Abstract
Pulmonary arterial hypertension (PAH) is a progressive potentially fatal disease. Multiple pharmacologic options are now available, which facilitated transitions between different therapeutic options, although the evidence for such transitions has not been well described. We sought to review the evidence supporting the safety and/or efficacy of transitioning between PAH-specific medications. We performed a systematic review of all published studies in the Medline database between 1 January 2000 and 30 June 2016 reporting on any transition between the currently Food and Drug Administration (FDA)-approved PAH-specific medications. Studies reporting on three or more adult patients published in the English language reporting on transitions between FDA-approved PAH medications were extracted and tabulated. Forty-one studies met the selection criteria, nine of which included less than eight patients (and thus were reported separately in the supplement), for a total of 32 studies. Transitioning from parenteral epoprostenol to parenteral treprostinil appears to be safe and efficacious in patients who have less severe disease and more favorable hemodynamics. Transitioning from a prostacyclin analogue to an oral medication may be successful in patients who have favorable hemodynamics and stable disease. There is conflicting evidence supporting the transition from a parenteral to an inhaled prostacyclin analogue, even in patients who are on background oral therapy. Currently, the only evidence in support of transitioning between oral PDE5 inhibitors is from sildenafil to tadalafil. Patients on higher doses of sildenafil are more likely to fail. In patients with liver abnormalities due to bosentan or sitaxentan, the transition to ambrisentan appears to be safe and can result in clinical improvement. Studies regarding PAH medication transitions are limited. Patients who have less severe disease, better functional status, and are on lower medications doses may be more successful at transitioning.
Keywords: pulmonary hypertension, transition, pulmonary arterial hypertension, pulmonary vascular disease, pharmacotherapy
Introduction
Pulmonary arterial hypertension (PAH) is a progressive disease of the pulmonary vasculature that, if left untreated, has a very poor prognosis.1–3 Since the introduction of infused epoprostenol in 1996, the number and routes of PAH-specific therapies have dramatically increased.4,5 Currently, there are 14 therapies for PAH approved by the United States Food and Drug Administration (FDA) that are available through the intravenous (IV), subcutaneous (SQ), inhaled (IH), and oral routes. These drugs target three main pathways: the nitric oxide, endothelin-1, and prostacyclin pathways; and they currently include five families of drugs: phosphodiesterase type-5 inhibitors (PDE5-I), guanylate cyclase stimulator, endothelin receptor antagonists (ERA), prostacyclin analogues, and selective prostacyclin receptor agonists.5–7 The availability of different classes of medications, different routes, and total number of available PAH-specific medications makes the number of potential transitions within the same class or between classes relatively large. These transitions are already occurring not uncommonly in clinical practice, and there may be pressure by external forces (i.e. third-party payers) to switch medications, often in the absence of high quality data about long-term clinical outcomes/consequences.
Despite the development of newer oral and IH therapies, most patients with advanced disease or rapidly progressive disease still require continuously infused parenteral prostacyclin analogues. Additionally, there is new interest in both upfront and sequential combination therapies.4 Over 50% of patients with PAH are on more than one PAH-specific therapy.8 At several large PAH centers, approximately 10% of patients on parenteral prostacyclin have attempted to transition to other therapies.8,9 Typically, patients will attempt to transition therapies because of complications such as line infections10 or vein stenosis in the case of IV therapies, site pain caused by SQ therapies, intolerable side effects from therapy, or to improve medication compliance relative to the simplicity of dosing with some newer agents.
To evaluate the evidence supporting the efficacy and safety of transitions between PAH-specific medications, we performed a systematic review of published studies of adult patients who were transitioned between the currently FDA-approved PAH therapies.
Materials and methods
Search and selection criteria
We utilized the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines11 to perform a systematic review of all published studies in the Medline database between 1 January 2000 and 30 June 2016 reporting on any transition between the currently FDA-approved PAH-specific medications. Studies were identified using the following search strategy: [“switch” or “switched” or “switching” or “conversion” or “converted” or “transition” or “transitioned” or “transitioning”] AND “pulmonary” AND “hypertension”; dates limit: 01/01/2000 through 06/30/2016.
We excluded case reports (reporting on less than three patients), studies including pediatric patients (age < 18 years), studies without a published English translation, and studies exclusively reporting on currently non-approved PAH medications (e.g. sitaxsentan) (e-Table 1). We report the studies that had less than eight patients in the supplement only, and we tabulate the studies separately based on their study design: retrospective versus prospective to reflect the differential quality level of the different studies presented. Three of the studies were reported as a “transition” from IH to infused prostacyclin analogues; however, we consider these “transitions” as escalation of care rather than transition, so we report them separately.
Table 1.
Intra-class PAH medication transitions: infused prostacyclin analogue to another infused prostacyclin analogue.
