Abstract
Translational research depends on the relevance of animal models and how well they replicate human disease. Here, we investigated plasma levels of three important pro-inflammatory cytokines (TNFα, IL-6, and MCP-1), known to be elevated in human pulmonary arterial hypertension (PAH), and systematically assessed their levels in PAH patients compared to five different rodent models of pulmonary hypertension (PH). A consistent immunoassay platform (Luminex xMAP) and source (Millipore) was used to measure all specimens. PAH patients (n = 29) exhibited significant elevations in all three cytokines (median [IQR] pg/mL; TNFα, 7.0 [4.8–11.7]; IL-6, 9.2 [3.8–17.2]; MCP-1, 109 [65–142]) versus healthy participants (n = 20) (median [IQR] pg/mL; TNFα, 3.0 [2.0–3.6]; IL-6, 1.7 [0.5–7.2]; MCP-1, 79 [49–93]. In contrast, mice with PH established after three weeks of hypoxia (n = 18) or SU5416 plus hypoxia (n = 20) showed no significant change in their plasma cytokine levels versus controls (n = 16), based on three to four independent experiments per group. Similarly, plasma cytokine levels were not elevated in rats with PH established three weeks after monocrotaline (n = 23), eight weeks after SU5416 alone (n = 10) or six to eight weeks after SU5416 plus hypoxia (n = 21) versus controls (n = 36 rats), based on three to eight independent experiments per group. Positive biologic control specimens from sepsis patients (n = 9), cecal-ligation and puncture (CLP)-induced septic mice (n = 6), and lipopolysaccharide-induced septic rats (n = 4) showed robust elevations in all three cytokines. This study suggests that animal models commonly used for the development of novel diagnostic and therapeutic approaches for PAH may have limited construct validity with respect to markers of systemic immune activation seen in human patients.
Keywords: inflammation, mouse, rat, animal models, monocrotaline, SU5416, hypoxia, circulating biomarkers
Introduction
Preclinical research, facilitated by animal models, is critical for interrogating the biology of human disease. In the study of pulmonary hypertension (PH), a number of different rodent models1 have been commonly used including rats or mice exposed to monocrotaline (MCT). These animal models recapitulate some, but not all, of the most salient pathophysiologic features observed in patients with pulmonary arterial hypertension (PAH), such as elevated pulmonary hemodynamics and vascular remodeling. Recently, newer models have been developed that reproduce the complex arterial remodeling that is thought to contribute to vascular obliteration and progressive increases in pulmonary vascular resistance in human patients, in particular, SU5416 plus chronic hypoxia, or SU5416 alone in specific rat strains.2 However, it remains unclear whether, and to what extent, the underlying pathobiological and molecular changes that contribute to PAH in humans can be faithfully reproduced in these various animal models.
In particular, there is increasing clinical and experimental evidence to support inflammation as an important factor contributing to the development and/or progression of PAH.3 Several independent clinical studies of idiopathic PAH (IPAH) have demonstrated significant elevations in the plasma/serum levels of the pro-inflammatory cytokines TNF-α, IL-6, and MCP-1,4–7 which in some cases have been shown to predict mortality.6,7 Targeted overexpression and knock-out models have also suggested that IL-6 plays a causal role in PH in transgenic mice.8–10 Nevertheless, there remains limited information on how the circulating levels of these cytokines change in response to experimentally induced PH in animal models.
In this study, we investigated whether alterations in systemic levels of pro-inflammatory cytokines were comparable between PAH patients and five of the most common rodent models. To minimize spurious correlations, we focused on three specific cytokines, TNF-α, IL-6, and MCP-1, that have been independently validated in one or more previous patient studies,4–7 and used plasma levels as a practical readout to facilitate direct comparisons between human and rodent PH. Construct validity is a prerequisite for successful translation of novel diagnostic and therapeutic strategies from bench to bedside. This study offers novel insight into the relevance of current experimental PH models for human disease with respect to systemic inflammation, and may help to inform new model development and/or guide strategic fit-for-purpose application of current models.
Methods
Inclusion/Exclusion criteria for patients and control participants
Peripheral blood samples from PAH patients and healthy participants were obtained with written informed consent during 2011–2014 at a single center. All methods were conducted in accordance with protocols, guidelines, and regulations approved by the Ottawa Hospital Research Ethics Board (#2011470-01H). PAH patients were outpatients with a clinical diagnosis of PAH (either idiopathic or associated) and able to provide free and informed consent. Associated PAH (APAH) included participants with congenital heart disease or scleroderma. Healthy participants were non-smokers (or had quit smoking ≥ 6 months prior to screening) and had no history of diabetes mellitus requiring medication (oral hypoglycemics or insulin), systemic hypertension requiring medication, hypercholesterolemia requiring medication, chronic asthma or chronic obstructive pulmonary disorders, scarring or fibrosis of the lung, or cancer in the previous five years (excluding superficial skin cancer). Peripheral blood samples from sepsis patients were obtained with informed written consent in 2009 in accordance with protocols, guidelines, and regulations approved by the respective research ethics boards in the multi-center Fluid Resuscitation with 5% Albumin versus Normal Saline in Early Septic Shock (PRECISE) pilot trial.11 Inclusion/exclusion criteria for these patients are as described previously.11
Human plasma isolation
Peripheral blood from PAH and healthy volunteers was first drawn into a 3 mL SST tube, which was subsequently discarded to eliminate blood that contacted tissue during venipuncture. Blood was drawn into Becton-Dickinson (BD) vacutainer (sodium citrate) tubes, and centrifuged at 200 × g for 15 min (4℃). The upper plasma phase was transferred into fresh microfuge tubes, and centrifuged twice at 11 000 × g for 2 min (4℃) to remove residual cells/platelets/cell debris, with transfer of the plasma supernatant into fresh microfuge tubes between each spin. Plasma was stored at −80℃. Peripheral blood from sepsis patients was initially drawn into BD vacutainers (sodium citrate), then transferred into a second vessel supplemented with sterile benzamidine (20 mM final concentration), and centrifuged at 1700 × g for 10 min (4℃). The upper plasma fraction was carefully removed (to within 0.2 mL of the plasma-cell interface) and transferred into cryo tubes for storage at −80℃.
