ABSTRACT
Electroporation is used in cancer treatment because of its ability to increase local cytotoxicity of e.g. bleomycin (electrochemotherapy) and calcium (calcium electroporation). Calcium electroporation is a novel anticancer treatment that selectively kills cancer cells by necrosis, a cell death pathway that stimulates the immune system due to high release of antigens and “danger signals.” In this exploratory study, we aimed to investigate whether calcium electroporation could initiate an anticancer immune response similar to electrochemotherapy. To this end, we treated immunocompetent balb/c mice with CT26 colon tumors with calcium electroporation, electrochemotherapy, or ultrasound-based delivery of calcium or bleomycin. High treatment efficiency was observed with 100% complete remission in all four groups (12/12 with complete remission in each treatment group). In addition, none of the surviving mice from these groups formed new tumors when re-challenged with CT26 cancer cells 100-d post treatment, whereas mice challenged with different cancer cells (4T1 breast cancer) all developed tumors. Treatment of immunodeficient mice with calcium electroporation and electrochemotherapy showed no long-lasting tumor response. Calcium electroporation and electrochemotherapy was associated with a release of High Mobility Group Box 1 protein (HMGB1) in vitro (p = 0.029) and a significant increase of the overall systemic level of pro-inflammatory cytokines in serum from the treated mice (p < 0.003). These findings indicate that calcium electroporation as well as electrochemotherapy could have a role as immune stimulators in future treatments.
KEYWORDS: Calcium electroporation, CT26 colon cancer model, cytokines, electrochemotherapy, immunity
Introduction
Electroporation is a method used to permeabilize cell membranes with high-voltage pulses, making it possible for molecules and ions to cross the cell membranes. Electroporation is extensively used in cancer treatments because of its ability to locally increase dose intensity of drugs (electrochemotherapy),1-12 in particular the chemotherapeutic drug bleomycin.
A novel perspective in electroporation treatments is the use of calcium instead of chemotherapy. Calcium is an essential intracellular signal molecule involved in vital cellular processes, such as transcription, proliferation, and metabolism.13 For cells to survive, the intracellular calcium concentration must be tightly regulated, and an overload of calcium induced by electroporation leads to ATP depletion and cell death by necrosis.14 Cells undergoing necrosis can be potent immune stimulators, due to the membrane rupture and great release of intracellular content, such as antigens and damage-associated molecular pattern molecules (DAMPS).15 Electroporation is in principle a non-thermal event and, therefore, released cancer antigens and DAMPS may be preserved to induce “danger signaling” alerting the immune system.16,17 Though an immunological response may not always be in favor of anticancer treatment, as the results can range from pro-carcinogenic, immunosuppression to anti-tumorigenic, the reasons for the different outcomes are complex and still not fully known.
Calcium electroporation is based on the procedures from electrochemotherapy, a treatment that has been used as cancer treatment for decades and has proven to cause acute and efficient cell death due to the dramatically increased toxicity of bleomycin. This is evident by the fact that once-only treatment can lead to a very high probability of complete tumor regression.1-12,18 Studies in murine models have shown that electrochemotherapy are able to induce anti-tumorgenic immune response, as evident by effect on distant tumors or prevent tumor growth in re-challenge studies.16,19,20 To reinforce the systemic effect, electrochemotherapy has been combined with immunotherapy, such as pro-inflammatory cytokines (IL-2 and IL-12)17,21,22 and checkpoint inhibitors,23 demonstrating encouraging results with systemic responses. Electroporation has also shown to be successful in gene therapy studies for immunotherapy, as a safe and effective cancer treatment.24
In this report, we aimed to investigate whether calcium electroporation can induce an immune response equally with electrochemotherapy in mice with CT26 colon tumors and examine whether the effect is dependent on the delivery method (electroporation) by also treating mice with calcium or bleomycin in combination with low-intensity ultrasound.25 The release of pro-inflammatory cytokines was detected in serum and tumor from the treated mice and the release of HMGB1, a “danger signal”, was measured in vitro.
Results
Tumor response after treatment with electroporation and ultrasound
CT26-bearing Balb/c mice were randomized into nine treatment groups: (1) calcium + electroporation; (2) bleomycin + electroporation (electrochemotherapy); (3) calcium + ultrasound; (4) bleomycin + ultrasound; (5) electroporation alone; (6) ultrasound alone; (7) calcium alone; (8) bleomycin alone; (9) untreated. Twenty mice were placed in each group, and 12 mice used for tumor response and survival analyses, while eight mice were used for cytokine and PCR analyses.
All combinations of electroporation or ultrasound with either bleomycin or calcium (calcium electroporation, calcium ultrasound, electrochemotherapy, or bleomycin ultrasound) induced complete remission within 12 d after treatment. In contrast, all mice in the control groups (calcium alone, bleomycin alone, ultrasound alone, electroporation alone, and untreated) were killed due to large tumor size within 12 d after treatment. In all the combination groups, the tumor volumes were significantly different from the untreated and the control treatment groups, p < 0.001 (Fig. 1).
Figure 1.