| No. | Original drug | Drug transitioned to | Publication year/ Authors | Study design | PAH patients | Time | Outcome (transition success) | Comments |
|---|---|---|---|---|---|---|---|---|
| Prospective | ||||||||
| 1 | IV epoprostenol | SQ treprostinil | 2007 / Rubenfire et al.12 | Prospective randomized placebo-controlled trial | 22 | 8 weeks | Successful in 13/14 patients randomized to transition to SQ treprostinil | Patients had stable WHO FC II or III disease 6MWD worsened by 35 m after transition to SQ treprostinil |
| 2 | IV epoprostenol | IV treprostinil | 2005 / Gomberg- Maitland et al.27 | Prospective open-label | 31 | 3 months | Successful in 27/31 patients | WHO FC, 6MWD were unchanged Hemodynamics worse |
| 3 | IV epoprostenol | IV treprostinil | 2007 / Sitbon et al.28 | Prospective open-label | 12 (NYHA class I or II) | 3 months | Successful in 12/12 patients | Epoprostenol dose 28 ± 14 ng/kg/min Treprostinil dose 62 ± 30 ng/kg/min Fewer adverse events and all remained on treprostinil |
| 4 | IV epoprostenol | IV treprostinil | 2013 / Benza et al.15 | Prospective open-label | 31 transition patients (out of total of 47 patients reported, 16 of which are de novo) | 11 months | Successful transition (defined as freedom from death, lung transplantation, atrial septostomy, or discontinuation of IV treprostinil) in 77% of transition patients | No change in exercise capacity, WHO FC or hemodynamics at 11 months Two transition patients died and six discontinued the study due to adverse events |
| 5 | IV epoprostenol | IV treprostinil | 2013 / Minai et al.29 | Prospective open-label | 10 | 8 weeks | Successful in 10/10 patients | No change in 6MWD; no worsening WHO FC; improved QOL and satisfaction, less time on drug prep activities |
| 6 | IV epoprostenol | IV thermostable epoprostenol | 2013 / Tamura et al.30 | Prospective open-label | 8 | 12 weeks | Successful in 8/8 patients | No safety events or change in hemodynamics, improved satisfaction scores |
| 7 | IV epoprostenol | IV thermostable epoprostenol | 2014 / Sitbon et al.31 | Prospective open-label | 41 | 3 months | Successful in 37/41 patients | TSQM scores showed an improvement in treatment convenience at 3 months |
| 8 | IV epoprostenol | IV thermostable epoprostenol | 2015 / Provencher el al.32 | Prospective open-label | 16 | 4 weeks | Successful in 16/16 patients | No change in SF-36 HRQoL, WHO FC, 6MWD, NT-proBNP Most patients preferred the thermostable product |
| 9 | IV epoprostenol | IV thermostable epoprostenol | 2015 / Frantz et al.14 | Prospective open-label registry | 189 transition patients (out of a total cohort of 336 patients) | 12 months | Successful in 132/189 PAH transition patients | Freedom from hospitalization: 57.1 ± 3.7%; 1-year survival: 87.7 ± 2.5% |
| Retrospective | ||||||||
| 10 | IV epoprostenol | SQ treprostinil | 2002 / Vachiéry et al.33 | Retrospective | 8 | 4–11 months | Successful in 7/8 patients | Transition achieved in 21–96 h, with no major adverse effects or worsening in clinical status All patients reported improved comfort at follow-up |
| 11 | SQ treprostinil | IV treprostinil or IV epoprostenol | 2014 / Alkukhun et al.34 | Retrospective | 9 (7 with PAH, 2 with CTEPH) | 12 months | Successful in 8/9 patients | Reasons for SQ to IV switch were site pain (n = 6), major surgery (n = 2) and septic shock (n = 1) SQ treprostinil to IV treprostinil: dose = 84.9 to 70.8 ng/kg/min SQ treprostinil to IV epoprostenol: dose = 24.5 to 13.3 ng/kg/min |
6MWD, 6-minute walk distance; FC, functional class; HRQoL, health-related quality of life; IV, intravenous; mPAP, mean pulmonary artery pressure; N/A, not applicable; NT-proBNP, N-terminal pro B-type natriuretic peptide; NYHA, New York Heart Association; QOL, quality of life; SF-36, short form (36) health survey; SQ, subcutaneous; TSQM, treatment satisfaction questionnaire for medication; WHO, World Health Organization.
Data extraction and assessment of risk of bias
To avoid bias, three investigators (WHF, AS, and JAW) independently reviewed the literature and identified the potential studies for inclusion in this systematic review (e-Table 1). All five investigators performed data extraction/ abstraction.
Results
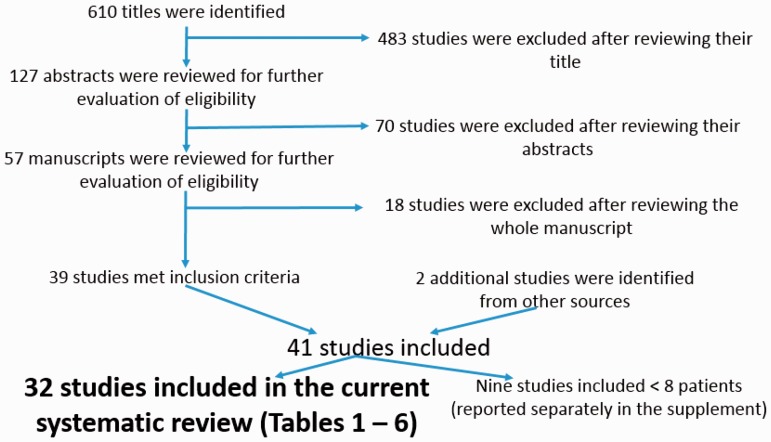
A total of 32 studies are included in the systematic review (Fig. 1). Additional nine studies had less than eight patients and thus were reported separately in the supplement (e-Tables 2–4).
Fig. 1.
PRISMA diagram of the selection of the studies included in the systematic review.
Intra-class PAH medication transitions: from one infused prostacyclin analogue to another infused prostacyclin analogue
There were 11 studies involving the transition from one infused prostacyclin analogue (either IV or SQ) to another infused prostacyclin analogue (Table 1), with a total of 377 patients studied. Two of the 11 studies were retrospective; eight of the studies were prospective open-label studies; and one study12 was a prospective, randomized controlled trial. The duration of the studies were in the range of 1–12 months. Five out of the nine prospective studies involved transition from IV epoprostenol to either IV or SQ treprostinil, with a total of 108 transition patients studied. The rate of successful transition in these five studies was 86/108 (80%). In general, patients who were successfully transitioned had less severe disease (New York Heart Association [NYHA]) functional class I or II, or World Health Organization [WHO] functional class II or III) and more favorable hemodynamics at baseline. Overall, there was no worsening in WHO functional class or significant differences in 6-minute walk distance (6MWD) in patients who completed the transition.
