Abstract
Objective:
To determine whether elevated β2-microglobulin (B2M) levels were associated with an increased risk of incident ischemic stroke events among women.
Methods:
We performed a nested case-control study among women enrolled in the Nurses' Health Study who provided blood samples between 1989 and 1990 and were free of prior stroke and cancer. We measured B2M levels in 473 ischemic strokes cases confirmed by medical record review and in 473 controls matched 1:1 to the cases on age, race, date of blood collection, menopausal status, postmenopausal hormone use, and smoking status. We analyzed the association between B2M and ischemic stroke using multivariable conditional logistic regression to adjust for traditional stroke risk factors.
Results:
Median levels of B2M were higher among cases (1.86 mg/L) than controls (1.80 mg/L, p = 0.009, Wilcoxon rank-sum test). Women in the highest B2M quartile had a multivariable-adjusted increased risk of ischemic stroke compared to those in the lowest quartile (odds ratio [OR] 1.56, 95% confidence interval [CI] 1.02–2.39). Results were similar when restricted to those without evidence of chronic kidney disease (estimated glomerular filtration rate ≥60 mL·min−1·1.73 m−2) (OR 1.49, 95% CI 1.08–2.06). In an exploratory analysis, the association between B2M and thrombotic stroke was similar to the overall ischemic stroke results, but no association was observed for embolic stroke risk.
Conclusion:
High levels of B2M were associated with an increased risk of ischemic stroke among women.
Given the high morbidity burden of stroke,1 there is great interest in finding novel markers that identify individuals at higher risk of stroke. One potential risk marker may be β2-microglobulin (B2M). B2M is a component of class I major histocompatibility molecules found on the surface of nucleated cells.2 B2M levels are elevated with systemic inflammation.3 In addition, B2M levels rise as glomerular filtration rate (GFR) decreases, prompting the evaluation of B2M as a marker of renal disease and function. Declining GFR may indicate subclinical vascular disease and has been associated with cardiovascular disease (CVD) events,4,5 leading to interest in B2M as a marker of CVD risk.
Recent studies have found associations between B2M and CVD or coronary heart disease (CHD) mortality in the general population.6–8 However, less is known about the association between B2M and ischemic stroke or ischemic stroke subtypes. One prior study using data from the Women's Health Initiative suggested that a 30% increase in B2M increases the risk of total stroke by 18%,9 but this study did not explore whether kidney function modified this association. In the Atherosclerosis Risk in Communities study, which enrolled both men and women, higher B2M levels were associated with increased risk of total CVD and total stroke among those with and without chronic kidney disease.7 Given the proposed mechanisms by which elevated B2M levels increase total stroke risk, we would expect to observe a similar, if not stronger, association between elevated B2M levels and risk of ischemic stroke. However, this has not been confirmed in prior studies, nor have these studies explored whether other CVD factors besides kidney function modify the association between B2M levels and stroke risk.
Using a nested case-control study design, we explored the association between B2M levels and risk of ischemic stroke after adjustment for CVD risk factors. In addition, we explored whether this association may be modified by kidney function, age, body mass index (BMI), smoking, postmenopausal hormone therapy, history of diabetes mellitus, and hypertension.
METHODS
The Nurses' Health Study (NHS) enrolled 121,700 registered female nurses 30 to 55 years of age in 1976. The NHS has been described in detail previously.10 Briefly, participants are sent biennial follow-up questionnaires asking about lifestyle factors and medical history, including stroke events. From 1989 to 1990, 32,826 participants provided blood samples that were processed, archived, and stored in liquid nitrogen freezers as previously described.11,12
We performed a nested case-control study of B2M levels and risk of ischemic stroke among participants with available blood samples. Both cases and controls did not have a history of cancer or stroke at the time of blood collection. Incident ischemic stroke cases, confirmed by medical record review, were matched 1:1 to controls without a history of stroke before the index date (i.e., the date the case experienced a stroke event) by age (±2 years), race/ethnicity (white/black/Asian/Hispanic/other/unknown), smoking (never, past, and current), date of blood draw (±3 months), fasting status, menopausal status, and hormone use (yes/no).
Standard protocol approvals, registrations, and patient consents.
The Institutional Review Board of Brigham and Women's Hospital approved this study. Informed consent was implied by completion of self-administered questionnaires and providing of blood samples.
Blood sample assay.
Case-control pairs were processed in the same manner, shipped together, and assayed in the same run at the laboratory. The laboratory is certified by the Centers for Disease Control and Prevention/National Heart, Lung, and Blood Institute Lipid Standardization Program with commercially available analytic systems. Laboratory precision was assessed with replicate blinded plasma samples. Drift samples standardized to specific biomarker values are used to assess and correct for laboratory assay drift.
