Abstract
The BREELIB nebulizer was developed for iloprost to reduce inhalation times for patients with pulmonary arterial hypertension (PAH). This multicenter, randomized, unblinded, four-part study compared inhalation time, pharmacokinetics, and acute tolerability of iloprost 5 µg at mouthpiece delivered via BREELIB versus the standard I-Neb nebulizer in 27 patients with PAH. The primary safety outcome was the proportion of patients with a maximum increase in heart rate (HR) ≥ 25% and/or a maximum decrease in systolic blood pressure ≥ 20% within 30 min after inhalation. Other safety outcomes included systolic, diastolic, and mean blood pressure, HR, oxygen saturation, and adverse events (AEs). Median inhalation times were considerably shorter with BREELIB versus I-Neb (2.6 versus 10.9 min; n = 24). Maximum iloprost plasma concentration and systemic exposure (area under the plasma concentration–time curve) were 77% and 42% higher, respectively, with BREELIB versus I-Neb. Five patients experienced a maximum systolic blood pressure decrease ≥ 20%, four with BREELIB (one mildly and transiently symptomatic), and one with I-Neb; none required medical intervention. AEs reported during the study were consistent with the known safety profile of iloprost. The BREELIB nebulizer offers reduced inhalation time, good tolerability, and may improve iloprost aerosol therapy convenience and thus compliance for patients with PAH.
Keywords: prostacyclin analog, inhalation time, patient convenience, treatment adherence
Iloprost is a stable prostacyclin analogue approved for the treatment of pulmonary arterial hypertension (PAH),1,2 a life-threatening condition characterized by vascular remodeling and increased pulmonary vascular resistance (PVR), ultimately leading to right heart failure.3–5 Prostacyclin analogues increase intracellular levels of cyclic adenosine monophosphate, thereby promoting arterial vasodilation and inhibiting cell proliferation, inflammation, and platelet aggregation.1,6,7
Iloprost is administered via inhalation for PAH.8–10 Inhaled iloprost has previously shown efficacy and safety in patients with PAH both as monotherapy11–14 and in combination with other PAH drugs.12,14–16 Treatment with inhaled iloprost in PAH requires multiple inhalations—usually six to nine times per day. The I-Neb Adaptive Aerosol Delivery nebulizer was approved for use with iloprost in 2006 and is now widely used. The estimated inhalation time for iloprost 5 µg at the mouthpiece is 6.5 min.1 However, clinical studies showed that inhalation times with this device were prolonged in some patients,1,8,17 thus introducing a risk of non-adherence.18
Based on recent progress in inhalation device technology,19 the BREELIB nebulizer (Vectura Group plc, Chippenham, UK) was developed to provide a device specifically for iloprost inhalation. It is designed to reduce inhalation times, improve convenience, and ensure precise, targeted drug delivery to the lungs. Each time the patient inhales, an aerosol bolus is released for 2 s followed by aerosol-free air for 1 s. The device guides the inhalation speed by LED feedback and a mechanical flow limitation valve. An air shut-off valve provides feedback when the target inhalation volume is reached. These features ensure that the exact dose is delivered, nebulized iloprost is not wasted, and suboptimal breathing patterns are reduced.
Here we report the findings of a phase 1/2 study comparing the acute tolerability and pharmacokinetics (PK) of iloprost administered by the BREELIB nebulizer within a markedly reduced inhalation time compared with inhalation via the standard I-Neb nebulizer. The objective of the study was to demonstrate the safety of rapid inhalation, which should improve the convenience of iloprost aerosol therapy and thus patient compliance.
Methods
Participants
Male or female patients with PAH (updated Dana Point Classification 1) aged ≥ 18 years were eligible for inclusion if they had a confirmed diagnosis of PAH (mean pulmonary arterial pressure [mPAP] > 25 mmHg, pulmonary arterial wedge pressure, or left ventricular end diastolic pressure < 15 mmHg and PVR > 4 Wood units) and were in World Health Organization (WHO) functional class III. All patients were receiving inhaled iloprost 5 µg using the I-Neb nebulizer. Patients receiving concomitant therapy with stable doses (≥4 weeks) of non-PAH-specific treatments (e.g., calcium channel blockers, nitrates, or diuretics) or stable doses (≥3 months) of PAH-specific therapies (endothelin receptor antagonists or phosphodiesterase-5 inhibitors) were eligible.
The study was carried out in accordance with Good Clinical Practice Guidelines and the Declaration of Helsinki. The protocol was approved by the ethics committees of all participating centers and all patients gave written informed consent. The study is registered at ClinicalTrials.gov (identifier NCT02032836).