Only three small studies involved the transition from either SQ or IV treprostinil to IV epoprostenol (Table 1 and e-Table 2), with a total of 18 patients studied and a success rate of 67%. In one of these studies,13 two of the four “transition” patients died; however, these four patients were transitioned (escalation of care) specifically to try to mitigate clinical worsening of their PAH, rather than for reasons of intractable medication side effects or non-adherence.
Table 2.
Intra-class PAH medication transitions: prostacyclin analogue from one route to another route (i.e. infused, inhaled, or oral) (other than switched from infused to another infused medication or route).
| No. | Original drug | Drug transitioned to | Publication year | Study design | PAH patients | Time | Measures | Outcome |
|---|---|---|---|---|---|---|---|---|
| Prospective | ||||||||
| 1 | IH iloprost | IH treprostinil | 2013 / Bourge et al.20 | Prospective open-label | 73 | 3–12 months | Likelihood of staying on treprostinil at 3 months, 6MWD, NT-proBNP, CAMPHOR, TSQM | Successful in 89% 6MWD ( + 16.0) NT proBNP (–74) Higher CAMPHOR/TSQM scores in treprostinil group |
| 2 | IV/SC treprostinil | Oral treprostinil | 2016 / Chakinala et al.18 | Prospective open-label | 33 | 24 weeks | Investigator-determined clinical stability at week 24, 6MWD, hemodynamics, QOL, safety, pharmacokinetics, treatment satisfaction | Successful transition in 31/33 participants within first 4 weeks No change in 6MWD, WHO FC, hemodynamics or symptoms at week 24 |
| Retrospective | ||||||||
| 3 | IV/SQ treprostinil or epoprostenol | IH treprostinil | 2012 / de Jesus Perez et al.17 | Retrospective | 18 15 IV/SQ treprostinil 3 IV epoprostenol | 7 months | WHO FC 6MWD Hemodynamics | Deterioration in WHO FC in minority |
| 4 | IV/SQ epoprostenol or treprostinil | IH iloprost | 2013 / Channick et al.9 | Retrospective | 37 | 12 months | Likelihood of staying on iloprost Clinical worsening | Successful in 78.4% 19% experienced clinical worsening Use of background oral PAH therapy was associated with success |
| 5 | IV / SQ / IH treprostinil | Oral treprostinil | 2016 / Coons et al.19 | Case series | 9 | 47 weeks (median follow-up) | Clinical symptoms, 6MWD, NT-proBNP level | Deemed successful in 6/9 patients (1 worsened, 1 could not tolerate side effects, and 1 transitioned to hospice care) |
CAMPHOR, Cambridge Pulmonary Hypertension Outcome Review; IH, inhaled. Other abbreviations per Table 1 footnote.
Four out of the 11 studies involved the transition from a traditional formulation of IV epoprostenol to a thermostable form, with a total of 254 patients studied and a success rate of 76%. Patients who transitioned successfully tended to have improved quality of life and treatment satisfaction scores. In one of these studies,14 which was a prospective, open-label registry, freedom from hospitalization rates were higher in patients who transitioned to thermostable IV epoprostenol.
There were no significant safety events in the majority of the studies. In the study by Benza et al.,15 in which 31 patients were transitioned from IV epoprostenol to IV treprostinil, a total of eight transition patients died or discontinued the drug; however, the authors concluded that none of the deaths were related to treprostinil.
Intra-class PAH medication transitions: from a prostacyclin analogue via one route to a prostacyclin analogue via another route
There were five studies involving the transition from a prostacyclin analogue via one route (i.e. infused, IH, or oral) to another route (other than from infused to another infused medication or route) (Table 2), with a total of 170 patients studied. Three of the five studies were retrospective studies; two were prospective, open-label studies. The duration of most of the studies was in the range of 6–12 months. Three additional studies (Table 3) involved the “transition” from an IH prostacyclin analogue to either IV or SQ prostanoid, with a total of 63 patients studied. These were not true transitions, as such a “transition” is considered an escalation of care. In general, these patients had worse baseline functional status and hemodynamic impairment and were transitioned from inhaled to parenteral prostanoids as a form of rescue therapy. The transition resulted in clinical and hemodynamic short-term stabilization in a majority of the patients, but did not prevent subsequent disease progression, as many patients still died and others required lung transplantation (Table 3).
Table 3.
Intra-class PAH medication escalations: prostacyclin analogue from inhaled route to infused route.
| No. | Original drug | Drug transitioned to | Publication year | Study design | PAH patients | Time | Measures | Outcome |
|---|---|---|---|---|---|---|---|---|
| Prospective | ||||||||
| 1 | IH iloprost | IV iloprost | 2002 / Hoeper et al.35 | Prospective open-label | 16 | 3–16 months | WHO FC 6MWD | 8 improved 1 alive but worsened 5 died 2 transplanted |
| 2 | IH iloprost | IV iloprost | 2007 / Ewert et al.36 | Prospective open-label | 24 | 1–44 months | WHO FC 6MWD Hemodynamics | WHO FC and hemodynamics improved in subset of patients 5 on long-term medical therapy 7 deaths 12 transplantation |
| Retrospective | ||||||||
| 3 | IH treprostinil | IV or SQ treprostinil | 2014 / Preston et al.37 | Retrospective | 23 (3 patients were WHO group 4 or 5) | 3–18 months | WHO FC 6MWD NT-proBNP Hemodynamics | 8 improved, 17 maintained their functional class, 1 deteriorated |
Two retrospective studies involved the transition from either IV or SQ prostacyclin analogue to an inhaled form, with 55 patients studied. The transition was considered successful in one of these studies.9,16 In a study by Channick et al.,9 in which 81% of patients remained free of clinical symptoms one year after transitioning, one of the major predictors of transition success was the use of background oral PAH therapy. In a small study by Enderby et al.16 (e-Table 3), in which the transition was considered successful in 3/3 patients, all three patients were on oral PAH therapy prior to the transition. By contrast, in the study by de Jesus Perez et al.,17 in which 18 patients on infused prostanoids as well as background oral PAH therapy were transitioned to IH treprostinil, there was a deterioration in WHO functional class as well as worsening of the 6MWD and NT-proBNP level in a minority of the patients. The authors raised concerns over the “amount of therapeutic control that can be achieved with inhaled therapies.”