B2M and creatinine were measured with the Roche P Modular system (Roche Diagnostics, Indianapolis, IN) via an immunoturbidimetric technique. Coefficient of variation (CV) for B2M was 1% and for creatinine was 2.2%. Total cholesterol was measured enzymatically (mean CV 4%) and high-density lipoprotein cholesterol (HDL-C) concentration was determined with a direct enzymatic colorimetric assay (mean CV 3%).
Ischemic stroke assessment.
Confirmed ischemic strokes that occurred after receipt of the blood sample through 2008 were included in these analyses. If a woman reported a nonfatal stroke, we asked for permission to review her medical records. Deaths were reported by next of kin or postal authorities or determined by searching the National Death Index. Fatal strokes were confirmed from death certificates, hospital records, or autopsy records. Physicians blinded to exposure status reviewed medical records and confirmed stroke events according to the National Survey of Stroke criteria, which require evidence of a neurologic deficit with sudden or rapid onset persisting for >24 hours or until death. Strokes with evidence of thrombotic or embolic occlusion of a cerebral artery were considered ischemic stroke events. Ninety-five percent of stroke events had imaging data available. Interobserver agreement for stroke classification was high.13 To gain further insights into the mechanisms behind the association between B2M levels and ischemic stroke, we performed an exploratory analysis using information on ischemic stroke subtypes. Thrombotic strokes were defined as “infarction involving the cortical artery regions in the cerebrum and cerebellum (cortex and subcortical areas)” or “focal, small, and deep areas such as the internal capsule, corona radiate, basal ganglia, and brain stem, without involvement of the cortex.”13 Strokes were defined as embolic “if evidence of an embolic source was present in the medical record and if imaging studies and neurology consult supported the diagnosis.”13 In the event of incomplete evidence or competing causes of stroke, strokes were considered to be unclassified ischemic stroke.
Statistical analysis.
A total of 473 cases and 473 matched controls had available information on B2M levels. We created quartiles of B2M levels based on the distribution in the controls and assigned cases to each quartile on the basis of their plasma B2M level. Baseline characteristics of individuals in each B2M quartile were compared by use of χ2 tests for categorical variables and proportions, except for race, for which the Fisher exact test was used, and Wilcoxon rank-sum tests for continuous variables.
We used lifestyle covariates from the 1990 questionnaire or the closest year before, except for height (collected only in 1976). We had complete information on all lifestyle covariates except for alcohol consumption, physical activity, smoking, and aspirin use. Those missing information on physical activity (n = 8) or alcohol consumption (n = 19) were assigned to the median value by case-control status. Those missing information on smoking (n = 2) or aspirin (n = 3) were assigned to never smoker and no use of aspirin, the most frequent category for each variable. Model 1 conditioned on age, race/ethnicity, smoking, menopausal status, hormone therapy use, and date of sample collection (all matching factors). In addition to conditioning on the matching factors, model 2 adjusted for physical activity (continuous in metabolic equivalents), BMI (continuous in kg/m2), aspirin use (none vs ≥1 tablets per week), alcohol consumption (continuous in g/d), history of diabetes mellitus (yes/no), history of high blood pressure (yes/no), history of CHD or revascularization (yes/no), and log-transformed ratio of total cholesterol to HDL-C (continuous). Thirteen matched pairs were missing information on cholesterol ratio, and median values were imputed by case-control status.
We conducted several sensitivity analyses to evaluate whether renal function modified our results. We calculated the estimated GFR (eGFR) using the Chronic Kidney Disease Epidemiology Collaboration equation.14 Creatinine levels were missing for 13 matched pairs, and median values were imputed by case-control status. First, in model 3, we controlled for continuous eGFR in addition to all the covariates in model 2. Second, we estimated the association between B2M levels and risk of ischemic stroke among those with eGFR ≥60 mL·min−1·1.73 m−2, the cutoff for abnormal renal function/chronic kidney disease.
A priori, we decided to examine effect modification of B2M (highest quartiles compared to all others) on risk of ischemic stroke by age (<65/≥65 years), postmenopausal hormone therapy (use vs no use), BMI (≥25 vs 18.5–24.9 kg/m2), smoking (current vs past/never), history of diabetes mellitus at baseline (yes vs no), and history of hypertension at baseline (yes vs no). Given the long duration of follow-up, we explored whether time since blood collection modified the association between B2M levels and risk of ischemic stroke by including an interaction term between median follow-up time (<9 vs ≥9 years) and our exposure. The likelihood ratio test was used to determine whether the interaction was statistically significant.
Finally, we explored the association between B2M levels and risk of ischemic stroke subtypes (thrombotic and embolic strokes).
We used SAS for UNIX statistical software (version 9.3, SAS Institute, Cary, NC) for all analyses. Statistical significance was assessed with 2-sided p values.
