Abstract
Essential tremor (ET) is one of the most common neurological diseases, with an estimated 7 million affected individuals in the United States. Postmortem studies in the past few years have resulted in new knowledge as well as a new formulation of disease pathophysiology. This new formulation centers on the notion that ET might be a disease of the cerebellum and, more specifically, the Purkinje cell (PC) population. Indeed, several investigators have proposed that ET may be a “Purkinjopathy.” Supporting this formulation are data from controlled postmortem studies demonstrating (1) a range of morphological changes in the PC axon, (2) abnormalities in the position and orientation of PC bodies, (3) reduction in the number of PCs in some studies, (4) morphological changes in and pruning of the PC dendritic arbor with loss of dendritic spines, and (5) alterations in both the PC-basket cell interface and the PC-climbing fiber interface in ET cases. This new formulation has engendered some controversy and raised additional questions. Whether the constellation of changes observed in ET differs from that seen in other degenerative disorders of the cerebellum remains to be determined, although initial studies suggest the likely presence of a distinct profile of changes in ET.
Keywords: essential tremor, cerebellum, neurodegeneration, Purkinje, biology
Introduction to Essential Tremor
Essential tremor (ET) is a chronic, progressive neurological disease (Putzke and others 2006). It is characterized by considerable clinical heterogeneity; indeed, it has been proposed that ET may represent a family of diseases rather than a single clinical-pathological entity (Louis 2014b). The most characteristic feature of ET is kinetic tremor in the hands or arms, which early in the disease process is mild and difficult to distinguish from enhanced physiological tremor (Louis 2013). As the disease advances, tremor (1) becomes more severe, resulting in functional difficulties; (2) may become more varied, with the development of rest and intention tremors; and (3) often becomes more anatomically widespread, involving cranial structures (e.g., head, voice, jaw) (Cohen and others 2003; Sternberg and others 2013). Aside from tremor, patients with ET may present with other motor features that indicate cerebellar dysfunction; these include gait ataxia as well as eye movement abnormalities (Gitchel and others 2013; Louis and others 2013c). A recent literature has also documented the presence of non-motor features, which can include cognitive, psychiatric, and sensory abnormalities (i.e., problems with hearing and olfaction) (Benito-Leon and others 2006; Louis and others 2002; Ondo and others 2003).
Greater interest in ET research in the recent decade has resulted in fresh knowledge of this entity and a new formulation of disease pathophysiology. This new formulation proposes that ET may be a disease of the cerebellum and, more specifically, the Purkinje cell (PC) population (Grimaldi and Manto 2013; Louis 2014d). Indeed, some investigators have even proposed that ET may be a “Purkinjopathy” (Grimaldi and Manto 2013). This new formulation has engendered some controversy and it has also raised additional questions. This review will present the postmortem data that link ET to the cerebellum and, more specifically, the PCs, and consider several issues that on the surface seem at variance with this notion.
Introduction to ET: Epidemiology
A discussion of ET should not exclude consideration of the fact that this is one of the most common neurological disorders and the most common tremor disorder (Louis and Ferreira 2010). In the United States, an estimated 7 million individuals are affected (Louis and Ottman 2014). The majority of affected individuals have some functional disability (Louis and others 2001). Hence, from a public health perspective, ET is of considerable importance.
ET as a Disorder of the Cerebellum or Cerebellar System
An ever-growing literature now provides support for the tenet that ET is a disorder of cerebellar or cerebellar system dysfunction. Furthermore, there is a new literature, still controversial (Deuschl and Elble 2009; Rajput and others 2012a; Rajput and others 2012b), which reveals the presence of structural, degenerative changes in the cerebellum itself (Louis 2014d; Louis and others 2012a).
The clinical literature on ET indicates the presence of “cerebellar signs” in many patients. Thus, nearly one half of patients have intention tremors in the arms, and 10% in the head (Leegwater-Kim and others 2006; Louis and others 2009b). Gait ataxia, though often mild in ET, can be more severe in some cases (Louis and others 2013c). Additional problems in motor timing and motor control point to a mild problem of cerebellar dysfunction in patients with ET (Bares and others 2012).
Neuroimaging studies, including both structural and functional, most consistently point to abnormalities in the cerebellum, with several of these revealing the presence of cerebellar atrophy and/or possible neuronal loss (Louis and others 2014a; Passamonti and others 2012).
The clinical pharmacology of ET also implicates the cerebellum. Nearly all of the commonly used prescription medications for ET enhance gamma-aminobutyric acid (GABA)-ergic transmission. These medications include primidone, phenobarbital, benzodiazepines, gabapentin, and topiramate (Rincon and Louis 2005). Ethanol, also an effective treatment for ET, acutely reduces the severity of tremor and, in low doses, ataxia. Among ethanol’s several actions is the enhancement of GABA-ergic transmission (Rincon and Louis 2005). This all suggests that there is a reduction in GABA-ergic tone in ET. Given the fact that PCs are the major GABA-ergic output of the cerebellum, this implies that a loss of PCs in ET is responsible for a reduction in GABA-ergic tone in that disease.
Postmortem studies in recent years have documented a growing number of structural changes in the ET cerebellum and none in other brain regions, further substantiating the notion that the seat of the disease is the cerebellum (Louis 2014c, 2014d). An older literature had attempted to link ET to a problem in the inferior olivary nucleus, although this notion is questionable, as it is supported by little empiric evidence (Louis 2014c).
ET as a Disorder of the PC: Postmortem Studies
Introduction
Although some ET cases exhibit brainstem Lewy bodies (Louis and others 2005), most ET cases do not exhibit such lesions (Louis and others 2007). The postmortem changes identified in ET have largely been restricted to the cerebellum. More specifically, a variety of morphological changes have been noted in the PC population or in neighboring neuronal populations that relate intimately to the PCs (Figs. 1–5). The presentation of these changes below is ordered in terms of the anatomical location of the lesion, with respect to the PC, as it is challenging to precisely designate some changes as primary and others as secondary remodeling.
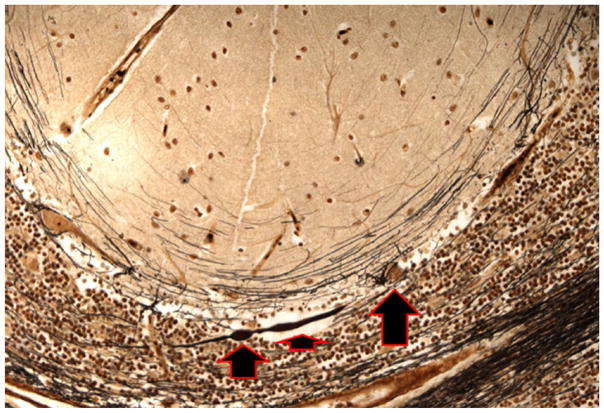
Figure 1.