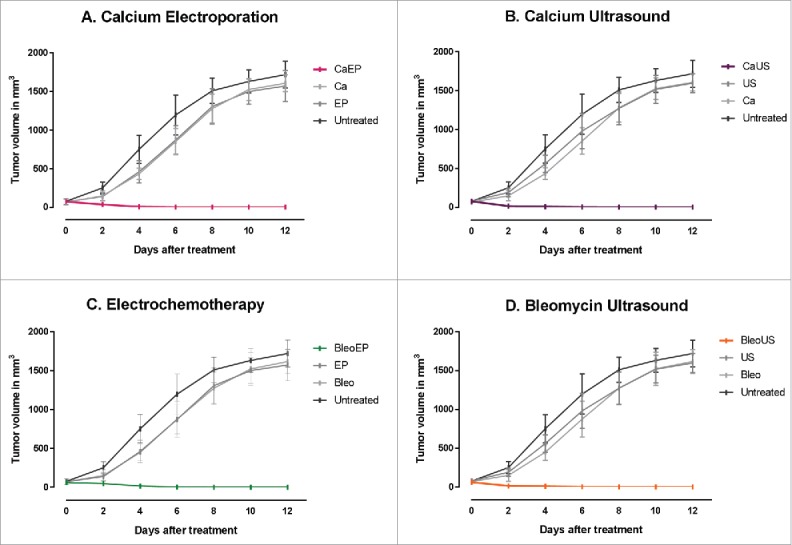
Tumor response over time. Immunocompetent CT26 balb/c mice randomized into treatment groups, CaEP = calcium electroporation, CaUS = calcium ultrasound, BleoEP = electrochemotherapy, BleoUS = bleomycin ultrasound, Ca = calcium alone, Bleo = bleomycin alone, EP = electroporation alone, US = ultrasound alone. After one treatment tumor volume was measured over time, n = 12 (means + SD). (A) Calcium electroporated compared with control groups p < 0.001 (B) Calcium ultrasound compared with control groups p < 0.001 (C) electrochemotherapy compared with control groups p < 0.001 (D) Bleomycin ultrasound compared with control groups p < 0.001.
Re-challenge of surviving mice with CT26 and 4T1 cancer cells
Naïve and surviving mice from the groups: calcium electroporation, calcium ultrasound, electrochemotherapy, and bleomycin ultrasound were re-challenged, 100 d after treatment, by injecting the same tumor cell line (CT26) or a different tumor cell line (4T1), respectively. The mice were inoculated with tumor cells at the opposite flank as previously treated.
None of the re-challenged survivors (5/5 in each treatment group) formed tumors when inoculated with the original cancer cell type (CT26). The naïve mice inoculated with CT26 cancer cells all formed new tumors (5 out of 5 inoculated mice). In each of the groups with surviving mice after the treatment of CT26 tumors that were challenged with a different cancer cell type (4T1 breast cancer) developed tumors (5/5 in each treatment group), p = 0.004–0.009 (Fig. 2).
Figure 2.
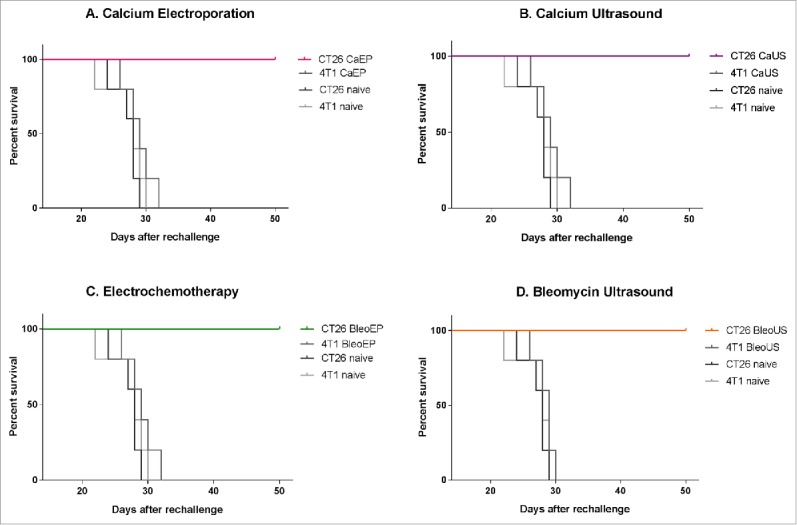
Survival curves after re-challenge. Surviving mice and naïve mice were re-challenged with the same tumor cell type (CT26) or a different tumor cell type (4T1) and observed for 50 d after inoculation. CT26 CaEP /4T1 CaEp = mice treated with calcium electroporation, inoculated with CT26 and 4T1, respectively; CT26 CaUS/4T1 CaUS = mice treated with calcium ultrasound, inoculated with CT26 and 4T1, respectively; CT26 BleoEP/4T1 BleoEP = mice treated with electrochemotherapy, inoculated with CT26 and 4T1, respectively; CT26 BleoUS/4T1 BleoUS = mice treated with bleomycin ultrasound, inoculated with CT26 and 4T1, respectively; CT26 naïve/4T1 naïve = naïve mice inoculated with CT26 and 4T1, respectively. N = 5 in each group. (A) Calcium electroporation compared with control groups, p = 0.004–0.008 (B) Calcium ultrasound compared with control groups, p = 0.006–0.008 (C) electrochemotherapy compared with control groups, p = 0.004–0.009 (D) Bleomycin ultrasound compared with control groups, p = 0.002–0.007.
Detection of systemic immune response by analyzing pro-inflammatory cytokine level in serum from the treated mice
The level of 23 pro-inflammatory cytokines was investigated in the nine groups and the treatment groups were compared with the group of tumor-bearing untreated mice (Fig. 3). Three cytokines were out of range in the analysis (IL-2, IL-4, and IL-9) and not included in the comparison. The mice treated with calcium electroporation showed a significantly increased level of pro-inflammatory cytokines at day 7 (p = 0.004), where no difference was noted at day 3. The same results were seen in mice treated with calcium alone and bleomycin alone. Conversely, an increased level was observed at day 3 in mice treated with electrochemotherapy (p = 0.003) and no significant difference at day 7. Electroporation alone increased the level of pro-inflammatory cytokines markedly, with the highest increase at day 3 (p < 0.0001, day 7 p = 0.006). Especially, the cytokine granulocyte-colony stimulating factor (G-CSF) stood out at day 3 with a mean difference of more than 26-fold higher for electroporated mice than untreated tumor-bearing mice.
Figure 3.