Two studies18,19 (Table 2) examined the transition from either IV, SQ, or IH treprostinil to oral treprostinil in a carefully selected, stable cohort of patients. The transition was deemed successful in 37/42 patients (88%).
One study (20) involved the transition from IH iloprost to IH treprostinil. The authors reported an 89% “success” rate and concluded that the transition was “safe and well-tolerated with no apparent loss of clinical status,” and resulted in an average time savings of approximately 1.4 h per day.
Inter-class PAH medication transitions: from a prostacyclin analogue to an oral non-prostacyclin analogue
There were seven studies involving the transition from a prostacyclin analogue to an oral non-prostacyclin analogue (Table 4). Two of the seven studies were prospective, open-label studies, five were retrospective studies. The duration of the studies was in the range of 3–60 months. All seven of these studies involved the transition from a parenterally administered prostanoid to either oral bosentan or sildenafil, with a total of 126 patients studied. In general, patients who were able to transition successfully had a more favorable hemodynamic profile and WHO functional class and were on lower doses of prostacyclin analogues prior to transitioning.
Table 4.
Inter-class PAH medication transitions: prostacyclin analogues to oral non-prostacyclin analogue agents.
| No. | Original drug | Drug transitioned to | Publication year | Study design | PAH patients | Time | Measures | Outcome |
|---|---|---|---|---|---|---|---|---|
| Prospective | ||||||||
| 1 | Epoprostenol or treprostinil | Oral bosentan | 2004 / Suleman et al.38 | Prospective open-label | 23 | 12 months | Successful transition WHO FC 6MWD Echocardiogram | Only 9 patients (39%) were successfully transitioned to bosentan. In failure group, there was a trend toward higher doses and duration of PG therapy and higher PAP, though not statistically significant Half of failures in initial 8 weeks and half in subsequent 3–12 months |
| 2 | IV epoprostenol or IV treprostinil | Oral bosentan | 2006 / Steiner et al.39 | Prospective open-label | 22 | 17.7 ± 5.3 months | Change 6MWD - primary changes in prostanoid dosing, BORG score, FC, changes in PAH therapy, RVSP by echo | 10/22 patients were able to complete transition over 6 months (2–12 range) 3/10 late failures 41 m fall in 6MWD in transitioned patients at 1 year Borg unchanged Successful patients with lower RVSP, mPAP, better 6MWD, and FC and lower PG analogue doses vs. those who failed |
| Retrospective | ||||||||
| 3 | SQ treprostinil | Oral sildenafil | 2007 / Keogh et al.21 | Retrospective | 14 | 3 months | Successful transition WHO FC 6MWD QoL Echocardiogram | 71% stayed on Sildenafil Improved QOL No other significant changes |
| 4 | IV epoprostenol | Oral bosentan or oral sildenafil | 2007 / Johnson et al.40 | Retrospective | 13 (2 failed epo wean and not transitioned) | 29.9 ± 11.6 months | RHC, FC, 6MWD | 9/13 unchanged FC 4/13 worse FC Normal pre-wean hemodynamics (mPAP < 30 mmHg or PVR < 4 WU) predicted successful transition 8 successful at end of study 1 death from SDH 4 worsened and restarted prostacyclin 6MWD unchanged All 4 who failed had abnormal hemodynamics pre-transition |
| 5 | Epoprostenol (17 patients) or treprostinil (4 patients) | Oral bosentan and/or sildenafil | 2008 / Diaz-Guzman et al.41 | Retrospective | 21 (15 successful; 6 failed transition) | 24.7 ± 13.6 months in ST 30 ± 5.6 in FT | 6MWD, FC, BNP | Successful in 15/21 (71.4%) Low doses of prostanoids, mPAP < 40 mmHg, 6MWD > 400 m, SLE-PH, and use of sildenafil could predict a higher likelihood of successful weaning |
| 6 | IV epoprostenol | Oral bosentan | 2009 / Safdar42 | Retrospective | 11 | 3 months | Successful transition Adverse events WHO FC 6MWD | 7/11 patients required resumption of infused prostanoid 57% remained stable for “substantial period of time” on oral therapy 2 discontinued due to abnormal LFTs |
| 7 | Parenteral epoprostenol or treprostinil | Oral ERA/PDE5i | 2013 / Escolar et al.43 | Retrospective | 22 | 60 months | Successful transition Adverse events WHO FC 6MWD NT-proBNP Hemodynamics | Successful in 50% Failure associated with: Age > 55 years, idiopathic PAH, combination therapy, abnormal hemodynamics (RAP > 5 mmHg, mPAP > 40 mmHg, PASP > 70 mmHg, PVR > 6.5 WU |
mPAP, mean pulmonary artery pressure; PG, prostaglandin; PASP, pulmonary artery systolic pressure; PVR, pulmonary vascular resistance; RAP, right atrial pressure; RVSP, right ventricular systolic pressure; SDH, subdural hematoma; WU, Wood units.
In a study21 examining transitioning from SQ treprostinil to oral sildenafil, the change was deemed successful in 71% of 14 patients based upon improvements in NHYA functional class and quality of life. In another study22 of six patients (e-Table 4) with PAH due to Eisenmenger’s syndrome who were transitioned from SQ treprostinil (n = 5) or oral beraprost (n = 1) to oral bosentan, patients had a non-significant decrease in 6MWD and no significant change in WHO functional class.