Cerebellar cortical section from an ET case. The PC axon is thickened proximally (short arrow) and there is also a single torpedo (medium arrow). The PC soma is also seen (long arrow). Bielschowsky ×200. ET = essential tremor; PC = Purkinje cell.
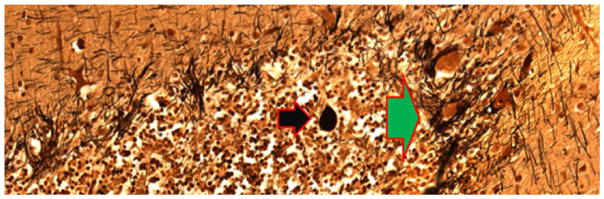
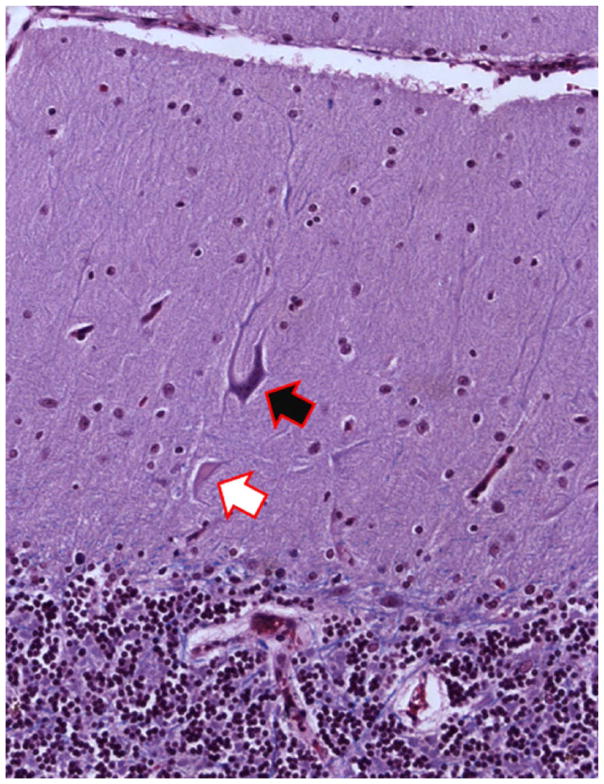
Figure 5.
Cerebellar cortical section from an ET case. A torpedo (black arrow) is seen in close proximity to PC soma surrounded by hypertrophic basket cell processes (green arrow). Bielschowsky ×200. ET = essential tremor; PC = Purkinje cell.
Morphological Changes in the PC Axon
A variety of changes have been observed in the PC axon, and these likely represent the effects of injury as well as attempts at reparation. Torpedoes (Figs. 1, 2, 4, and 5), which are abnormal ovoid swellings of the proximal portion of the PC axon, have been observed to be approximately seven times more common in ET brains compared with brains from age-matched controls (Louis and others 2006b; Louis and others 2007). Moreover, a single PC axon in ET may contain several such torpedoes. Torpedoes contain a massive accumulation of abnormally phosphorylated and disorganized neurofilaments and other disrupted organelles (Louis and others 2009c; Mann and others 1980). In general, such neurofilament mis-accumulations and resulting axonal swellings are thought to inhibit both anterograde and retrograde axonal transport, ultimately leading to cell strangulation. This strangulation may lead to the selective degeneration and, in some instances, death of neurons (Louis 2014a). This will be discussed further below. Of interest is the observation that the reduction in numbers of PCs is greatest in ET brains that contain the most torpedoes (Louis and others 2007). Torpedoes are present in the brains of patients with other neurodegenerative disorders (e.g., Parkinson’s disease, Alzheimer’s), but not nearly to the same degree as occurs in ET (Louis and others 2009a). Torpedoes also occur in other diseases of the cerebellum (e.g., some forms of spinocerebellar ataxia), although in these diseases the number of such torpedoes is numerically greater than that seen in patients with ET, and the relationship of torpedoes to PC loss seems to follow a different pattern than that seen in ET (Louis and others 2014b). More specifically, in these other diseases, the dramatic loss of PCs eventually leads to a paradoxical reduction in observed torpedoes.
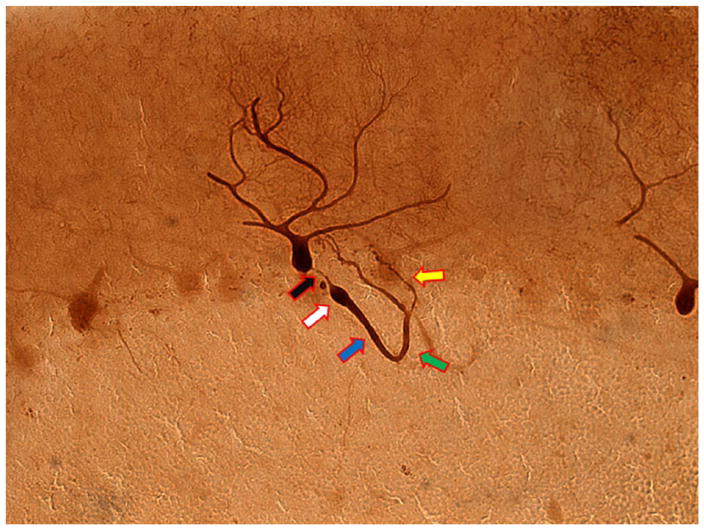
Figure 2.
Cerebellar cortical section from an ET case. The normal appearance of the proximal portion of the PC axon (black arrow) is followed by a torpedo (white arrow), a post-torpedo thickened axon (blue arrow), an axonal recurrent collateral (green arrow), and axonal branching (yellow arrow). Calbindin ×200. ET = essential tremor; PC = Purkinje cell.
Figure 4.
Cerebellar cortical section from an ET case. A PC dendritic swelling (black arrow) is adjacent to a torpedo (green arrow). The PC soma is also seen (blue arrow). Luxol fast blue hematoxylin and eosin stain ×200. ET = essential tremor; PC = Purkinje cell.