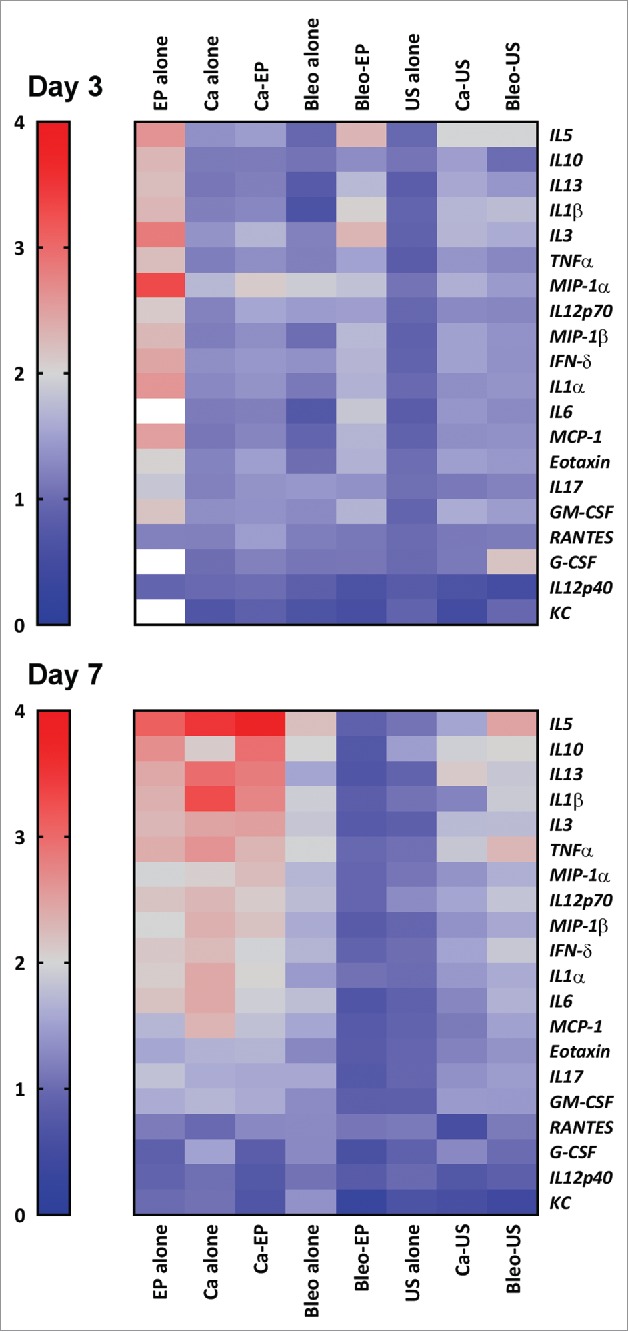
Heat map demonstrating the level of proinflammatory cytokines in serum 3 and 7 d after treatment. The color bar on the left represent values fold increase relative to untreated control. Values above 4-fold are represented as white. (1) Calcium electroporation showed no difference in cytokine level day 3 (p = 0.5) but a significant increased level day 7 (p<0.0001). (2) Electrochemotherapy showed a significant increase in cytokine level day 3 (p = 0.003) but no difference at day 7 (p = 1). (3) Electroporation alone showed a significant increase in cytokines both day 3 (p<0.0001) and day 7 (p<0.0001) and a noticeable increase at day 3 of G-CSF. (4) Calcium alone and bleomycin alone showed no difference in cytokine level day 3 (p = 1) but a significantly increased level day 7 (p<0.0001). (5) treatment with ultrasound showed no significant difference day 3 or 7. More detailed graph is shown in supplementary 1.
The three groups treated with ultrasound (ultrasound alone, calcium ultrasound, and bleomycin ultrasound) did not induce any significant difference in pro-inflammatory cytokines for any of the time points.
PCR analysis for markers of immune cells and pro-inflammatory cytokines in treated tumor tissue
At day 3, tumors were collected from all nine treatment groups and analyzed with PCR for gene expression of markers of immune cells (natural killer cells (NK-cells), macrophages, regulatory T-lymphocytes, B-lymphocytes), pro-inflammatory cytokines and programmed death ligand 1 (PD-L1). The results from the eight treatment groups were compared with results from the untreated mice (Figs. S2 and S3). The electroporated groups (electroporation alone, calcium electroporation, and electrochemotherapy) all showed a tendency for increased levels of the pro-inflammatory interleukins: IL-1β, IL-10, IL-6, TNF-α (tumor necrosis factor), and IFNγ (interferon gamma), as well as tendency of increased expression of markers for NK-cells and macrophages. The same tendency was seen in tumor tissue from mice treated with bleomycin alone and calcium alone. In contrast, we could not detect any difference in gene expression levels of cytokines or markers of immune cells in tumor tissue from the three groups treated with ultrasound.
No long-lasting tumor response detected in immunodeficient mice
NMRI-Foxn1nu mice were randomized into six treatment groups: (1) calcium electroporation; (2) electrochemotherapy; (3) calcium alone; (4) bleomycin alone; (5) electroporation alone; (6) untreated. No treatment with ultrasound was performed. Ten–eleven mice were placed in each group all used for tumor response and survival analyses.
None of the mice had complete remission, and 19 d after treatment only one mouse was alive (treated with electrochemotherapy). There was no significant difference in tumor volume over time between any of the six groups (p = 0.07–1.0), and the survival difference was not significant between untreated mice and mice from the five treatment groups (p = 0.06–0.8) (Fig. 4).
Figure 4.
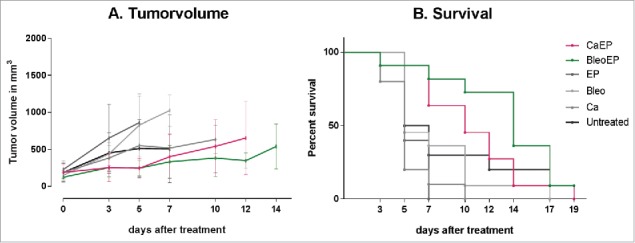
(A) Tumor response over time. NMRI-Foxn1nu mice randomized into treatment groups, CaEP = calcium electroporation, BleoEP = electrochemotherapy, Ca = calcium alone, Bleo = bleomycin alone, EP = electroporation alone, n = 11–12 (means + SD). After one treatment tumor volume was measured over time. The individual graphs terminates when n < 4. The difference in tumor volume between the six groups was not statistical significant (p = 0.07–1.0). (B) Survival curves from the same treatment groups show no statistical difference in survival between untreated mice and the five treatment groups (p = 0.06–0.8).