RESULTS
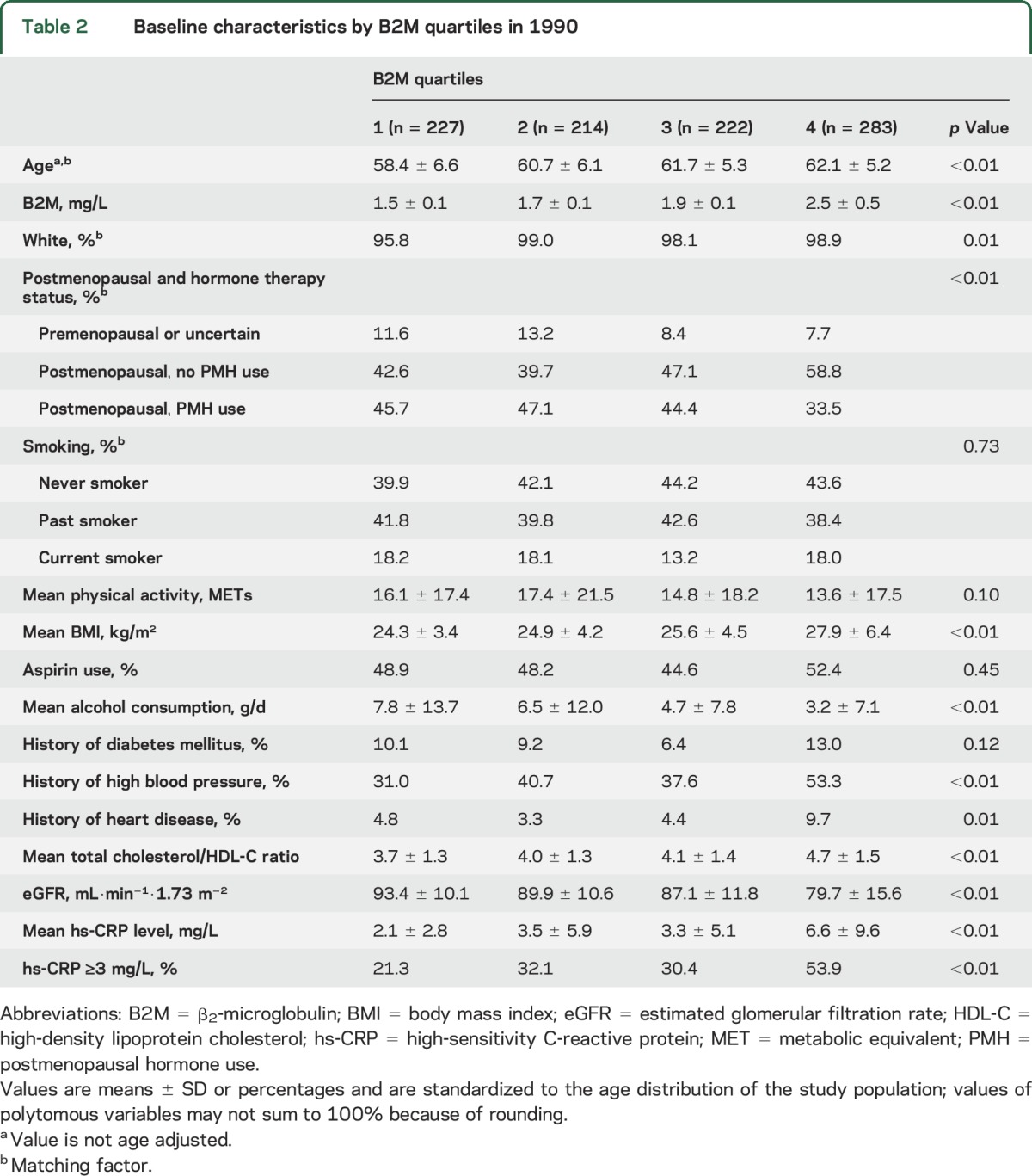
The mean age at baseline was 60.8 years, with a median of 9.0 years to stroke event. Baseline median levels of B2M were higher among cases (1.86 mg/L) than controls (1.80 mg/L, p = 0.009, Wilcoxon rank-sum test). The baseline characteristics are presented in table 1. As expected, women who later developed an ischemic stroke had a higher ratio of total cholesterol to HDL-C ratio and were more likely to have a history of diabetes mellitus or high blood pressure than controls. Table 2 presents the baseline characteristics of the participants by quartile of B2M. Women in the highest B2M quartile had more adverse cardiovascular profiles; they were older and had higher BMI, high-sensitivity C-reactive protein (hs-CRP) levels, total cholesterol/HDL-C ratio, and prevalence of hypertension and prior CHD.
Table 1.