As noted above, the neurofilament mis-accumulations in PC axons in ET seem at times to produce ovoid swellings (torpedoes), whereas in other instances result in other aberrant morphologies. Most notable among these is a linear thickening of the PC axonal profile (Figs. 1 and 2). Such linear thickenings occur to a greater degree in ET cases than in age-matched controls (Babij and others 2013). These lesions appear as more elongated expansions of the diameter of the PC axon in contrast to the ovoid expansions of the PC axon that characterize the torpedo (Babij and others 2013). On a mechanistic level, these linear thickenings probably represent a similar event or perhaps an even earlier axonal event prior to the formation of a full, ovoid, out-pouching or torpedo. Of interest is that in ET, PCs with torpedoes were 5.3 times more likely to also have a thickened axonal profile when compared with PCs without torpedoes (Babij and others 2013).
Aside from torpedoes and thickened axonal profiles, a series of more extensive changes have also been observed in PC axons in patients with ET relative to age-matched controls (Babij and others 2013; Louis 2014c). These changes are highly correlated with one another (Babij and others 2013), suggesting that they represent a series of parallel expressions of an underlying pathomechanistic problem or closely related events in a pathomechanistic cascade. On a physiological level, this axonal remodeling also likely results in the formation of aberrant synapses, and the creation of new/abnormal cerebellar cortical circuits in ET (Dusart and others 1999; Louis 2014c). Before reviewing these changes, however, it is important to point out that the general response of PCs to stress involves a range of reparative axonal changes (Louis 2014c; Rossi and others 1995a). For example, fetal PCs in organotypic cultures are able to regenerate their axons on mature cerebellar slices, and these regrowing axons invade all cerebellar regions of the apposed mature slices, including cerebellar white matter (Carulli and others 2004). We also know that, in contrast to most neurons in the central nervous system, PCs often still survive despite marked injury (Dusart and Sotelo 1994; Louis 2014c; Rossi and others 1995b), exhibiting a vigorous inclination toward sprouting and other changes both along the intracortical segment and the distal stump (Carulli and others 2004; Chan-Palay 1971; Dusart and others 1999; Louis 2014c). It has been hypothesized that these reparative axonal changes represent the PC’s attempt to access trophic factors by establishing additional connections with other PCs or other granule layer neurons (Babij and others 2013).
The axonal changes noted above are of several types. First, there is an increased number of arciform axons compared to age-matched controls (Babij and others 2013). These are axons that gradually curve back toward the PC layer rather than continuing downward to the deep cerebellar nuclei. In other words, these axons seem to be heading in a cortical direction rather than a normal corticofugal direction. Second, there is an increase in the number of axon recurrent collaterals (Fig. 2) compared to age-matched controls (Babij and others 2013). These are axons or axon branches that, in contrast to arciform axons, make at least a 90° turn back toward the PC layer. Third, there is an increase in axonal branching (Fig. 2) (Babij and others 2013). These are axons with at least one branch point and, sometimes, multiple bifurcations. Fourth, there is an increase in terminal axonal sprouting compared to age-matched controls (Babij and others 2013). In other words, there is a fraying of the terminal Purkinje axon near to the PC layer. As noted above, on a mechanistic level, these structural abnormalities indicate a likely increase in aberrant Purkinje–Purkinje interactions and/or aberrant PC interactions with other neurons, with an implied alteration in the normal cerebellar cortical circuitry and output (Babij and others 2013).
Reduction in PCs
As noted above, in cerebellar disease, neurofilament mis-accumulations and resulting PC axonal swellings are thought to inhibit both anterograde and retrograde PC axonal transport, ultimately leading to neuronal strangulation. This inhibition could lead to the selective degeneration and, in some instances, death of PCs (Louis 2014a). There is a debate in the current literature as to the presence and extent of PC loss in ET, some of which could be attributable to methodological issues (Axelrad and others 2008; Louis and others 2007; Louis and others 2012a; Louis and others 2013b; Rajput and others 2012a; Rajput and others 2012b; Symanski and others 2014). Nonetheless, several studies, using a range of methods, have demonstrated PC loss in patients with ET (Axelrad and others 2008; Louis and others 2007; Louis and others 2013b), with the number of PCs being inversely related to the number of torpedoes. This issue is further discussed below.
While on the topic of PCs, brief mention should be made that PCs are highly vulnerable to excitotoxic stress (Gugger and Kapfhammer 2010). Glutamatergic excitatory signals are transmitted to PCs via climbing fibers as well as parallel fibers, and one postulated mechanism for ET is an overexcitation of glutamatergic fibers, leading to excitotoxic death of PCs. Excitatory amino acid transporter type 2 (EAAT2), expressed in glial cells, normally clears glutamate from the synaptic cleft. A significant reduction in cerebellar cortical EAAT2 protein levels in ET, reported in a recent study (Lee and others 2014), suggests that PCs in ET might be even more vulnerable to excitotoxic damage than those of controls. Additional studies are needed.
Changes in the Positioning/Orientation of PCs
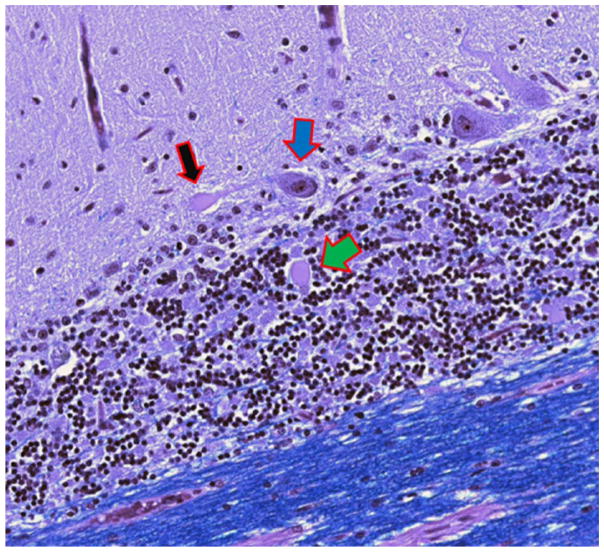
Previous paragraphs discussed changes that are likely to result in alterations in local cerebellar cortical circuitry in ET. In addition, a reduction in the number of PCs in ET, discussed above, as well as pruning of the extensive PC dendritic arbor, as will be discussed below, likely also result in changes in the local cerebellar cortical architecture, with an untethering and rearrangement of structures. For example, one sees an increase in the number of heterotopic PCs in the ET relative to age-matched control brains (Kuo and others 2011). These PCs are displaced (Fig. 3), with their soma in the molecular layer rather than the PC layer. In some instances, the orientation of these PCs is abnormal, with the dendritic arbor paradoxically facing downward, toward the granular layer, and the PC axon emerging and traveling upward into the molecular layer, away from the PC layer. The effect(s) that this might have on local cortical circuitry is unknown.
Figure 3.
