Abstract
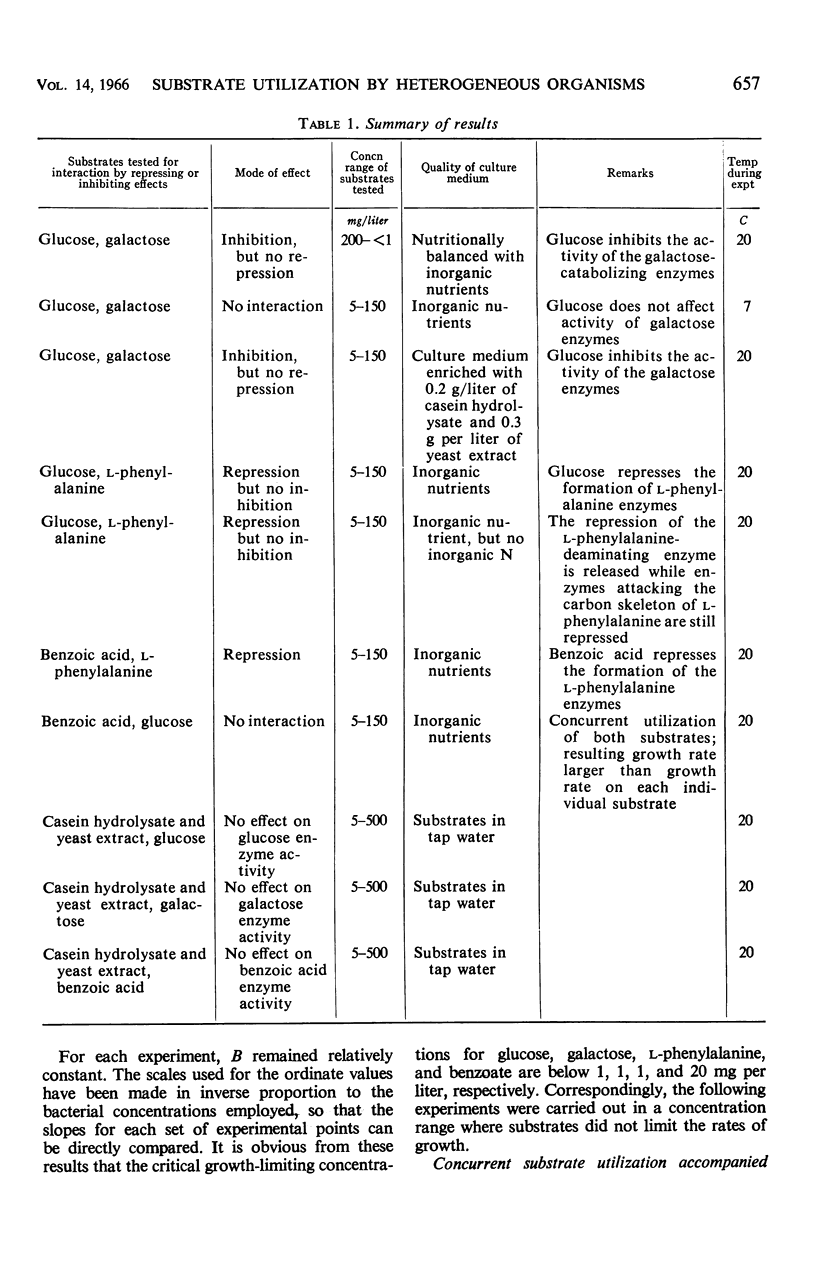
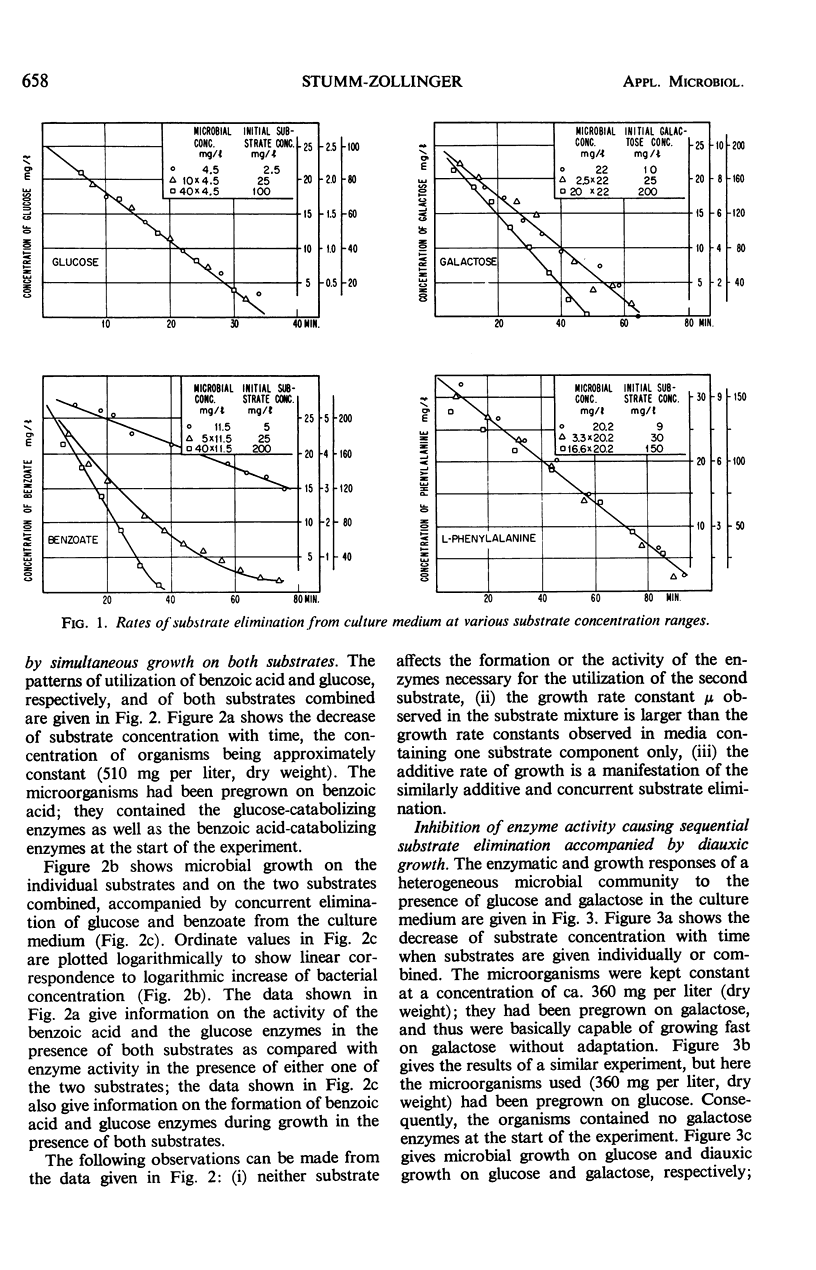
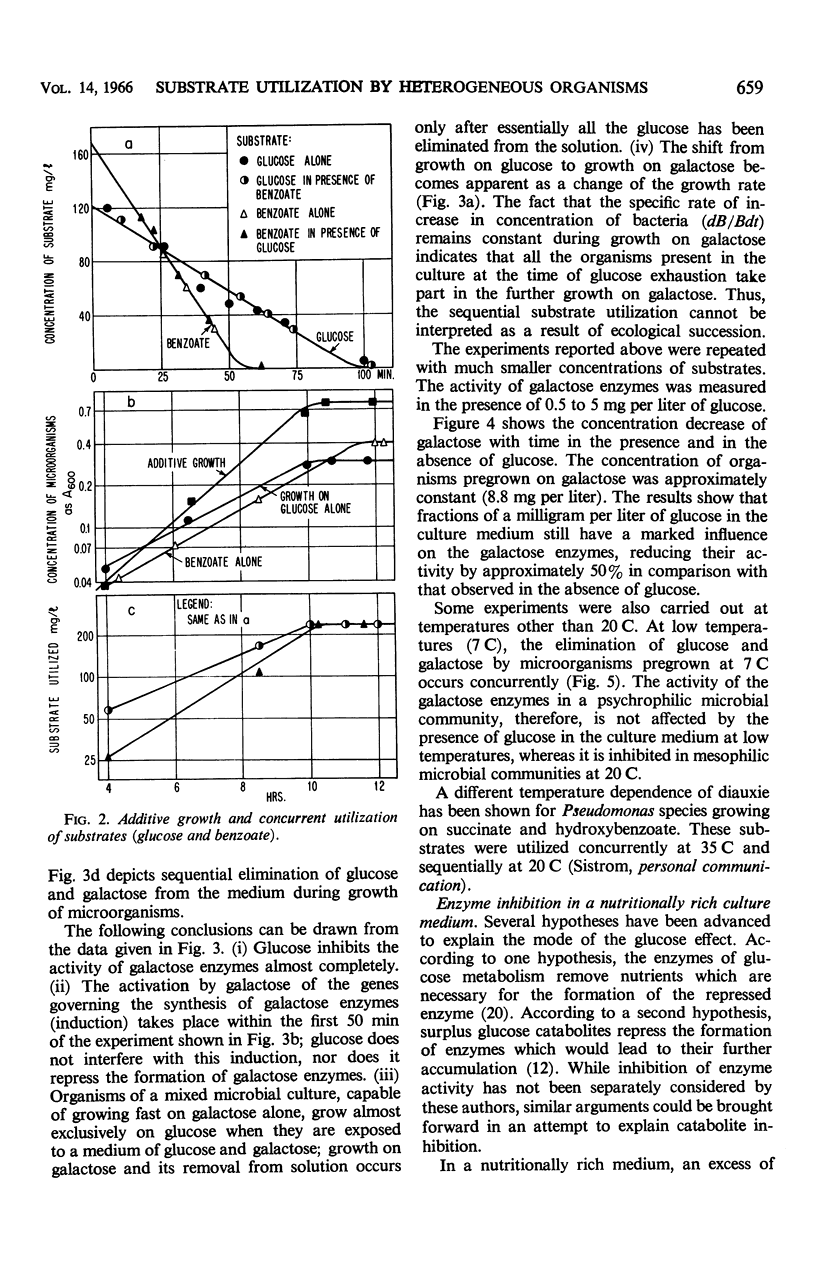
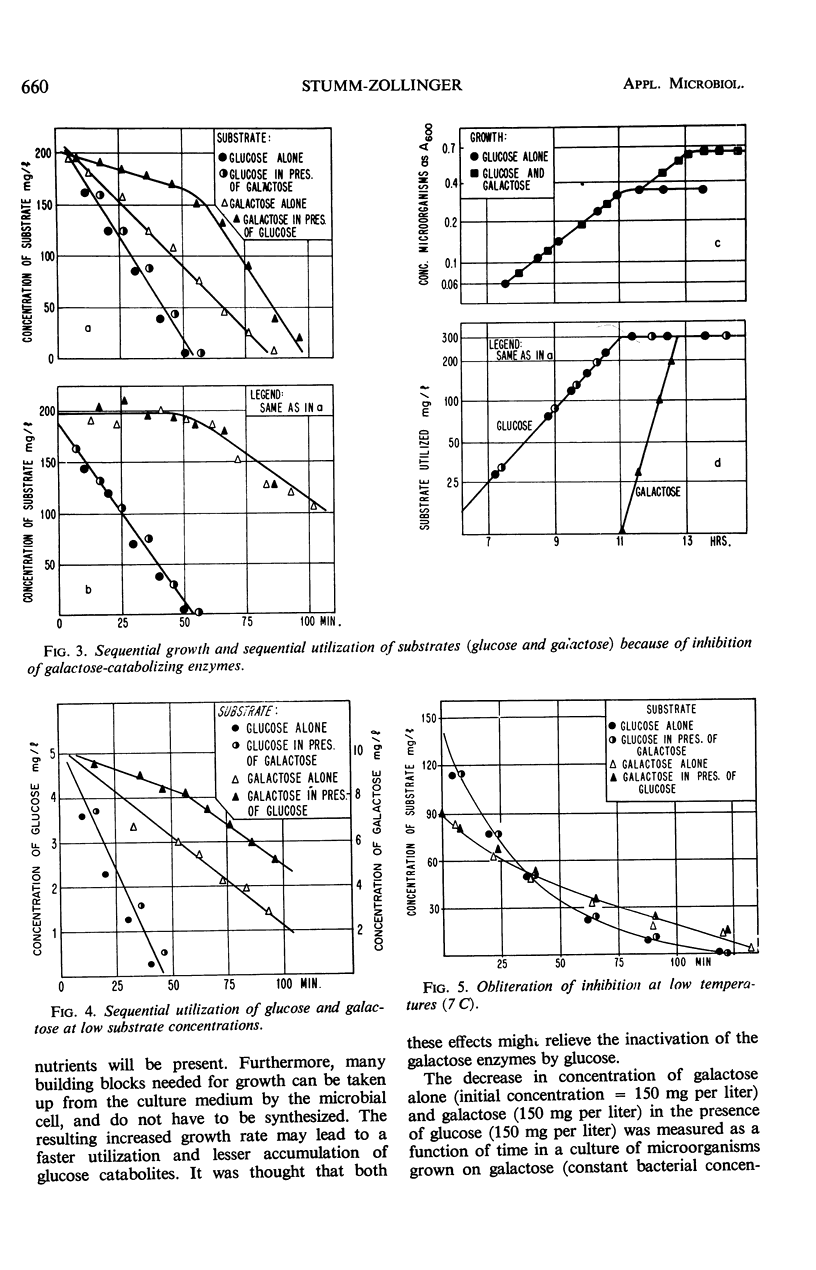
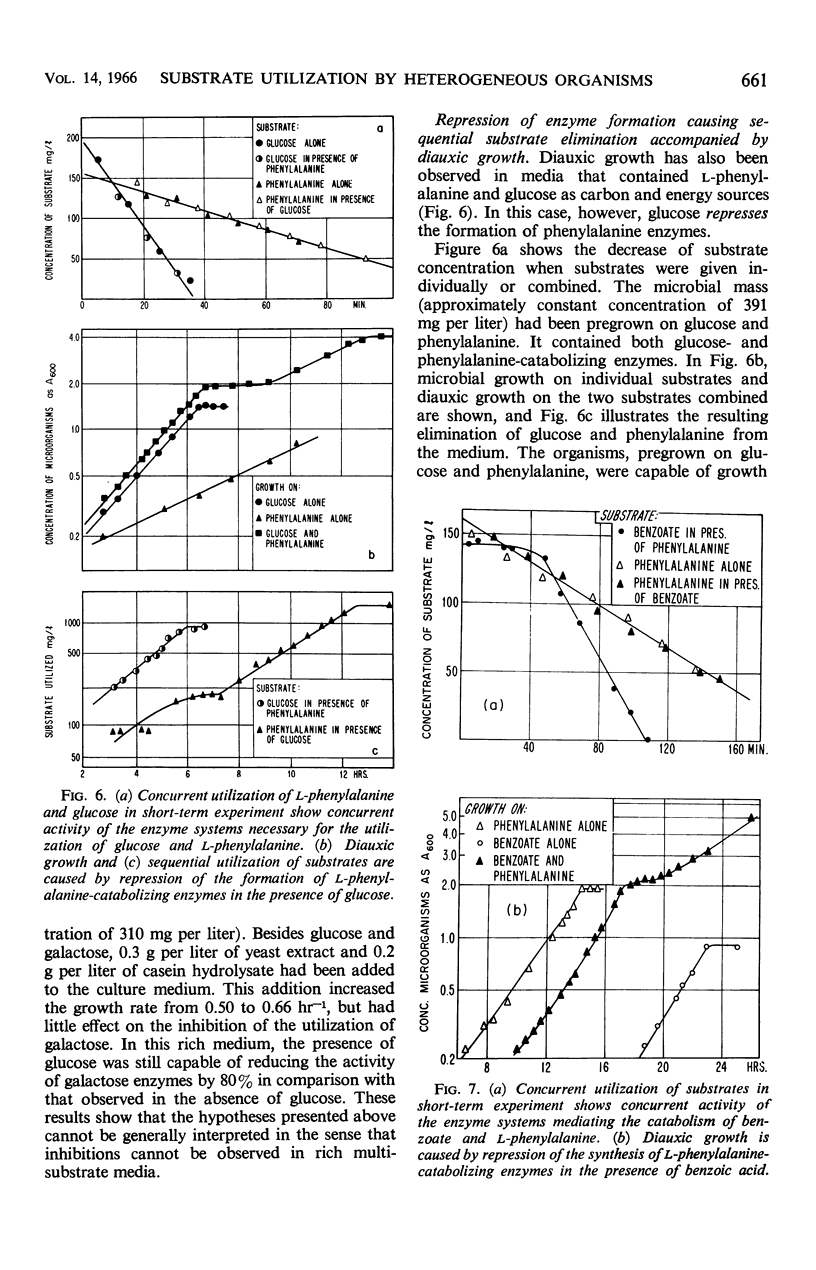
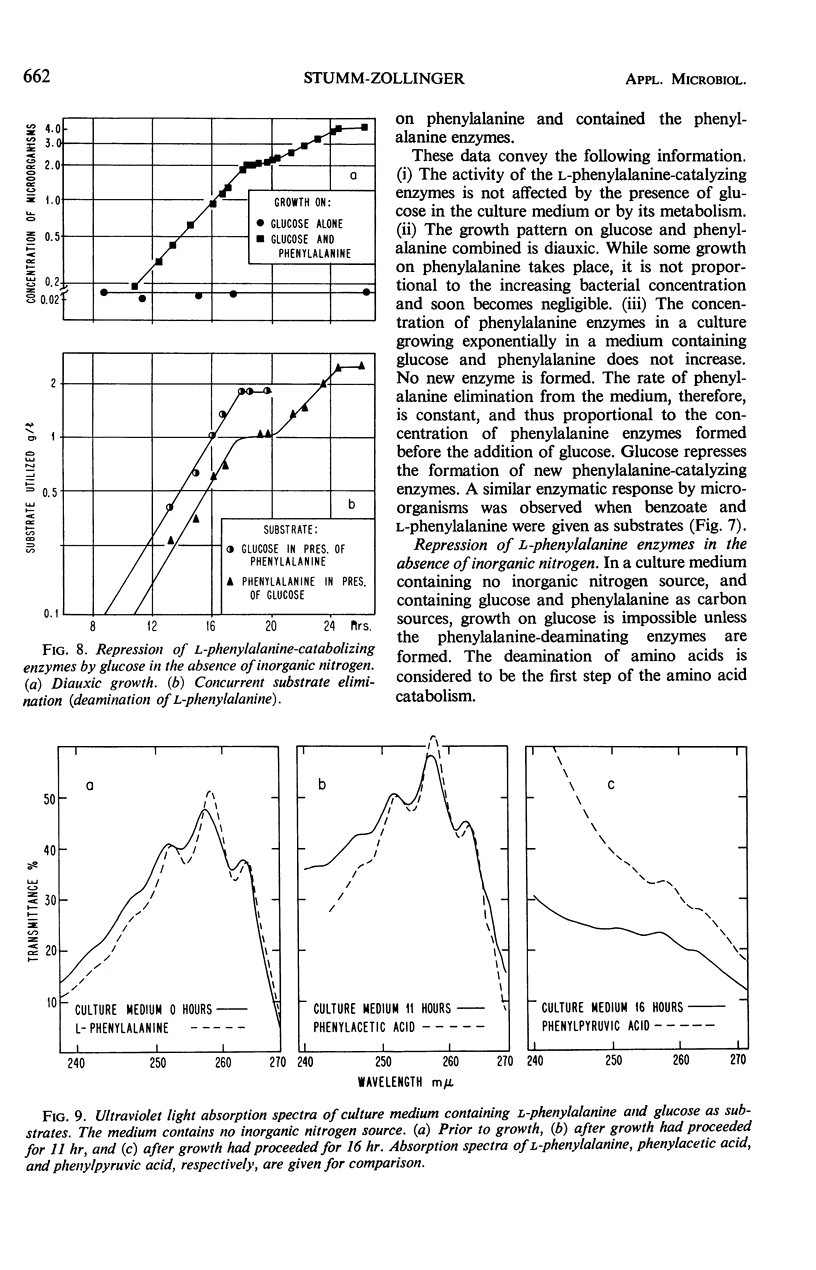
This investigation attempts to evaluate to what extent enzyme inhibition and repression by metabolites, indigenous to the cell, are significant phenomena in natural microbial communities. Three case histories of the kinetics of substrate utilization and growth in multisubstrate media by heterogeneous bacterial populations are presented: (i) concurrent substrate utilization and growth on both substrates simultaneously (glucose plus benzoate); (ii) sequential substrate elimination accompanied by diauxic growth as a result of