In PH patients, all scans were performed within 48 h of right heart catheterization. Prior to ds-MRI, all participants were weighed to allow calculation of a dobutamine infusion rate (see the “Dobutamine Infusion” subsection). The dobutamine infusion was connected (but not started) and a brachial artery sphygmomanometer and three-lead ECG monitoring were attached before the participant was moved inside the magnet.
Baseline measurements
Baseline MRI measurements were recorded as previously reported.16,17 MR velocity flow mapping was used to determine RV stroke volume (RVSV) from flow in the main PA. Flow maps were planned perpendicular to the target vessel in a double-oblique plane, at least 1.5 cm distal to the pulmonary valve. A velocity-encoded k-space segmented gradient-echo sequence was used (imaging parameters: echo time/repetition time [TE/TR] = 3.1/16 ms, flip angle [FA] = 15°, slice thickness = 6 mm, temporal resolution = limited by TR, image matrix = 256, field of view [FOV] = 380 mm, in-plane resolution = 1.9 × 1.5 mm, velocity encoding range = 150 cm/s) to generate 45 matched pairs of anatomical and velocity images. TrueFISP cine images of the main PA were then acquired utilizing copied sliced positions from the flow map sequence. Retrospective ECG-gating was used to ensure coverage of the complete cardiac cycle. Participants were instructed to breathe freely throughout this section of the protocol. The average acquisition time was 2–3 min.
Dobutamine infusion
Dobutamine was administered using a digital syringe driver loaded with a 50-mL syringe containing 50 mg of dobutamine hydrochloride (Posiject, Boehringer Ingelheim) diluted in 0.9% saline. The syringe driver was located in the control room, away from the magnet, and attached to the participant by a length of tubing fed through a wave-guide conduit. The infusion was started at 5 mcg/kg/min and increased in 5 mcg steps every 5 min to achieve the maximum tolerated dose, up to a ceiling of 20 mcg/kg/min. Heart rate, symptoms, and a three-lead ECG trace were monitored throughout and systemic BP was measured at every 3-min interval. The following predefined criteria were used as triggers to defer any further increase in dobutamine dose, and if persistent prompt a 5 mcg/kg/min reduction in infusion rate: (1) rise in HR > 50%; (2) fall in systolic BP > 20%; (3) intolerable side-effects or participant’s request.
Stress measurements
Stress measurements were initiated once the maximum tolerated dose of dobutamine had been reached and a steady state had been achieved (i.e. after at least 3 min on this dose). No specific alterations were made to the imaging protocol for stress imaging. However, the velocity-encoding gradient was modified to accommodate higher stroke volume velocities (maximum 300 cm/s) in approximately half of the PH patients and all of the younger volunteers.
MRI analysis
All images were analyzed by a single experienced operator (KGB) using the Argus analysis software (Siemens, Germany). Scans were coded by number and analyzed in batches. KGB was blinded to the identity and hemodynamic results of the patients. A second experienced operator (GJ) contoured the MPA at rest and stress to provide inter-observer data for ΔRAC. GJ was blinded to all clinical data and KGB’s results.
Pulmonary artery area and determination of relative area change
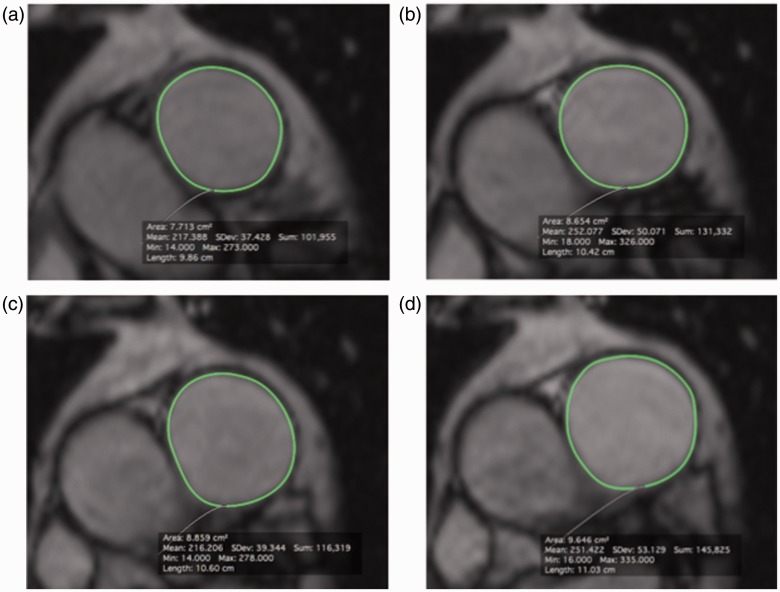
Maximum PA area (AMAX) and minimum PA area (AMIN) were measured at rest (AMAX REST and AMIN REST) and stress (AMAX STRESS and AMIN STRESS) on ds-MRI images by manual planimetry using Osirix for Mac v5.8 (Pixmeo, Bernex, Switzerland). Example images are shown in Fig. 1. The change in AMAX (ΔAMAX) and AMIN (ΔAMIN) between rest and stress were calculated as (AMAX or MIN STRESS – AMAX or MIN REST). RAC at rest (RACREST) and RAC during stress (RACSTRESS) were calculated as RAC = (AMAX – AMIN)/AMIN, using PA measurements recorded under appropriate conditions, as in earlier publications. The change in RAC between rest and stress (ΔRAC) was calculated as (RACSTRESS – RACREST).
Fig. 1.
Representative MRI images of the main pulmonary artery (MPA) acquired at rest (a, b) and at stress (c, d) in PH patient 1. The intima of the MPA was manually contoured at all time points using Osirix for Mac v5.8 (Pixmeo, Bernex, Switzerland) allowing measurement of the minimum PA area (AMIN (a, c)) and maximum PA area (AMAX (b, d)). These data were used to calculate RACREST, RACSTRESS, and ΔRAC, which were 12%, 9%, and −3% for this patient, respectively.