Animal models
All animal procedures were approved by the University of Ottawa’s Animal Care Ethics Committee and complied with the principles and guidelines of the Canadian Council on Animal Care. Right ventricular systolic pressure (RVSP) was measured using a pressure catheter transducer and readings were determined by observing sequential pressure loops recorded on LabScribe2 software (Transonic Scisense, Inc.). Right ventricle hypertrophy was assessed as a weight ratio of the right ventricle (RV) over the left ventricle plus septum (LV + S). For the MCT model of PH, male Fischer rats (two original experiments; 161 ± 14 g, CDF strain from Charles River) or male Spraque-Dawley rats (one validation experiment: 176 ± 5 g, SD strain from Harlan) were injected with a single intraperitoneal dose (70 mg/kg body weight) of MCT (Sigma) or saline as vehicle control. End of study measurements were conducted three weeks after MCT injection. For the SU5416 plus hypoxia rat model and SU5416 alone rat model, male Sprague Dawley rats (four original experiments: 220 ± 54 g, Charles River) or male Sprague-Dawley rats (one validation experiment: 187 ± 7 g, Harlan) were subcutaneously injected with 20 mg/kg SU5416 (Sigma) suspended in 0.5% CMC (0.5% carboxymethyl cellulose, 0.9% sodium chloride, 0.4% Tween 80, 0.9% benzyl alcohol in deionized water) or 0.5% CMC as vehicle control. Rats were housed in a ventilated hypoxia chamber (10% O2, Biospherix) for the first three weeks, and then housed under normoxic conditions until the study endpoint at six or eight weeks post SU5416 injection (as noted in relevant figure legend). Rats that received SU5416 only were housed under normoxia for eight weeks. For the lipopolysaccharide (LPS) rat model of sepsis, male Sprague-Dawley rats (270 ± 20 g, Charles River) received a single intraperitoneal injection of LPS (5 mg/kg bw; Sigma, E. coli serotype 055:B5) and were sacrificed 3 h post LPS exposure. For the chronic hypoxia and SU5416 plus hypoxia mouse models of PH, male C57Bl6J mice (six weeks old, 22 ± 3 g, Jackson Laboratories) were housed in a ventilated hypoxia chamber (10% O2, Biospherix). Normoxic mice were kept in the same room and on the same light–dark cycle. End of study measurements were conducted at three weeks. For the SU5416 plus hypoxia mouse model of PH, mice received one, three (one injection per week) or six (two injections per week) doses of 20 mg/kg SU5416 (Sigma) suspended in 0.5% CMC, and were housed in a ventilated hypoxia chamber (10% O2, Biospherix) for three weeks. Cecal-ligation and puncture (CLP) model of sepsis: Male mice (aged 10–12 week, C57Bl6J, Jackson Laboratories) were anesthetized with 200 mg/kg ketamine (Ketalean, 100 mg/mL; Bimeda-MTC Animal Health Inc., Cambridge, ON, Canada) and 10 mg/kg xylazine hydrochloride (Rompun, 20 mg/mL; Bayer Inc., Toronto, ON, Canada) by intraperitoneal injection. The mice were positioned in dorsal recumbency, and the ventrums were shaved and prepared with 70% ethanol. A ventral midline incision (∼1 cm) was made to allow exteriorization of the cecum. The cecum was ligated 1 cm from the apex with 3–0 silk suture and penetrated through-and-through with a 22-gauge needle. The abdominal incision was then closed in two layers with 4–0 nylon suture and then staples. Sham surgery, in which the cecum was exteriorized and manipulated as described but not ligated or punctured, was performed in control animals. Immediately after surgery, animals were fluid resuscitated with 50 mL/kg saline injected subcutaneously. Animals were euthanized at 12 h and 24 h post surgery to collect plasma.
Animal plasma isolation
Rat and mouse blood was drawn from the inferior vena cava into syringe's containing 50 mM EDTA (pH 8.0) at one-tenth the final volume of drawn blood, which was subsequently centrifuged at 1700 × g for 10 min at 4℃. The upper plasma phase was transferred to a fresh microfuge tube (with care taken not to disturb the lower cell phase), centrifuged again at 11 000 x g for 2 min (4℃), followed by transfer of the supernatant into fresh tubes prior to storage at −80℃.