Electrochemotherapy and calcium electroporation liberate HMGB1
The release of HMGB1 was measured in the CT26 cell culture supernatants 30 min and 72 h after treatment with, respectively: 1. calcium + electroporation; 2. bleomycin + electroporation; 3. calcium alone; 4. bleomycin alone; 5. electroporation alone; 6. untreated. At 30 min after treatment no difference in HMGB1 release was seen between the groups, whereas at 72 h after treatment calcium electroporation, electrochemotherapy, and electroporation alone all stimulated more than 7-fold increase compared with untreated (p = 0.029). (Fig. 5)
Figure 5.
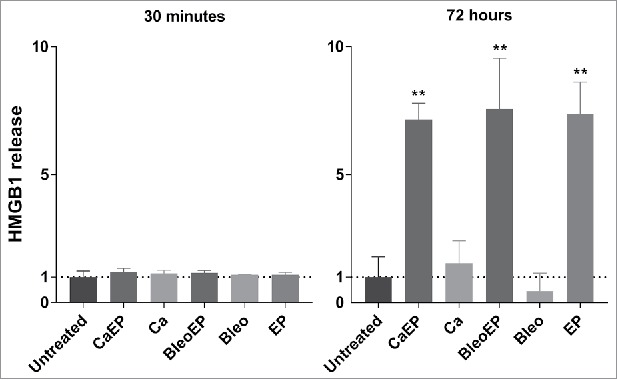
HMGB1 release measured in the supernatant of CT26 cells treated with CaEP = calcium electroporation, BleoEP = electrochemotherapy, Ca = calcium alone, Bleo = bleomycin alone, EP = electroporation alone, n = 4 (normalized means + SD). The supernatants was collected 30 min and 72 hafter treatment. After 30 min there no significant difference in extracellular HMGB1 concentration between the groups. At 72 h an 7-fold increase is observed for Calcium electroporation, electrochemotherapy and electroporation alone (p = 0.029) there was no statistical increase in calcium and bleomycin alone (p = 0.2).
Discussion
This is an exploratory study demonstrating that calcium electroporation effectively eradicates tumors and induced protective immunity in a CT26 colon tumor-bearing mouse model equal to the effect obtained with electrochemotherapy. Similar results of tumor eradication were seen when calcium and bleomycin were internalized by ultrasound. Both calcium electroporation and electrochemotherapy showed to liberate HMGB1 in vitro and the treatments were associated with increased levels of pro-inflammatory cytokines in serum and tumor tissue from the treated mice. The importance of a functioning immune system was also indicated as long-term tumor response could not be achieved in immunodeficient CT26-bearing mice after treatment with either calcium electroporation or electrochemotherapy.
Electrochemotherapy has been used in cancer treatment for more than 25 y as a local treatment of solid tumors due to its ability to locally increase cytotoxicity of drugs.26 Studies have indicated an immunogenic effect of electrochemotherapy,27 based on changes in the plasma membrane, antigen release,16 and increased infiltration of cytotoxic T-lymphocytes.28 In this study, we confirm that electrochemotherapy by itself can induce a protective immune memory in CT26 tumor-bearing balb/c mice. This validates a previous study where 12 out of 22 CT26-bearing mice treated with electrochemotherapy did not develop tumors when re-challenged with similar CT26 cells.19 That study also noted the appearance of cytotoxic T-lymphocytes specific for CT26 cells in the spleens 90-d post treatment. The results were confirmed in 2003 by Miyazaki et al,20 who also demonstrated that the protection was lost in nude balb/c mice, lacking T-lymphocytes. In 2014, Calvet et al16 showed that electrochemotherapy displays characteristics of immunogenic cell death with release of antigens and DAMPs, including calrecticulin, HMGB1, and ATP from CT26 cells treated with electrochemotherapy. In the same study, dying electrochemotherapy-treated CT26 cells were injected into mice, which were re-challenged with viable CT26 cells a week after. Only 8% of the re-challenged mice grew tumor demonstrating a “vaccination” effect. All three studies showed an immune involvement after electrochemotherapy, which may indicate a role for T-lymphocytes in the effect of electrochemotherapy. One study has shown that repetitive treatment with bleomycin alone in CT26-bearing mice can induce an immune response with expansion of regulatory T-cells,29 contrary to a recent study in patients with malignant melanoma treated with bleomycin and electroporation, where an increase in the presence of cytotoxic T-cells alongside a decrease in the presence of regulatory T-cells was observed.28
In this study, we demonstrate similar immunogenic effect when bleomycin is replaced by calcium. Calcium electroporation is a novel treatment, which is proving to be an effective local cancer treatment in different cancer histologies.14,30 The method utilizes that calcium overload induces acute ATP depletion in cancer cells.14,31 Here, we support these data, as complete tumor remission of 100% in colon tumor-bearing mice after only one treatment with calcium electroporation was observed (Fig. 1). Additionally, this study is the first to demonstrate that calcium electroporation not only has a cytotoxic effect on cancer cells, but also has the ability to generate an immunologic memory response in mice. The immunologic memory seems to be immune specific as mice treated and cured by calcium electroporation all grew tumors when challenged with a different cancer cell type, as did naïve mice (Fig. 2). For calcium, as well as bleomycin, the cytotoxic effect and capability of immunity protection was not dependent on the method of internalization when it came to electroporation or ultrasound, as all four treatments seemed to be equally effective in tumor eradication and protection. Mice treated with bleomycin alone, calcium alone, electroporation alone, or ultrasound alone did not show tumor regression.