Study design
This was a multicenter, randomized, unblinded study in four parts comparing the inhalation time, PK, and acute tolerability of iloprost inhaled as a 20 µg/mL solution through the BREELIB or as a 10 µg/mL solution through the I-Neb, to give a 5 µg dose at the mouthpiece. Iloprost dosing for Parts 1–4 is shown in Table 1. Briefly, in Part 1 patients received single doses of iloprost 1.25 µg and 2.5 µg via BREELIB; any patients not tolerating these doses were to be withdrawn. In Part 2, patients were randomized to receive a single dose of iloprost 5 µg via each nebulizer in a cross-over design. Inhalation times for each nebulizer were determined. In Part 3, patients received 2 weeks of treatment with each of the nebulizers in the same order as they were randomized to in Part 2. Patients inhaled iloprost approximately 6–9 times per day during each 2-week period. Part 4 was an optional long-term extension (LTE) where patients received continuous iloprost treatment with the BREELIB for a planned duration of up to 30 months. At the time of manuscript preparation, Part 4 was ongoing.
Table 1.
Study design and dosing with iloprost in each treatment period.
| Study part | Study period | Study treatment |
|---|---|---|
| 1 | Day 1 | Single doses of iloprost 1.25 µg (first 11 patients) and 2.5 µg using the BREELIB nebulizer* |
| 2 | Day 2 | Two doses of iloprost 5 µg (1 with the BREELIB and 1 with the I-Neb in a randomized order, with a 2-h washout in between)* |
| 3 | Days 2–29 | Multiple inhalations of iloprost 5 µg as needed (approximately 6–9 inhalations per day); 2 weeks with the BREELIB and 2 weeks with the I-Neb, in the same order as in Part 2 |
| 4 | Months 2–30 | Up to 30-month long-term extension with inhalation of iloprost 5 µg using the BREELIB as needed (approximately 6–9 inhalations per day) |
During Parts 1 and 2 of the study, patients continued their usual background therapy with the I-Neb nebulizer.
Outcome measures
The primary safety outcome was the proportion of patients with a maximum increase in heart rate (HR) of ≥25% and/or a maximum decrease in systolic blood pressure (SBP) of ≥20% within 30 min of completing inhalation. Other safety outcomes included non-invasive measurement of systolic, diastolic, and mean arterial blood pressure, HR, oxygen saturation, adverse events (AEs), and laboratory variables. Safety was assessed in all four parts of the study.
The main PK outcome was the area under the plasma concentration–time curve (AUC) until last measurement (AUC[0-tlast]) of iloprost as administered by the two nebulizers. Other PK parameters included the maximum drug concentration in plasma (Cmax), time to maximum concentration (tmax), and terminal elimination half-life (t1/2). For comparison of dose linearity, the PK parameters AUC/dose (D), AUC(0-tlast)/D, and Cmax/D of iloprost were derived.
Measurement of pharmacokinetic parameters
PK was assessed in Parts 1 and 2 of the study; blood samples were taken before inhalation, at the end of inhalation, and 2, 5, 10, 20, 30, and 45 min (Parts 1 and 2) and 60 min (Part 2) after the end of inhalation. Owing to the high variability of inhalation duration, and to avoid interference with the inhalation procedure, no blood sampling was performed during inhalation.
Quantitative analysis of iloprost in plasma was performed by high-pressure liquid chromatography and tandem mass-spectrometric detection (LC-MS/MS) at Nuvisan GmbH, Bioanalytics, Germany. The validation of the method and analysis of the study samples were performed in compliance with the relevant guidelines of the European Medicines Agency,20,21 the Food and Drug Administration,22 and the Japanese authority.23 The calibration range was from 20.0 ng/L (lower limit of quantification [LLOQ]) to 250 ng/L (upper limit of quantification). Mean inter-assay accuracy was in the range of 97.3–102% and precision was ≤ 6.1%. All samples were stored at or below −20 ± 5℃ and analyzed within 260 days after sample withdrawal.
Statistical analysis
Safety data were analyzed descriptively. All patients who received any study drug (with either or both the I-Neb and BREELIB) were included in the safety analysis set to assess AEs. Hemodynamic variables were evaluated in patients completing both treatment periods of Part 2 without major protocol deviations, with quantifiable iloprost plasma concentrations and pre- and post-inhalation data on SBP and HR for both periods. Hemodynamic parameters in Part 2 were analyzed by analysis of variance (ANOVA) including sequence, period, subject, and treatment effects using SAS (version 9.2, SAS Institute Inc.).
PK data were analyzed by a standard non-compartmental PK analysis method using WinNonlin (Version 5.3, Pharsight Corporation) and WinAE 2.80 (Bayer AG) software. All patients who completed Part 1 with two valid PK profiles using the BREELIB nebulizer were included in the dose-linearity analysis of iloprost with the BREELIB nebulizer. The comparison of PK parameters from BREELIB and I-Neb was analyzed by ANOVA including sequence, period, subject, and treatment effects using SAS.
Results
Participants
Of 28 patients screened and randomized, 27 were treated and completed Parts 1 and 2 of the study. Of these, 26 completed Part 3 (Fig. 1). Twenty-four patients had valid data for PK evaluation; three patients in Part 2 had missing or invalid data or major protocol deviations affecting PK evaluation and were excluded from the analysis. Patient characteristics and concomitant medications at baseline are shown in Table 2.
Fig. 1.

Patient disposition. x/y values indicate that x participants were treated in the sequence I-Neb–BREELIB and y in the sequence BREELIB–I-Neb. AE, adverse event.