Intra-class oral PAH medication transitions: from one phosphodiesterase type5 (PDE5) inhibitor to another
There were five studies examining the transition between different PDE5 inhibitors and all involved the transition from sildenafil to tadalafil (Table 5). Three of the five studies were retrospective studies and two were prospective, open-label studies, with a total of 193 patients studied. The duration of follow-up was in the range of 3–12 months. The majority of the transitions were deemed successful (success rate = 86–97%), which was generally defined as the successful continuation of tadalafil without clinical deterioration. Patients who failed to transition successfully in one study23 were noted to have more severe disease and were on a higher dose of sildenafil at baseline (180 versus 115.5 mg per day, P = 0.06).
Table 5.
Intra-class oral PAH medication transitions: PDE5 inhibitors.
| No. | Original drug | Drug transitioned to | Publication year | Study design | PAH patients | Time | Measures | Outcome |
|---|---|---|---|---|---|---|---|---|
| Prospective | ||||||||
| 1 | Sildenafil | Tadalafil | 2008 / Tay et al.44 | Prospective open-label | 12 | 3–6 months | 6MWD, Borg Dyspnea index, cardiac index, SF-36 score – physical function | No significant differences in 6MWD, NYHA class, Borg index and SF-36 physical function scores after transition to tadalafil. No significant adverse effects at 6 months |
| 2 | Sildenafil | Tadalafil | 2014 / Frantz et al.45 | Prospective open-label | 35 (56% were receiving ≥ 2 PAH therapies) | 6 months | Treatment Satisfaction Questionnaire for medication Adverse events | Successful in 86% Only 55% “satisfied” at 90 days. Patients taking > 20 mg three times a day of sildenafil were successfully transitioned to tadalafil 40 mg once daily |
| Retrospective | ||||||||
| 3 | Sildenafil | Tadalafil | 2012 / Shlobin et al.23 | Retrospective | 35 (5 non-group 1 PAH) | 12 months | Likelihood of staying on therapy 6MWD | 86% stayed tadalafil 6MWD + 37.04 m Failure group had higher sildenafil dose (180 vs. 115.5 mg/day; p = 0.06) |
| 4 | Sildenafil | Tadalafil | 2013 / Shapiro et al.46 | Retrospective | 98 (The majority of patients [78%] were receiving sildenafil 80–100 mg three time a day) | 8 ± 4.5 months | Likelihood of staying on therapy 6MWD BNP | 97% stayed on tadalafil No changes. There was no pattern of response observed for the patients whose 6MWD improved or worsened in relation to the dose of sildenafil at the time of transition |
| 5 | Sildenafil | Tadalafil | 2015 / Lichtblau et al.24 | Retrospective | 13 patients who did not tolerate side effects of sildenafil | 11 ± 3 months | WHO FC 6MWD Echocardiogram NT-proBNP | Successful in 54% 5/13 patients had adverse events leading to discontinuation |
In the study by Lichtblau et al.,24 in which 13 patients who could not tolerate the side effects of sildenafil attempted to transition to tadalafil, the transition success rate was only 54%. Patients who were unable to transition successfully had discontinued the drug due to adverse events. The authors noted that “in almost half of these cases, adverse reactions were similar to those with sildenafil, in the other half, different side effects led to discontinuation of treatment.”
Intra-class oral PAH medication transitions: from one endothelin receptor antagonist (ERA) to another
Transitioning from one ERA to another was reviewed in an uncontrolled, prospective open-label study25 of 36 patients who had elevated liver function tests due to bosentan or sitaxsentan (Table 6). Patients were switched to ambrisentan with a follow-up period of 12 weeks. Only one patient had an elevation of liver function tests (>3 times upper limit of normal) at the end of the study. Additionally, there were significant improvements in several clinical parameters in patients transitioned to ambrisentan. The authors concluded that ambrisentan may be a “viable treatment option for patients with PAH who have previously had liver abnormalities during bosentan or sitaxsentan therapy.”
Table 6.
Intra-class oral PAH medication transitions: endothelin receptor antagonists.
| No. | Original drug | Drug transitioned to | Publication year | Study | PAH patients | Time | Measures | Outcome |
|---|---|---|---|---|---|---|---|---|
| 1 | Bosentan or sitaxsentan | Ambrisentan | 2009 / McGoon et al.25 | Prospective open-label | 36 (prior abnormal LFTs with therapy) | 12 weeks | LFTs > 3x’s ULN; Tx discontinuation, Δ in 6MWD) Borg dyspnea index, WHO FC functional class, health survey score | One out of 36 with LFTs > 3x’s ULN; no discontinuations; improvements in other clinical endpoints |
LFT, liver function tests; ULN, upper limit of normal.
Discussion
Certain PAH therapies, particularly those requiring parenteral administration, can pose a significant burden to patients. With the availability of newer and more convenient treatment options for PAH, there has been an increase in the number of patients transitioned between therapies. Despite this increase, there have been a limited number of studies examining the safety and/or efficacy of these transitions. To our knowledge, this is the first systematic review examining transitions between PAH-specific therapies.
The majority of the studies we reviewed involved the transition from a prostacyclin analogue to another medication (Tables 1–4). The studies consisted of both retrospective and prospective open-label studies. The transition from parenteral epoprostenol to parenteral treprostinil was successful in 80% of patients and generally well tolerated. Patients who were successfully transitioned tended to have less severe disease and more favorable hemodynamics at baseline. Overall, there was no worsening in WHO functional class or significant differences in 6MWD in patients who completed the transition. Conversely, there were only few studies reporting on the transition from parenteral treprostinil to parenteral epoprostenol; these studies had a lower success rate for transitioning; however, some of the patients were transitioned (from parenteral treprostinil to parenteral epoprostenol) to address clinical worsening rather than mitigation of drug side effects.
