Abstract
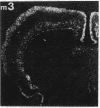
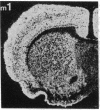
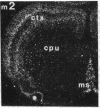
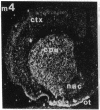
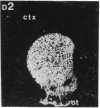
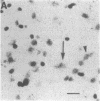
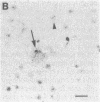
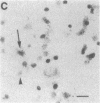
Within the basal ganglia, acetylcholine and dopamine play a central role in the extrapyramidal control of motor function. The physiologic effects of these neurotransmitters are mediated by a diversity of receptor subtypes, several of which have now been cloned. Muscarinic acetylcholine receptors are encoded by five genes (m1-m5), and of the two known dopamine receptor subtypes (D1 and D2) the D2 receptor gene has been characterized. To gain insight into the physiological roles of each of these receptor subtypes, we prepared oligodeoxynucleotide probes to localize receptor subtype mRNAs within the rat striatum and substantia nigra by in situ hybridization histochemistry. Within the striatum, three muscarinic (m1, m2, m4) receptor mRNAs and the D2 receptor mRNA were detected. The m1 mRNA was expressed in most neurons (greater than 80%); the m2 mRNA, in neurons which were both very large and rare; and the m4 and D2 mRNAs, in 40-50% of the neurons, one-third of which express both mRNAs. Within the substantia nigra, pars compacta, only the m5 and D2 mRNAs were detected, and most neurons expressed both mRNAs. These data provide anatomical evidence for the identity of the receptor subtypes which mediate the diverse effects of muscarinic and dopaminergic drugs on basal ganglia function.
Full text
PDF
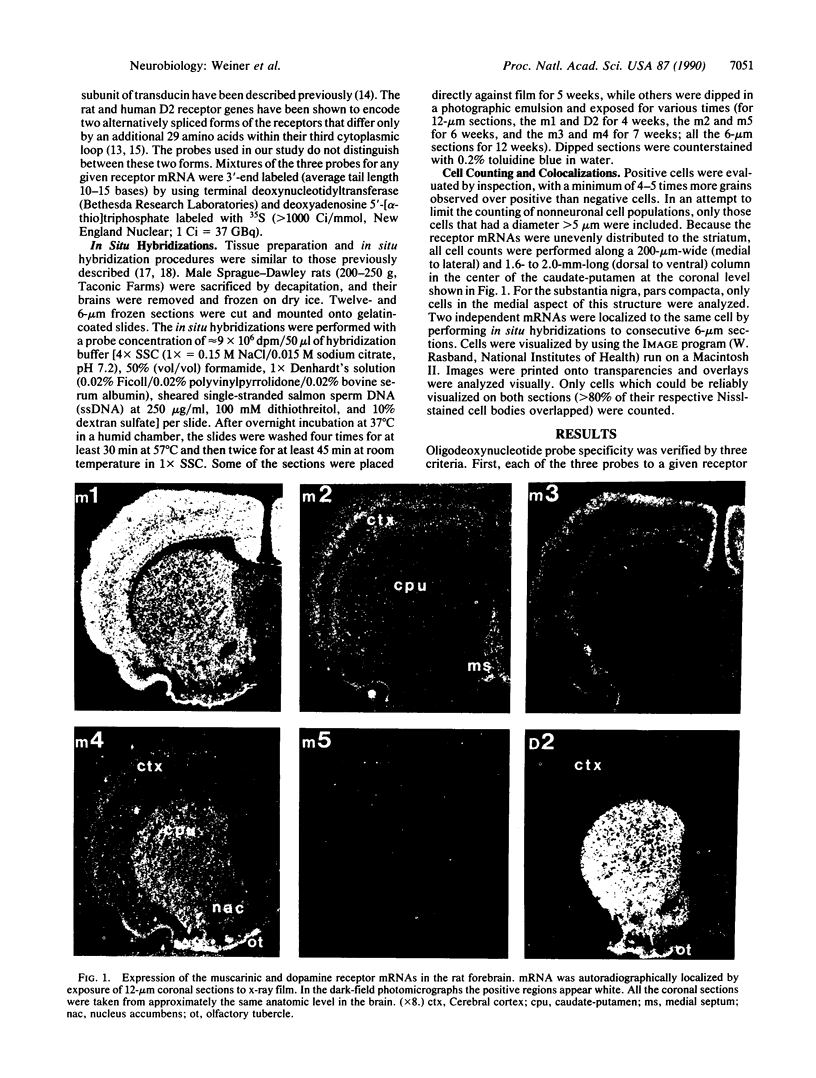
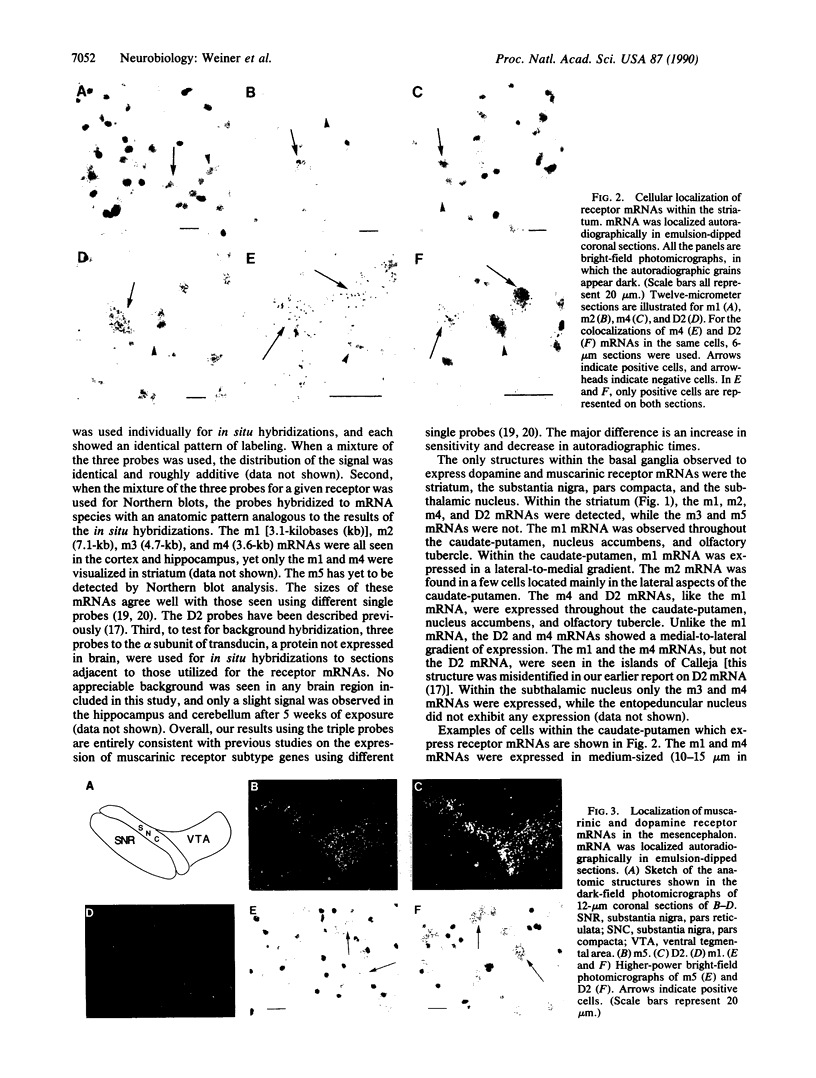


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchine J. R. Drug therapy of parkinsonism. N Engl J Med. 1976 Oct 7;295(15):814–818. doi: 10.1056/NEJM197610072951505. [DOI] [PubMed] [Google Scholar]
- Bolam J. P., Wainer B. H., Smith A. D. Characterization of cholinergic neurons in the rat neostriatum. A combination of choline acetyltransferase immunocytochemistry, Golgi-impregnation and electron microscopy. Neuroscience. 1984 Jul;12(3):711–718. doi: 10.1016/0306-4522(84)90165-9. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Buckley N. J., Young A. C., Brann M. R. Identification of a family of muscarinic acetylcholine receptor genes. Science. 1987 Jul 31;237(4814):527–532. doi: 10.1126/science.3037705. [DOI] [PubMed] [Google Scholar]
- Bonner T. I., Young A. C., Brann M. R., Buckley N. J. Cloning and expression of the human and rat m5 muscarinic acetylcholine receptor genes. Neuron. 1988 Jul;1(5):403–410. doi: 10.1016/0896-6273(88)90190-0. [DOI] [PubMed] [Google Scholar]