Baseline characteristics by case-control status in 1990
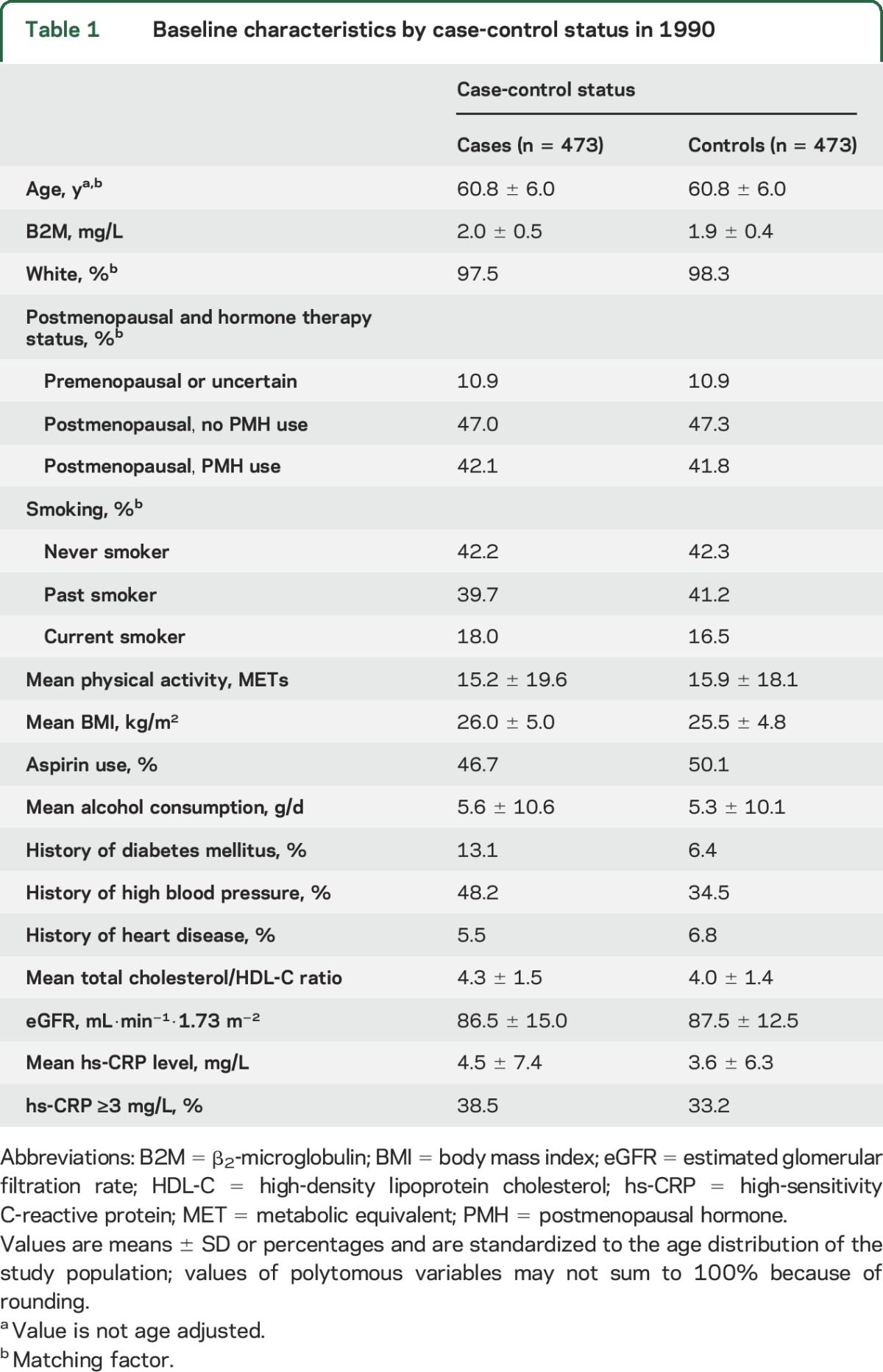
Table 2.
Baseline characteristics by B2M quartiles in 1990
Conditional on the matching factors, women in the highest quartile of B2M had a statistically significant increase in the odds of developing an ischemic stroke (odds ratio [OR] 1.71, 95% confidence interval [CI] 1.14–2.56) compared to those in the lowest quartile. Other quartiles were not associated with an increased risk of ischemic stroke compared to the lowest quartile (table 3 and figure). Adjustment for traditional stroke risk factors, including BMI, physical activity, alcohol intake, history of diabetes mellitus, history of hypertension, history of CHD, aspirin use, and total cholesterol/HDL-C ratio, attenuated the effect estimate (model 2: OR 1.56, 95% CI 1.02–2.39). Additional adjustment for eGFR slightly attenuated the results, and the effect estimate was no longer statistically significant (model 3: OR 1.53, 95% CI 0.98–2.41). Additional adjustment for hypertension severity (use of antihypertension medication and blood pressure categories before blood collection) also slightly attenuated our results (OR 1.49, 95% CI 0.95–2.35, highest vs lowest quartile).
Table 3.