Right ventricular volume, ejection fraction, and RV-PA coupling
Planimetry of selected short axis images was used to measure RV volumes as described previously.18 Each value was indexed against body surface areas and is reported as RV end-diastolic volume index (RVEDVI), RV end-systolic volume index (RVESVI). Velocity encoded flow maps were analyzed to determine RVSV at rest and during stress using techniques described in earlier publications.10 The Argus analysis software was utilized, including background subtraction to correct for velocity measurement error (or “baseline drift”) by assessing a region of zero flow at each point in the cardiac cycle. Prior to analysis, each image set was inspected to verify accurate depiction of the target vessel and the absence of artefacts including aliasing on the velocity images. The software automatically computed the flow rate (in mL/s) during each time point within the target vessel. These data were integrated with the area of the target vessel and the positive values summed to produce RVSV. These values were indexed against body surface area to compute RV stroke volume index (RVSVI). Right ventricular ejection fraction (RVEF (%)) was determined as (stroke volume/end-diastolic volume) × 100. Changes in RV volumes, RVEF, and heart rate between rest and stress were calculated as a percentage (e.g. ΔRVSVI = ((RVSVISTRESS – RVSVIREST)/RVSVIREST) × 100).
RV-PA coupling efficiency is most accurately described by the ratio maximal systolic ventricular elastance (Emax,) to effective arterial elastance (Ea), with these data being measured invasively. However, Sanz et al. recently demonstrated that Emax/Ea can be approximated non-invasively using the parameter η’VV, which is the ratio of RV stroke volume/RV end-systolic volume, measured by MRI.11 Previous studies demonstrated that optimal ventriculo-arterial coupling occurs when Emax/Ea (or the non-invasive equivalent η’VV) is close to unity,19 with lower values occurring in pulmonary vascular disease.11,20 η’VV was determined in the current study as RV stroke volume/RV end-systolic volume. In the current study, the change in η’VV between rest and stress (Δη’VV) was calculated as (η’VV STRESS – η’VV REST).
Statistics
A sample size calculation indicated that 15 paired observations in PH patients were required to reliably detect a reduction in median ΔRAC in the PH group of at least 28% using the Wilcoxon matched-pairs signed rank test, assuming a one-sided α = 0.05 and 80% power. This calculation used data on resting RAC collected in healthy controls and patients with Acquired Immunodeficiency Syndrome (AIDS) and observed proximal PA stiffening and RV remodeling on MRI.21 These data were chosen because no stress data had been reported when the study was designed. Echocardiographic systolic PAP estimates were only available in 5/13 AIDS patients reported in this study (mean systolic PAP = 40.4 (±25) mmHg) but the observed MRI abnormalities were consistent with elevated RV afterload, likely associated with Human Immunodeficiency Virus (HIV)-associated pulmonary vascular disease.21 Given the small sample size, non-parametric tests were used where possible. All values are presented as median (interquartile range) unless otherwise stated. In PH patients, comparisons between resting and stress MRI measurements were made using the Wilcoxon matched-pairs signed rank test. Since this test cannot be used in samples smaller than six, the paired t-test was used for these comparisons in the younger volunteer population and in comparisons of measurements in low and high ΔRAC subgroups. Correlation was assessed using Spearman’s rho test and P values were adjusted by Bonferroni’s method (0.05/number of comparisons). Agreement between MRI reporters regarding RAC in PH patients was assessed using the intraclass correlation coefficient (ICC, two-way mixed effects model). The PH population was dichotomized either side of the median values of RACREST, RACSTRESS, and ΔRAC. Kaplan–Meier mortality curves were generated for these dichotomized groups and any inequalities in mortality rates were assessed by log rank testing. Differences in the risk of death between groups were quantified by hazard ratios (95% confidence interval [CI]). Differences in RV parameters or hemodynamic measurements within each group were assessed using the Mann–Whitney test. All statistical analyses were performed using GraphPad Prism 6 for Mac (GraphPad, San Diego, CA, USA) or SPSS v.22 (SPSS, Chicago, IL, USA). All tests were two-sided and assumed a significance level of 0.05.
Results
Population demographics and clinical findings
PAH or CTEPH was confirmed in all 15 patients. Clinical and hemodynamic results for this cohort are summarized in Table 1. The five volunteers were all men, had no history of cardiorespiratory disease, and were significantly younger than PH patients (median age = 33 years, age range = 31–49 years; PH patients: median age = 54 years, age range = 45–60 years; P = 0.013). Seven of 12 PH patients died over the follow-up period. The median survival time was 3045 days. Patient 7 (see Table 1) was excluded from long-term survival analyses having died from surgical complications directly related to attempted pulmonary endarterectomy (PEA). No other patients with CTEPH were referred for PEA.
Table 1.
Clinical and hemodynamic results in 15 patients with PH.