- Brann M. R., Buckley N. J., Bonner T. I. The striatum and cerebral cortex express different muscarinic receptor mRNAs. FEBS Lett. 1988 Mar 28;230(1-2):90–94. doi: 10.1016/0014-5793(88)80648-3. [DOI] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Brann M. R. Localization of a family of muscarinic receptor mRNAs in rat brain. J Neurosci. 1988 Dec;8(12):4646–4652. doi: 10.1523/JNEUROSCI.08-12-04646.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley N. J., Bonner T. I., Buckley C. M., Brann M. R. Antagonist binding properties of five cloned muscarinic receptors expressed in CHO-K1 cells. Mol Pharmacol. 1989 Apr;35(4):469–476. [PubMed] [Google Scholar]
- Bunzow J. R., Van Tol H. H., Grandy D. K., Albert P., Salon J., Christie M., Machida C. A., Neve K. A., Civelli O. Cloning and expression of a rat D2 dopamine receptor cDNA. Nature. 1988 Dec 22;336(6201):783–787. doi: 10.1038/336783a0. [DOI] [PubMed] [Google Scholar]
- Charuchinda C., Supavilai P., Karobath M., Palacios J. M. Dopamine D2 receptors in the rat brain: autoradiographic visualization using a high-affinity selective agonist ligand. J Neurosci. 1987 May;7(5):1352–1360. doi: 10.1523/JNEUROSCI.07-05-01352.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesselet M. F. Presynaptic regulation of neurotransmitter release in the brain: facts and hypothesis. Neuroscience. 1984 Jun;12(2):347–375. doi: 10.1016/0306-4522(84)90058-7. [DOI] [PubMed] [Google Scholar]
- Cortés R., Palacios J. M. Muscarinic cholinergic receptor subtypes in the rat brain. I. Quantitative autoradiographic studies. Brain Res. 1986 Jan 8;362(2):227–238. doi: 10.1016/0006-8993(86)90448-8. [DOI] [PubMed] [Google Scholar]
- Dal Toso R., Sommer B., Ewert M., Herb A., Pritchett D. B., Bach A., Shivers B. D., Seeburg P. H. The dopamine D2 receptor: two molecular forms generated by alternative splicing. EMBO J. 1989 Dec 20;8(13):4025–4034. doi: 10.1002/j.1460-2075.1989.tb08585.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duvoisin R. C. Cholinergic-anticholinergic antagonism in parkinsonism. Arch Neurol. 1967 Aug;17(2):124–136. doi: 10.1001/archneur.1967.00470260014002. [DOI] [PubMed] [Google Scholar]
- Dwoskin L. P., Zahniser N. R. Robust modulation of [3H]dopamine release from rat striatal slices by D-2 dopamine receptors. J Pharmacol Exp Ther. 1986 Nov;239(2):442–453. [PubMed] [Google Scholar]
- Edley S. M., Graybiel A. M. The afferent and efferent connections of the feline nucleus tegmenti pedunculopontinus, pars compacta. J Comp Neurol. 1983 Jun 20;217(2):187–215. doi: 10.1002/cne.902170207. [DOI] [PubMed] [Google Scholar]
- Giorguieff M. F., Le Floc'h M. L., Glowinski J., Besson M. J. Involvement of cholinergic presynaptic receptors of nicotinic and muscarinic types in the control of the spontaneous release of dopamine from striatal dopaminergic terminals in the rat. J Pharmacol Exp Ther. 1977 Mar;200(3):535–544. [PubMed] [Google Scholar]