Multivariable-adjusted ORs (95% CIs) of ischemic stroke by quartiles of B2M
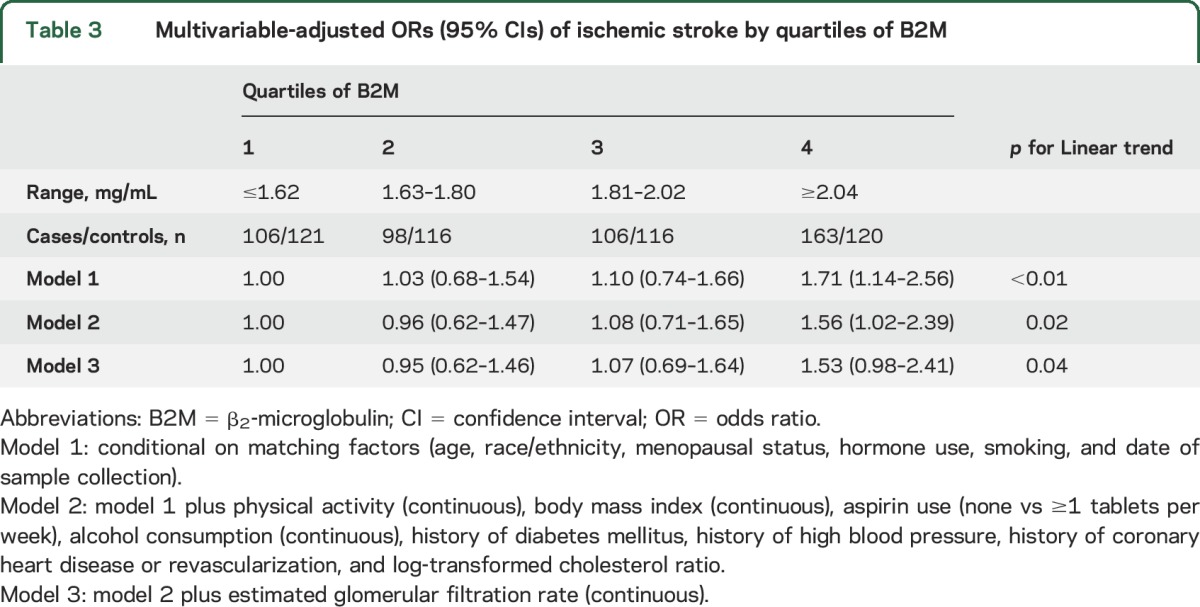
Figure. Multivariable-adjusted OR (95% CI) of ischemic stroke by quartiles of B2M.
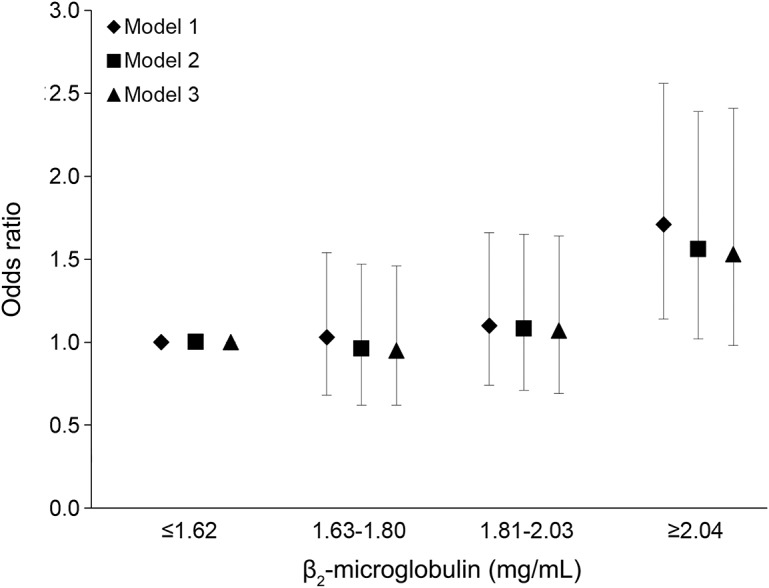
Model 1: conditional on matching factors (age, race/ethnicity, menopausal status, hormone use, smoking, and date of sample collection). Model 2: model 1 plus physical activity (continuous), body mass index (continuous), aspirin use (none vs ≥1 tablets per week), alcohol consumption (continuous), history of diabetes mellitus, history of high blood pressure, history of coronary heart disease or revascularization, and log-transformed cholesterol ratio. Model 3: model 2 plus eGFR (continuous). B2M = β2-microglobulin; CI = confidence interval; eGFR = estimated glomerular filtration rate; OR = odds ratio.
When B2M levels were dichotomized (highest quartile vs all other quartiles), high B2M levels were associated with a statistically significant increase in the multivariate-adjusted odds of developing an ischemic stroke (model 2: OR 1.54, 95% CI 1.11–2.12) compared to lower B2M levels. We also examined risk within the highest quartiles by dividing the highest quartiles into tertiles. We did not observe a clear pattern between increasing tertiles within the highest quartile and increasing risk of ischemic stroke (results not shown).