Cytokine measurements
Cytokine levels in humans, rats, and mice were measured in a fixed volume of plasma (25 µL) using Luminex xMAP technology with the Milliplex brand of magnetic-bead fluorescent immunoassays (Millipore EMD) specific for each species, according to manufacturer’s instructions. The ProcartaPlex brand of Luminex xMAP magnetic-bead fluorescent immunoassays (Affymetrix) was also used to validate cytokine levels in select rat plasma specimens. All assays were run on a Bioplex 200 system (Biorad). Data were fit to a seven-point standard curve generated using five-parameter logistic regression with Bioplex manager software. Extrapolated values were used for samples that exhibited fluorescent signals above background (i.e., above blank samples) but below the standard curve range. Samples that were below the standard curve range and below background levels of fluorescence were set to the lowest extrapolated value observed among all samples in a given assay run, or the manufacturer defined minimum detectable concentration, whichever was lower. Immunoassays were confirmed to work within manufacturer’s specifications, evidenced by positive internal quality control standards run in parallel with biologic samples. All human and animal plasma samples were limited to one to two freeze-thaw cycles at the time of measurement (except sepsis plasma with two to four freeze-thaw cycles). Statistical tests were performed with Graphpad Prism V7.0, and are reported in the figure legends.
Results
Plasma levels of TNF-α, IL-6, and MCP-1 are elevated in PAH patients
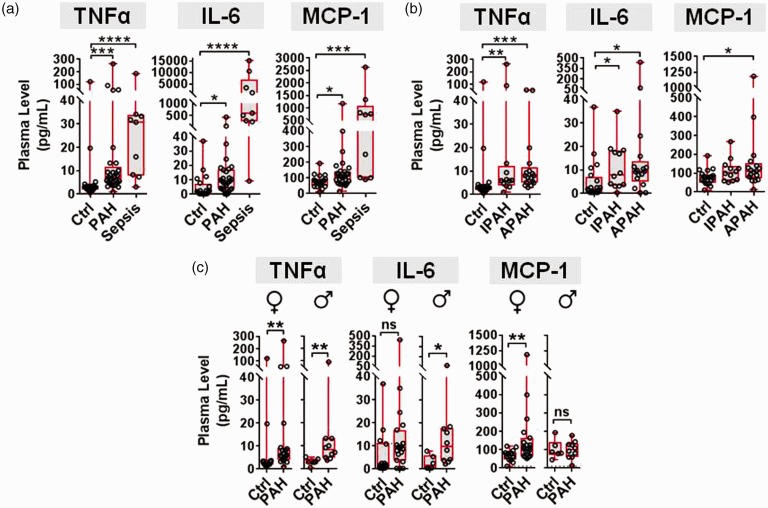
Demographic and clinical characteristics of patients are presented in Table 1. The plasma levels of TNF-α, IL-6 and MCP-1 were significantly elevated in PAH patients versus healthy control participants (Fig. 1a, Table 1). The change in median cytokine levels in PAH patients ranged from 1.4 (MCP-1) to 5.2 (IL-6) fold above basal levels in healthy participants, which is consistent with levels reported previously in IPAH.4–7 As a positive control and comparator, median cytokine levels in patients with a prototypical inflammatory condition such as sepsis ranged from 9.4 (MCP-1) to 356 (IL-6) fold above basal levels (Fig. 1a, Table 1). Plasma cytokine levels were also assessed after stratification of participants by type of PAH and sex, to facilitate comparison with the animal models, most of which are used as preclinical models of IPAH and typically conducted with male animals. When PAH participants were divided into idiopathic (n = 13) and associated (n = 16) forms of the disease, TNF-α and IL-6 remained significantly elevated in both groups of PAH patients compared to controls (Fig. 1b). MCP-1 was significantly increased in the APAH group, but did not quite reach statistical significance in the IPAH group. When participants were stratified according to sex, TNFα levels were significantly elevated in both female and male PAH patients, IL-6 was only significantly elevated in males (though a non-significant trend toward higher levels was observed in female patients), and MCP-1 was only significantly increased in female patients (Fig. 1c).
Table 1.
Clinical characteristics of healthy (control) participants, PAH, and sepsis patients.
| Characteristic | Control | PAH | Sepsis |
|---|---|---|---|
| Sample size (n) | 20 | 29 | 9 |
| Age* (years) | 46 ± 12 | 62 ± 11† | 55 ± 9 |
| Female (n (%)) | 14 (70) | 19 (66) | 4 (44) |
| APACHE score | N/A | N/A | 21 ± 9 |
| Cause of PAH (n (%)) | |||
| Idiopathic | N/A | 13 (45) | |
| Associated | N/A | 16 (55) | |
| Connective tissue disease; scleroderma | 14 (48) | ||
| Congenital heart disease | 2 (7) | ||
| WHO functional class (n (%)) | |||
| Class I | 1 (3) | ||
| Class II | 18 (62) | ||
| Class III | 8 (28) | ||
| Class IV | 2 (7) | ||
| PAH medications (n (%)) | 28 (97) | ||
| Diuretics | 16 (57) | ||
| Calcium channel blockers | 2 (7) | ||
| Prostacylins | 3 (11) | ||
| Endothelin receptor blockers | 21 (75) | ||
| Phosphodiesterase inhibitors | 6 (21) | ||
| Hemodynamic parameters* (n) | |||
| mPAP (mmHg) | 46 ± 15 (28) | ||
| PVR (dsc) | 603 ± 293 (26) | ||
| PCWP (mmHg) | 11 ± 6 (27) | ||
| Plasma cytokine levels (pg/mL)‡ | |||
| TNFα | 3.0 (2.0–3.6) | 7.0 (4.8–11.7)§ | 30.8 (7.8–33.8)** |
| IL-6 | 1.7 (0.5–7.2) | 9.2 [3.8–17.2]*** | 605 [269–7042]** |
| MCP-1 | 79 (49–93) | 109 [65–142]*** | 742 [101–1083]§ |
Mean ± Std Dev.