| Patient | Age (years) | Sex | Diagnosis | NYHA class | mPAP (mmHg) | CI (L/min/m2) | PVR (mmHg/L/min) | RAP (mmHg) | MVO2 (%) | PCWP (mmHg) | NT-proBNP (ng/L) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 62 | F | CTEPH | III | 49 | 2.1 | 10 | 5 | 60 | 8 | 3870 |
| 2 | 45 | F | iPAH | III | 49 | 1.6 | 16 | 9 | n/a | 4 | 669 |
| 3 | 51 | F | PAH-CTD | III | 49 | 2.4 | 12 | 5 | 72 | 7 | 126 |
| 4 | 43 | F | PAH-CTD | III | 53 | 3.3 | 9 | 2 | 60 | 8 | 803 |
| 5 | 45 | F | CTEPH | III | 45 | 3.6 | 7 | 1 | 70 | 5 | 254 |
| 6 | 56 | F | PAH-CTD | III | 58 | 1.7 | 18 | 6 | 63 | 7 | 2715 |
| 7 | 67 | M | CTEPH | III | 42 | 2.3 | 8 | 5 | 61 | 8 | 1544 |
| 8 | 60 | F | iPAH | III | 65 | 1.6 | 23 | 7 | 63 | 6 | 852 |
| 9 | 54 | M | iPAH | II | 51 | 2.5 | 8 | 9 | n/a | 10 | 40 |
| 10 | 59 | M | iPAH | II | 30 | 2.3 | 7 | 5 | 68 | 8 | 106 |
| 11 | 74 | F | CTEPH | III | 69 | 2.5 | 14 | 2 | 64 | 10 | n/a |
| 12 | 50 | F | PAH-CTD | III | 43 | 2 | 12 | 13 | 53 | 5 | 4672 |
| 13 | 54 | F | iPAH | III | 47 | 2.5 | 8 | 8 | 76 | 11 | 81 |
| 14 | 38 | F | iPAH | II | 25 | 2.2 | 7 | 2 | 58 | n/a | 33 |
| 15 | 56 | M | CTEPH | III | 79 | 1.9 | 19 | 18 | 61 | 7 | 1641 |
CTEPH, chronic thrombo-embolic PH; iPAH, idiopathic pulmonary arterial hypertension; PAH-CTD, PAH related to connective tissue disease; PAP, pulmonary artery pressure; CI, Cardiac Index; PVR, pulmonary vascular resistance; RAP, right atrial pressure; MVO2, mixed venous oxygen saturation; PCWP, pulmonary capillary wedge pressure; NT-proBNP, N terminal pro-B type natriuretic peptide.
Dobutamine dose and symptoms
There were no serious adverse events and all participants completed the study. Of the 15 PH patients, five experienced breathlessness, one reported light-headedness, and one reported headache. None of these symptoms were intolerable or necessitated a reduction in dobutamine dose. Excessive tachycardia (>50% of resting HR) necessitated a reduction in the dobutamine dose to 15 mcg/kg/min in 4/15 patients (patients 3, 7, 8, and 9) before stress-MRI was initiated. The maximum tolerated dose was, therefore, 20 mcg/kg/min in 11 patients and 15 mcg/kg/min in four patients.
Pulmonary artery area and RAC
Data are described in detail in Table 2. AMIN REST was significantly larger in PH patients (median = 8.3 cm2, range = 5.9–10.4 cm2) than in younger volunteers (median = 5.2 cm2, range = 3.6–5.7 cm2; P = 0.0037). Similarly, AMAX REST was larger in PH patients (median = 9.3 cm2, range = 7.3–11.4 cm2) than younger volunteers (median = 6.8 cm2, range = 5.9–7.9 cm2; P = 0.0499). RACREST was significantly lower in PH patients (12.6% [range = 9.4–19.2%]) than in younger volunteers (43.6% [range = 33.1–74.6%]; P = 0.0001).
Table 2.
MRI was performed at rest and during dobutamine stress in 15 patients with PH and five younger volunteers. Values are median (interquartile range).
| PH patients |
Younger Volunteers |
|||
|---|---|---|---|---|
| Rest | Stress | Rest | Stress | |
| Pulmonary artery | ||||
| AMAX (cm2) | 9.3 (7.1–11.0) | 9.6 (7.2–11.5)* | 6.8 (6.0–7.9)† | 7.1 (6.5–8.0) |
| AMIN (cm2) | 8.2 (5.9–9.9) | 8.3 (6.0–10.7)** | 5.2 (3.6–5.7)‡ | 4.1 (3.6–5.0) |
| RAC (%) | 12.6 (9.4–19.2) | 11.0 (9.2–16.5) | 43.6 (33.1–74.6)‡ | 67.5 (29.4–85.4)# |
| Right ventricle | ||||
| SVI (mL/m2) | 23 (17–37) | 26 (22–34) | 47 (44–49)‡ | 52 (49–58)# |
| EDVI (mL/m2) | 98 (68–113) | 81 (60–108) | 67 (61–87) | 64 (58–76) |
| ESVI (mL/m2) | 56 (38–73) | 42 (24–54)* | 29 (23–42)† | 11 (9–22)# |
| EF (%) | 34 (18–43) | 40 (23–46)* | 67 (52–81)‡ | 85 (82–93)# |
| CI (L/min/m2) | 1.9 (1.5–2.4) | 2.8 (2.4–3.5)** | 2.9 (2.4–2.9) | 5.2 (4.4–5.9)# |
| HR (bpm) | 75 (65–87) | 109 (88–121)** | 60 (50–66) | 87 (84–94)# |
| η'vv | 0.6 (0.2–0.8) | 0.8 (0.4–1.3)** | 1.7 (1.0–2.0)‡ | 4.9 (2.9–5.9)# |
AMAX, maximum PA area; AMIN, minimum PA area; RAC, Relative area change; RV, right ventricle; LV, left ventricle; SVI, stroke volume index; EDVI, end-diastolic volume index; ESVI, end-systolic volume index; EF, ejection fraction; CI, Cardiac Index; HR, heart rate; η'vv, RV SV/ESV. *P < 0.05. **P < 0.01 (PH Stress vs. Rest). †P < 0.05. ‡P < 0.01 (PH vs. Volunteers at rest). #P < 0.05 (Volunteers Stress vs. Rest).