inhibition of enzyme activity (glucose plus galactose); (iii) sequential substrate utilization accompanied by diauxic growth caused by repression of enzyme formation (glucose plus l-phenylalanine, benzoate plus l-phenylalanine). It is shown that enzyme inhibition was observed in two-substrate media as well as in multisubstrate media and was maintained at low substrate concentrations (few milligrams per liter). A special attempt has been made to maintain the diversity of the experimental microbial population during the adaptation and enrichment period. All substrates were determined with sensitive analytical methods specific for the individual substrates. The results obtained confirm that catabolite repression and the resulting sequential substrate utilization are observed in heterogeneous bacterial populations.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- COCKING E. C., YEMM E. W. Estimation of amino acids by ninhydrin. Biochem J. 1954 Jun 19;58(330TH):xii–xii. [PubMed] [Google Scholar]
- DAVIS B. D. The teleonomic significance of biosynthetic control mechanisms. Cold Spring Harb Symp Quant Biol. 1961;26:1–10. doi: 10.1101/sqb.1961.026.01.005. [DOI] [PubMed] [Google Scholar]
- GAUDY A. F., Jr, GAUDY E. T., KOMOLRIT K. Multicomponent substrate utilization by natural populations and a pure culture of Escherichia coli. Appl Microbiol. 1963 Mar;11:157–162. doi: 10.1128/am.11.2.157-162.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gale E. F. FACTORS INFLUENCING THE ENZYMIC ACTIVITIES OF BACTERIA. Bacteriol Rev. 1943 Sep;7(3):139–173. doi: 10.1128/br.7.3.139-173.