To confirm the immunogenic effect of calcium electroporation, the procedure was replicated on immunodeficient mice with lack of T-cells. A clear difference was observed as both mice treated with calcium electroporation and electrochemotherapy did not obtain long-lasting tumor response. To further detect a systemic immune response created by the treatments, we analyzed the levels of 20 different pro-inflammatory cytokines in serum at day 3 and 7 after treatment. As this was an exploratory study, we chose a wide range of cytokines. We observed an increased level of pro-inflammatory cytokines in mice treated with calcium electroporation and electrochemotherapy, but with different peaking times in the two groups. In mice treated with calcium electroporation, the cytokine level was significantly increased at day 7, whereas the increase was seen already at day 3 for electrochemotherapy (Fig. 3). An increase in pro-inflammatory cytokines was observed in mice treated with electroporation alone with an overall increase of all cytokines but a particularly high increase of G-CSF at day 3 (26-fold increase). G-CSF is known to stimulate the survival, proliferation, and differentiation of granulocytes and is secreted by different tissues and immune cells. This finding supports the theory of electroporation as a usable method for immunotherapeutic treatments with both internalization of drugs and genes, and studies using gene electrotransfer of IL-12 have used similar pulsing parameters.24
PCR from treated tumor tissue, collected 3 d after treatment, showed a tendency of increased levels of markers for NK-cells, macrophages, as well as pro-inflammatory cytokines in tumor tissue treated with calcium electroporation, electrochemotherapy, and electroporation alone. But as the number of samples was low, no firm conclusions can be made (Figs. S2 and S3).
HMGB1 is a nuclear protein, which is released from necrotic cells, it activates the release of pro-inflammatory cytokines from innate immune cells32 and is classified as one of the “key DAMPS” in immunogenic cell death. This study demonstrates an increased liberation of HMGB1 in CT26 cells after calcium electroporation with a 7-fold increase in extracellular HMGB1 concentration 72 h after treatment. A similar increase is seen in CT26 cells treated with electrochemotherapy, which supports findings in the previous study by Calvet et al.16
Although ultrasound combined with calcium or bleomycin showed ability to generate a protective immune memory against the same tumor type, indicating an activation of the adaptive immune system, we could not detect any systemic immunological response in the serum of the treated mice (calcium ultrasound, bleomycin ultrasound, and ultrasound alone) at day 3 or 7 or in the tumor tissue at day 3, in contrary to electroporation combined with calcium or bleomycin. Electroporation and ultrasound are both used for drug delivery, although the mechanisms are different.25 Electroporation uses high-voltage pulses to induce structural changes in the lipid membrane allowing formation of hydrophilic pores, unlike ultrasound that uses acoustic pressure and heat to impair the membrane integrity. These different effects on the cell membrane may explain the differences in activation of pro-inflammatory cytokines.
The results are of great interest as electrochemotherapy already now is being investigated in combination with immunotherapeutic treatments, such as checkpoint inhibitors.23 Here, we suggest that not only electrochemotherapy could be considered as an immune activator but also calcium electroporation. Calcium is beneficial as it is a cheap, accessible drug already in clinical trials. This is an exploratory study that invites to further investigations into the mechanisms behind the difference in activation of immune response by calcium electroporation and electrochemotherapy as well as the difference between immune response activation in electroporation treatments versus ultrasound treatments.
Material and methods
Ethics
In vivo experiments on immunocompetent balb/c mice were performed at Cork Cancer Research Center, University College Cork, Cork, Ireland and approved by the University College Cork Animal Experimentation Ethics Committee and performed under licenses from the department of Health Ireland as directed by the Cruelty to Animal Act Ireland and EU statutory Instructions.
In vivo experiments on NMRI-Foxn1nu mice were performed at the department of oncology, Herlev and Gentofte hospital, Denmark and approved by the European Convention for the Protection of Vertebrate Animals used for Experimentation and with approval from the Danish Animal Experiments Inspectorate.
Cell tissue culture
The two cell lines, CT26 a murine colon adenocarcinoma, and 4T1 a murine breast carcinoma were obtained from the American Type Cell Collection (Manassas, VA, USA). The CT26 cells were cultured with Dulbecco's modified Eagle's medium supplemented with 10% fetal calf serum and 4T1 cells were cultured in RPMI-1640 supplemented with 10% fetal calf serum and 300 µg L-glutamine.
Animals and tumor induction
Female balb/c mice (6–8 weeks) were obtained from Harlan Laboratories (Oxfordshire, UK). For tumor induction 5× 105 tumor cells, suspended in 200 µL serum-free Dulbecco's modified Eagle's medium, were inoculated into the flank of the mice. CT26 cells were inoculated into the right flank of the mice and tumors were allowed to establish and grow until they reached 0.4–0.8 cm in diameter and were ready to treat. 180 mice were randomized into nine treatment groups, 20 mice in each group, 12 used for survival analysis and 8 used for cytokine and PCR analyses. Day 100 post treatment surviving mice were re-challenged with either the same CT26 cancer cells or the 4T1 cancer cell. At re-challenge cells were injected under the same procedure as described above, but into opposite flank as baseline.
NMRI-Foxn1nu mice were obtained from Harlan Laboratories (Oxfordshire, UK) and bred at the laboratory in the department of oncology, Herlev and Gentofte hospital. CT26 cells were inoculated into the right flank of the mice and tumors were allowed to establish and grow until they reach 0.4–1 cm in diameter and were ready to treat. 63 mice were randomized into six groups (calcium electroporation, electrochemotherapy, and controls). All mice were used for tumor volume and survival.
Treatment procedures
Prior to treatment, the mice were anesthetized by i.p. injection of a mixture of ketamine and Xylapan in phosphate-buffered Saline. Bleomycin 1000 IE/mL or calcium chloride 168 mmol was injected intratumorally with volumes according to tumor size (200 µL calcium/bleomycin in tumors ≥ 0.5 cm in diameter and 100 µL calcium/bleomycin in tumors < 0.5 cm in diameter). In treatment groups with the combination of a drug and a delivery method, electroporation or ultrasound were performed immediately after drug injection.