To minimize the influence of abnormal renal function on our results, we also restricted our analyses to those with eGFR ≥60 mL·min−1·1.73 m−2 at baseline (448 cases and 459 controls). We observed a significant increase in the risk of ischemic stroke for those without chronic kidney disease in the highest quartile of B2M compared to those in the lower quartiles (OR 1.49, 95% CI 1.08–2.06) (table e-1 at Neurology.org). We were unable to examine the association between elevated B2M and risk of ischemic stroke among individuals with eGFR <60 mL·min−1·1.73 m−2 because all of the cases in this group (n = 25) had elevated B2M levels.
We observed no evidence of effect modification by age, postmenopausal hormone therapy, BMI, history of hypertension at baseline, or follow-up time (all p >0.05) (table e-1). We observed some evidence that current smoking may modify the association of B2M with risk of ischemic stroke (p = 0.02). However, given the low prevalence of smoking in this study (≈17%), this analysis should be interpreted with caution.
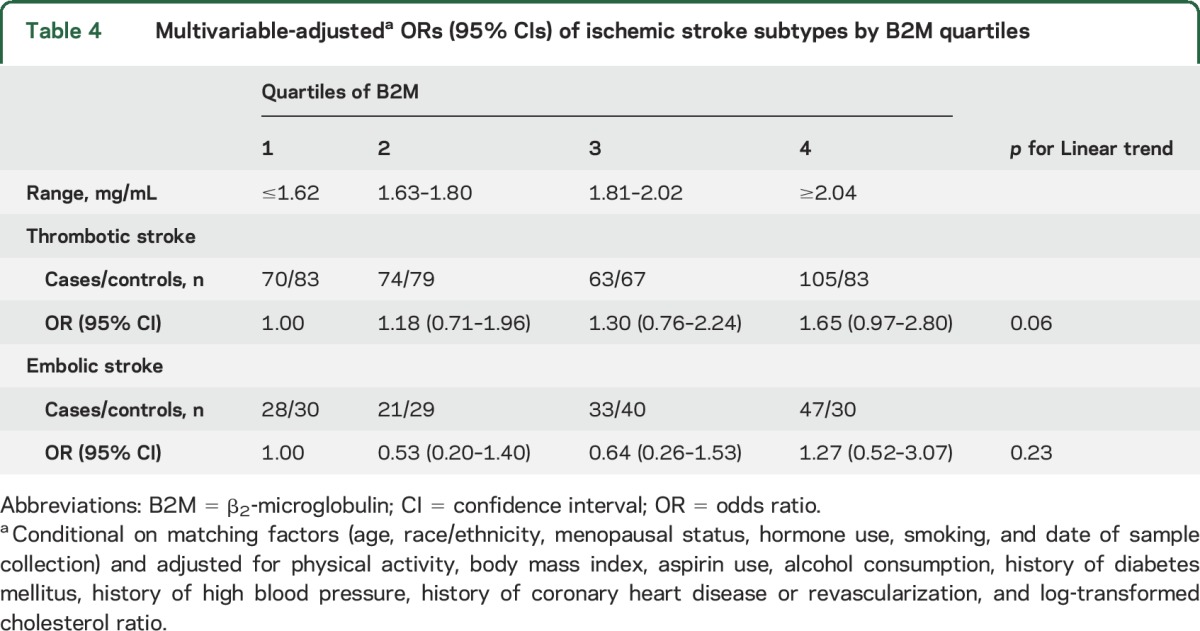
Next, we explored the association between B2M quartiles and risk of ischemic stroke subtypes (312 thrombotic strokes and 129 embolic strokes). The association between B2M and thrombotic ischemic stroke was similar to the overall ischemic stroke results (table 4). Those in the highest quartile of B2M had an increase in the risk of thrombotic stroke compared to those in the lowest quartile (OR 1.65, 95% CI 0.97–2.80). However, we observed no association between B2M and embolic stroke risk (table 4).
Table 4.
Multivariable-adjusteda ORs (95% CIs) of ischemic stroke subtypes by B2M quartiles
DISCUSSION
In this sample of women without a history of stroke at baseline, being in the highest quartile of B2M was associated with a 56% increased risk of incident ischemic stroke. This association may be specific to ischemic strokes of thrombotic origin. When we additionally controlled for eGFR, our effect estimates were slightly attenuated and no longer statistically significant. However, among women without chronic kidney disease function (eGFR >60 mL·min−1·1.73 m−2), those in the highest B2M quartile were at a 49% higher risk of ischemic stroke. This suggests that some of the original association may have been driven by the individuals with higher B2M levels and impaired kidney function but that residual risk remains even among individuals with normal kidney function.