The transition from parenteral epoprostenol to a thermostable form was generally successful, and patients who transitioned tended to have improved quality of life, treatment satisfaction scores, and freedom from hospitalization.
There were limited studies examining the transition from an IH prostacyclin analogue to a parenteral prostanoid. Patients in these studies generally had more severe disease and were “transitioned” as a form of rescue therapy. Most of these patients were able to achieve short-term clinical and hemodynamic stabilization but had subsequent disease progression, including death or lung transplantation.
There is conflicting evidence involving the transition from a parenteral prostacyclin analogue to an IH form. Two studies examining this transition9,16 in patients who were on background oral PAH therapy reported success, whereas one study17 reported clinical deterioration in a minority of patients. Therefore, clinicians should carefully discuss the risks and benefits of this particular transition with their patients and ensure close monitoring during and after the transition period. The transition from parenteral to oral treprostinil was evaluated in two studies18,19 and it appears to be feasible and safe in low-risk, clinically stable patients. The transition from inhaled iloprost to inhaled treprostinil was evaluated in one study20 and appears to be safe and well tolerated, and can result in modest time savings.
The transition from a parenteral prostacyclin analogue to an oral non-prostacyclin analogue was evaluated in seven studies (Table 4), with variable success rates. Most of these patients were transitioned from either epoprostenol or treprostinil to bosentan or sildenafil. Patients who successfully transitioned tended to have more favorable hemodynamics, better functional class and higher 6MWD at baseline compared with those who failed, and were on lower doses of prostacyclin analogues prior to transitioning.
In terms of intra-class oral PAH medication transitions, only sildenafil to tadalafil and bosentan or sitaxsentan to ambrisentan are supported by the available evidence. Most patients successfully transitioned from oral sildenafil to once daily tadalafil; patients who failed to do so were on a higher dose of sildenafil at baseline, thus raising concerns about the safety and efficacy of transitioning patients on higher doses of sildenafil. Other patients who may not transition successfully are those who have experienced intolerable side effects from sildenafil.
Limitations and potential biases
One major limitation of this systematic review is the heterogeneity of the studies reviewed and the heterogeneity of the patients included in these studies; including reasons for transitioning, inconsistency in defining “successful transition,” variable length of follow-up, and lack of standardized protocols for transitioning patients from one drug to another, especially in the case of infused prostanoids. For example, patients may have transitioned to a different medication as a form of rescue therapy in some studies rather than to reduce unwanted side effects of initial therapy, which would lead to significant bias. In addition, the PAH subtype and severity of disease, as well as background therapy and combination therapy are confounders that could not be accounted for in this analysis as they were not well described or characterized in many of the above studies. Additionally, most of these studies were retrospective reviews with small sample sizes (only one study was a prospective trial) limiting the generalizability of the data. It is also difficult to generalize our findings to the pediatric population, in which studies are severely limited. In addition, publication bias could not be accounted for, as unsuccessful transitions would be unlikely to be published.
Implications for clinical practice
Patients and clinicians may wish to transition between PAH therapies for a variety of reasons. We have provided a comprehensive review of the available evidence to guide clinicians in discussing transitions between PAH-specific therapies with their patients, as well as patient-specific characteristics that may help predict the likelihood of a successful transition. More studies26 are expected to further guide clinicians when considering transitions between PAH medications.
Conclusion
In summary, data regarding transitions between PAH-specific therapies are limited and the quality of the available evidence is variable. Certain patient characteristics, such as severity of disease as well as baseline hemodynamic profile and clinical parameters may help clinicians predict the likelihood of a successful transition between different PAH therapies. Patients will require careful monitoring during and after the transition period to ensure clinical stability and to monitor for adverse effects.
Supplementary Material
Conflict of interest
WHF is on the Speakers Bureau of Actelion, Gilead, & United Therapeutics/Lung; and advisory Board of Actelion, Bayer, Gilead, and United Therapeutics. JAW (Principal Investigator for Actelion, Bayer, Gilead, Lung LLC, and United Therapeutics). RJT serves as a hemodynamic core-lab for a Actelion phase II study.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Benza RL, Gomberg-Maitland M, Miller DP, et al. The REVEAL Registry risk score calculator in patients newly diagnosed with pulmonary arterial hypertension. Chest 2012; 141(2): 354–362. [DOI] [PubMed] [Google Scholar]