Electroporation was performed with 8 pulses of 100 µs at 1.0 kV/cm at 1 Hz, using a needle-based electrode and a square wave electroporator (ePORE, CCRC, Cork, Ireland, respectively, Cliniporator, IGEA, Italy). Ultrasound was performed with a frequency of 1 mHz and intensity of 3.5 W/cm2 for 2 min using (Sonitron 2000, Rich-Mar Corp., Inola, OK, USA) based on the protocol described in Larkin et al.25
The mice were only treated once and tumor size monitored three times per week. When the tumor diameter reached ethically approved limit of (Cork 1.5 cm, Herlev 1.2 cm) the mice was culled. Tumors were measured in two dimensions using Vernier calipers and tumor volume calculated as ab2π/6, where a is the longest diameter of the tumor and b is the longest diameter perpendicular to a.
Analysis of cytokine levels in serum from treated mice
At, respectively, day 3 and 7 blood samples from all nine treatment groups were collected by heart puncture under anesthesia (n = 3–4 per treatment group at day 3 and 7, respectively), serum was isolated by clot and centrifugation and analyzed using Luminex 200 system (Ramcon, Birkerød, Denmark) with a standard Bio-Plex Pro™ Mouse Cytokine 23-plex Assay (Interleukins: IL-1α, IL-1β, IL-2, IL-3, IL-4, IL-5, IL-6, IL-9, IL-10, IL-12p40, IL-12p70, IL-13, 1L-17α and Eotaxin, G-CSF, GM-CSF (granulocyte macrophage colony stimulating factor), IFNγ, KC (keratinocyte chemoattractant), MCP-1 (monocyte chemoattractant protein), macrophage inflammatory proteins: MIP-1α, MIP-1β, and RANTES and TNF-α) from Bio-Rad Laboratories, Copenhagen, Denmark. The assays were performed according to manufacturer's instructions.
PCR in treated tumor tissue
Tumor tissues were collected at day 3 and snap frozen to −80°C. Prior to RNA isolation, tissues were homogenized at −80°C using a mortar and then lysed in the Tissuelyser (Qiagen Retsch MM300) at 25 Hz suspended in Trizol. RNA was isolated from the tissue lysate by addition of chloroform followed by centrifugation at 12.000 g for 15 min at 4°C, and the RNA was precipitated from the resulting aqueous phase by adding this to isopropyl alcohol (1:1 reaction) for incubation for 10 min. at room temperature. The precipitated RNA was spun down at 12.000 g for 15 min at 4°C, washed twice with ice cold 75% ethanol (centrifugation at 8.000 g for 5 min. at 4°C) and resuspended in Ultrapure RNase/DNase free water. RNA concentrations were determined using the NanoDrop 1000 spectrophotometer (Thermo Scientific).
cDNA preparations were synthesized from 250 ng RNA using the high capacity cDNA transcription kit (Applied Biosystems™) in a total reaction volume of 20 µL. The reactions were submitted to the following PCR program: 25°C for 10 min, 37°C for 120 min, and 85°C for 5 min followed by cooling to 4°C.
The resulting cDNA was then used for qPCR detecting the genes encoding the following immune cell specific markers or cytokines: CD335/NKp46 and NKG2D (NK cells), CD68 (macrophages), CD209 (dendritic cells/T cells), FOXP3 (regulatory T cells), CD19 (B cells), IL-6, IL-1β, IL-10, TNF-α, and IFNγ. All samples were run in triplicates using Power Up SYBR® Green PCR Master Mix (Life Technologies), 7.5 ng cDNA, 300 nM forward primer, and 300 nM reverse primer in a total reaction volume of 10 µL pr. well in MicroAmp® Optical 384-well reaction plates (Life Technologies). The plates were covered with adhesive film and spun down for 2 min at 1000 rpm. Before submission to the SYBR® Green qPCR program using the ViiA™7 system (Thermo Scientific): 50°C for 2 min, 95°C for 2 min, and 42 cycles of 95°C for 15 sec followed by 60°C for 1 min.
Primer sequences were designed using the Primer-BLAST tool from NCBI and ordered from TAG Copenhagen A/S.
Calcium electroporation in vitro and HMGB1 assays
HMGB1 was measured in supernatants from CT26 cells treated accordingly to the six treatment groups: 1. calcium electroporation; 2. calcium alone; 3. bleomycin electroporation; 4. bleomycin alone; 5. electroporation alone; 6. untreated. Cells were washed in 10 mL of PBS, trypsinated and centrifuged at 1,000 rpm for 3 min. The pellet was resuspended in HEPES buffer. A solution containing 6.1 × 106 cells/mL was used. Cells were treated with 5 mM calcium or 1,000 IE/mL bleomycin accordingly, and HEPES buffer for control. The cells were electroporated in 4 mm cuvettes with eight pulses of 0.48 kV for 99 µs and 1 Hz using a square wave electroporator (BTXT820). After treatment the cells were incubated at 37°C for 20 min or 72 h, respectively. Cells were then centrifuged at 1,000 rpm for 5 min and the supernatant collected for HMGB1 assays. The analyses were performed using HMGB1 detection Elisa kit (Mybiosource) and analyzed in 96-well plates according to the manufacturer's instruction.
Statistical analyses
Permutation test of survival curves was performed using the R-packet “Clinfun”. The remaining of the statistical analyses was done using SPSS v 22. Tumor growth curves were analyzed using mixed model. For cytokine assays, analysis was performed with two-way ANOVA with treatment and cytokines as fixed factors, and with Bonferroni correction. HMGB1 measurements were analyzed using Mann–Whitney–Wilcoxon test. A p value of 0.05 or less was considered statistically significant. For PCR analysis only descriptive statistics (mean and range) were used.
Supplementary Material
Disclosure of potential conflicts of interest
Julie Gehl reports a conflict of interest regarding a pending patent on calcium electroporation (PCT/DK2012/050496).
Funding
This work was supported by Danish Cancer Society under grant number R110-A6996 and COST Action TD1104 under grant number COST-STSM-ECOST-STSM-TD1104–160315–049034.