P < 0.01 versus controls.
Median (interquartile range).
P < 0.001 versus controls. ***P < 0.05 versus controls.
P < 0.0001 versus controls.
mPAP, mean pulmonary arterial pressure; PAH, pulmonary arterial hypertension; PCWP, pulmonary capillary wedge pressure; PVR, pulmonary vascular resistance.
Fig. 1.
Plasma cytokine levels in PAH patients. (a) Plasma levels of target cytokines in healthy participants (Ctrl, n = 20), PAH patients (n = 29), and sepsis patients (n = 9). (b) Plasma cytokine levels stratified according to IPAH (n = 13) and APAH (n = 16). (c) Cytokine changes between Ctrl and PAH participants are also shown stratified by sex (female n = 14 Ctrl, n = 19 PAH; male n = 6 Ctrl, n = 10 PAH). Boxplots denote median and interquartile range, and whiskers denote min/max values. Individual participants are shown as circles. Axes are split on graphs to show distribution of data. Statistical significance of cytokine changes was determined by non-parametric Kruskal–Wallis with Dunn’s multiple comparison test for three or more groups, and Mann–Whitney test for two groups. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus Ctrl group.
Plasma levels of TNF-α, IL-6, and MCP-1 are not elevated in two different mouse models of PH
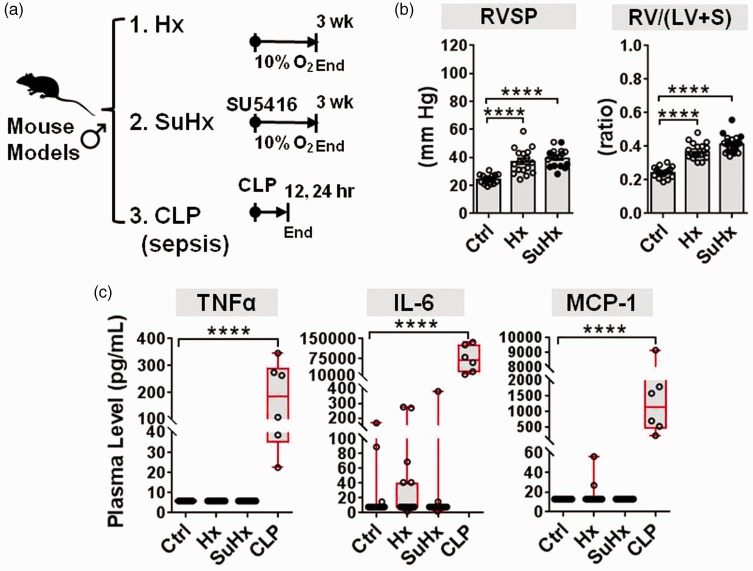
Plasma cytokine levels were assessed in mice after three weeks of chronic hypoxia with and without SU5416, and in mice 12–24 h following CLP to induce sepsis as a positive control (Fig. 2a). The development of PH was confirmed in both PH models just before plasma sampling, as evidenced by significant increases in right ventricular systolic pressure (RVSP) and the right ventricle (RV) to left ventricle plus septum (LV + S) weight ratio (Fig. 2b). TNF-α, IL-6, and MCP-1 plasma levels were not significantly elevated in either PH mouse model versus controls (Fig. 2c). However, mice with experimental sepsis exhibited significant increases in median plasma levels of TNF-α (23-fold), MCP-1 (88-fold), and IL-6 (8795-fold) (all P < 0.0001 versus controls, Fig. 2c), consistent with the marked elevations observed in sepsis patients.
Fig. 2.
Plasma cytokine levels in mouse models of PH. (a) Schematic overview of experimental timelines used for two different mouse models of PH and one mouse model of sepsis. PH was induced in mice by either hypoxia alone (Hx n = 18 mice from four independent experiments) or in combination with SU5416 (SuHx n = 20 mice from three independent experiments). Cytokine levels were measured after three weeks versus controls (Ctrl n = 16 mice from four independent experiments). As a positive control comparator, mice with CLP-induced sepsis were assessed 12–24 h post procedure (CLP n = 6, Ctrl n = 3 mice from one experiment). (b) RVSP and the RV/(LV + S) weight ratio confirm establishment of PH (bar charts denote mean ± SEM). SuHx mice denoted by open circles in bar graphs received three to six doses of SU5416 in an effort to increase PH severity. (c) Plasma levels of target cytokines measured by Milliplex immunoassay. Boxplots denote median and interquartile range, and whiskers denote min/max values. Individual animals are shown as circles. Axes are split on graphs to show distribution of data. Statistical significance of RVSP and RV/(LV + S) was determined by one-way ANOVA and Dunnett’s multiple comparison test. Statistical significance of cytokine changes was determined by non-parametric Kruskal–Wallis with Dunn’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus Ctrl group.