B2M is freely filtered by the glomerulus and is reabsorbed and metabolized by the proximal tubule. As GFR declines, B2M levels retained in the blood rise, which has led to studies of B2M as marker of renal function.6,15–17 Higher B2M levels have been associated with CVD among individuals with kidney disease and among the general population.6–8,16 For example, elevated levels of B2M predicted incident CVD among individuals with chronic kidney disease.16 In addition, in a cohort of middle-aged US adults, B2M was associated with incident CHD, and this association persisted after the sample was restricted to individuals without chronic kidney disease.6 Among those without chronic kidney disease in the nationally representative National Health and Nutrition Examination Survey, B2M levels >2.86 mg/L were associated with a >2-fold increase in the risk of CVD mortality.8 Another study among individuals with asymptomatic carotid atherosclerosis observed that those in the highest B2M quartile had an 88% increased risk of CVD events compared to those in the lowest quartile.18
Some studies have also explored stroke as a separate outcome from total CVD. In the Atherosclerosis Risk in Community Study, B2M levels were associated with significantly increased risk of total CVD and total stroke in both those with chronic kidney disease and those without, although the relationship appeared slightly stronger for those without chronic kidney disease.7 This suggests that even among those with “normal” kidney function as indicated by creatinine-based eGFR, B2M levels may predict risk of CVD and stroke. A prior study from the Women's Health Initiative observed that elevated baseline levels of plasma B2M were associated with an increased risk of both total stroke and CHD during an average follow-up of at least 4 years.9 Our results are consistent with these prior studies of B2M levels and risk of total stroke among individuals without kidney disease but expand on these prior findings by demonstrating that baseline B2M levels are associated with ischemic stroke risk, particularly thrombotic stroke, during a median follow-up of 9 years.
Several mechanisms have been proposed to explain why B2M is associated with an increased risk of CVD. As discussed above, B2M may be a marker of subclinical renal dysfunction and small vessel disease.6,15–17 In addition, B2M may be a marker of systemic inflammation.3,19 A prior cross-sectional study observed an association between higher B2M levels and higher CRP levels.15 Similarly, in our study, we observed a strong trend of higher hs-CRP levels and hs-CRP >3 mg/L across B2M quartiles (p < 0.01 for each, table 2). Adjusting for elevated hs-CRP levels modestly attenuated our results (model 2: OR 1.51, 95% CI 0.98–2.33), suggesting that inflammation may explain some, but not all, of the association between B2M and ischemic stroke risk. Higher B2M levels have also been associated with incident peripheral artery disease20 and severity of peripheral artery disease,21 suggesting that B2M may act through atherosclerosis. Our finding that B2M levels were associated with ischemic stroke of thrombotic origin but not embolic origin may further support the hypothesis that B2M levels are influenced by atherosclerosis.
Some limitations and strengths should be noted. Unfortunately, we could not examine the association between B2M change and risk of ischemic stroke. Prior studies have suggested that a rise in B2M levels may be associated with an increased risk of stroke.9,22 In addition, this study was performed primarily among white women. Other studies have suggested that the B2M levels may vary by ethnicity but not sex.15 We were able to classify ischemic strokes only into broad subtypes (embolic vs thrombotic), which limited our ability to determine the association between B2M levels and specific ischemic stroke subtypes. There is also the potential for residual confounding by factors such as atrial fibrillation; however, we know of no studies that observed an association between atrial fibrillation and elevated B2M levels. Finally, while this case-control study demonstrated that elevated B2M levels are associated with an increased risk of stroke after controlling for CVD risk factors, future cohort studies will be needed to explore whether adding B2M levels to existing cerebrovascular disease risk prediction scores improves risk prediction because assays to measure B2M are already clinically available.
Strengths include the nested case-control design, which allowed us to measure B2M levels before ischemic stroke events, and the use of medical records to verify stroke events and to classify ischemic stroke subtypes. We explored stroke subtypes and potential effect modifiers of the association between B2M and ischemic stroke, although the power for these analyses was limited. The long length of follow-up in this study allowed us to demonstrate that B2M levels can predict risk of stroke a median of 9 years later.
High B2M levels were associated with an increased risk of ischemic stroke in this study of middle-aged women. Future studies are needed to further elucidate the mechanisms by which B2M increases the risk of ischemic stroke and to determine whether B2M levels can be modified through lifestyle interventions.
Supplementary Material
GLOSSARY
- B2M
β2-microglobulin
- BMI
body mass index
- CHD
coronary heart disease
- CI
confidence interval
- CRP
C-reactive protein
- CV
coefficient of variation
- CVD
cardiovascular disease
- eGFR
estimated glomerular filtration rate
- GFR
glomerular filtration rate
- HDL-C
high-density lipoprotein cholesterol
- hs-CRP
high-sensitivity C-reactive protein
- NHS
Nurses' Health Study
- OR
odds ratio
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Pamela M. Rist: analysis and interpretation of data, statistical analysis, and drafting the manuscript for intellectual content. Monik C. Jiménez: interpretation of data and critical revision of manuscript for intellectual content. Kathryn M. Rexrode: acquisition of data, study concept and design, interpretation of data, critical revision of manuscript for intellectual content, study supervision, and funding.