Table 2.
Baseline / pre-dose characteristics of the study population.
| Characteristic | All patients (n = 27) |
|---|---|
| Age (years) | 58 (16) |
| Sex (n (%)) | |
| Male | 6 (22) |
| Female | 21 (78) |
| Race (n (%)) | |
| Caucasian | 27 (100) |
| BMI (kg/m2) | 27 (7) |
| SBP (screening) (mmHg) | 120 (20) |
| DBP (screening) (mmHg) | 70 (10) |
| Patients with SBP < 100 mmHg immediately prior to dosing (n) | 7 |
| Concomitant medications | |
| ERA (n) | 25 |
| Bosentan | 17 |
| Ambrisentan | 7 |
| Macitentan | 1 |
| PDE5i (n) | 22 |
| Sildenafil | 17 |
| Tadalafil | 5 |
| Anti-thrombotic agents (n) | 27 |
| Phenprocoumon | 16 |
| Rivaroxaban | 3 |
| Acetylsalicylic acid | 3 |
| Warfarin | 2 |
| Apixaban | 1 |
| Enoxaparin | 1 |
| Heparin | 1 |
Data are mean (standard deviation) unless otherwise indicated.
BMI, body mass index; ERA, endothelin receptor antagonist; PDE5i, phosphodiesterase-5 inhibitor.
Inhalation time
Inhalation time with the BREELIB was approximately one-quarter that observed with the I-Neb, with median inhalation durations of 2.6 min (range = 1.6–3.4 min) and 10.9 min (range = 4.3–22.1 min), respectively (n = 24). Inhalation time was reduced for all patients with the BREELIB compared with the I-Neb.
PK analysis
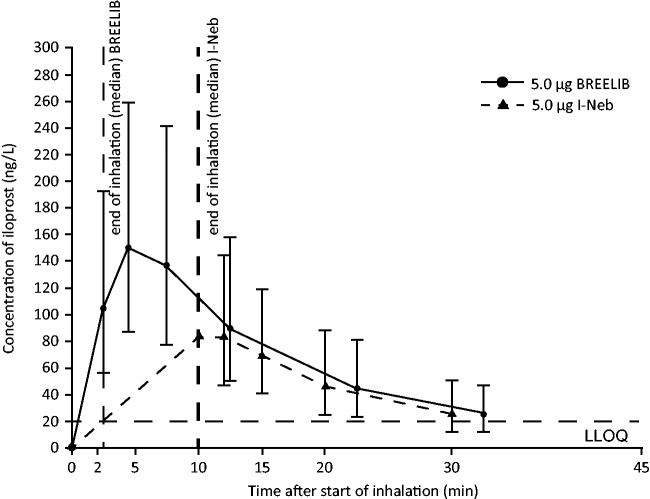
The PK of iloprost were dose proportional for the 2.5 µg and 5 µg doses at the mouthpiece following inhalation via the BREELIB (Table 3). Table 3 shows the PK parameters for iloprost 5 µg administered by the I-Neb and BREELIB. The plasma concentration–time profile of iloprost following inhalation with either nebulizer could be followed for up to 30 min after the end of inhalation. Cmax was reached within 2–4 min after the end of inhalation for each nebulizer, followed by an exponential decrease until the LLOQ was reached (Fig. 2). Geometric mean Cmax values were 77% higher for the BREELIB compared with the I-Neb (Table 3). As the total AUC could not be reliably determined in all patients owing to the rapid decline in iloprost plasma concentrations below the LLOQ after the end of inhalation, the AUC(0-tlast) of iloprost was used to determine systemic exposure. Geometric mean AUC(0-tlast) was 42% higher with the BREELIB compared with the I-Neb (Table 3). Although exposure values were higher with the BREELIB, there was considerable overlap between the ranges of individual exposure values for the two nebulizers. The higher overall AUC(0-tlast) observed with the BREELIB was mainly related to the high exposure fraction during the first 10 min after the start of inhalation, although it should be noted that exposure was not measured during inhalation with the I-Neb or BREELIB.
Table 3.
Pharmacokinetics and dose-linearity analysis for single-dose iloprost 2.5 µg and 5 µg administered with the BREELIB nebulizer and multiple-dose iloprost 5 µg administered with the BREELIB and I-Neb nebulizers in Parts 1 and 2 of the study.
