Abstract
We present a 60-year-old man with biopsy-proven metastatic squamous cell carcinoma of the right inguinal and external iliac lymph nodes with unknown primary. Hypermetabolic soft tissue masses were identified in bilateral subscapular regions on follow-up positron emission tomography (PET)–computed tomography (CT) after completion of chemoradiation. The right subscapular mass was biopsied under CT guidance, and histopathology showed it to be elastofibroma dorsi. Elastofibroma dorsi is a benign tumor with no malignant potential; due to its ill-defined appearance and tracer uptake on PET-CT, it can be misdiagnosed as soft tissue sarcoma. This report describes the typical location and imaging features of this incidental hypermetabolic mass.
Elastofibroma dorsi (EFD) is an uncommon, slow-growing, noncapsulated, ill-defined, benign soft tissue pseudotumor without well-defined boundaries and is commonly located in the infra- or periscapular area (1). It is classified as a benign fibroblast/myofibroblast tumor. Patients are usually asymptomatic, with a few presenting with shoulder pain, swelling, or scapular clunk (2). Here we present a case of EFD to highlight its status as a benign “incidentaloma.”
CASE REPORT
A 60-year-old man who had biopsy-proven metastatic squamous cell carcinoma of the right inguinal and external iliac nodes of unknown primary improved after completion of chemoradiation therapy. Three months after he completed therapy, positron emission tomography (PET)–computed tomography (CT) revealed bilateral soft tissue masses deep to the latissimus dorsi, more prominent on the right (SUV max of 2.9) and less prominent on the left (SUV max of 2.1) (Figure 1). Based on the location and imaging appearance, a presumptive diagnosis of EFD was made; however, in view of the patient's history of squamous cell carcinoma, biopsy was suggested (Figure 2). The biopsy confirmed EFD (Figure 3).
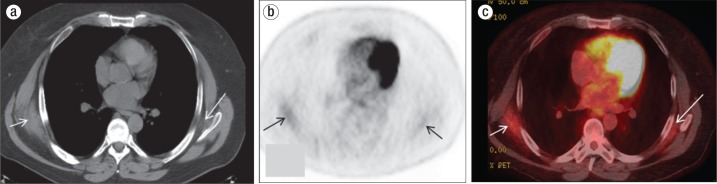
Figure 1.
Posttreatment positron emission tomography (PET)–computed tomography (CT) in a 60-year-old man. (a) Axial CT scan at the level of the left atrium shows crescent-shaped soft tissue masses in both subscapular lesions deep to the latissimus dorsi muscles, with the right bigger than the left (white arrows). (b) Axial nonattenuation corrected PET image shows tracer uptake in the bilateral posterolateral chest (black arrows). (c) The fused PET-CT image, in which the area of hypermetabolic activity corresponds to the soft tissue masses (white arrows), shows an SUV max of 2.7 in the right mass and 2.1 in the left.
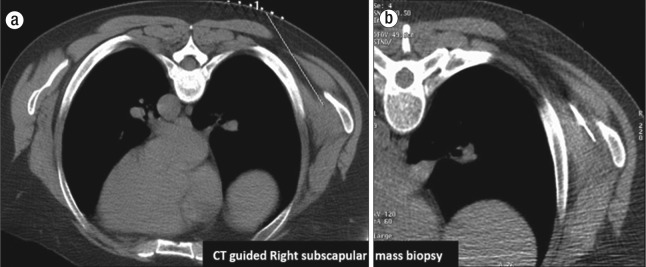
Figure 2.
Limited prone CT images show grid placement for (a) preprocedure planning and (b) subsequent biopsy needle in the right subscapular mass.
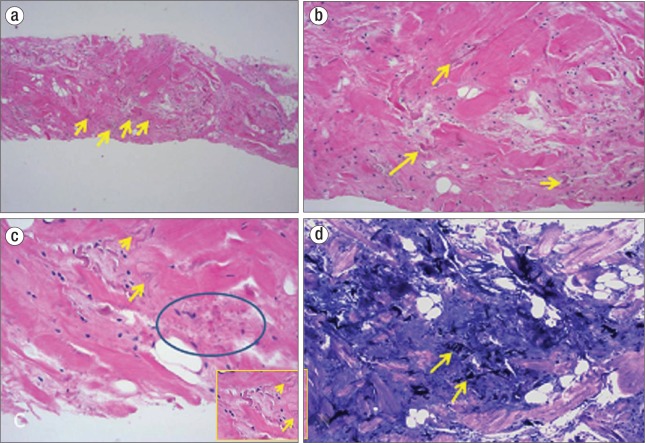
Figure 3.