Plasma levels of TNF-α, IL-6, and MCP-1 are not elevated in three different rat models of PH
Plasma cytokine levels were evaluated in three different rat models of PH including MCT rats three weeks after exposure, SU5416 plus chronic hypoxia (SuHx) rats eight weeks after exposure, and SU5146 alone (Su) rats eight weeks after exposure (Fig. 3a). The establishment of PH was verified in all three models, which showed significant elevations in RVSP and RV/(LV + S) ranging from 67 ± 4 mmHg and 0.36 ± 0.02 weight ratio in the MCT model to 98 ± 8 mmHg and 0.7 ± 0.02 weight ratio in the Su model (versus control group 28 ± 0.3 mmHg and 0.25 ±0.006 fulton index) (Fig. 3b). Plasma levels of TNF-α, IL-6, and MCP-1 were not significantly elevated in any of the rat models (Fig. 3c). The only change in plasma cytokine levels was a modest (1.7-fold) decrease in MCP-1 levels observed in rats with MCT-induced PH.
Fig. 3.
Plasma cytokine levels in rat models of PH. (a) Schematic overview of experimental timelines used for three different rat models. PH was induced in rats by either MCT (n = 15 rats from two independent experiments), SU5416 alone (Su; n = 10 rats from four independent experiments) or in combination with three weeks of hypoxia (SuHx; n = 9 rats from two independent experiments), and compared to controls (Ctrl; n = 28 rats from six independent experiments) after weeks 3 or 8. (b) RVSP and the right ventricle to left ventricle plus septum weight ratio confirm establishment of PH (bar charts denote mean ± SEM). (c) Plasma levels of target cytokines measured by Milliplex immunoassay. Boxplots denote median and interquartile range, and whiskers denote min/max values. Individual animals are shown as circles. Axes are split on graphs to show distribution of data. Statistical significance of RVSP and RV/(LV + S) was determined by one-way ANOVA and Dunnett’s multiple comparison test. Statistical significance of cytokine changes was determined by non-parametric Kruskal–Wallis with Dunn’s multiple comparison test. *P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 versus Ctrl group.
Archived plasma specimens show no evidence of cytokine degradation
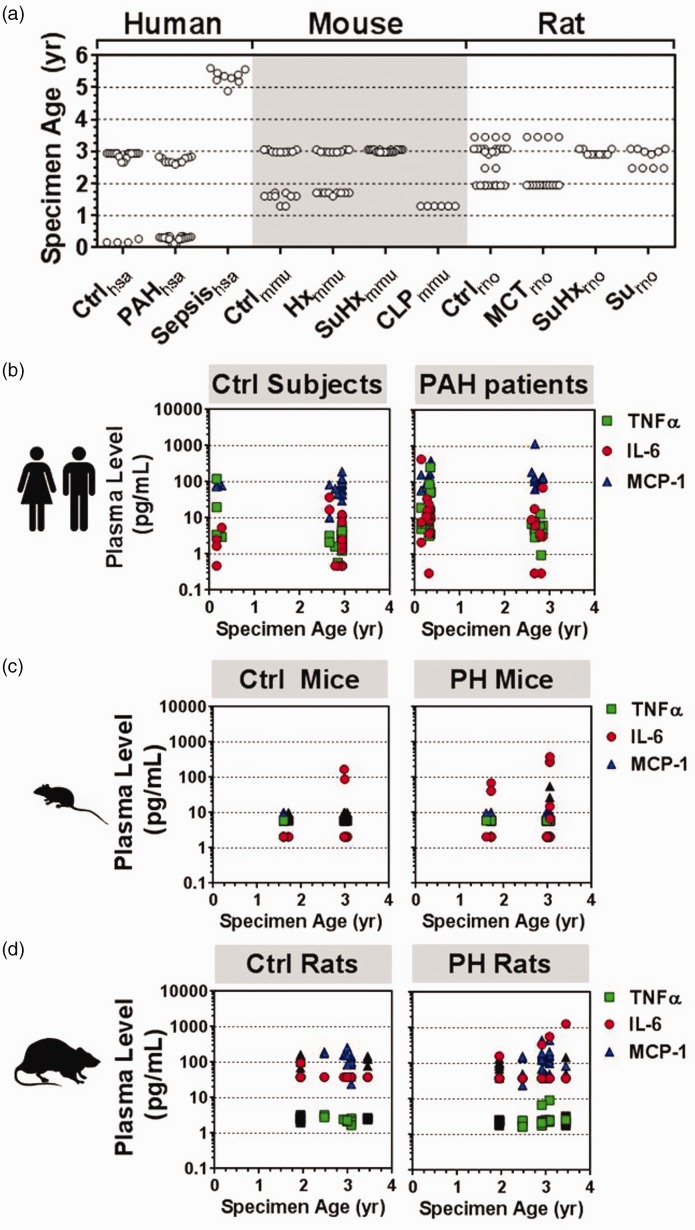
Since prolonged storage of plasma specimens can potentially lead to some level of cytokine degradation,12 we analyzed the age of the plasma specimens used in this study to facilitate robust data interpretation. For perspective, a previous study using purposefully designed cytokine stability experiments has shown that more than 50% of IL-6 and TNF-α plasma levels remain detectable even after four years of storage at −80℃.12 By comparison, the majority of plasma specimens in the current study were aged under three years at the time of cytokine measurement (Fig. 4a), minimizing the likelihood of false-negative results. Moreover, specimen age was generally similar between the human (mean ± SD; 2.2 ± 1.8 years, n = 58), rat (2.7 ± 0.6 years, n = 62), and mouse samples (2.4 ± 0.7 years, n = 62). The oldest samples, representing sepsis patients, were aged 4.9–5.6 years and still exhibited among the highest cytokine levels measured in the study. Specimen age was also represented relatively evenly between control and disease groups (Fig. 4a). Thus, any potential cytokine degradation owing to the age of samples would likely be reflected systematically in both groups, limiting false-positive differential effects. Importantly, no significant negative correlation between cytokine level and specimen age was observed in the human (Fig. 4b), mouse (Fig. 4c), or rat (Fig. 4d) samples.