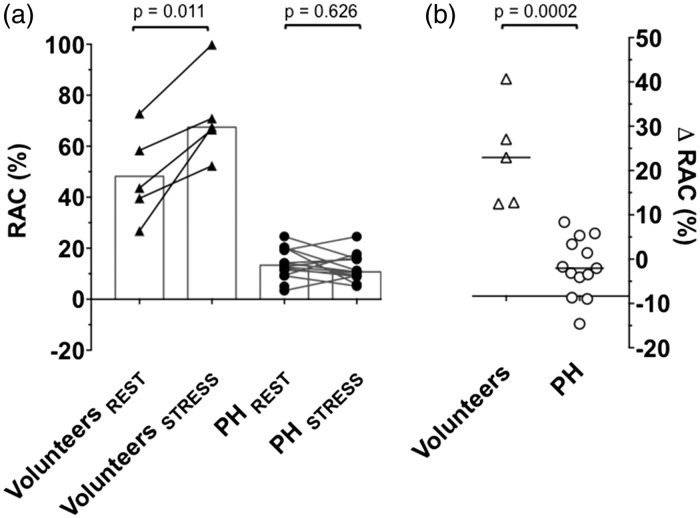
In 2/15 PH patients, the stress MPA cine images were significantly degraded by cardiac motion artefact. Data from 13/15 patients were therefore available for analyses. These data are presented in detail in Table 2. In 11/13 PH patients, AMIN increased (i.e. ΔAMIN was positive). As a result, there was a small but statistically significant increase in median AMIN during stress (8.2 [5.9–9.9] cm2 to 8.3 [6.0–10.7] cm2; P = 0.0034). ΔAMAX was also positive in 11/13 patients and median AMAX also increased by a small but significant amount (9.3 [7.1–11.0] cm2 to 9.6 [7.2–11.5] cm2; P = 0.025). Median RACSTRESS (12.8 [10.4–19.8]%) was not different from median RACREST (10.8 [9.1–16.0]%); P = 0.626), since the interquartile range of ΔRAC traversed zero (median ΔRAC = [−2.1 (−6.4 – 4.4)%]). This was likely due to significant heterogeneity in the effect of stress on RAC in PH patients, with increments seen in some patients and decrements in others (see Fig. 2).
Fig. 2.
Dobutamine stress-MRI was performed in 15 PH patients and five younger volunteers. Pulmonary artery RAC was measured at rest and during stress (a), allowing calculation of ΔRAC (b). In all younger volunteers (depicted by triangles), RACSTRESS was greater than RACREST; ΔRAC was therefore positive in all. In PH patients (depicted by circles) there was no difference in median RACREST (10.8 [9.1–16.0]%) vs. RACSTRESS (12.8 [10.4–19.8]%, P = 0.626) but responses were variable. ΔRAC was negative in 8/13 evaluable PH patients. Median ΔRAC was lower in PH patients (−2.1 [−6.4 – 4.4]%) than younger volunteers (+22.9 [12.7–33.9]%; P = 0.0002).
In younger volunteers, there was a trend towards a reduction in median AMIN and increment in AMAX, but this was not statistically significant. There was, however, evidence of increased RAC during stress (RACREST = 44 [33–75]%, RACSTRESS = 68 [29–85]%; P = 0.0112). Contrary to PH patients, ΔRAC was positive in all younger volunteers with no heterogeneity and median ΔRAC was higher in younger volunteers (+22.9 [12.7–33.9]%) than in PH patients (−2.1 [−6.4 – 4.4]%; P = 0.0002), see Fig. 2.
Inter-observer variability of RAC measurements
There was no statistically significant difference between median ΔRAC measured in PH patients by Operators 1 and 2 (−2.6% versus −0.31%; P = 0.436) and these data showed good correlation (r = 0.731, P = 0.006). The variance of the data appeared similar (Operator 1 SD = 6.6, Operator 2 SD = 7.2). There was good intra-observer agreement between operator regarding ΔRAC (ICC = 0.862 [0.570–0.957], P = 0.001). The level of agreement was superior for RACREST 0.900 ([0.690–0.969]; P < 0.001) relative to RACSTRESS (0.864 [0.46–0.961]; P < 0.001).
RV measurements and RV-PA coupling (η’VV)
As shown in Table 2, η’VV REST was significantly lower in PH patients (0.62 [0.24–0.79]) than in younger volunteers (1.69 [1.071–2.03], P = 0.0005), in whom it exceeded unity. In PH patients, there was evidence of RV systolic dysfunction, with statistically significant differences in RVSVI, RVEF, and RVESVI, relative to younger volunteers. All RV measurements were normal in volunteers.
In PH patients, η’VV increased significantly with dobutamine stress (median Δη’VV: +0.2, P < 0.001), but median η’VV STRESS remained below unity (0.8). Dobutamine stress resulted in a statistically significant fall in RVESVI (median ΔRVESVI = −14 mL, P = 0.0353) and increment in RVEF (median ΔRVEF = +6%, P = 0.0181). Cardiac index (CI) increased significantly (median ΔCI = +0.8 L/min/m2, P = 0.0001), but since RVSVI did not change (median ΔRVSVI = +3 mL, P = 0.208) this was mediated purely by increased HR (median ΔHR = +39 beats per minute [bpm], P < 0.0001).
In younger volunteers, dobutamine resulted in significantly increased η’VV (median Δη’VV = +3.2, P = 0.004), RVSVI (median ΔRVSVI = +5 mL, P = 0.0312), RVEF (median ΔRVEF = +18%, P = 0.0343), HR (median ΔHR = +27 bpm, P = 0.0035) and CI (median ΔCI = +2.3 L/min/m2, P = 0.0037) and significantly decreased RVESVI (median ΔRVESVI = −18 mL, P = 0.0012).