Cerebellar cortical section from an ET case. Heterotopic PC with an elongated and rotated soma in the molecular layer (black arrow). There is an adjacent dendritic swelling (white arrow). Luxol fast blue hematoxylin and eosin ×200. ET = essential tremor; PC = Purkinje cell.
Changes in the PC Dendritic Arbor
Changes in the PCs in ET may be found in the dendritic arbor. Indeed, the number of abnormal swellings (Figs. 3 and 4) on PC dendrites is increased in comparison to age-matched control brains (Yu and others 2012), indicating a more widespread involvement of the PC, extending beyond the axonal compartment. Furthermore, recent data show a significant pruning of the PC dendritic arbor with a reduction in the number of dendritic spines in ET cases compared to age-matched controls (Louis and others 2014c). In neurological diseases, in general, pruning of the dendritic arbor is a more proximate structural change that may precede frank neuronal death (Ferrer and others 1984). Such pruning is by no means specific to ET, and it has been noted in PCs in other disorders in which there is a loss of PCs, ranging from patients with hereditary ataxias (Shintaku and Kaneda 2009) to chronic alcoholics and patients with Alzheimer’s disease (Ferrer and others 1984; Mavroudis and others 2013).
Changes in the PC–Basket Cell Interface
The changes in the PC described above, including the loss of PCs themselves, likely lead to a remodeling of the surrounding basket cell processes. Basket cells are GABA-ergic inhibitory interneurons. In humans, up to 50 axon collaterals from neighboring basket cells descend from the molecular layer and combine to form a complex basket-shaped structure around the PC body (Leclerc and others 1985). We have observed a dense and tangled appearance (hypertrophy) of the basket cell axonal plexuses that surround PC soma in Bielschowsky preparations of cerebellar cortex in ET brains (Fig. 5); this occurs to a greater extent in ET than in age-matched control brains, and brains with greater reduction in PCs had greater hypertrophy of basket cell axonal processes (Erickson-Davis and others 2010). It has been postulated that this hypertrophy represents an accumulation of converging basket cell processes recruited from neighboring PCs that have been damaged or died. Although there has been little investigation of such a phenomenon in the human cerebellum, selective preservation and reorganization of basket cell axonal processes has been demonstrated in basket cells in the CA1 and CA3 regions of the hippocampus (Erickson-Davis and others 2010). These neurons form baskets around hippocampal pyramidal cells and function as local inhibitory GABA-ergic interneurons, analogous to the relationship between cerebellar basket cells and PCs. These hippocampal basket cells undergo extensive reorganization in the setting of pyramidal cell death (Arellano and others 2004; Erickson-Davis and others 2010).
Changes in the PC–Climbing Fiber Interface
Climbing fibers are excitatory neurons that project from the inferior olivary nucleus to the cerebellar cortex, where they form synaptic connections with PCs. In a recent study (Lin and others 2014), ET cases were observed to have decreased climbing fiber–PC synaptic density, with more climbing fibers extending to the outer portion of the molecular layer, and more climbing fiber–PC synapses on the thin PC spiny branchlets. The mechanism that underlies these abnormal climbing fiber–PC synaptic connections in ET is unclear. However, climbing fiber synaptic density and distribution is highly plastic and is dynamically regulated in the context of PC degeneration. In mouse models of spinocerebellar ataxias, for example, expression of mutant proteins in PCs causes PC degeneration and abnormal climbing fiber–PC connections, which include changes in the climbing fiber–PC synaptic territory, abnormal climbing fiber–PC synapses on PC soma, and defective climbing fiber–PC synaptic transmission (Lin and others 2014). Therefore, it is possible that abnormal climbing fiber–PC synaptic connections in ET could be secondary to PC degeneration.
Changes in the Cerebellar Dentate Nucleus
In most ET cases, changes have not been detected in the cerebellar dentate nucleus, although in one case, there were abnormalities in the dentate nucleus, in the form of neuronal loss, neuronal atrophy, microglial clusters, and reduction in the number of efferent fibers (i.e., pallor of the hilum) (Louis and others 2006a). A recent study reported a reduction in both GABAA and GABAB receptors in the dentate nucleus of ET patients (Paris-Robidas and others 2012). This finding has yet to be reproduced and its interpretation is uncertain. Whether the reduction in both receptors restricts the postsynaptic action of GABA released from PC axons is not known, but has been postulated (Paris-Robidas and others 2012). Additional studies are needed.
Other Changes
The presence of gliosis in the cerebellar cortex has been noted in several postmortem studies of ET (Louis and others 2006b; Shill and others 2008); however, this has not been quantified.
ET Not as a Disorder of Other Brain Regions: Postmortem Studies
The section above reviewed the pathological changes in ET that have been observed in the cerebellum. It is important to point out that systematic postmortem study of the other brain regions that form loop connections with the cerebellum (i.e., the thalamus, inferior olivary nucleus, red nucleus, and motor cortex), as well as other brain regions, have not noted any significant pathologic changes in these brain regions (Rajput and others 2004; Shill and others 2008). Aside from the presence of brainstem Lewy bodies in some cases, brain changes are not evident in other brain regions in postmortem studies of ET (Louis and others 2005; Louis and others 2006a; Louis and others 2007). Most worth emphasizing is a detailed and systematic postmortem study of the inferior olivary nucleus in ET cases versus controls, in which a series of metrics was used to quantify microscopic neuronal and glial changes in the inferior olivary nucleus and its input and output tracts (Louis and others 2013a). Olivary linear neuronal density also was assessed. The study did not detect any differences between cases and controls (Louis and others 2013a). These data serve to reinforce the notion that the cerebellum is the focal point of interest in ET (Louis 2014d).
Heterogeneity of Postmortem Findings in the ET Cerebellum
It is important to point out that not all ET cases exhibit precisely the same set of changes in the cerebellum on postmortem examination. In this way, postmortem findings reflect the clinical findings in this disorder, and both serve to support the notion that ET itself is likely to be a family of diseases (Louis 2014b). Thus, a small number of ET cases exhibit ubiquitin-positive intranuclear inclusions in the PCs and elsewhere (Louis and others 2010; Louis and others 2012b) and others exhibit pronounced changes in the cerebellar dentate nucleus, as noted above (Louis and others 2006a). Some also exhibit Lewy bodies in the brainstem and, particularly, in the locus ceruleus (Louis and others 2005; Louis and others 2007), a structure whose neurons synapse directly with PCs. Despite the presence of this heterogeneity, in the majority of ET cases, the postmortem changes that have been observed to date are the changes described in previous sections.
