Abstract
Approaches to locally deliver drugs to specific regions of the body are being developed for many clinical applications, including treating hemorrhage. Increasing the concentration of therapeutic coagulants in areas where clots are forming and growing can be achieved by applying them directly to the injury, such as with catheters or external delivery devices, or by systemically administering therapeutics that target molecular signals of vascular damage. Treating severe hemorrhage by external measures is challenging because blood flow pushes hemostatic agents outward, reducing their efficacy. This review explains that self-propelling particles may be used for delivering therapeutics, such as coagulation factors, small molecules, or other chemical or biological agents, deep into wounds during hemorrhage. A recent example of self-propelling particles is highlighted, where propulsion enhanced the efficacy of a formulation of thrombin and tranexamic acid in treating bleeding in two murine models of hemorrhage and a porcine model of fatal, non-compressible hemorrhage. Many agents exist which modulate clotting, and novel approaches that facilitate their safe delivery to sites of vascular injury could reduce the enormous number of deaths from hemorrhage that occur globally.
Main text
Convective and diffusive transport regulate blood coagulation through delivery of coagulation factors to growing hemostatic plugs and through removal of activated factors to prevent thrombosis. Methods for specifically transporting and maintaining high concentrations of coagulation factors at sites of vascular damage are useful treatment strategies for stopping bleeding and preventing hypovolemic shock and death. Many agents have been developed that can initiate and stabilize clot growth during bleeding, including biologics such as thrombin, small molecules such as tranexamic acid (TXA), and inorganic materials such as kaolin [1–3]. Topical application of such hemostatic agents can expedite their delivery to damaged blood vessels [4]. However, topical hemostatic agents have limited efficacy in multiple clinical scenarios, such as when bleeding originates deep within a wound, when damaged vessels cannot be located, or when wounds cannot be compressed, which together are a leading cause of death of young people world-wide [5]. In these situations, blood flow rapidly transports external agents away, preventing their delivery and delaying initiation of clotting at compromised vessels. Instead, superficial clots form at wound surfaces, which are susceptible to rupture during patient transport and resuscitation, causing rebleeding [6–8], which is correlated with poor clinical outcomes [9, 10]. Some intravenously administered coagulants, such as TXA and recombinant factor VIIa, are often effective, whereas many other coagulants carry major risks of thrombosis when their action cannot be adequately localized after injection [11–14].
Enhanced local drug delivery for managing hemorrhage
Enhancing the targeted delivery of hemostatic agents specifically to sites of bleeding could greatly increase their safety and efficacy, and many technologies are being developed to achieve this. Among these are agents that mimic endogenous components of coagulation by responding to biochemical signals and localizing at sites of bleeding. Some of these agents, which can be soluble or particles, bind extracellular matrix components such as collagen, and to plasma components such as von Willebrand factor and fibrin, to mediate platelet clustering and clot initiation and adhesion [13, 15–18]. Synthetic polymers have also been described that are activated specifically by coagulation enzymes and mediate coagulation at sites of bleeding and thrombosis [19]. Similarly, particle-based agents have been designed which respond to mechanical stimuli, such as changes in shear rate, to release bioactive molecules which mediate coagulation [18, 20]. For example, particles have been developed that release fibrinolytic enzymes at sites of thrombosis in response to high shear [20]; this approach may be useful for targeted release of coagulants at sites of hemorrhage. Agents that sense low shear, such as where hemorrhaged blood pools, have not been reported to our knowledge, but could also be useful [21]. Many endoscope- and catheter-based delivery vehicles are also being developed to enhance delivery of hemostatic agents, such as catheter-based embolic agents and hemostatic sprays [22–24]. A wide array of creative and sometimes exotic drug delivery technologies have been produced for non-hemostatic indications and these may potentially be useful for treating hemorrhage in the future, such as technologies that can be systemically administered and then controllably triggered to release therapeutic cargos [25–28]. This review focuses on one delivery vehicle in particular, self-propelling particles that can transport cargo upstream against blood flow, which holds promise as a powerful addition to hemostatic treatments because it delivers coagulants deep into wounds when applied to leaking blood.