(a, b) Hematoxylin and eosin staining of the biopsy specimen shows altered elastin fibers (yellow arrows) and interspersed mature adipose tissue in a fibrous background. (c) Note the characteristic wavy, serrated edges (yellow arrow) and beaded appearance (blue circle) of the elastic fibers. The inset shows a magnified view. (d) Elastin stains show deeply staining branched and unbranched elastin fibers (yellow arrows).
DISCUSSION
EFD is a slow-growing lesion that typically arises from the connective tissue between the chest wall from the sixth to the eighth ribs and the lower portion of the scapula beneath the serratus anterior and the latissimus dorsi muscles. Rarely, it can also occur in extrascapular locations such as the greater trochanter, ischial tuberosity, olecranon, axilla, elbows, hand, foot, tricuspid valve, stomach, rectum, eye, and inguinal region (3–6). Bilateral lesions are common, seen in up to 66% of patients (1, 3, 6), as in our case. Subscapular EFD is seen on the right side in up to 60% of cases (6). The size as well as associated SUV max on PET-CT is higher on the right side than the left side for bilateral lesions (7), possibly because the right side is exposed to greater mechanical friction due to right-handedness. The size of the lesion varies from a few centimeters to 15 to 20 cm (3).
EFD was classified as a benign fibroblast/myofibroblast tumor by the World Health Organization in 2000. It is characterized by the presence of elastic fibers at various degrees of maturation in a stroma of collagen and fatty connective tissue. On pathology, wavy, string bead, serrated, and globular-shaped elastic fibers and interspersed mature adipose cells in the fibrous background are the diagnostic evidence for this disease, with positive elastic fiber staining (Figure 3). The etiology of EFD is still unclear, and its development either results from a reactive hyperproliferation of fibroblastic tissue or from continued mechanical friction between the scapula and the ribs, which occurs in certain repetitive manual work, and includes a genetic predisposition (2). Studies have suggested that EFD has a monoclonal neoplastic process with genomic instability (8). One study suggested an integral role of CD34+ fibroblast monoclonal proliferation (9). Thus, EFD originates from either a neoplastic or reactive process that develops very slowly.
The prevalence of EFD is 2% in an asymptomatic elderly population; however, for subclinical EFD (<3 cm), a higher prevalence was found in an autopsy series, with rates of 24% for women and 11% for men (1, 10). Most cases (>50%) of EFD are incidental, as in our case, or patients may complain of a slow-growing, palpable subscapular mass, back discomfort, or a scapular clunk. Symptoms related to brachial plexus impingement are possible (2).
On imaging, EFD appears as an ill-defined nonencapsulated mass, classically occupying a subscapular position adjacent to the rib cage. CT shows density similar to skeletal muscle, possibly with low-density linear infiltrating areas due to adipose tissue (11). On MRI, EFD is T1 and T2 intermediate, similar to skeletal muscle, with high signal intensity for linear strands on T1- and T2-weighted images representing interspersed foci of fatty tissue, with variable contrast enhancement (11). On PET-CT, there is variable low-level fluorodeoxyglucose (FDG) uptake, with the most common pattern (55%) uptake less than that of liver and second pattern (33%) uptake equal to or slightly higher than that of the liver (12, 13). Increased 18F-FDG activity is likely due to a combination of high vascularity, abnormal fibroblastic proliferation, and an inflammatory process within the mass (13).
The differential diagnoses of periscapular lesions include desmoid tumors, neurofibroma, liposarcoma, soft tissue sarcoma, aggressive fibromatosis, and malignant histiocytofibroma (14). Enhancement of these tumors is usually substantial or heterogeneous due to neovascularization and is significantly higher than that of EFD (2, 11, 15). In general, subscapular topography, bilateralism, and typical imaging features including FDG uptake pattern in the elderly are sufficient for diagnosis of EFD, thus avoiding biopsies and unnecessary surgical resection. Occasionally, image-guided biopsy is required if doubt persists in oncologic patients after imaging exams. Otherwise, stable patterns of 18F-FDG uptake and mass size on follow-up PET-CT may virtually eliminate malignancy from the differential diagnosis. If the lesion is asymptomatic, simple observation suffices. Complete surgical resection may be considered in symptomatic patients or for esthetic reasons (≥5 cm) (10, 15). Local tumor recurrence has been reported after incomplete excision (2, 16). As no cases of malignant transformation have been described in the literature (17), there is no need for follow-up with EFD.