ET as a Disorder of the PC: Important Issues to Consider
A new pathomechanistic formulation centers on the notion that ET might be a disease of the cerebellum and, more specifically, a disease of the PC population (Grimaldi and Manto 2013; Louis 2014d), with some investigators even proposing that ET may be a “Purkinjopathy” (Grimaldi and Manto 2013). This formulation contrasts to the older olivary model of ET, put forward in the 1970s, which posited that ET might be the result of a disordered pacemaker in the inferior olivary nucleus. As reviewed elsewhere, that model is problematic for many reasons and its utility as a model for ET is questionable (Louis 2014a). This reformulation of ET as a disorder of PC degeneration has engendered some controversy (Deuschl and Elble 2009), and as discussed below, there are several observations that on the surface seem to be at variance with this notion. These merit elaboration.
First, several studies have not been able to reproduce the finding of PC loss in ET. There are several possible explanations. One is the methodological problems with these studies, which include both insufficient study power (Louis and others 2012a) and problems with case definition (Louis and Faust 2014). Another possibility, in the absence of a study that has used an unbiased sampling scheme, is sampling bias (Jellinger 2014). A more recent approach, which used a stereological-based approach to eliminate potential field selection bias, continues to find a reduction in PC counts in ET cases compared to controls (Louis and others 2015). A third possibility is that PC loss is a mild and/or late finding in ET and not appreciable across all samples. Additional studies are clearly needed to study this phenomenon.
A second, related concern about the notion that ET is a Purkinjopathy, raised by some (Rajput and others 2012a; Rajput and others 2012b; Symanski and others 2014), is the contention that if this were the case, that PC loss should uniformly be present across all ET cases, even before the development of tremor. That is, the presence of some ET cases without PC loss is of concern if one is proposing that ET is a Purkinjopathy. On further consideration, however, this is not concerning. For example, if PC loss in ET were a byproduct of long-standing pathological molecular/cellular processes involving the PC in ET, it might not be the earliest finding. Rather, it could be a relatively late finding and might long follow the onset of tremor. Indeed, as noted above, frank PC loss in ET may be a relatively late event, with earlier markers (e.g., changes in the dendritic or axonal compartment) serving as markers of more proximate metabolic and molecular disarray in the PC (Louis and Faust 2014).
In addition, one might expect that the longer the duration of symptoms, the more pronounced the morphological changes in the cerebellum (Louis 2014c). Although some of the postmortem morphological changes in the PC in ET have been shown to correlate with disease duration (Babij and others 2013), others have not. While at first glance this observation is somewhat concerning, there are a number of possible plausible explanations. As a general principle, cerebellar response to injury leads to changes that are likely to be partly regenerative and compensatory while others are regressive and degenerative (Louis 2014a, 2014c; Rossi and others 1995b). For example, as has been observed in PCs in various settings (Dusart and others 1999) some of the observed axonal changes in ET likely represent either successful or aborted regenerative attempts that are later followed by degeneration (Louis 2014c). Therefore, it is important to recognize that at any one time, the observed morphological changes in a given brain region likely represent a complex mélange of regenerative (both aborted and successful) and degenerative events (Louis 2014a, 2014c). This mixed array of ambi-directional events is likely to make simple linear models that attempt to regress morphological counts on disease duration unrevealing (Louis 2014c).
Fourth, PC loss has been reported in a variety of disorders other than ET (e.g., Alzheimer’s disease and Huntington’s disease) (Mavroudis and others 2013; Rub and others 2013), yet action tremor is not a prominent feature of either of these diseases. On the surface, this seems at variance with the notion that PC loss is at the root of ET. However, it is important to keep in mind that PC loss does not occur in isolation in ET but is accompanied by a variety of additional changes in PC morphology, as noted above. Furthermore, the time course of unfolding PC changes in ET is important. It is not acute/sudden (as in a stroke); indeed, it is very slow (in many cases, 20–60 years), which is far longer than the time course observed in patients with Alzheimer’s disease and Huntington’s disease. Aside from PC loss, it is the extent of local GABA-ergic circuit reorganization (e.g., recurrent collateral formation, terminal axonal sprouting) that is likely of equal or greater importance in ET as the frank PC loss. The primary molecular disturbance, the ordering and nature of what is likely to be a complex cascade of unfolding events in the ET cerebellum, the time course of such changes, as well as the extent of local cerebellar cortical circuit reorganization are likely to be key importance in defining this disease.
ET and Cerebellar Degeneration: Is There an ET-Specific Set of Changes?
The presence of postmortem changes in the ET cerebellum naturally raises several intriguing questions. For one, are the morphologic changes that have been observed in the ET cerebellum specific to ET or merely of a garden-variety nature (i.e., generic features of cerebellar injury)? Furthermore, one may ask whether there is a particular “profile” of morphological changes in the ET cerebellum, which distinguishes ET from other degenerative diseases of the cerebellum. Stated in another way, is there a disease signature? Current data that may be used to address this are limited. Despite this, there is evidence that cerebellar diseases, including ET, do not all display a uniform set of postmortem changes. The various spinocerebellar ataxias themselves differ from one another in terms of the relative involvement of the cerebellar cortex, inferior olivary nucleus and dentate nucleus, and the extent of PC loss is not uniform across these disorders (Koeppen 2005). Torpedo formation is a feature of cerebellar diseases; however, the relationship between torpedo and PC counts has been shown to be heterogeneous across these diseases. With more severe cerebellar disease, torpedoes can be quite numerous and are likely a common feature of surviving PCs, but eventually, in diseases in which there is a dramatic loss of PCs, there is a paradoxical reduction in observable torpedoes (Louis and others 2014b); thus, the ET and the spinocerebellar ataxia brain differ from one another with respect to the PC–torpedo ratio. We have also demonstrated that the extensive degree of PC loss in patients with spinocerebellar ataxia-1 seems to result in an increase in molecular layer cellular density that is not apparent in patients with ET, in whom PC loss is less dramatic (Louis RJ and others 2014). Lengthening of the basket cell processes in the region of the PC initial axonal segment, as observed in ET, is not present in patients with spinocerebellar ataxia (Kuo and others 2013). These are among some of the emerging data that are demonstrating that the changes seen in the ET brain are not identical to those seen in patients with other forms of cerebellar degeneration. Additional studies are currently underway to better characterize the ET signature.
Summary
In summary, studies of the pathophysiology of ET have shifted interest in recent years from the inferior olivary nucleus to the cerebellum. These recent studies, which are tissue-based, have revealed a host of changes in the cerebellum of ET cases and these changes are not present to the same degree in the brains of age-matched controls, indicating that they are in excess of what is observed in normal aging. Based on these studies, a new formulation of ET centers on the notion that ET may be a disease of the cerebellum and, more specifically, the PC population. This formulation has engendered some controversy and raised additional questions, serving to further drive research in this area. How the described changes differ from those seen in other disorders of the cerebellum remains to be determined, although initial studies suggest that there is a distinct set of events in the ET brain.