| Dose linearity (Parts 1 and 2)* | 2.5 µg iloprost, BREELIB nebulizer |
5 µg iloprost, BREELIB nebulizer |
||||
|---|---|---|---|---|---|---|
| n | Geometric mean | Geometric CV% | n | Geometric mean | Geometric CV% | |
| AUC(0–tlast) (ng·h/L) | 24 | 23.5 | 77 | 24 | 46.6 | 78 |
| AUC (ng·h/L) | 7† | 45.0 | 12 | 8† | 63.1 | 42 |
| AUC(0–tlast)/D (h/L) | 24 | 0.0094 | 77 | 24 | 0.0093 | 78 |
| Cmax (ng/L) | 24 | 89.1 | 47 | 24 | 176 | 58 |
| Cmax/D (/L) | 24 | 0.0356 | 47 | 24 | 0.0353 | 58 |
| t1/2 (h) | 11† | 0.204 | 27 | 10† | 0.175 | 24 |
| 5 µg iloprost, I-Neb nebulizer |
5 µg iloprost, BREELIB nebulizer | |||||
| Nebulizer comparison (Part 2)* |
n |
Geometric mean |
Geometric CV% |
n |
Geometric mean |
Geometric CV% |
| AUC(0–tlast) (ng·h/L) | 24 | 29.1 | 67 | 24 | 40.9 | 81 |
| AUC (ng·h/L) | 5† | 44.6 | 27 | 7† | 61.8 | 46 |
| AUC(0–tlast)/D (h/L) | 24 | 0.0058 | 67 | 24 | 0.0082 | 81 |
| Cmax (ng/L) | 24 | 90.2 | 55 | 24 | 159 | 60 |
| Cmax/D (/L) | 24 | 0.0180 | 55 | 24 | 0.0317 | 60 |
| t1/2 (h) | 5† | 0.148 | 28 | 9† | 0.170 | 23 |
The population for dose linearity (upper half of table) consists of patients completing the inhalation of 2.5 µg and 5 µg iloprost in study Parts 1 and 2. The population for the nebulizer comparison (lower half of table) consists of patients completing the cross-over in study Part 2.
AUC and t1/2 could be determined reliably only for a minority of patients due to the rapid decrease of plasma concentrations below LLOQ.
AUC, area under the concentration–time curve; AUC(0-tlast), AUC from time 0 to the last data point above LLOQ; CV, coefficient of variation; Cmax, maximum drug concentration in plasma after single dose; D, dose; LLOQ, lower limit of quantification; t1/2, terminal elimination half-life.
Fig. 2.
Geometric mean concentration–time curves of iloprost (linear scale; bars, geometric standard deviation) obtained after a single inhalation of iloprost 5 µg using the BREELIB and I-Neb nebulizers. Note that blood samples were not taken for the duration of inhalation with either nebulizer. LLOQ, lower limit of quantification.
Safety
Part 1
The first 11 patients tolerated inhalation of iloprost 1.25 µg and 2.5 µg well. In accordance with the protocol, the remaining patients received only iloprost 2.5 µg. The AEs experienced were mild, transient, and consistent with the known safety profile of iloprost (Table 4).
Table 4.
Adverse events reported in Parts 1–3 of the study.
| AE (n (%)) |
Iloprost dose and nebulizer |
||
|---|---|---|---|
| Part 1 | 1.25 µg BREELIB (n = 11) | 2.5 µg BREELIB (n = 27) | Overall (n = 27) |
| Any AE | 3 (27) | 6 (22) | 9 (33) |
| Hypotension* | 2 (18) | 3 (11) | 5 (19) |
| Headache | 1 (9) | 1 (4) | 2 (7) |
| Angina pectoris | 0 | 1 (4) | 1 (4) |
| Cough | 0 | 1 (4) | 1 (4) |
| Dizziness | 0 | 1 (4) | 1 (4) |
| Hematoma | 0 | 1 (4) | 1 (4) |
| Nasopharyngitis | 0 | 1 (4) | 1 (4) |
| Part 2 |
5 µg I-Neb (n = 26) |
5 µg BREELIB (n = 27) |
Overall (n = 27) |
| Decrease in SBP ≥ 20% within 30 min of inhalation | 1 (4) | 4 (15) | 5 (19) |
| Increase in HR ≥ 25% within 30 min of inhalation | 0 (0) | 0 (0) | 0 (0) |
| Any AE | 3 (12)† | 4 (15)† | 6 (22)† |
| Hypotension* | 1 (4) | 4 (15) | 5 (19) |
| Cough | 1 (4) | 0 | 1 (4) |
| Dizziness | 0 | 1 (4) | 1 (4) |
| Feeling abnormal | 0 | 1 (4) | 1 (4) |
| Head discomfort | 0 | 1 (4) | 1 (4) |
| Hypertensive crisis | 1 (4) | 0 | 1 (4) |
| Part 3 |
5 µg I-Neb (n = 26) |
5 µg BREELIB (n = 27) |
Overall (n = 27) |
| Any AE | 7 (27)† | 14 (52)† | 14 (52)† |
| AEs occurring in > 4% of patients | |||
| Headache | 2 (8) | 4 (15) | 5 (19) |
| Cough | 0 | 3 (11) | 3 (11) |
| Atrioventricular block first degree | 0 | 2 (7) | 2 (7) |
| Hot flush | 0 | 2 (7) | 2 (7) |
| Palpitations | 0 | 2 (7) | 2 (7) |
| Respiratory tract infection | 1 (4) | 1 (4) | 2 (7) |
| Any serious AE | 2 (8)† | 2 (7)† | 4 (15)† |
| Pneumonia | 0 | 1 (4) | 1 (4) |
| Gastrointestinal hemorrhage | 0 | 1 (4) | 1 (4) |
| Syncope | 0 | 1 (4) | 1 (4) |
| Hyperglycemia | 1 (4) | 0 | 1 (4) |
| Hypokalemia | 1 (4) | 0 | 1 (4) |
| Diabetes mellitus | 1 (4) | 0 | 1 (4) |
| Colonoscopy | 1 (4) | 0 | 1 (4) |
Hypotension was pre-defined as SBP ≤ 90 mmHg, irrespective of hypotensive symptoms.