References
- 1.Belehradek M, Domenge C, Luboinski B, Orlowski S, Belehradek J Jr, Mir LM. Electrochemotherapy, a new antitumor treatment. First clinical phase I-II trial. Cancer 1993; 72:3694-700; PMID:7504576; https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 2.Matthiessen LW, Chalmers RL, Sainsbury DC, Veeramani S, Kessell G, Humphreys AC, Bond JE, Muir T, Gehl J. Management of cutaneous metastases using electrochemotherapy. Acta Oncol 2011; 50:621-9; PMID:21574833; https://doi.org/ 10.3109/0284186X.2011.573626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Matthiessen LW, Johannesen HH, Hendel HW, Moss T, Kamby C, Gehl J. Electrochemotherapy for large cutaneous recurrence of breast cancer: a phase II clinical trial. Acta Oncol 2012; 51:713-21; PMID:22731832; https://doi.org/ 10.3109/0284186X.2012.685524 [DOI] [PubMed] [Google Scholar]
- 4.Marty M, Sersa G, Garbay JR, Gehl J, Collins CG, Snoj M, Billard V, Geertsen PF, Larkin JO, Miklavcic D et al.. Electrochemotherapy – An easy, highly effective and safe treatment of cutaneous and subcutaneous metastases: results of ESOPE (European Standard Operating Procedures of Electrochemotherapy) study. Ejc Supplements 2006; 4:3-13; https://doi.org/ 10.1016/j.ejcsup.2006.08.002 [DOI] [Google Scholar]
- 5.Campana LG, Mocellin S, Basso M, Puccetti O, De Salvo GL, Chiarion-Sileni V, Vecchiato A, Corti L, Rossi CR, Nitti D. Bleomycin-based electrochemotherapy: clinical outcome from a single institution's experience with 52 patients. Ann Surg Oncol 2009; 16:191-9; PMID:18987914; https://doi.org/ 10.1245/s10434-008-0204-8 [DOI] [PubMed] [Google Scholar]
- 6.Campana LG, Valpione S, Falci C, Mocellin S, Basso M, Corti L, Balestrieri N, Marchet A, Rossi CR. The activity and safety of electrochemotherapy in persistent chest wall recurrence from breast cancer after mastectomy: a phase-II study. Breast Cancer Res Treat 2012; 134:1169-78; PMID:22821399; https://doi.org/ 10.1007/s10549-012-2095-4 [DOI] [PubMed] [Google Scholar]
- 7.Campana LG, Valpione S, Mocellin S, Sundararajan R, Granziera E, Sartore L, Chiarion-Sileni V, Rossi CR. Electrochemotherapy for disseminated superficial metastases from malignant melanoma. Br J Surg 2012; 99:821-30; PMID:22508342; https://doi.org/ 10.1002/bjs.8749 [DOI] [PubMed] [Google Scholar]
- 8.Curatolo P, Quaglino P, Marenco F, Mancini M, Nardo T, Mortera C, Rotunno R, Calvieri S, Bernengo MG. Electrochemotherapy in the treatment of Kaposi sarcoma cutaneous lesions: a two-center prospective phase II trial. Ann Surg Oncol 2012; 19:192-8; PMID:21822561; https://doi.org/ 10.1245/s10434-011-1860-7 [DOI] [PubMed] [Google Scholar]
- 9.Heller R, Jaroszeski MJ, Reintgen DS, Puleo CA, DeConti RC, Gilbert RA, Glass LF. Treatment of cutaneous and subcutaneous tumors with electrochemotherapy using intralesional bleomycin. Cancer 1998; 83:148-57; PMID:9655305; https://doi.org/ [DOI] [PubMed] [Google Scholar]
- 10.Kis E, Olah J, Ocsai H, Baltas E, Gyulai R, Kemeny L, Horvath AR. Electrochemotherapy of cutaneous metastases of melanoma–a case series study and systematic review of the evidence. Dermatol Surg 2011; 37:816-24; PMID:21605245; https://doi.org/ 10.1111/j.1524-4725.2011.01951.x [DOI] [PubMed] [Google Scholar]
- 11.Larkin JO, Collins CG, Aarons S, Tangney M, Whelan M, O'Reily S, Breathnach O, Soden DM, O'Sullivan GC. Electrochemotherapy – aspects of preclinical development and early clinical experience. Ann Surgery 2007; 245:469-79; PMID:17435555; https://doi.org/ 10.1097/01.sla.0000250419.36053.33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mir LM, Glass LF, Sersa G, Teissie J, Domenge C, Miklavcic D, Jaroszeski MJ, Orlowski S, Reintgen DS, Rudolf Z et al.. Effective treatment of cutaneous and subcutaneous malignant tumours by electrochemotherapy. Br J Cancer 1998; 77:2336-42; PMID:9649155; https://doi.org/ 10.1038/bjc.1998.388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berridge MJ, Bootman MD, Roderick HL. Calcium signalling: dynamics, homeostasis and remodelling. Nat Rev Mol Cell Biol 2003; 4:517-29; PMID:12838335; https://doi.org/ 10.1038/nrm1155 [DOI] [PubMed] [Google Scholar]
- 14.Frandsen SK, Gissel H, Hojman P, Tramm T, Eriksen J, Gehl J. Direct therapeutic applications of calcium electroporation to effectively induce tumor necrosis. Cancer Res 2012; 72:1336-41; PMID:22282658; https://doi.org/ 10.1158/0008-5472.CAN-11-3782 [DOI] [PubMed] [Google Scholar]
- 15.Garg AD, Nowis D, Golab J, Vandenabeele P, Krysko DV, Agostinis P. Immunogenic cell death, DAMPs and anticancer therapeutics: an emerging amalgamation. Biochim Biophys Acta 2010; 1805:53-71; PMID:19720113; https://doi.org/ 10.1016/j.bbcan.2009.08.003 [DOI] [PubMed] [Google Scholar]
- 16.Calvet CY, Famin D, Andre FM, Mir LM. Electrochemotherapy with bleomycin induces hallmarks of immunogenic cell death in murine colon cancer cells. Oncoimmunology 2014; 3:e28131; PMID:25083316; https://doi.org/ 10.4161/onci.28131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Andersen MH, Gehl J, Reker S, Pedersen LO, Becker JC, Geertsen P, thor Straten P. Dynamic changes of specific T cell responses to melanoma correlate with IL-2 administration. Semin Cancer Biol 2003; 13:449-59; PMID:15001164; https://doi.org/ 10.1016/j.semcancer.2003.09.009 [DOI] [PubMed] [Google Scholar]