Acknowledgments
Funding
The author disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Louis has received research support from the National Institutes of Health: NINDS #R01 NS042859 (principal investigator), NINDS #R01 NS39422 (principal investigator), NINDS #R01 NS086736 (principal investigator), NINDS #R01 NS073872 (principal investigator), NINDS #R01 NS085136 (principal investigator), and NINDS #R01 NS088257 (principal investigator). He has also received support from the Claire O’Neil Essential Tremor Research Fund (Yale University).
Footnotes
Declaration of Conflicting Interests
The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- Arellano JI, Munoz A, Ballesteros-Yanez I, Sola RG, DeFelipe J. Histopathology and reorganization of chandelier cells in the human epileptic sclerotic hippocampus. Brain. 2004;127(pt 1):45–64. doi: 10.1093/brain/awh004. [DOI] [PubMed] [Google Scholar]
- Axelrad JE, Louis ED, Honig LS, Flores I, Ross GW, Pahwa R, et al. Reduced Purkinje cell number in essential tremor: a postmortem study. Arch Neurol. 2008;65(1):101–7. doi: 10.1001/archneurol.2007.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babij R, Lee M, Cortes E, Vonsattel JP, Faust PL, Louis ED. Purkinje cell axonal anatomy: quantifying morphometric changes in essential tremor versus control brains. Brain. 2013;136(pt 10):3051–61. doi: 10.1093/brain/awt238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bares M, Husarova I, Lungu OV. Essential tremor, the cerebellum, and motor timing: towards integrating them into one complex entity. Tremor Other Hyperkinet Mov (N Y) 2012;2 doi: 10.7916/D89G5KH9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benito-Leon J, Louis ED, Bermejo-Pareja F. Population-based case-control study of cognitive function in essential tremor. Neurology. 2006;66(1):69–74. doi: 10.1212/01.wnl.0000192393.05850.ec. [DOI] [PubMed] [Google Scholar]
- Carulli D, Buffo A, Strata P. Reparative mechanisms in the cerebellar cortex. Prog Neurobiol. 2004;72(6):373–98. doi: 10.1016/j.pneurobio.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Chan-Palay V. The recurrent collaterals of Purkinje cell axons: a correlated study of the rat’s cerebellar cortex with electron microscopy and the Golgi method. Z Anat Entwicklungsgesch. 1971;134(2):200–34. doi: 10.1007/BF00519300. [DOI] [PubMed] [Google Scholar]
- Cohen O, Pullman S, Jurewicz E, Watner E, Louis ED. Rest tremor in patients with essential tremor: prevalence, clinical correlates, and electrophysiologic characteristics. Arch Neurol. 2003;60(3):405–10. doi: 10.1001/archneur.60.3.405. [DOI] [PubMed] [Google Scholar]
- Deuschl G, Elble R. Essential tremor—neurodegenerative or nondegenerative disease towards a working definition of ET. Mov Disord. 2009;24(14):2033–41. doi: 10.1002/mds.22755. [DOI] [PubMed] [Google Scholar]
- Dusart I, Morel MP, Wehrle R, Sotelo C. Late axonal sprouting of injured Purkinje cells and its temporal correlation with permissive changes in the glial scar. J Comp Neurol. 1999;408(3):399–418. [PubMed] [Google Scholar]
- Dusart I, Sotelo C. Lack of Purkinje cell loss in adult rat cerebellum following protracted axotomy: degenerative changes and regenerative attempts of the severed axons. J Comp Neurol. 1994;347(2):211–32. doi: 10.1002/cne.903470206. [DOI] [PubMed] [Google Scholar]
- Erickson-Davis CR, Faust PL, Vonsattel JP, Gupta S, Honig LS, Louis ED. “Hairy baskets” associated with degenerative Purkinje cell changes in essential tremor. J Neuropathol Exp Neurol. 2010;69(3):262–71. doi: 10.1097/NEN.0b013e3181d1ad04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrer I, Fabregues I, Pineda M, Gracia I, Ribalta T. A Golgi study of cerebellar atrophy in human chronic alcoholism. Neuropathol Appl Neurobiol. 1984;10(4):245–53. doi: 10.1111/j.1365-2990.1984.tb00357.x. [DOI] [PubMed] [Google Scholar]
- Gitchel GT, Wetzel PA, Baron MS. Slowed saccades and increased square wave jerks in essential tremor. Tremor Other Hyperkinet Mov (N Y) 2013;3 doi: 10.7916/D8251GXN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimaldi G, Manto M. Is essential tremor a Purkinjopathy? The role of the cerebellar cortex in its pathogenesis. Mov Disord. 2013;28(13):1759–61. doi: 10.1002/mds.25645. [DOI] [PubMed] [Google Scholar]
- Gugger OS, Kapfhammer JP. Reduced size of the dendritic tree does not protect Purkinje cells from excitotoxic death. J Neurosci Res. 2010;88(4):774–83. doi: 10.1002/jnr.22247. [DOI] [PubMed] [Google Scholar]
- Jellinger KA. Is there cerebellar pathology in essential tremor? Mov Disord. 2014;29(4):435–6. doi: 10.1002/mds.25852. [DOI] [PubMed] [Google Scholar]
- Koeppen AH. The pathogenesis of spinocerebellar ataxia. Cerebellum. 2005;4(1):62–73. doi: 10.1080/14734220510007950. [DOI] [PubMed] [Google Scholar]
- Kuo SH, Erickson-Davis C, Gillman A, Faust PL, Vonsattel JP, Louis ED. Increased number of heterotopic Purkinje cells in essential tremor. J Neurol Neurosurg Psychiatry. 2011;82(9):1038–40. doi: 10.1136/jnnp.2010.213330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo SH, Tang G, Louis ED, Ma K, Babji R, Balatbat M, et al. Lingo-1 expression is increased in essential tremor cerebellum and is present in the basket cell pinceau. Acta Neuropathol. 2013;125(6):879–89. doi: 10.1007/s00401-013-1108-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leclerc N, Gravel C, Plioplys A, Hawkes R. Basket cell development in the normal and hypothyroid rat cerebellar cortex studied with a monoclonal anti-neurofilament antibody. Can J Biochem Cell Biol. 1985;63(6):564–76. doi: 10.1139/o85-075. [DOI] [PubMed] [Google Scholar]
- Lee M, Chang MM, Lin CY, Louis ED, Faust PL, Kuo SH. Decreased EAAT2 protein expression in the essential tremor cerebellar cortex. Acta Neuropathal Comm. 2014;2(1):157. doi: 10.1186/s40478-014-0157-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leegwater-Kim J, Louis ED, Pullman SL, Floyd AG, Borden S, Moskowitz CB, et al. Intention tremor of the head in patients with essential tremor. Mov Disord. 2006;21(11):2001–5. doi: 10.1002/mds.21079. [DOI] [PubMed] [Google Scholar]