Patients can have experienced more than one AE.
AE, adverse event; HR, heart rate; SBP, systolic blood pressure.
Part 2
Twenty-seven patients participated in Part 2 and were included in the safety analysis, of whom 24 received single doses of iloprost 5 µg with each nebulizer and had valid data for both study periods. Predefined hemodynamic events (a maximum increase in HR of ≥ 25% and/or a maximum decrease in SBP of ≥ 20% within 30 min of completing inhalation) or AEs of hypotension were observed more often with the BREELIB than the I-Neb (Table 4). Four patients (15%) experienced a decrease in SBP ≥ 20% with the BREELIB compared with one patient (4%) with the I-Neb. No patient had an increase in HR ≥ 25%.
AEs of hypotension (SBP ≤ 90 mmHg, irrespective of symptoms) were reported in four patients (15%) after inhalation with the BREELIB (two of whom also experienced a predefined hemodynamic event of a maximum decrease in SBP of ≥ 20% within 30 min of inhalation) and in one patient with the I-Neb. In total, eight patients (30%) experienced a hemodynamic event or hypotension: six with the BREELIB and two with the I-Neb. Of these, only one (4%, treated with the BREELIB) experienced symptomatic hypotension (transient symptoms for 5–32 min, mild in severity). None of the eight patients required medical intervention for decreased SBP. All eight patients continued treatment with the BREELIB and none experienced hypotension in Parts 3 or 4. One patient (4%) experienced local irritation (cough, mild in severity, not leading to discontinuation of inhalation) while using the I-Neb; no local irritation events were reported with the BREELIB.
The maximum increase in HR was 6.5 beats per minute (bpm) (90% confidence interval [CI] 5.2–7.9 bpm) for the BREELIB versus 2.4 bpm (90% CI 1.1–3.7 bpm) with the I-Neb. The mean maximum decrease in SBP was 12.9 mmHg (90% CI 10.3–15.5 mmHg) with the BREELIB versus 10.1 mmHg (90% CI 7.5–12.7 mmHg) with the I-Neb. These changes were mild and transient, and did not require medical intervention. No serious AEs (SAEs) were reported in Parts 1 and 2.
Part 3
Twenty-six patients received multiple doses of iloprost with the I-Neb and 27 with the BREELIB in Part 3. More AEs were reported with the BREELIB (52%) compared with the I-Neb (27%) in Part 3 (Table 4). SAEs were reported in two patients (7%) with the BREELIB and two patients (8%) with the I-Neb (Table 4). No SAE was considered study drug-related by the investigators. No AEs of hypotension were reported in Part 3.
Local irritation was observed more frequently with the BREELIB than the I-Neb in Part 3; three patients (11%) experienced cough and one (4%) experienced oropharyngeal pain with the BREELIB, while no events were reported with the I-Neb. All local irritations were mild in severity and did not interfere with inhalation.
There were no deaths during Parts 1–3 of the study, and no treatment-emergent AEs led to discontinuation of treatment.
Part 4
Of the 26 patients completing Part 3 of the study, 25 entered the LTE. At the October 2015 data cutoff, 18 patients were ongoing in the study. The median treatment duration was 330 days (range = 38–555 days) and the total treatment exposure of the LTE population was 8287 days. Of the seven patients (28%) who discontinued from the study in Part 4, two patients died (both due to right ventricular failure as a result of worsening PAH), four withdrew due to AEs (lung transplantation [n = 3] and worsening PAH [n = 1]), and one withdrew due to lack of efficacy. The AEs leading to death or drug withdrawal were assessed by the investigators to be unrelated to iloprost. Twenty-two patients experienced AEs (Table 5), six of whom were judged to be study drug-related. All study drug-related AEs were non-serious and were known adverse reactions to iloprost with the exception of extrasystoles, which occurred in a patient with a history of atrial fibrillation and extrasystoles. Hypotension was reported in two patients (not study drug-related); one patient had a history of intermittent hypotension, with baseline SBP < 90 mmHg, and the other was receiving concomitant antihypertensives. Syncope was reported in five patients (not study drug-related). The causes of syncope were physical stress, intercurrent infections, diabetes, orthostatic hypotension, and progression of underlying PAH. Five patients experienced AEs of cough, two of whom were judged to be study drug-related and three of which were not. The reported AEs were consistent with the known safety profile of iloprost and the nature of PAH. Thirteen patients (52%) experienced SAEs; none were considered study drug-related. The most frequent SAEs were right heart failure, syncope, worsening PAH, and lung transplant (Table 5).
Table 5.