- 2.D’Alonzo GE, Barst RJ, Ayres SM, et al. Survival in patients with primary pulmonary hypertension. Results from a national prospective registry. Ann Intern Med 1991; 115(5): 343–349. [DOI] [PubMed] [Google Scholar]
- 3.McGoon MD, Benza RL, Escribano-Subias P, et al. Pulmonary arterial hypertension: epidemiology and registries. J Am Coll Cardiol 2013; 62(25 Suppl): D51–59. [DOI] [PubMed] [Google Scholar]
- 4.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46(4): 903–975. [DOI] [PubMed] [Google Scholar]
- 5.Galie N, Corris PA, Frost A, et al. Updated treatment algorithm of pulmonary arterial hypertension. J Am Coll Cardiol 2013; 62(25 Suppl): D60–72. [DOI] [PubMed] [Google Scholar]
- 6.Fares WH. Pushing the envelope on the indications and doses of pulmonary arterial hypertension medications: what is reasonable? J Cardiovasc Pharmacol 2016; 67(4): 319–321. [DOI] [PubMed] [Google Scholar]
- 7.Sitbon O, Channick R, Chin KM, et al. Selexipag for the treatment of pulmonary arterial hypertension. N Engl J Med 2015; 373(26): 2522–2533. [DOI] [PubMed] [Google Scholar]
- 8.Farber HW, Miller DP, Meltzer LA, et al. Treatment of patients with pulmonary arterial hypertension at the time of death or deterioration to functional class IV: insights from the REVEAL Registry. J Heart Lung Transplant 2013; 32(11): 1114–1122. [DOI] [PubMed] [Google Scholar]
- 9.Channick RN, Frantz RP, Kawut SM, et al. A multicenter, retrospective study of patients with pulmonary arterial hypertension transitioned from parenteral prostacyclin therapy to inhaled iloprost. Pulm Circ 2013; 3(2): 381–388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Barst RJ, Rubin LJ, Long WA, et al. A comparison of continuous intravenous epoprostenol (prostacyclin) with conventional therapy for primary pulmonary hypertension. The Primary Pulmonary Hypertension Study Group. N Engl J Med 1996; 334(5): 296–302. [DOI] [PubMed] [Google Scholar]
- 11.Moher D, Liberati A, Tetzlaff J, et al. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med 2009; 6(7): e1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rubenfire M, McLaughlin VV, Allen RP, et al. Transition from IV epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension: a controlled trial. Chest 2007; 132(3): 757–763. [DOI] [PubMed] [Google Scholar]
- 13.Lang I, Gomez-Sanchez M, Kneussl M, et al. Efficacy of long-term subcutaneous treprostinil sodium therapy in pulmonary hypertension. Chest 2006; 129(6): 1636–1643. [DOI] [PubMed] [Google Scholar]
- 14.Frantz RP, Schilz RJ, Chakinala MM, et al. Hospitalization and survival in patients using epoprostenol for injection in the PROSPECT observational study. Chest 2015; 147(2): 484–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Benza RL, Tapson VF, Gomberg-Maitland M, et al. One-year experience with intravenous treprostinil for pulmonary arterial hypertension. J Heart Lung Transplant 2013; 32(9): 889–896. [DOI] [PubMed] [Google Scholar]
- 16.Enderby CY, Soukup M, Al Omari M, et al. Transition from intravenous or subcutaneous prostacyclin therapy to inhaled treprostinil in patients with pulmonary arterial hypertension: a retrospective case series. J Clin Pharm Ther 2014; 39(5): 496–500. [DOI] [PubMed] [Google Scholar]
- 17.de Jesus Perez VA, Rosenzweig E, Rubin LJ, et al. Safety and efficacy of transition from systemic prostanoids to inhaled treprostinil in pulmonary arterial hypertension. Am J Cardiol 2012; 110(10): 1546–1550. [DOI] [PubMed] [Google Scholar]
- 18.Chakinala MM, Feldman JP, Rischard F, et al. Transition from parenteral to oral treprostinil in pulmonary arterial hypertension. J Heart Lung Transplant 2017; 36(2): 193–201. [DOI] [PubMed] [Google Scholar]
- 19.Coons JC, Miller T, Simon MA, et al. Oral treprostinil for the treatment of pulmonary arterial hypertension in patients transitioned from parenteral or inhaled prostacyclins: case series and treatment protocol. Pulm Circ 2016; 6(1): 132–135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bourge RC, Tapson VF, Safdar Z, et al. Rapid transition from inhaled iloprost to inhaled treprostinil in patients with pulmonary arterial hypertension. Cardiovasc Ther 2013; 31(1): 38–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Keogh AM, Jabbour A, Weintraub R, et al. Safety and efficacy of transition from subcutaneous treprostinil to oral sildenafil in patients with pulmonary arterial hypertension. J Heart Lung Transplant 2007; 26(11): 1079–1083. [DOI] [PubMed] [Google Scholar]
- 22.Kotlyar E, Sy R, Keogh AM, et al. Bosentan for the treatment of pulmonary arterial hypertension associated with congenital cardiac disease. Cardiol Young 2006; 16(3): 268–274. [DOI] [PubMed] [Google Scholar]
- 23.Shlobin OA, Brown AW, Weir N, et al. Transition of PH patients from sildenafil to tadalafil: feasibility and practical considerations. Lung 2012; 190(5): 573–578. [DOI] [PubMed] [Google Scholar]
- 24.Lichtblau M, Harzheim D, Ehlken N, et al. Safety and long-term efficacy of transition from sildenafil to tadalafil due to side effects in patients with pulmonary arterial hypertension. Lung 2015; 193(1): 105–112. [DOI] [PubMed] [Google Scholar]
- 25.McGoon MD, Frost AE, Oudiz RJ, et al. Ambrisentan therapy in patients with pulmonary arterial hypertension who discontinued bosentan or sitaxsentan due to liver function test abnormalities. Chest 2009; 135(1): 122–129. [DOI] [PubMed] [Google Scholar]
- 26.ClinicalTrials.gov. Available at: https://clinicaltrials.gov/ct2/results?term=pulmonary+hypertension+transition&Search=Search.