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaudy A. F. Studies on Induction and Repression in Activated Sludge Systems. Appl Microbiol. 1962 May;10(3):264–271. doi: 10.1128/am.10.3.264-271.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- IMSANDE J., EPHRUSSI B. REEVALUATION OF ASSAYS USED TO SHOW RNA INDUCTION OF GLUCOSE-6-PHOSPHATASE IN ASCITES CELLS. Science. 1964 May 15;144(3620):854–856. doi: 10.1126/science.144.3620.854. [DOI] [PubMed] [Google Scholar]
- KENNELL D., MAGASANIK B. THE CONTROL OF THE RATE OF ENZYME SYNTHESIS IN AEROBACTER AEROGENES. Biochim Biophys Acta. 1964 Mar 9;81:418–434. doi: 10.1016/0926-6569(64)90127-0. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B. Catabolite repression. Cold Spring Harb Symp Quant Biol. 1961;26:249–256. doi: 10.1101/sqb.1961.026.01.031. [DOI] [PubMed] [Google Scholar]
- MAGASANIK B., NEIDHARDT F. C. The effect of glucose on the induced biosynthesis of bacterial enzymes in the presence and absence of inducing agents. Biochim Biophys Acta. 1956 Aug;21(2):324–334. doi: 10.1016/0006-3002(56)90016-6. [DOI] [PubMed] [Google Scholar]
- MANDELSTAM J. Factors affecting the passage of basic amino acids into coliform bacteria. Biochim Biophys Acta. 1956 Nov;22(2):313–323. doi: 10.1016/0006-3002(56)90157-3. [DOI] [PubMed] [Google Scholar]
- McKINNEY R. E., WEICHLEIN R. G. Isolation of floc-producing bacteria from activated sludge. Appl Microbiol. 1953 Sep;1(5):259–261. doi: 10.1128/am.1.5.259-261.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NEIDHARDT F. C., MAGASANIK B. Reversal of the glucose inhibition of histidase biosynthesis in Aerobacter aerogenes. J Bacteriol. 1957 Feb;73(2):253–259. doi: 10.1128/jb.73.2.253-259.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- PARDEE A. B., PRESTIDGE L. S. Induced formation of serine and threonine deaminases by Escherichia coli. J Bacteriol. 1955 Dec;70(6):667–674. doi: 10.1128/jb.70.6.667-674.1955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephenson M., Gale E. F. The adaptability of glucozymase and galactozymase in Bacterium coli. Biochem J. 1937 Aug;31(8):1311–1315. doi: 10.1042/bj0311311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WASHKO M. E., RICE E. W. Determination of glucose by an improved enzymatic procedure. Clin Chem. 1961 Oct;7:542–545. [PubMed] [Google Scholar]