Summary of AEs in the long-term extension (Part 4).
| AE (n (%)) | All patients (n = 25) |
|---|---|
| Any AE | 22 (88) |
| AEs occurring in ≥ 20% of patients | |
| Respiratory tract infection | 8 (32) |
| Hypokalemia | 8 (32) |
| Nasopharyngitis | 6 (24) |
| Dyspnea | 6 (24) |
| Dizziness | 6 (24) |
| Anemia | 6 (24) |
| Cough | 5 (20) |
| Syncope | 5 (20) |
| Palpitations | 5 (20) |
| Peripheral edema | 5 (20) |
| Nausea | 5 (20) |
| Any AE related to the study drug | 6 (24) |
| Any AE leading to discontinuation of treatment | 4 (16) |
| Death | 2 (8) |
| SAE | |
| Any SAE | 13 (52) |
| Any SAE, study drug-related | 0 |
| SAEs occurring in ≥ 5% of patients | |
| Right heat failure | 4 (16) |
| Syncope | 5 (20) |
| Lung transplant | 3 (12) |
| Worsening PAH | 2 (8) |
AE, adverse event; PAH, pulmonary arterial hypertension; SAE, serious adverse event.
Discussion
The BREELIB nebulizer was developed to provide a device specifically for iloprost inhalation, to reduce inhalation times and thereby to improve convenience for patients. This phase 1/2 study compared inhalation time, PK, and safety of iloprost 5 µg administered via the BREELIB nebulizer and the standard I-Neb nebulizer.
These data show that inhalation time was substantially reduced with the BREELIB compared with the I-Neb, with no overlap between the ranges for the median inhalation times of the two nebulizers. Patients with PAH are required to inhale iloprost 6–9 times daily according to individual need and tolerability.1 The estimated inhalation time for iloprost 5 µg is 6.5 min with the I-Neb.1 The median duration of inhalation for delivery of iloprost 5 µg with the I-Neb in the present study was 10.9 min, consistent with reports from other clinical studies.1,8,17 Therefore, net daily inhalation time with the I-Neb in this study equates to 65–98 min, excluding time for preparation and cleaning of the nebulizer. Such inhalation times may be inconvenient for patients and could result in treatment non-compliance, as seen with inhalation therapies in other indications.24 A key characteristic and potential advantage of the BREELIB is reduced inhalation times, as shown in this study, with a median inhalation time of 2.6 min (equating to a net daily inhalation time of 16–23 min), a reduction of 76% compared with the l–Neb. This may improve convenience for the patient and thus their adherence to treatment, ultimately leading to improvements in efficacy of therapy and quality of life. Interestingly, the time required for delivery of an adequate iloprost dose with the BREELIB is in the range seen for current treprostinil aerosol therapy for PAH.25,26
Following inhalation with the BREELIB, the PK of iloprost were dose proportional in the range of 2.5–5 µg. A comparison of the 5 µg dose inhaled via the two nebulizers showed higher AUC(0-tlast) and Cmax for iloprost with the BREELIB compared with the I-Neb. This is consistent with the shorter inhalation time—and thus higher dose rate—with the BREELIB, resulting in higher mean iloprost plasma concentrations in the first 10 min after the start of inhalation. However, it should also be noted that no PK samples were taken during inhalation, which may have impacted AUC evaluation. The PK of iloprost with the BREELIB were comparable to those reported for other iloprost nebulizers,27 suggesting similar pharmacodynamic effects. The Cmax and AUC(0-tlast) of iloprost with the BREELIB were 159 ng/L and 40.9 ng·h/L, respectively. Olschewski et al. previously compared inhalation by three jet nebulizers. This study demonstrated decreases in PVR and mPAP with a Cmax of 155–158 ng/L and an AUC(0-tlast) of 47.8–54.2 ng·h/L.27
The shorter inhalation times using the BREELIB were anticipated to lead to higher Cmax values. Consequently, acute tolerability, inferred from the incidence rate of predefined hemodynamic events, and AEs were the primary outcomes of this study. A higher incidence of hemodynamic events was observed with the BREELIB versus the I-Neb: four (15%) versus one (4%), respectively. The difference in incidences is within the expected range, based on data from the pivotal phase 3 AIR study,11 in which the incidence of hemodynamic events was 3% (90% CI 1–8%) in patients receiving iloprost. Thus, for this study with 24 patients, a difference of up to three incidents was not considered to be of significance when comparing both treatments.