Prognostic impact of RAC in PH patients
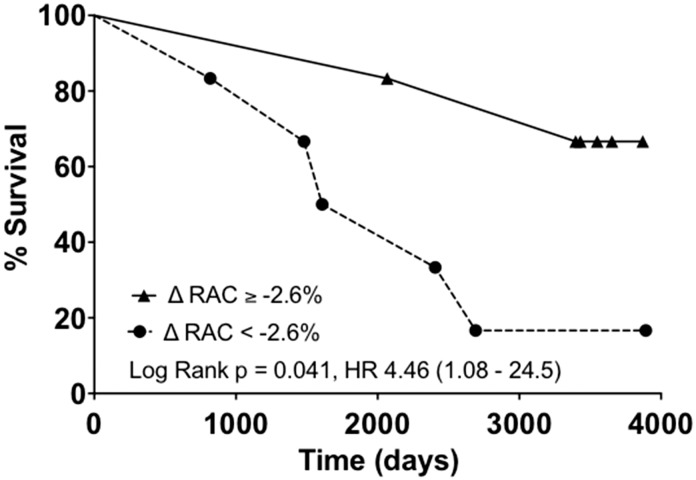
Survival data need to be interpreted with caution in a study of this size. With this caveat, long-term survival appeared significantly poorer in patients with low ΔRAC, defined by a ΔRAC below the median value (−2.6%) (log-rank Chi square = 4.0, P = 0.045), see Fig. 3. The hazard ratio for death in the low ΔRAC group was 4.46 although the lower end of the 95% CI was close to 1 (95% CI = 1.08–24.5). In all but one of the better prognosis (higher ΔRAC) groups, ΔRAC was positive, although the magnitude of this increase was much lower than that seen in healthy younger volunteers.
Fig. 3.
Pulmonary artery RAC was measured at rest and during stress in 15 patients with PH using ds-MRI. Patients with a below median change in RAC with stress (ΔRAC < −2.6%) had a poorer ten-year survival relative to patients with an above median ΔRAC. Twelve of 15 patients were included in survival analyses.
There was no statistically significant difference in survival between groups dichotomized around median values of RACREST (log-rank P = 0.47) or RACSTRESS (log-rank P = 0.59). Similarly, η’VV REST, η’VV STRESS, and Δη’VV yielded no prognostic information (log-rank P values = 0.77, 0.95, and 0.62, respectively). Likewise, MRI-derived RVEF, RVSVI (at rest or stress), and invasive hemodynamic values provided no statistically significant prognostic information.
Correlates of RAC and η’VV
PH patients
RACREST, RACSTRESS, and ΔRAC were each correlated against 19 other variables, including resting hemodynamics and MRI measurements of RV and PA function Therefore, the adjusted level of significance for these comparisons was P < 0.002. ΔRAC correlated inversely with ΔAMIN (r = −0.84, P < 0.0006). ΔRAC did not correlate with ΔAMAX or any other data, including those indicative of RV dysfunction (e.g. RVEFREST, ΔRVEF, RVSVIREST, or ΔRVSVI) or RV-PA coupling (e.g. η’VV at rest or stress). There were no statistically significant correlations between RACREST or RACSTRESS and resting hemodynamic data or any correlations with rest or stress MRI measurements. However, consistent relationships approaching statistical significance were observed between RACSTRESS and measures of stress RV-PA coupling, as η’VV STRESS (r = 0.75, P = 0.004) and Δη’VV (r = 0.75, P = 0.005). A similar association, of borderline significance was observed between RACSTRESS and ΔRVEF (r = 0.73, P = 0.006).
η’VV REST, η’VV STRESS, and Δη’VV were correlated against 18 other variables, including resting hemodynamics and MRI measurements of RV and PA function. Therefore, the adjusted level of significance for these comparisons was P < 0.003. η’VV REST correlated with RVEFREST (r = 0.99, P < 0.0001) and RVMI (r = 0.81, P = 0.005). η’VV STRESS correlated less strongly, but still significantly with RVEFSTRESS (r = 0.90, P < 0.0001), and with RVMI (r = 0.79, P = 0.0007). Δη’VV did not correlate significantly with any variable.
Younger volunteers
In younger volunteers, there were no statistically significant relationships identified between RACREST, RACSTRESS, ΔRAC, η’VV REST, η’VV STRESS, and Δη’VV and MRI-derived RV measurements. Invasive hemodynamics were not recorded in younger volunteers.
Discussion
In this study, there was considerable heterogeneity in the effect of dobutamine stress on RAC (ΔRAC) in 15 patients with PH (see Fig. 2). This was in contrast to the effect in younger volunteers, in whom ΔRAC was universally positive (median ΔRAC = +23%). In 8/13 PH patients, ΔRAC was negative, indicating that the PA became less distensible during stress. In others, there was a marginal increase, but this was not of the same magnitude to that seen in younger volunteers. ΔRAC proved to be the only prognostically significant variable in this study but these results must be interpreted with caution in a study of this size. Notwithstanding this, patients with a below median ΔRAC (<−2.6%) had a hazard ratio for death over the ten-year follow-up period of 4.46 (95% CI = 1.08–24.5, see Fig. 3). These data provide a potentially intriguing insight into the pathophysiology of adverse outcomes in PH from which hypotheses for future studies can be generated.
Associations with ΔRAC and potential mechanisms
Possible explanations for the potential prognostic importance of ΔRAC can be found in the relationships identified between it and other data (Table 3). The only statistically significant correlation identified, after adjusting for the multiple comparisons involved, was with ΔAMIN. We found no association between ΔRAC and ΔAMAX. This suggests that increasing diastolic PA area (AMIN) during stress was the principal reason for the fall in RAC in the poor prognosis PH subgroup. In the younger volunteer population, this behavior was not observed. Instead, the systolic area of the main PA (AMAX) tended to increase, while AMIN tended to decrease; resulting in a universally positive ΔRAC. Conversely, in PH patients, both AMAX and AMIN increased with no overall change in median RAC, but highly variable ΔRAC in individual patients. The factors responsible for this variation are likely to be complex, and include the combined effects of PA stiffening, RV systolic dysfunction, and abnormal RV pressure loading.