- 27.Gomberg-Maitland M, Tapson VF, Benza RL, et al. Transition from intravenous epoprostenol to intravenous treprostinil in pulmonary hypertension. Am J Respir Crit Care Med 2005; 172(12): 1586–1589. [DOI] [PubMed] [Google Scholar]
- 28.Sitbon O, Manes A, Jais X, et al. Rapid switch from intravenous epoprostenol to intravenous treprostinil in patients with pulmonary arterial hypertension. J Cardiovasc Pharmacol 2007; 49(1): 1–5. [DOI] [PubMed] [Google Scholar]
- 29.Minai OA, Parambil J, Dweik RA, et al. Impact of switching from epoprostenol to IV treprostinil on treatment satisfaction and quality of life in patients with pulmonary hypertension. Respir Med 2013; 107(3): 458–465. [DOI] [PubMed] [Google Scholar]
- 30.Tamura Y, Ono T, Fukuda K, et al. Evaluation of a new formulation of epoprostenol sodium in Japanese patients with pulmonary arterial hypertension (EPITOME4). Adv Ther 2013; 30(5): 459–471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sitbon O, Delcroix M, Bergot E, et al. EPITOME-2: An open-label study assessing the transition to a new formulation of intravenous epoprostenol in patients with pulmonary arterial hypertension. Am Heart J 2014; 167(2): 210–217. [DOI] [PubMed] [Google Scholar]
- 32.Provencher S, Paruchuru P, Spezzi A, et al. Quality of life, safety and efficacy profile of thermostable flolan in pulmonary arterial hypertension. PLoS One 2015; 10(3): e0120657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Vachiery JL, Hill N, Zwicke D, et al. Transitioning from i.v. epoprostenol to subcutaneous treprostinil in pulmonary arterial hypertension. Chest 2002; 121(5): 1561–1565. [DOI] [PubMed] [Google Scholar]
- 34.Alkukhun L, Bair ND, Dweik RA, et al. Subcutaneous to intravenous prostacyclin analog transition in pulmonary hypertension. J Cardiovasc Pharmacol 2014; 63(1): 4–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoeper MM, Spiekerkoetter E, Westerkamp V, et al. Intravenous iloprost for treatment failure of aerosolised iloprost in pulmonary arterial hypertension. Eur Respir J 2002; 20(2): 339–343. [DOI] [PubMed] [Google Scholar]
- 36.Ewert R, Opitz CF, Wensel R, et al. Continuous intravenous iloprost to revert treatment failure of first-line inhaled iloprost therapy in patients with idiopathic pulmonary arterial hypertension. Clin Res Cardiol 2007; 96(4): 211–217. [DOI] [PubMed] [Google Scholar]
- 37.Preston IR, Feldman J, White J, et al. Safety and efficacy of transition from inhaled treprostinil to parenteral treprostinil in selected patients with pulmonary arterial hypertension. Pulm Circ 2014; 4(3): 456–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suleman N, Frost AE. Transition from epoprostenol and treprostinil to the oral endothelin receptor antagonist bosentan in patients with pulmonary hypertension. Chest 2004; 126(3): 808–815. [DOI] [PubMed] [Google Scholar]
- 39.Steiner MK, Preston IR, Klinger JR, et al. Conversion to bosentan from prostacyclin infusion therapy in pulmonary arterial hypertension: a pilot study. Chest 2006; 130(5): 1471–1480. [DOI] [PubMed] [Google Scholar]
- 40.Johnson RF, Loyd JE, Mullican AL, et al. Long-term follow-up after conversion from intravenous epoprostenol to oral therapy with bosentan or sildenafil in 13 patients with pulmonary arterial hypertension. J Heart Lung Transplant 2007; 26(4): 363–369. [DOI] [PubMed] [Google Scholar]
- 41.Diaz-Guzman E, Heresi GA, Dweik RA, et al. Long-term experience after transition from parenteral prostanoids to oral agents in patients with pulmonary hypertension. Respir Med 2008; 102(5): 681–689. [DOI] [PubMed] [Google Scholar]
- 42.Safdar Z. Outcome of pulmonary hypertension subjects transitioned from intravenous prostacyclin to oral bosentan. Respir Med 2009; 103(11): 1688–1692. [DOI] [PubMed] [Google Scholar]
- 43.Escolar E, Pineda AM, Correal B, et al. Transition from prostacyclin analogue infusion to oral therapy in patients with pulmonary arterial hypertension: a 5-year follow-up. Pulm Circ 2013; 3(4): 880–888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tay EL, Geok-Mui MK, Poh-Hoon MC, et al. Sustained benefit of tadalafil in patients with pulmonary arterial hypertension with prior response to sildenafil: a case series of 12 patients. Int J Cardiol 2008; 125(3): 416–417. [DOI] [PubMed] [Google Scholar]
- 45.Frantz RP, Durst L, Burger CD, et al. Conversion from sildenafil to tadalafil: results from the sildenafil to tadalafil in pulmonary arterial hypertension (SITAR) study. J Cardiovasc Pharmacol Ther 2014; 19(6): 550–557. [DOI] [PubMed] [Google Scholar]
- 46.Shapiro S, Traiger G, Hill W, et al. Safety, tolerability, and efficacy of overnight switching from sildenafil to tadalafil in patients with pulmonary arterial hypertension. Cardiovasc Ther 2013; 31(5): 274–279. [DOI] [PubMed] [Google Scholar]
- 47.Escribano Subias P, Cea-Calvo L, Tello de Menesses R, et al. [Transition from intravenous to subcutaneous prostacyclin in pulmonary hypertension]. Rev Esp Cardiol 2003; 56(8): 818–821. [DOI] [PubMed] [Google Scholar]
- 48.Park K, Ostrow D, Levy RD, et al. Transition from intravenous epoprostenol to oral or subcutaneous therapy in pulmonary arterial hypertension: a retrospective case series and systematic review. Can Respir J 2011; 18(3): 157–162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Walkey AJ, Fein D, Horbowicz KJ, et al. Differential response to intravenous prostacyclin analog therapy in patients with pulmonary arterial hypertension. Pulm Pharmacol Ther 2011; 24(4): 421–425. [DOI] [PubMed] [Google Scholar]
- 50.Schenk P, Petkov V, Madl C, et al. Aerosolized iloprost therapy could not replace long-term IV epoprostenol (prostacyclin) administration in severe pulmonary hypertension. Chest 2001; 119(1): 296–300. [DOI] [PubMed] [Google Scholar]
- 51.Kim NH, Channick RN, Rubin LJ. Successful withdrawal of long-term epoprostenol therapy for pulmonary arterial hypertension. Chest 2003; 124(4): 1612–1615. [PubMed] [Google Scholar]
- 52.Flox Camacho A, Escribano Subias P, Tello de Meneses R, et al. [Transition from prostacyclin to bosentan in five patients with severe pulmonary hypertension: the switch is possible]. Rev Esp Cardiol 2006; 59(7): 737–739. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.