- Lin CY, Louis ED, Faust PL, Koeppen AH, Vonsattel JP, Kuo SH. Abnormal climbing fibre-Purkinje cell synaptic connections in the essential tremor cerebellum. Brain. 2014;137(pt 12):3149–59. doi: 10.1093/brain/awu281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. The primary type of tremor in essential tremor is kinetic rather than postural: cross-sectional observation of tremor phenomenology in 369 cases. Eur J Neurol. 2013;20(4):725–7. doi: 10.1111/j.1468-1331.2012.03855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. Re-thinking the biology of essential tremor: from models to morphology. Parkinsonism Related Disord. 2014a;20(suppl 1):S88–93. doi: 10.1016/S1353-8020(13)70023-3. [DOI] [PubMed] [Google Scholar]
- Louis ED. “Essential tremor” or “the essential tremors”: is this one disease or a family of diseases? Neuroepidemiology. 2014b;42(2):81–9. doi: 10.1159/000356351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. From neurons to neuron neighborhoods: the rewiring of the cerebellar cortex in essential tremor. Cerebellum. 2014c;13(4):501–12. doi: 10.1007/s12311-013-0545-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED. Understanding essential tremor: progress on the biological front. Curr Neurol Neurosci Rep. 2014d;14(6):450. doi: 10.1007/s11910-014-0450-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Babij R, Cortés E, Vonsattel JPG, Faust PL. The inferior olivary nucleus: a postmortem examination of essential tremor cases vs. controls. Mov Disord. 2013a;28:779–86. doi: 10.1002/mds.25400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Babij R, Lee M, Cortes E, Vonsattel JP. Quantification of cerebellar hemispheric Purkinje cell linear density: 32 ET cases versus 16 controls. Mov Disord. 2013b;28(13):1854–9. doi: 10.1002/mds.25629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Bromley SM, Jurewicz EC, Watner D. Olfactory dysfunction in essential tremor: a deficit unrelated to disease duration or severity. Neurology. 2002;59(10):1631–3. doi: 10.1212/01.wnl.0000033798.85208.f2. [DOI] [PubMed] [Google Scholar]
- Louis ED, Choe M, Faust PL. Reduced Purkinje cell counts in essential tremor cases vs. controls: further support for the neurodegenerative hypothesis of essential tremor (Abstract) Neurology. 2015;84(14) Suppl P5.278. [Google Scholar]
- Louis ED, Erickson-Davis C, Pahwa R, Lyons KE, Garber A, Moskowitz CB, et al. Essential tremor with ubiquitinated Purkinje cell intranuclear inclusions. Acta Neuropathol. 2010;119(3):375–7. doi: 10.1007/s00401-010-0641-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Faust PL. Purkinje cell loss in essential tremor. Mov Disord. 2014;29(10):1329–30. doi: 10.1002/mds.25965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel JP. Purkinje cell loss is a characteristic of essential tremor: towards a more mature understanding of pathogenesis. Parkinsonism Relat Disord. 2012a;18(8):1003–4. doi: 10.1016/j.parkreldis.2012.06.017. [DOI] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Pahwa R, et al. Torpedoes in Parkinson’s disease, Alzheimer’s disease, essential tremor, and control brains. Mov Disord. 2009a;24(11):1600–5. doi: 10.1002/mds.22567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Faust PL, Vonsattel JP, Honig LS, Rajput A, Robinson CA, et al. Neuropathological changes in essential tremor: 33 cases compared with 21 controls. Brain. 2007;130(pt 12):3297–307. doi: 10.1093/brain/awm266. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ferreira JJ. How common is the most common adult movement disorder? Update on the worldwide prevalence of essential tremor. Mov Disord. 2010;25(5):534–41. doi: 10.1002/mds.22838. [DOI] [PubMed] [Google Scholar]
- Louis ED, Ford B, Frucht S, Barnes LF, Tang XM, Ottman R. Risk of tremor and impairment from tremor in relatives of patients with essential tremor: a community-based family study. Ann Neurol. 2001;49(6):761–9. doi: 10.1002/ana.1022. [DOI] [PubMed] [Google Scholar]
- Louis ED, Frucht SJ, Rios E. Intention tremor in essential tremor: prevalence and association with disease duration. Mov Disord. 2009b;24(4):626–7. doi: 10.1002/mds.22370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Galecki M, Rao AK. Four essential tremor cases with moderately impaired gait: how impaired can gait be in this disease? Tremor Other Hyperkinet Mov (N Y) 2013c;3 doi: 10.7916/D8QV3K7G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Honig LS, Vonsattel JP, Maraganore DM, Borden S, Moskowitz CB. Essential tremor associated with focal nonnigral Lewy bodies: a clinicopathologic study. Arch Neurol. 2005;62(6):1004–7. doi: 10.1001/archneur.62.6.1004. [DOI] [PubMed] [Google Scholar]
- Louis ED, Huang CC, Dyke JP, Long Z, Dydak U. Neuroimaging studies of essential tremor: how well do these studies support/refute the neurodegenerative hypothesis? Tremor Other Hyperkinet Mov (N Y) 2014a;4:235. doi: 10.7916/D8DF6PB8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Kuo SH, Vonsattel JP, Faust PL. Torpedo formation and Purkinje cell loss: modeling their relationship in cerebellar disease. Cerebellum. 2014b;13(4):433–9. doi: 10.1007/s12311-014-0556-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Lee M, Babij R, Ma K, Cortes C, Vonsattel JP, et al. Reduced Purkinje cell dendritic arborization and loss of dendritic spines in essential tremor. Brain. 2014c;137(pt 12):3142–8. doi: 10.1093/brain/awu314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Mazzoni P, Ma KJ, Moskowitz CB, Lawton A, Garber A, et al. Essential tremor with ubiquitinated intranuclear inclusions and cerebellar degeneration. Clin Neuropathol. 2012b;31(3):119–26. doi: 10.5414/NP300414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Ottman R. How many people in the USA have essential tremor? Deriving a population estimate based on epidemiological data. Tremor Other Hyperkinet Mov (N Y) 2014;4:259. doi: 10.7916/D8TT4P4B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis ED, Vonsattel JP, Honig LS, Lawton A, Moskowitz C, Ford B, et al. Essential tremor associated with pathologic changes in the cerebellum. Arch Neurol. 2006a;63(8):1189–93. doi: 10.1001/archneur.63.8.1189. [DOI] [PubMed] [Google Scholar]