Table 3.
MRI was performed at rest and during dobutamine stress in 15 patients with PH and five younger volunteers. Thirteen 15 PH participants with evaluable ΔRAC are dichotomized around the median (−2.6%). Values are median (range). Statistically significant differences between low and high ΔRAC groups are highlighted.
| Low ΔRAC | High ΔRAC | Younger volunteers | |
|---|---|---|---|
| Stress PA | |||
| ΔRAC (%) | −6.4 (−14.6–−3.1)* | 3.4 (−2.1–8.4) | 37.2 (21.4–152.0) |
| ΔAMAX (cm2) | 0.23 (−0.53–0.78) | 0.32 (0.01–0.54) | 0.82 (−1.04–1.33) |
| ΔAMIN (cm2) | 0.67 (0.36–1.09)* | 0.04 (−0.01–0.52) | −0.01 (−1.80–0.22) |
| Stress RV | |||
| ΔSVI (%) | −2 (−21–43) | 15 (−3–116) | 22 (0–43) |
| ΔEF (%) | 1 (−25–54) | 17 (−8–227) | 46 (4–62) |
| ΔHR (%) | 51 (30–114) | 30 (10–56) | 49 (28–89) |
| RV-PA coupling | |||
| η'vv REST | 0.51 (0.11–1.26) | 0.46 (0.16–0.89) | 1.69 (1.01–2.30) |
| η'vv STRESS | 0.80 (0.09–2.52) | 0.66 (0.21–1.69) | 4.86 (2.81–6.13) |
| Resting hemodynamics | |||
| PVR (mmHg/L/min) | 11 (8–19) | 9.5 (7–23) | NR |
| sPAP (mmHg) | 92 (76–132) | 70 (38–112) | NR |
| PAPP (mmHg) | 61 (52–84) | 40 (26–76) | NR |
| MVO2 (%) | 63 (60–76) | 63 (53–70) | NR |
PA, pulmonary artery; RV, right ventricle; RAC, relative area change; RV, right ventricle; AMAX; maximum PA area; AMIN, minimum PA area; SVI, stroke volume index; EF, ejection fraction; HR, heart rate; PVR, pulmonary vascular resistance; sPAP, systolic pulmonary artery pressure; MVO2, mixed venous oxygen saturation; η'vv, RV stroke volume/RV end-systolic volume ratio; NR, not recorded.
P < 0.01 (low ΔRAC vs. high ΔRAC).
We identified consistent trends to association between RACSTRESS and RV-PA coupling efficiency during stress (η’VV STRESS [r = 0.75, P = 0.004] and Δη’VV [r = 0.75, P = 0.005]). Although these associations did not reach our adjusted level of statistical significance, they suggest that increased RV-PA coupling inefficiency may be an important mediator of reduced ΔRAC and might contribute to adverse outcomes. However, we found no detectable differences in coupling efficiency (as η’VV) between the high and low ΔRAC groups, so cannot draw any definitive conclusions in this area. We did find that RV-PA coupling efficiency was less tightly correlated to RV systolic function during stress than at rest, since the correlation coefficient between RVEF and η’VV fell from 0.99 to 0.90 (P value for both <0.0001). This suggests that additional factor(s), likely including proximal PA stiffening, may become more influential under stress conditions. Importantly, we did not identify any significant relationship between RAC and either mean PAPP or PAPP. Mean PAP is a reflection of steady flow conditions and resistance in the small pulmonary arteries, while PAPP is linearly correlated with the pulmonary circulation distensibility co-efficient, α.7,22
Translational potential: mechanisms of stress-induced PA stiffening
Although our data suggest that the principal determinant of low ΔRAC, and potentially a poorer outcome in this study, was proximal PA stiffening, these findings are not sufficient to precisely resolve the mechanism(s) involved. A better understanding from future studies is clearly desirable. If the prognostic impact of ΔRAC is validated elsewhere, these mechanisms might be targetable by novel PH therapies specific to the proximal PA. Potential mechanisms are discussed here.
In PH, the distal vasculature is already fully recruited and distended at rest. Dobutamine stress results in abnormal RV pressure loading, with pressure-induced stiffening of the proximal arteries and early return of reflected pressure waves with transmission of these forces onto the still-ejecting RV.23 This would generate extreme trans-mural diastolic PAP and an increase in AMIN and may have resulted in the fall in RAC is some PH patients during stress. An alternative mechanism would be an inability to increase proximal vascular tone during stress. Also, thickening and stiffening of the proximal PA could result in a decrease in diastolic elastic recoil. Remodeling of the PA due to chronic PH would explain why, even in the presence of a dobutamine-induced increase in CI, AMIN was unchanged or mildly increased compared to resting conditions in the PH patients studied. The relative importance of these mechanisms should be explored in future studies, which if large enough might also be able to define specific mechanisms in PH groups. For example, differing biomechanics are highly plausible in idiopathic PAH versus CTEPH and in sub-types of CTEPH, but could not be meaningfully explored in this study.