References
- 1.Jarvi O, Saxen E. Elastofibroma dorsi. Acta Pathol Microbiol Scand Suppl. 1961;51(Suppl 144):83–84. [PubMed] [Google Scholar]
- 2.Fibla J, Molins L, Marco V, Pérez J, Vidal G. Bilateral elastofibroma dorsi. Joint Bone Spine. 2007;74(2):194–196. doi: 10.1016/j.jbspin.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 3.Nagamine N, Nohara Y, Ito E. Elastofibroma in Okinawa. A clinicopathologic study of 170 cases. Cancer. 1982;50(9):1794–1805. doi: 10.1002/1097-0142(19821101)50:9<1794::aid-cncr2820500925>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 4.Mirra JM, Straub LR, Jarvi OH. Elastofibroma of the deltoid. A case report. Cancer. 1974;33(1):234–238. doi: 10.1002/1097-0142(197401)33:1<234::aid-cncr2820330135>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Enjoji M, Sumiyoshi K, Sueyoshi K. Elastofibromatous lesion of the stomach in a patient with elastofibroma dorsi. Am J Surg Pathol. 1985;9(3):233–237. doi: 10.1097/00000478-198503000-00007. [DOI] [PubMed] [Google Scholar]
- 6.Hoffman JK, Klein MH, McInerney VK. Bilateral elastofibroma: a case report and review of the literature. Clin Orthop Relat Res. 1996;325:245–250. [PubMed] [Google Scholar]
- 7.Fang N, Wang YL, Zeng L, Wu Z, Cui X, Wang Q, Gao S, Ding W. Characteristics of elastofibroma dorsi on PET/CT imaging with 18F-FDG. Clin Imaging. 2016;40(1):110–113. doi: 10.1016/j.clinimag.2015.08.009. [DOI] [PubMed] [Google Scholar]
- 8.Mortman KD, Hochheiser GM, Giblin EM, Manon-Matos Y, Frankel KM. Elastofibroma dorsi: clinicopathologic review of 6 cases. Ann Thorac Surg. 2007;83(5):1894–1897. doi: 10.1016/j.athoracsur.2006.11.050. [DOI] [PubMed] [Google Scholar]
- 9.Hisaoka M, Hashimoto H. Elastofibroma: clonal fibrous proliferation with predominant CD34-positive cells. Virchows Arch. 2006;448(2):195–199. doi: 10.1007/s00428-005-0053-9. [DOI] [PubMed] [Google Scholar]
- 10.Chandrasekar CR, Grimer RJ, Carter SR, Tillman RM, Abudu A, Davies AM, Sumathi VP. Elastofibroma dorsi: an uncommon benign pseudotumour. Sarcoma. 2008;2008:756565. doi: 10.1155/2008/756565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kransdorf MJ, Meis JM, Montgomery E. Elastofibroma: MR and CT appearance with radiologic-pathologic correlation. AJR Am J Roentgenol. 1992;159(3):575–579. doi: 10.2214/ajr.159.3.1503030. [DOI] [PubMed] [Google Scholar]
- 12.Onishi Y, Kitajima K, Senda M, Sakamoto S, Suzuki K, Maeda T, Yoshikawa T, Ohno Y, Sugimura K. FDG-PET/CT imaging of elastofibroma dorsi. Skeletal Radiol. 2011;40(7):849–853. doi: 10.1007/s00256-010-1057-3. [DOI] [PubMed] [Google Scholar]
- 13.Metser U, Miller E, Lerman H, Even-Sapir E. Benign nonphysiologic lesions with increased 18F-FDG uptake on PET/CT: characterization and incidence. AJR Am J Roentgenol. 2007;189(5):1203–1210. doi: 10.2214/AJR.07.2083. [DOI] [PubMed] [Google Scholar]
- 14.Daigeler A, Vogt PM, Busch K, Pennekamp W, Weyhe D, Lehnhardt M, Steinstrasser L, Steinau HU, Kuhnen C. Elastofibroma dorsi—differential diagnosis in chest wall tumours. World J Surg Oncol. 2007;5(1):15. doi: 10.1186/1477-7819-5-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alouini R, Allani M, Harzallah L, Bahri M, Kraiem C, Tlili-Graies K. Elastofibroma: imaging features. J Radiol. 2005;86(11):1712–1715. doi: 10.1016/s0221-0363(05)81513-6. [DOI] [PubMed] [Google Scholar]
- 16.Brandser EA, Goree JC, El-Khoury GY. Elastofibroma dorsi: prevalence in an elderly patient population as revealed by CT. AJR Am J Roentgenol. 1998;171(4):977–980. doi: 10.2214/ajr.171.4.9762978. [DOI] [PubMed] [Google Scholar]
- 17.Muramatsu K, Ihara K, Hashimoto T, Seto S, Taguchi T. Elastofibroma dorsi: diagnosis and treatment. J Shoulder Elbow Surg. 2007;16(5):591–595. doi: 10.1016/j.jse.2006.12.010. [DOI] [PubMed] [Google Scholar]