Fig. 4.
Plasma specimen age at time of cytokine measurement. (a) Distribution of specimen age according to species (hsa, mmu, rno) and specific exposure condition (n = 58–62 participants/animals per species group). Ctrl, PAH, MCT, SU5416 (Su), SU5416 and hypoxia (SuHx), hypoxia (Hx), CLP model of sepsis. (b–d) Plasma cytokine levels versus specimen age. No significant correlations between cytokine level and specimen age were observed in human, mouse or rat samples.
Validation experiments limit potential false-negative results due to specimen age or assay sensitivity
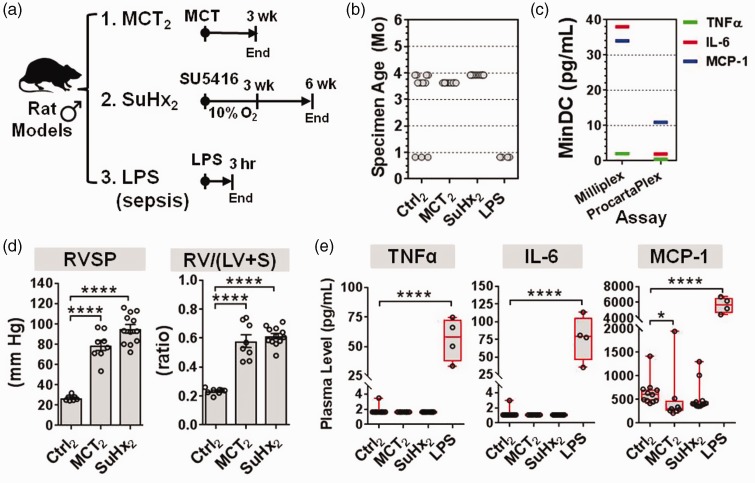
Several lines of evidence argue against the likelihood of false-negative results in our initial assessment of archived plasma specimens. Nevertheless, this potential limitation was addressed further in two rat models by evaluating cytokine levels (1) in substantially newer plasma samples, (2) using a more sensitive immunoassay, and (3) in parallel with a new biologic positive control, based on rats with sepsis caused by exposure to the bacterial endotoxin, LPS. We focused on the MCT and SuHx rat models because these represent the two most commonly used rat models of IPAH (Fig. 5a). All plasma specimens from this new set of experiments were aged less than four months at the time of cytokine measurement (Fig. 5b). In addition, we used the ProcartaPlex brand (Affymetrix) of Luminex-based immunoassays that offered a lower cytokine detection limit compared to the Milliplex assays (Millipore) used in our original experiments (Fig. 5c). Significant elevations in pulmonary hemodynamics and RV hypertrophy, indicative of PH development, were confirmed in rats three weeks after MCT and six weeks after SuHx (Fig. 5d). Consistent with previous results, the plasma levels of TNF-α, IL-6, and MCP-1 were not significantly elevated in either rat PH model. The only significant change was a decrease in MCP-1 levels in MCT rats (consistent with prior observations, Fig. 3c). In addition, positive control plasma specimens from LPS-exposed rats exhibited significant increases in all three cytokines (Fig. 5e). Of note, MCP-1 levels were systematically higher in the validation experiment compared to the original set of experiments presented in Fig. 3; however, this can be attributed to assay-specific variability rather than the difference in specimen age, as evidenced by further evaluation of the Milliplex and ProcartaPlex assays with identical plasma samples (Supplementary Fig. 1).
Fig. 5.