Nevertheless, the data presented here point to a slightly more pronounced impact of the BREELIB on systemic hemodynamics compared with the I-Neb during the first 10 min after inhalation, consistent with the higher exposure immediately following inhalation via BREELIB. However, these changes were mild, mostly asymptomatic and transient, and did not require medical intervention. Further analysis of patients with elevated HR or tachycardia at baseline did not suggest a higher risk (i.e. more pronounced treatment-emergent HR increases), and patients with a low SBP at baseline did not have a higher risk of experiencing hypotension (data not shown). Taking these data into account, it is recommended that at initiation of iloprost treatment or when switching from another device, the first inhalation should be performed with 1 mL of iloprost 10 µg/mL (delivering iloprost 2.5 µg at the mouthpiece); if 2.5 µg is well tolerated, the dose should be increased by using iloprost 20 µg/mL (delivering iloprost 5 µg at the mouthpiece).1
AEs with the BREELIB were consistent with the known safety profile of iloprost, with no unexpected findings. More AEs were reported with the BREELIB than the I-Neb, especially in the 2-week cross-over period of Part 3. The higher rate of hypotension and local irritations experienced with the BREELIB may be related to the higher initial exposure to iloprost with the BREELIB; however, these events were mild, transient, and well tolerated. Syncope, which was reported in one patient in Part 3 and five patients in the LTE, is a known adverse drug reaction associated with iloprost; however, it is also a frequently observed symptom of PAH,28 and in this study no episode of syncope was assessed as study drug-related. No patient discontinued use of the BREELIB during Parts 1–3, and 25 of 26 eligible patients decided to enter the LTE with the BREELIB despite being used to the I-Neb before study entry. Data from the LTE support the safety and tolerability of iloprost administered using the BREELIB; after a median treatment duration of 330 days, there have been no reports of study drug-related SAEs.
Limitations of the study include the fact that blinding was not possible due to the different design and appearance of the nebulizers. Therefore, a bias in safety reporting cannot be excluded due to patients’ familiarity with the I-Neb. In addition, as the study population was pre-treated with iloprost, tolerance or conditioning to the side effects of prostanoids may have resulted in under-reporting of AEs in comparison with treatment-naïve patients. The recommendation for initiation of therapy with iloprost 2.5 µg at the mouthpiece is intended to mitigate this possibility.
Conclusions
The BREELIB considerably reduced inhalation time compared with the I-Neb. As a result, higher iloprost plasma concentrations were observed, especially within the first 10 min after commencing inhalation with the BREELIB. AEs following inhalation of iloprost with either nebulizer were consistent with the known safety profile of iloprost. These PK and safety data suggest that the BREELIB represents a viable option for iloprost inhalation, potentially enhancing acceptance by patients and thereby improving treatment compliance.
Acknowledgments
The BREELIB nebulizer was developed and manufactured by Vectura Group plc. Medical writing assistance was provided by Adelphi Communications Ltd, funded by Bayer AG.
Declaration of conflicting interests
TG reports non-financial support from Bayer AG during the conduct of the study. In addition, TG has a patent WO002014067699A1 with royalties paid to ACTIVAERO GmbH, and a patent WO002012146642A1 with royalties paid to ACTIVAERO GmbH. H-AG reports personal fees from Actelion, Bayer, GSK, Novartis, Pfizer, Bellerophon Therapeutics, and United Therapeutics; grants from Actelion, Bayer, Pfizer, and Novartis. MH reports grants from Actelion, honoraria for lectures from Actelion, Bayer Healthcare, Berlin Chemie, Boehringer Ingelheim, GSK, MSD, Novartis, Pfizer; honoraria for advisory board activities from Actelion, Bayer, Boehringer, GSK, and MSD. HK has nothing to disclose. HL reports consulting and personal fees from Actelion Pharmaceuticals; speaker honoraria, and personal fees from Bayer AG; consulting and speaker honoraria and non-financial support from Bayer AG; travel support and personal fees from MSD; consulting and speaker honoraria and personal fees from GSK; consulting and speaker honoraria and personal fees from Pfizer. HO reports grants and personal fees from Actelion and Bayer; personal fees from Gilead, GSK, Novartis, Pfizer; personal fees from Bellerophon. SR reports grants and personal fees from Actelion, Bayer, Novartis, Pfizer, and United Therapeutics; personal fees from GSK. LF, NL, DR, AK, M-HS-M, and BR are employees of Bayer AG. BM is an employee of Vectura GmbH. WS has nothing to disclose. ACTIVAERO GmbH was acquired by Vectura Group plc. in March 2014.
Funding
This study was funded by Bayer AG.
References
- 1.Bayer Pharma AG. Ventavis10 SmPC. Available at: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000474/WC500048691.pdf (2016, accessed on 19 April 2016).
- 2.Actelion Pharma Ltd. Ventavis Prescribing information. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2008/021779s008lbl.pdf (2015, accessed on 13 July 2015).