- Gocayne J., Robinson D. A., FitzGerald M. G., Chung F. Z., Kerlavage A. R., Lentes K. U., Lai J., Wang C. D., Fraser C. M., Venter J. C. Primary structure of rat cardiac beta-adrenergic and muscarinic cholinergic receptors obtained by automated DNA sequence analysis: further evidence for a multigene family. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8296–8300. doi: 10.1073/pnas.84.23.8296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grandy D. K., Marchionni M. A., Makam H., Stofko R. E., Alfano M., Frothingham L., Fischer J. B., Burke-Howie K. J., Bunzow J. R., Server A. C. Cloning of the cDNA and gene for a human D2 dopamine receptor. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9762–9766. doi: 10.1073/pnas.86.24.9762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagan J. J., Tonnaer J. A., Rijk H., Broekkamp C. L., van Delft A. M. Facilitation of amphetamine-induced rotation by muscarinic antagonists is correlated with M2 receptor affinity. Brain Res. 1987 Apr 28;410(1):69–73. doi: 10.1016/s0006-8993(87)80021-5. [DOI] [PubMed] [Google Scholar]
- Kebabian J. W., Calne D. B. Multiple receptors for dopamine. Nature. 1979 Jan 11;277(5692):93–96. doi: 10.1038/277093a0. [DOI] [PubMed] [Google Scholar]
- Kemel M. L., Desban M., Glowinski J., Gauchy C. Distinct presynaptic control of dopamine release in striosomal and matrix areas of the cat caudate nucleus. Proc Natl Acad Sci U S A. 1989 Nov;86(22):9006–9010. doi: 10.1073/pnas.86.22.9006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo T., Fukuda K., Mikami A., Maeda A., Takahashi H., Mishina M., Haga T., Haga K., Ichiyama A., Kangawa K. Cloning, sequencing and expression of complementary DNA encoding the muscarinic acetylcholine receptor. Nature. 1986 Oct 2;323(6087):411–416. doi: 10.1038/323411a0. [DOI] [PubMed] [Google Scholar]
- Le Moine C., Normand E., Guitteny A. F., Fouque B., Teoule R., Bloch B. Dopamine receptor gene expression by enkephalin neurons in rat forebrain. Proc Natl Acad Sci U S A. 1990 Jan;87(1):230–234. doi: 10.1073/pnas.87.1.230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. J., Rye D. B., Hallanger A. E., Levey A. I., Wainer B. H. Cholinergic vs. noncholinergic efferents from the mesopontine tegmentum to the extrapyramidal motor system nuclei. J Comp Neurol. 1988 Sep 22;275(4):469–492. doi: 10.1002/cne.902750402. [DOI] [PubMed] [Google Scholar]
- Levey A. I., Wainer B. H., Mufson E. J., Mesulam M. M. Co-localization of acetylcholinesterase and choline acetyltransferase in the rat cerebrum. Neuroscience. 1983 May;9(1):9–22. doi: 10.1016/0306-4522(83)90042-8. [DOI] [PubMed] [Google Scholar]
- Mash D. C., Potter L. T. Autoradiographic localization of M1 and M2 muscarine receptors in the rat brain. Neuroscience. 1986 Oct;19(2):551–564. doi: 10.1016/0306-4522(86)90280-0. [DOI] [PubMed] [Google Scholar]