Validation of plasma cytokine levels in select rat models of PH. (a) Schematic overview of two different rat models of PH, and a LPS model of sepsis. PH was induced in rats by either MCT (n = 8 rats from one experiment), or SU5416 plus three weeks of hypoxia (SuHx; n = 12 rats from one experiment), and compared to controls at weeks 3 or 6 (Ctrl; n = 8 rats from two independent experiments). As a positive control, rats with LPS-induced sepsis were assessed 3 h post exposure (LPS n = 4, Ctrl n = 3 rats from one experiment). (b) Specimen age at the time of cytokine measurement was under 4 months. (c) Manufacturer’s specifications of minimum detectable concentrations (MinDC) for the Milliplex and ProcartaPlex brands of Luminex-based immunoassays. (d) RVSP and the right ventricle to left ventricle plus septum weight ratio confirm establishment of pulmonary hypertension (bar charts denote mean ± SEM). (e) Plasma levels of target cytokines measured by ProcartaPlex immunoassay. Boxplots denote median and interquartile range, and whiskers denote min/max values. Individual animals are shown as circles. Axes are split on graphs to more clearly show distribution of data. Statistical significance of RVSP and RV/(LV + S) was determined by one-way ANOVA and Dunnett’s multiple comparison test. Statistical significance of cytokine changes was determined by non-parametric Kruskal–Wallis with Dunn’s multiple comparison test. *P < 0.05, ****P < 0.0001 versus Ctrl group. Subscript 2 denotes new set of animals used in this validation experiment.
Discussion
In this study, we investigated whether circulating levels of the pro-inflammatory cytokines TNF-α, IL-6, and MCP-1, were concordantly altered in PAH patients and five of the most common rodent models of PH. Our results showed that in PAH patients, plasma levels of TNF-α, IL-6, and MCP-1 were significantly elevated; however, these changes were not reproduced in common rodent models including three different rat models induced by either MCT, SU5416/hypoxia, or SU5416 alone, and two different mouse models induced by either hypoxia or SU5416/hypoxia.
Our finding that the plasma levels of TNF-α, IL-6, and MCP-1 were significantly elevated in PAH patients but not in rodent models is supported by several lines of evidence. First, the increase in plasma cytokine levels observed in PAH patients in this study is consistent with results from several previous independent studies.4–7 Second, reproducibility of the results from our animal experiments are supported by relatively large sample sizes, and a total of at least three independent experiments per exposure group in each model. Third, commercially validated immunoassays were used to quantify cytokine levels, and their performance was supported not only by internal quality control standards (provided by the manufacturer), but also by external biologic positive controls representing a prototypical inflammatory condition such as sepsis. These biologic comparators showed concordant and very robust elevations in all three cytokines in plasma (ranging from ∼ 1–3 orders of magnitude) across sepsis patients, mice with CLP-induced sepsis, and rats with LPS-induced sepsis. Finally, technical variables such as specimen age (while difficult to control in archived retrospective specimens) were generally quite similar between human, mouse, and rat samples, and the same type and source of immunoassay platform was used consistently for all cytokine measurements. Furthermore, key findings were validated in two rat models using relatively new plasma specimens, and a second immunoassay with a superior detection limit.
The difference in plasma cytokine alterations observed between clinical and experimental forms of PH may reflect underlying differences in the mechanisms of disease activity, or could potentially be due to fundamental epigenetic/genetic differences between humans and animals. Indeed, there is increasing evidence that the molecular regulation of disease in animal models may differ from human PH,13 and this may extend more generally to other human diseases.14 Seok et al. previously reported that the genomic response to various acute inflammatory stresses in humans correlated poorly with corresponding mouse models, based on transcriptional profiling of total blood leukocytes.14 These authors suggested that the differences could be attributed to multiple considerations including the evolutional distance between mice and humans, the complexity of human disease compared to relatively simple models based on single mechanisms, and the inbred nature of the mouse model. Of note, another consideration in our study was that males were used exclusively in the animal models, whereas both women and men were included in the patient cohort (∼2:1 ratio). However, when cytokine levels were assessed in only male patients, two of three cytokines remained significantly elevated, suggesting that sex-specific effects were not a major determinant in the different results between patients and animal models. Another possible explanation for the discordant outcomes, which was not specifically assessed in this study, is dynamic spatio-temporal regulation of cytokine levels. For instance, pulmonary transcript levels of IL-6 in mice have previously been reported to peak after just two days of hypoxia, and return to baseline levels within one week.8 Nevertheless, our finding that plasma levels of TNF-α, IL-6, and MCP1 were not elevated in animals with established PH suggests that current models might induce only transient and/or possibly more lung-specific changes in inflammatory cytokine levels, which contrasts with the chronic systemic inflammation suggested by the plasma cytokine profile in PAH patients. This represents a distinction with potentially significant implications for how circulating inflammatory mediators in experimental versus clinical PH, may contribute differentially toward the development versus progression of PH.