The influence of RV function
In the current study, we did not observe any statistically significant difference in RV performance parameters between low and high ΔRAC sub-groups (Table 3). However, the numbers involved were small, making meaningful sub-group comparisons difficult. We did observe a trend towards lower RV function in the poor prognosis, low ΔRAC group (median ΔRVEF = 1% versus 17%; median ΔRVSVI = −2% versus 15%, see Table 3). These observations would be in keeping with multiple previous associations between adverse survival and reduced RV performance in PH.1–3 Detection of a relationship between ΔRAC and survival, where none is observed with RV parameters, may reflect a Type II error due to the small sample size, but may also indicate that the prognostic impact of the vascular response to stress (as ΔRAC) exceeds that of the ventricular response (as ΔRVEF, ΔRVSV). The latter hypothesis is supported by recent data reported by Vanderpool et al., which describe a hierarchy of prognostic biomarkers in PH patients.24 In that study, RV-PA coupling outperformed RVEF (both measured by MRI) and invasive hemodynamics in multivariable prognostic models. We did not perform a multivariable analysis to determine the relative prognostic value of RAC and RV responses because the small samples size precludes this. However, we doubt that any additional prognostic information derived from ΔRAC would justify the additional cost and burden of ds-MRI in clinical practice. RV performance is well established as the primary prognostic tool in PH and can be easily assessed by resting CMR.14 Instead, the point of this study is primarily conceptual and hypothesis generating. Specifically, our data suggest (but do not prove) that proximal PA function may be a more important determinant of outcome, or response to particular PH therapies, than RV function in some PH patients. The predictive value of vascular response was previously demonstrated in an MRI pilot study, reported by Jardim, in which a RAC threshold of 10% differentiated responders from non-responders to calcium channel blockers with 100% sensitivity and 56% specificity.25
Comparison with previous studies
Swift et al. recently demonstrated that RACREST was prognostically important in patients with early-stage disease, as defined by minor elevations in PVR (<4 WU increase) and normal RV function.13 Although our study was conducted in patients with more advanced disease (median PVR = 10 [8–16] mmHg/L/min, median RVEF = 34 [18–43]%), our findings are in agreement with this work.
In an earlier study, we reported a non-linear, complex relationship between PAPP and survival in 67 patients with PH.7 In that study, although PAPP correlated linearly with distal pulmonary arterial distensibility, as described by α, mortality was associated in a non-linear fashion. Patients with intermediate levels of PAPP had the highest mortality, likely reflecting greater RV dysfunction in that cohort. Unlike PAPP, ΔRAC may be capable of integrating information regarding pulmonary arterial stiffening and RV function in patients with PH, resulting in a linear relationship with survival, similar to that previously reported by Mahapatra et al. regarding pulmonary arterial capacitance (RVSV/PAPP).26 Although we found no statistically significant differences in measures of RV performance in ΔRAC sub-groups (Table 3) a future, larger study would be able to determine these factors more adequately.
Our results differ from previous literature in that we did not find a correlation between RACREST and invasive PA pressures, or any prognostic influence of RACREST. We did, however, demonstrate a relationship between ΔRAC and survival. The most likely explanation for this apparent discordance is the small sample size of the current study. The previous studies investigating RACREST involved 8612 and 13413 patients, respectively. It may be that the additional sensitivity provided by stress imaging allowed detection of a prognostic relationship with ΔRAC but not RACREST. We therefore do not believe that our findings conflict with these earlier studies. Instead, we think it is more likely that our sample was simply too small to detect the relationships they observed without stress.
Study limitations
It is essential to interpret the survival data in the current study with caution given the small sample size. However, this was necessitated by the relatively low incidence of PH and the complexity and invasive nature of the measurements. The sample size calculation for the study used data recorded at rest because no stress data had been published at that time. However, a post-hoc power calculation using the actual difference in median ΔRAC observed between rest and stress in PH participants indicates that the sample recruited (n = 15) delivered 98.8% power. Nevertheless, the small sample means it is possible that additional factors involved in adverse RAC behavior have not been detected, including a more significant impact of RV dysfunction, which appears possible from our data. A further limitation is the poor age matching of volunteer and PH populations (median age of volunteers = 33 [31–49] years, PH patients = 54 [45–60] years; P = 0.013). This reflected difficulty in recruiting age-matched normal controls not taking any cardiac medications. Nevertheless, it is possible that the differences in response to dobutamine between cohorts were purely due to age. However, this seems unlikely since previous authors have reported similar differences in RAC between PH patients and age matched controls at rest.12,13 Furthermore, we found no difference in the age of PH patients with high ΔRAC (48 [43–59] years) versus low ΔRAC (55 [53–58] years; P = 0.227). In addition, there was no correlation between age and ΔRAC (r = −0.138, P = 0.64) or RACSTRESS (r = −0.135, P = 0.645). Importantly, the poor age matching between PH patients and volunteers would not affect our conclusions regarding the influence of Δ RAC on survival in the PH cohort.
Conclusions
In this study, low ΔRAC was associated with proximal PA stiffening during dobutamine stress. A low (and frequently negative) ΔRAC in response to dobutamine was also associated with an adverse clinical outcome, although these results need to be interpreted with caution given the size of the study. Interestingly, RV performance was not strongly associated with low ΔRAC, nor predictive of long-term survival, suggesting that the vascular response to stress may have more prognostic value than the ventricular response in some PH patients. A larger, prospective study is required to validate the potential prognostic impact of ΔRAC during dobutamine-stress MRI in patients with PH. If this confirms the data reported here, and better defines the biomechanics involved, it may be worth pursuing disease modifying therapies targeted to the proximal PA circulation.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This study was supported by a project grant from Chest, Heart & Stroke Scotland. KGB is currently supported by a NHS Research Scotland Senior Research Fellowship. Support from NIH 1R01HL105598 (to NCC) is also gratefully acknowledged.