- Louis ED, Vonsattel JP, Honig LS, Ross GW, Lyons KE, Pahwa R. Neuropathologic findings in essential tremor. Neurology. 2006b;66(11):1756–9. doi: 10.1212/01.wnl.0000218162.80315.b9. [DOI] [PubMed] [Google Scholar]
- Louis ED, Yi H, Erickson-Davis C, Vonsattel JPG, Faust PL. Structural study of Purkinje cell axonal torpedoes in essential tremor. Neurosci Lett. 2009c;450(3):287–91. doi: 10.1016/j.neulet.2008.11.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Louis RJ, Lee M, Kuo SH, Vonsattel JP, Louis ED, Faust PL. Cellular density in the cerebellar molecular layer in essential tremor, spinocerebellar ataxia, and controls. Parkinsonism Relat Disord. 2014;20(11):1270–3. doi: 10.1016/j.parkreldis.2014.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann DM, Stamp JE, Yates PO, Bannister CM. The fine structure of the axonal torpedo in Purkinje cells of the human cerebellum. Neurol Res. 1980;1(4):369–78. doi: 10.1080/01616412.1980.11739567. [DOI] [PubMed] [Google Scholar]
- Mavroudis IA, Manani MG, Petrides F, Petsoglou K, Njau SD, Costa VG, et al. Dendritic and spinal pathology of the Purkinje cells from the human cerebellar vermis in Alzheimer’s disease. Psychiatr Danub. 2013;25(3):221–6. [PubMed] [Google Scholar]
- Ondo WG, Sutton L, Dat Vuong K, Lai D, Jankovic J. Hearing impairment in essential tremor. Neurology. 2003;61(8):1093–7. doi: 10.1212/01.wnl.0000086376.40750.af. [DOI] [PubMed] [Google Scholar]
- Paris-Robidas S, Brochu E, Sintes M, Emond V, Bousquet M, Vandal M, et al. Defective dentate nucleus GABA receptors in essential tremor. Brain. 2012;135(pt 1):105–16. doi: 10.1093/brain/awr301. [DOI] [PubMed] [Google Scholar]
- Passamonti L, Cerasa A, Quattrone A. Neuroimaging of essential tremor: what is the evidence for cerebellar involvement? Tremor Other Hyperkinet Mov (N Y) 2012;2 doi: 10.7916/D8F76B8G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putzke JD, Whaley NR, Baba Y, Wszolek ZK, Uitti RJ. Essential tremor: predictors of disease progression in a clinical cohort. J Neurol Neurosurg Psychiatry. 2006;77(11):1235–7. doi: 10.1136/jnnp.2006.086579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput A, Robinson CA, Rajput AH. Essential tremor course and disability: a clinicopathologic study of 20 cases. Neurology. 2004;62(6):932–6. doi: 10.1212/01.wnl.0000115145.18830.1a. [DOI] [PubMed] [Google Scholar]
- Rajput AH, Adler CH, Shill HA, Rajput A. Essential tremor is not a neurodegenerative disease. Neurodegener Dis Manag. 2012a;2(3):259–68. doi: 10.2217/nmt.12.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajput AH, Robinson CA, Rajput ML, Robinson SL, Rajput A. Essential tremor is not dependent upon cerebellar Purkinje cell loss. Parkinsonism Relat Disord. 2012b;18(5):626–8. doi: 10.1016/j.parkreldis.2012.01.013. [DOI] [PubMed] [Google Scholar]
- Rincon F, Louis ED. Benefits and risks of pharmacological and surgical treatments for essential tremor: disease mechanisms and current management. Expert Opin Drug Saf. 2005;4:899–913. doi: 10.1517/14740338.4.5.899. [DOI] [PubMed] [Google Scholar]
- Rossi F, Jankovski A, Sotelo C. Differential regenerative response of Purkinje cell and inferior olivary axons confronted with embryonic grafts: environmental cues versus intrinsic neuronal determinants. J Comp Neurol. 1995a;359(4):663–77. doi: 10.1002/cne.903590412. [DOI] [PubMed] [Google Scholar]
- Rossi F, Jankovski A, Sotelo C. Target neuron controls the integrity of afferent axon phenotype: a study on the Purkinje cell-climbing fiber system in cerebellar mutant mice. J Neurosci. 1995b;15(3 pt 1):2040–56. doi: 10.1523/JNEUROSCI.15-03-02040.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rub U, Hoche F, Brunt ER, Heinsen H, Seidel K, Del Turco D, et al. Degeneration of the cerebellum in Huntington’s disease (HD): possible relevance for the clinical picture and potential gateway to pathological mechanisms of the disease process. Brain Pathol. 2013;23(2):165–77. doi: 10.1111/j.1750-3639.2012.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shill HA, Adler CH, Sabbagh MN, Connor DJ, Caviness JN, Hentz JG, et al. Pathologic findings in prospectively ascertained essential tremor subjects. Neurology. 2008;70(16 pt 2):1452–5. doi: 10.1212/01.wnl.0000310425.76205.02. [DOI] [PubMed] [Google Scholar]
- Shintaku M, Kaneda D. Chromosome 16q22.1-linked autosomal dominant cerebellar ataxia: an autopsy case report with some new observations on cerebellar pathology. Neuropathology. 2009;29(3):285–92. doi: 10.1111/j.1440-1789.2008.00947.x. [DOI] [PubMed] [Google Scholar]
- Sternberg EJ, Alcalay RN, Levy OA, Louis ED. Postural and intention tremors: a detailed clinical study of essential tremor vs. Parkinson’s disease. Front Neurol. 2013;4:51. doi: 10.3389/fneur.2013.00051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Symanski C, Shill HA, Dugger B, Hentz JG, Adler CH, Jacobson SA, et al. Essential tremor is not associated with cerebellar Purkinje cell loss. Mov Disord. 2014;29(4):496–500. doi: 10.1002/mds.25845. [DOI] [PubMed] [Google Scholar]
- Yu M, Ma K, Faust PL, Honig LS, Cortes E, Vonsattel JP, et al. Increased number of Purkinje cell dendritic swellings in essential tremor. Eur J Neurol. 2012;19(4):625–30. doi: 10.1111/j.1468-1331.2011.03598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]