One limitation of this study is that the inflammatory phenotype of PAH patients and animal models was assessed only in the context of circulating cytokines, and therefore does not provide direct insight into other important inflammatory mechanisms, such as inflammatory cell infiltrates in lung tissue. However, plasma cytokine levels provided a very practical readout to quantify inflammation consistently and robustly across a large number of archived specimens, and circumvented the difficulty in obtaining relevant lung tissue specimens from patients (which were not available). Another limitation is that plasma cytokine levels were not assessed serially over time. Thus, we cannot discount the possibility that systemic cytokine levels may increase earlier (or later) in the time course of experimental PH in the various animal models that were investigated. Our experiments, however, were designed based on common experimental endpoints when PH is clearly established in these models. In addition, among the five different models that were examined, cytokine levels were assessed from three to eight weeks after the initial PH insult, providing insight on cytokine changes associated with various levels of disease severity, including a broad range of physiologic derangements in pulmonary hemodynamic and RV remodeling (i.e., RVSP range ∼40–100 mmHg and Fulton index range ∼0.35–0.7).
Our demonstration of a marked discrepancy between the circulating inflammatory cytokine profiles of PAH patients and five different animal models provides insight into limitations that should be considered when gauging the translational value of different experimental models. However, identification of such limitations represents an important step toward informing the development of new models that not only replicate the characteristic abnormalities in pulmonary structure and function of human PH, but also the underlying molecular perturbations that may contribute to disease activity. Previous patient studies have also highlighted the potential utility of plasma cytokines as biomarkers to help guide clinical decisions in PAH. Thus, another important question addressed by this study is whether these biomarker candidates, could likewise be used as a practical readout to facilitate preclinical studies, such as for monitoring the anti-inflammatory effects of new drug candidates. However, we now know that plasma levels of TNF-α, IL-6, and MCP-1 would likely not be effective biomarkers in these animal models, since levels appeared to be unaffected even in models of severe PH. Of note, while no increase in the plasma levels of these pro-inflammatory cytokines was observed across five distinct animal models, these data do not diminish the importance of inflammation in the pulmonary vasculopathy of experimental PH demonstrated previously.8–10 Instead, this study offers a broader perspective on the potential context-dependent roles of inflammation in PH, and contributes toward a stronger conceptual framework to better understand the translational value of preclinical animal experiments.
Supplementary Material
Acknowledgments
The authors thank Leslie Carling and Irene Watpool for technical support with collection of human blood samples.
Conflict of interest
The author(s) declare that there is no conflict of interest.
Funding
This work was supported by the Canadian Institutes of Health Research (MOP57726 to DJS), and the Entelligence Young Investigator’s grant from Actelion Pharmaceuticals US, Inc. (KS).
References
- 1.Maarman G, Lecour S, Butrous G, et al. A comprehensive review: the evolution of animal models in pulmonary hypertension research; are we there yet? Pulm Circ 2013; 3: 739–756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jiang B, Deng Y, Suen C, et al. Marked strain-specific differences in the SU5416 rat model of severe pulmonary arterial hypertension. Am J Respir Cell Mol Biol 2016; 54: 461–468. [DOI] [PubMed] [Google Scholar]
- 3.Groth A, Vrugt B, Brock M, et al. Inflammatory cytokines in pulmonary hypertension. Respir Res 2014; 15: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Humbert M, Monti G, Brenot F, et al. Increased interleukin-1 and interleukin-6 serum concentrations in severe primary pulmonary hypertension. Am J Respir Crit Care Med 1995; 151: 1628–1631. [DOI] [PubMed] [Google Scholar]
- 5.Itoh T, Nagaya N, Ishibashi-Ueda H, et al. Increased plasma monocyte chemoattractant protein-1 level in idiopathic pulmonary arterial hypertension. Respirology 2006; 11: 158–163. [DOI] [PubMed] [Google Scholar]
- 6.Soon E, Holmes AM, Treacy CM, et al. Elevated levels of inflammatory cytokines predict survival in idiopathic and familial pulmonary arterial hypertension. Circulation 2010; 122: 920–927. [DOI] [PubMed] [Google Scholar]
- 7.Selimovic N, Bergh CH, Andersson B, et al. Growth factors and interleukin-6 across the lung circulation in pulmonary hypertension. Eur Respir J 2009; 34: 662–668. [DOI] [PubMed] [Google Scholar]
- 8.Hashimoto-Kataoka T, Hosen N, Sonobe T, et al. Interleukin-6/interleukin-21 signaling axis is critical in the pathogenesis of pulmonary arterial hypertension. Proc Natl Acad Sci U S A 2015; 112: E2677–2686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Savale L, Tu L, Rideau D, et al. Impact of interleukin-6 on hypoxia-induced pulmonary hypertension and lung inflammation in mice. Respir Res 2009; 10: 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steiner MK, Syrkina OL, Kolliputi N, et al. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res 2009; 104: 236–244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McIntyre LA, Fergusson DA, Cook DJ, et al. Fluid resuscitation with 5% albumin versus normal saline in early septic shock: a pilot randomized, controlled trial. J Crit Care 2012; 27: 317. [DOI] [PubMed] [Google Scholar]
- 12.de Jager W, Bourcier K, Rijkers GT, et al. Prerequisites for cytokine measurements in clinical trials with multiplex immunoassays. BMC Immunol 2009; 10: 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schlosser K, Taha M, Deng Y, et al. Discordant regulation of microRNA between multiple experimental models and human pulmonary hypertension. Chest 2015; 148: 481–490. [DOI] [PubMed] [Google Scholar]
- 14.Seok J, Warren HS, Cuenca AG, et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc Natl Acad Sci U S A 2013; 110: 3507–3512. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.