References
- 1.van Wolferen SA, Marcus JT, Boonstra A, et al. Prognostic value of right ventricular mass, volume, and function in idiopathic pulmonary arterial hypertension. Eur Heart J 2007; 28: 1250–1257. [DOI] [PubMed] [Google Scholar]
- 2.Sandoval J, Bauerle O, Palomar A, et al. Survival in primary pulmonary hypertension. Validation of a prognostic equation. Circulation 1994; 89: 1733–1744. [DOI] [PubMed] [Google Scholar]
- 3.Fijalkowska A, Kurzyna M, Torbicki A, et al. Serum N-terminal brain natriuretic peptide as a prognostic parameter in patients with pulmonary hypertension. Chest 2006; 129: 1313–1321. [DOI] [PubMed] [Google Scholar]
- 4.Sitbon O, Humbert M, Nunes H, et al. Long-term intravenous epoprostenol infusion in primary pulmonary hypertension: prognostic factors and survival. J Am Coll Cardiol 2002; 40: 780–788. [DOI] [PubMed] [Google Scholar]
- 5.Eysmann SB, Palevsky HI, Reichek N, et al. Two-dimensional and Doppler-echocardiographic and cardiac catheterization correlates of survival in primary pulmonary hypertension. Circulation 1989; 80: 353–360. [DOI] [PubMed] [Google Scholar]
- 6.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115: 343–349. [DOI] [PubMed] [Google Scholar]
- 7.Blyth KG, Syyed R, Chalmers J, et al. Pulmonary arterial pulse pressure and mortality in pulmonary arterial hypertension. Respir Med 2007; 101: 2495–2501. [DOI] [PubMed] [Google Scholar]
- 8.Sharma T, Lau EMT, Choudhary P, et al. Dobutamine stress for evaluation of right ventricular reserve in pulmonary arterial hypertension. Eur Respir J 2015; 45: 700–708. [DOI] [PubMed] [Google Scholar]
- 9.Bellofiore A, Roldán-Alzate A, Besse M, et al. Impact of acute pulmonary embolization on arterial stiffening and right ventricular function in dogs. Ann Biomed Eng 2013; 41: 195–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lotz J, Meier C, Leppert A, et al. Cardiovascular flow measurement with phase-contrast MR imaging: basic facts and implementation. Radiographics 2002; 22: 651–671. [DOI] [PubMed] [Google Scholar]
- 11.Sanz J, García-Alvarez A, Fernández-Friera L, et al. Right ventriculo-arterial coupling in pulmonary hypertension: a magnetic resonance study. Heart 2012; 98: 238–243. [DOI] [PubMed] [Google Scholar]
- 12.Gan CT-J, Lankhaar J-W, Westerhof N, et al. Noninvasively assessed pulmonary artery stiffness predicts mortality in pulmonary arterial hypertension. Chest 2007; 132: 1906–1912. [DOI] [PubMed] [Google Scholar]
- 13.Swift AJ, Rajaram S, Condliffe R, et al. Pulmonary artery relative area change detects mild elevations in pulmonary vascular resistance and predicts adverse outcome in pulmonary hypertension. Invest Radiol 2012; 47: 571–577. [DOI] [PubMed] [Google Scholar]
- 14.Benza R, Biederman R, Murali S, et al. Role of cardiac magnetic resonance imaging in the management of patients with pulmonary arterial hypertension. J Am Coll Cardiol 2008; 52: 1683–1692. [DOI] [PubMed] [Google Scholar]
- 15.Galiè N, Torbicki A, Barst R, et al. Guidelines on diagnosis and treatment of pulmonary arterial hypertension. The Task Force on Diagnosis and Treatment of Pulmonary Arterial Hypertension of the European Society of Cardiology. Eur Heart J 2004; 25: 2243–2278. [DOI] [PubMed] [Google Scholar]
- 16.Blyth KG, Groenning BA, Martin TN, et al. Contrast enhanced-cardiovascular magnetic resonance imaging in patients with pulmonary hypertension. Eur Heart J 2005; 26: 1993–1999. [DOI] [PubMed] [Google Scholar]
- 17.Blyth KG, Groenning BA, Mark PB, et al. NT-proBNP can be used to detect right ventricular systolic dysfunction in pulmonary hypertension. Eur Respir J 2007; 29: 737–744. [DOI] [PubMed] [Google Scholar]
- 18.Lorenz CH, Walker ES, Morgan VL, et al. Normal human right and left ventricular mass, systolic function, and gender differences by cine magnetic resonance imaging. J Cardiovasc Magn Reson 1999; 1: 7–21. [DOI] [PubMed] [Google Scholar]
- 19.Sunagawa K, Maughan WL, Sagawa K. Optimal arterial resistance for the maximal stroke work studied in isolated canine left ventricle. Circ Res 1985; 56: 586–595. [DOI] [PubMed] [Google Scholar]
- 20.Fourie PR, Coetzee AR, Bolliger CT. Pulmonary artery compliance: its role in right ventricular-arterial coupling. Cardiovasc Res 1992; 26: 839–844. [DOI] [PubMed] [Google Scholar]
- 21.Casalino E, Laissy JP, Soyer P, et al. Assessment of right ventricle function and pulmonary artery circulation by cine-MRI in patients with AIDS. Chest 1996; 110: 1243–1247. [DOI] [PubMed] [Google Scholar]
- 22.Reeves JT, Linehan JH, Stenmark KR. Distensibility of the normal human lung circulation during exercise. Am J Physiol Lung Cell Mol Physiol 2005; 288: L419–25. [DOI] [PubMed] [Google Scholar]
- 23.Ha B, Lucas CL, Henry GW, et al. Effects of chronically elevated pulmonary arterial pressure and flow on right ventricular afterload. Am J Physiol 1994; 267: H155–65. [DOI] [PubMed] [Google Scholar]
- 24.Vanderpool RR, Pinsky MR, Naeije R, et al. RV-pulmonary arterial coupling predicts outcome in patients referred for pulmonary hypertension. Heart 2015; 101: 37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jardim C, Rochitte CE, Humbert M, et al. Pulmonary artery distensibility in pulmonary arterial hypertension: an MRI pilot study. Eur Respir J 2007; 29: 476–481. [DOI] [PubMed] [Google Scholar]
- 26.Mahapatra S, Nishimura RA, Sorajja P, et al. Relationship of pulmonary arterial capacitance and mortality in idiopathic pulmonary arterial hypertension. J Am Coll Cardiol 2006; 47: 799–803. [DOI] [PubMed] [Google Scholar]