- 18.Spratt DE, Gordon Spratt EA, Wu S, DeRosa A, Lee NY, Lacouture ME, Barker CA. Efficacy of skin-directed therapy for cutaneous metastases from advanced cancer: a meta-analysis. J Clin Oncol 2014; 32:3144-55; PMID:25154827; https://doi.org/ 10.1200/JCO.2014.55.4634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kuriyama S, Mitoro A, Tsujinoue H, Toyokawa Y, Nakatani T, Yoshiji H, Tsujimoto T, Okuda H, Nagao S, Fukui H. Electrochemotherapy can eradicate established colorectal carcinoma and leaves a systemic protective memory in mice. Int J Oncol 2000; 16:979-85; PMID:10762634; https://doi.org/ 10.3892/ijo.16.5.979 [DOI] [PubMed] [Google Scholar]
- 20.Miyazaki S, Gunji Y, Matsubara H, Shimada H, Uesato M, Suzuki T, Kouzu T, Ochiai T. Possible involvement of antitumor immunity in the eradication of colon 26 induced by low-voltage electrochemotherapy with bleomycin. Surg Today 2003; 33:39-44; PMID:12560905; https://doi.org/ 10.1007/s005950300006 [DOI] [PubMed] [Google Scholar]
- 21.Mir LM, Roth C, Orlowski S, Quintin-Colonna F, Fradelizi D, Belehradek J Jr, Kourilsky P. Systemic antitumor effects of electrochemotherapy combined with histoincompatible cells secreting interleukin-2. J Immunother Emphasis Tumor Immunol 1995; 17:30-8; PMID:7537154 [DOI] [PubMed] [Google Scholar]
- 22.Cemazar M, Ambrozic Avgustin J, Pavlin D, Sersa G, Poli A, Krhac Levacic A, Tesic N, Lampreht Tratar U, Rak M, Tozon N. Efficacy and safety of electrochemotherapy combined with peritumoral IL-12 gene electrotransfer of canine mast cell tumours. Vet Comp Oncol 2016; PMID:26840222; https://doi.org/ 10.1111/vco.12208 [DOI] [PubMed] [Google Scholar]
- 23.Heppt MV, Eigentler TK, Kahler KC, Herbst RA, Goppner D, Gambichler T, Ulrich J, Dippel E, Loquai C, Schell B et al.. Immune checkpoint blockade with concurrent electrochemotherapy in advanced melanoma: a retrospective multicenter analysis. Cancer Immunol Immunother 2016; 65:951-9; PMID:27294607; https://doi.org/ 10.1007/s00262-016-1856-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Daud AI, DeConti RC, Andrews S, Urbas P, Riker AI, Sondak VK, Munster PN, Sullivan DM, Ugen KE, Messina JL et al.. Phase I trial of interleukin-12 plasmid electroporation in patients with metastatic melanoma. J Clin Oncol 2008; 26:5896-903; PMID:19029422; https://doi.org/ 10.1200/JCO.2007.15.6794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larkin JO, Casey GD, Tangney M, Cashman J, Collins CG, Soden DM, O'Sullivan GC. Effective tumor treatment using optimized ultrasound-mediated delivery of bleomycin. Ultrasound Med Biol 2008; 34:406-13; PMID:17988788; https://doi.org/ 10.1016/j.ultrasmedbio.2007.09.005 [DOI] [PubMed] [Google Scholar]
- 26.Mir LM, Belehradek M, Domenge C, Orlowski S, Poddevin B, Belehradek J Jr, Schwaab G, Luboinski B, Paoletti C. Electrochemotherapy, a new antitumor treatment: first clinical trial. C R Acad Sci III 1991; 313:613-8; PMID:1723647 [PubMed] [Google Scholar]
- 27.Falk H, Lambaa S, Johannessen HH, Wooler G, Venzo A, Gehl J. Electrochemotherapy and calcium electroporation inducing a systemic immune response with local and distant remission of tumors in a patient with malignant melanoma – a case report. Acta Oncol; 2017; [Published online]; https://doi.org/ 10.1080/0284186X.2017.1290274 [DOI] [PubMed] [Google Scholar]
- 28.Di Gennaro P, Gerlini G, Urso C, Sestini S, Brandani P, Pimpinelli N, Borgognoni L. CD4+FOXP3+ T regulatory cells decrease and CD3+CD8+ T cells recruitment in TILs from melanoma metastases after electrochemotherapy. Clin Exp Metastasis 2016; 33(8):787-98 [DOI] [PubMed] [Google Scholar]
- 29.Bugaut H, Bruchard M, Berger H, Derangere V, Odoul L, Euvrard R, Ladoire S, Chalmin F, Végran F, Rébé C et al.. Bleomycin exerts ambivalent antitumor immune effect by triggering both immunogenic cell death and proliferation of regulatory T cells. PLoS One 2013; 8:e65181; PMID:23762310; https://doi.org/ 10.1371/journal.pone.0065181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frandsen SK, Gibot L, Madi M, Gehl J, Rols MP. Calcium electroporation: evidence for differential effects in normal and malignant cell lines, evaluated in a 3D spheroid model. PLoS One 2015; 10:e0144028; PMID:26633834; https://doi.org/ 10.1371/journal.pone.0144028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hansen EL, Sozer EB, Romeo S, Frandsen SK, Vernier PT, Gehl J. Dose-dependent ATP depletion and cancer cell death following calcium electroporation, relative effect of calcium concentration and electric field strength. PLoS One 2015; 10:e0122973; PMID:25853661; https://doi.org/ 10.1371/journal.pone.0122973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raucci A, Palumbo R, Bianchi ME. HMGB1: a signal of necrosis. Autoimmunity 2007; 40:285-9; PMID:17516211; https://doi.org/ 10.1080/08916930701356978 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