STUDY FUNDING
The NHS is funded by UM1 CA186107, R01 CA49449, and R01 HL088521. Dr. Rist is funded by K01 HL128791. Dr. Jiménez is funded by K01 HL124391.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Thrift AG, Cadilhac DA, Thayabaranathan T, et al. Global stroke statistics. Int J Stroke 2014;9:6–18. [DOI] [PubMed] [Google Scholar]
- 2.Schardijn GH, Statius van Eps LW. Beta 2-microglobulin: its significance in the evaluation of renal function. Kidney Int 1987;32:635–641. [DOI] [PubMed] [Google Scholar]
- 3.Cooper EH, Forbes MA, Hambling MH. Serum beta 2-microglobulin and C reactive protein concentrations in viral infections. J Clin Pathol 1984;37:1140–1143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chronic Kidney Disease Prognosis C, Matsushita K, van der Velde M, et al. Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 2010;375:2073–2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Matsushita K, Coresh J, Sang Y, et al. Estimated glomerular filtration rate and albuminuria for prediction of cardiovascular outcomes: a collaborative meta-analysis of individual participant data. Lancet Diabetes Endocrinol 2015;3:514–525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Astor BC, Shafi T, Hoogeveen RC, et al. Novel markers of kidney function as predictors of ESRD, cardiovascular disease, and mortality in the general population. Am J Kidney Dis 2012;59:653–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Matsushita K, Sang Y, Ballew SH, et al. Cardiac and kidney markers for cardiovascular prediction in individuals with chronic kidney disease: the Atherosclerosis Risk in Communities study. Arterioscler Thromb Vasc Biol 2014;34:1770–1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Foster MC, Inker LA, Levey AS, et al. Novel filtration markers as predictors of all-cause and cardiovascular mortality in US adults. Am J Kidney Dis 2013;62:42–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prentice RL, Zhao S, Johnson M, et al. Proteomic risk markers for coronary heart disease and stroke: validation and mediation of randomized trial hormone therapy effects on these diseases. Genome Med 2013;5:112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stampfer MJ, Colditz GA, Willett WC, Speizer FE, Hennekens CH. A prospective study of moderate alcohol consumption and the risk of coronary disease and stroke in women. N Engl J Med 1988;319:267–273. [DOI] [PubMed] [Google Scholar]
- 11.Hankinson SE, Manson JE, Spiegelman D, Willett WC, Longcope C, Speizer FE. Reproducibility of plasma hormone levels in postmenopausal women over a 2-3-year period. Cancer Epidemiol Biomarkers Prev 1995;4:649–654. [PubMed] [Google Scholar]
- 12.Larsson SC, Virtamo J, Wolk A. Potassium, calcium, and magnesium intakes and risk of stroke in women. Am J Epidemiol 2011;174:35–43. [DOI] [PubMed] [Google Scholar]
- 13.Iso H, Rexrode K, Hennekens CH, Manson JE. Application of computer tomography-oriented criteria for stroke subtype classification in a prospective study. Ann Epidemiol 2000;10:81–87. [DOI] [PubMed] [Google Scholar]
- 14.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Juraschek SP, Coresh J, Inker LA, et al. Comparison of serum concentrations of beta-trace protein, beta2-microglobulin, cystatin C, and creatinine in the US population. Clin J Am Soc Nephrol 2013;8:584–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Foster MC, Coresh J, Hsu CY, et al. Serum beta-trace protein and beta2-microglobulin as predictors of ESRD, mortality, and cardiovascular disease in adults with CKD in the Chronic Renal Insufficiency Cohort (CRIC) study. Am J Kidney Dis 2016;68:68–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Inker LA, Tighiouart H, Coresh J, et al. GFR estimation using beta-trace protein and beta2-microglobulin in CKD. Am J Kidney Dis 2016;67:40–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Amighi J, Hoke M, Mlekusch W, et al. Beta 2 microglobulin and the risk for cardiovascular events in patients with asymptomatic carotid atherosclerosis. Stroke 2011;42:1826–1833. [DOI] [PubMed] [Google Scholar]
- 19.Shinkai S, Chaves PH, Fujiwara Y, et al. Beta2-microglobulin for risk stratification of total mortality in the elderly population: comparison with cystatin C and C-reactive protein. Arch Intern Med 2008;168:200–206. [DOI] [PubMed] [Google Scholar]
- 20.Joosten MM, Pai JK, Bertoia ML, et al. Beta2-microglobulin, cystatin C, and creatinine and risk of symptomatic peripheral artery disease. J Am Heart Assoc 2014:3:e000803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wilson AM, Kimura E, Harada RK, et al. Beta2-microglobulin as a biomarker in peripheral arterial disease: proteomic profiling and clinical studies. Circulation 2007;116:1396–1403. [DOI] [PubMed] [Google Scholar]
- 22.Rebholz CM, Grams ME, Matsushita K, et al. Change in multiple filtration markers and subsequent risk of cardiovascular disease and mortality. Clin J Am Soc Nephrol 2015;10:941–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