- Peralta E. G., Ashkenazi A., Winslow J. W., Smith D. H., Ramachandran J., Capon D. J. Distinct primary structures, ligand-binding properties and tissue-specific expression of four human muscarinic acetylcholine receptors. EMBO J. 1987 Dec 20;6(13):3923–3929. doi: 10.1002/j.1460-2075.1987.tb02733.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pycock C., Milson J., Tarsy D., Marsden C. D. The effect of manipulation of cholinergic mechanisms on turning behaviour in mice with unilateral destruction of the nigro-neostriatal dopaminergic system. Neuropharmacology. 1978 Mar;17(3):175–183. doi: 10.1016/0028-3908(78)90097-7. [DOI] [PubMed] [Google Scholar]
- Raiteri M., Leardi R., Marchi M. Heterogeneity of presynaptic muscarinic receptors regulating neurotransmitter release in the rat brain. J Pharmacol Exp Ther. 1984 Jan;228(1):209–214. [PubMed] [Google Scholar]
- Scatton B. Further evidence for the involvement of D2, but not D1 dopamine receptors in dopaminergic control of striatal cholinergic transmission. Life Sci. 1982 Dec 20;31(25):2883–2890. doi: 10.1016/0024-3205(82)90679-8. [DOI] [PubMed] [Google Scholar]
- Stoof J. C., Kebabian J. W. Two dopamine receptors: biochemistry, physiology and pharmacology. Life Sci. 1984 Dec 3;35(23):2281–2296. doi: 10.1016/0024-3205(84)90519-8. [DOI] [PubMed] [Google Scholar]
- Stormann T. M., Gdula D. C., Weiner D. M., Brann M. R. Molecular cloning and expression of a dopamine D2 receptor from human retina. Mol Pharmacol. 1990 Jan;37(1):1–6. [PubMed] [Google Scholar]
- Trugman J. M., Geary W. A., 2nd, Wooten G. F. Localization of D-2 dopamine receptors to intrinsic striatal neurones by quantitative autoradiography. Nature. 1986 Sep 18;323(6085):267–269. doi: 10.1038/323267a0. [DOI] [PubMed] [Google Scholar]
- Weiner D. M., Brann M. R. The distribution of a dopamine D2 receptor mRNA in rat brain. FEBS Lett. 1989 Aug 14;253(1-2):207–213. doi: 10.1016/0014-5793(89)80960-3. [DOI] [PubMed] [Google Scholar]
- White F. J., Wang R. Y. Pharmacological characterization of dopamine autoreceptors in the rat ventral tegmental area: microiontophoretic studies. J Pharmacol Exp Ther. 1984 Nov;231(2):275–280. [PubMed] [Google Scholar]
- Xu M., Mizobe F., Yamamoto T., Kato T. Differential effects of M1- and M2-muscarinic drugs on striatal dopamine release and metabolism in freely moving rats. Brain Res. 1989 Aug 28;495(2):232–242. doi: 10.1016/0006-8993(89)90217-5. [DOI] [PubMed] [Google Scholar]
- Young W. S., 3rd, Bonner T. I., Brann M. R. Mesencephalic dopamine neurons regulate the expression of neuropeptide mRNAs in the rat forebrain. Proc Natl Acad Sci U S A. 1986 Dec;83(24):9827–9831. doi: 10.1073/pnas.83.24.9827. [DOI] [PMC free article] [PubMed] [Google Scholar]