- 3.Schermuly RT, Ghofrani HA, Wilkins MR, et al. Mechanisms of disease: pulmonary arterial hypertension. Nat Rev Cardiol 2011; 8(8): 443–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Montani D, Gunther S, Dorfmuller P, et al. Pulmonary arterial hypertension. Orphanet J Rare Dis 2013; 8: 97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Galiè N, Muller K, Scalise AV, et al. PATENT PLUS: a blinded, randomised and extension study of riociguat plus sildenafil in PAH. Eur Respir J 2015; 45(5): 1314–1322. [DOI] [PubMed] [Google Scholar]
- 6.LeVarge BL. Prostanoid therapies in the management of pulmonary arterial hypertension. Ther Clin Risk Manag 2015; 11: 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gomberg-Maitland M, Olschewski H. Prostacyclin therapies for the treatment of pulmonary arterial hypertension. Eur Respir J 2008; 31(4): 891–901. [DOI] [PubMed] [Google Scholar]
- 8.Hill NS, Preston IR, Roberts KE. Inhaled therapies for pulmonary hypertension. Respir Care 2015; 60(6): 794–802. [DOI] [PubMed] [Google Scholar]
- 9.Olschewski H, Walmrath D, Schermuly R, et al. Aerosolized prostacyclin and iloprost in severe pulmonary hypertension. Ann Intern Med 1996; 124(9): 820–824. [DOI] [PubMed] [Google Scholar]
- 10.Galie N, Humbert M, Vachiery JL, et al. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Respir J 2015; 46(4): 903–975. [DOI] [PubMed] [Google Scholar]
- 11.Olschewski H, Simonneau G, Galiè N, et al. Inhaled iloprost for severe pulmonary hypertension. N Engl J Med 2002; 347(5): 322–329. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin VV, Oudiz RJ, Frost A, et al. Randomized study of adding inhaled iloprost to existing bosentan in pulmonary arterial hypertension. Am J Respir Crit Care Med 2006; 174(11): 1257–1263. [DOI] [PubMed] [Google Scholar]
- 13.Olschewski H, Hoeper MM, Behr J, et al. Long-term therapy with inhaled iloprost in patients with pulmonary hypertension. Respir Med 2010; 104(5): 731–740. [DOI] [PubMed] [Google Scholar]
- 14.Saji T, Myoishi M, Sugimura K, et al. Efficacy and safety of inhaled iloprost in Japanese patients with pulmonary arterial hypertension – insights from the IBUKI and AIR studies. Circ J 2016; 80(4): 835–842. [DOI] [PubMed] [Google Scholar]
- 15.Gall H, Sommer N, Milger K, et al. Survival with sildenafil and inhaled iloprost in a cohort with pulmonary hypertension: an observational study. BMC Pulm Med 2016; 16: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ghofrani HA, Galiè N, Grimminger F, et al. Riociguat for the treatment of pulmonary arterial hypertension. N Engl J Med 2013; 369(4): 330–340. [DOI] [PubMed] [Google Scholar]
- 17.Actelion. The “Power 15 Study”: Safety Study of Inhalation of Ventavis With the Power Disc-15 Setting. Available at: https://clinicaltrials.gov/ct2/show/results/NCT00467896 2016 [accessed on 19 April 2016].
- 18.Lareau SC, Yawn BP. Improving adherence with inhaler therapy in COPD. Int J Chron Obstruct Pulmon Dis 2010; 5: 401–406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fischer A, Stegemann J, Scheuch G, et al. Novel devices for individualized controlled inhalation can optimize aerosol therapy in efficacy, patient care and power of clinical trials. Eur J Med Res 2009; 14(Suppl. 4): 71–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.European Medicines Agency. Guideline on bioanalytical method validation. Available at: http://www.ema.europa.eu/ema/index.jsp?curl=pages/includes/document/document_detail.jsp?webContentId=WC500109686%26mid=WC0b01ac058009a3dc (2011, accessed on 1 August 2016).
- 21.European Medicines Agency. Reflection paper for laboratories that perform the analysis or evaluation of clinical trial samples. Available at: www.ema.europa.eu/docs/en_GB/document_library/…and…/WC500127124.pdf (2012, accessed on 1 August 2016).
- 22.Food and Drug Administration. Guidance for Industry “Bioanalytical Method Validation”. Available at: www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf (2001, accessed on 1 August 2016).
- 23.Ministry of Health and Welfare J. Guideline on Bioanalytical Method Validation in Pharmaceutical Development. Available at: https://www.pmda.go.jp/files/000206209.pdf (2013, accessed on 1 August 2016).
- 24.Makela MJ, Backer V, Hedegaard M, et al. Adherence to inhaled therapies, health outcomes and costs in patients with asthma and COPD. Respir Med 2013; 107(10): 1481–1490. [DOI] [PubMed] [Google Scholar]
- 25.McLaughlin VV, Benza RL, Rubin LJ, et al. Addition of inhaled treprostinil to oral therapy for pulmonary arterial hypertension: a randomized controlled clinical trial. J Am Coll Cardiol 2010; 55(18): 1915–1922. [DOI] [PubMed] [Google Scholar]
- 26.Voswinckel R, Enke B, Reichenberger F, et al. Favorable effects of inhaled treprostinil in severe pulmonary hypertension: results from randomized controlled pilot studies. J Am Coll Cardiol 2006; 48(8): 1672–1681. [DOI] [PubMed] [Google Scholar]
- 27.Olschewski H, Rohde B, Behr J, et al. Pharmacodynamics and pharmacokinetics of inhaled iloprost, aerosolized by three different devices, in severe pulmonary hypertension. Chest 2003; 124(4): 1294–1304. [DOI] [PubMed] [Google Scholar]
- 28.Rich S, Dantzker DR, Ayres SM, et al. Primary pulmonary hypertension. A national prospective study. Ann Intern Med 1987; 107(2): 216–223. [DOI] [PubMed] [Google Scholar]