Self-propelling particles can transport cargo through liquids and blood
Many self-propelling particle systems have been developed, with proposed applications in targeted drug delivery (Figure 1a). Though few of these systems have yet advanced to in vivo testing, their diversity and ingenuity make self-propelling particles promising candidates for biomedical applications [29–31]. The first reports of self-propelling microparticles used catalytic degradation of aqueous hydrogen peroxide to generate gas and thrust [32–34]. Since then, particles have been developed which utilize multiple forms of propulsion, such as magnetic swimming, ultrasound-driver motion, and bioelectrochemical reactions [30, 35–37]. Self-propelling particles have been loaded with a range of cargoes, including sugars, drugs, such as doxorubicin, and whole cells [29, 38–41]. Self-propelled nanomotors have been developed which can localize at sites of damage in electrical circuits, and this yields interesting future prospects for delivering therapeutics to wound sites [42]. Some reports previously suggested that propulsion of micromotors through biological fluids, such as blood, may be very difficult or impossible to achieve, but this was recently accomplished with simple self-propelling particles [43–46].
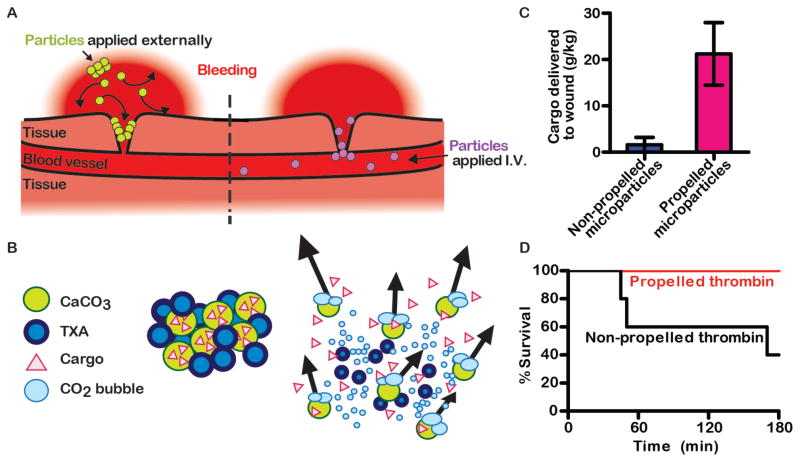
Figure 1. Enhanced local drug delivery to wounds increases survival from hemorrhage.
(A) Schematic shows two strategies for locally delivering hemostatic drugs to wounds. Self-propelling particles can push externally administered drugs deeper into wounds (left). Targeting ligands can bind intravenously-administered (I.V.) drugs to sites of vascular damage (right). (B) Schematic of how a formulation of self-propelling particles works in blood. CaCO3 particles loaded with a cargo, such as thrombin, are mixed with an organic acid, such as TXA. When this powder contacts an aqueous solution, it reacts to produce CO2 gas. CO2 bubbles propel the particles through direct force and by generating convection in the medium. (C) In a murine model of liver laceration, self-propelling microparticles delivered more cargo, in this case fluorescent nanoparticles, into wounds than non-propelling microparticles. (D) In a porcine model of incompressible hemorrhage from the femoral artery, more animals survived when treated with thrombin and TXA loaded onto self-propelling particles than a similar formulation that did not propel. Images in panels B-D were modified from figures in [46]. These images are licensed under CC BY-NC (http://creativecommons.org/licenses/by-nc/4.0/).
Drug delivery in vivo with self-propelling particles
Recently, the first reports have emerged showing that self-propelling particles can function in vivo. Zinc-based micromotors can react in gastric acid to produce gas and enhance penetration of the particles into the stomach linings of mice [44]. We have developed self-propelling particles that function in blood in vivo (Figure 1B,C). The formulation utilizes carbonate salts, which release CO2 when mixed with a solid organic acid and upon contact with aqueous solutions, such as a blood. For the organic acid, we used TXA, because it is used clinically to stabilize clots during trauma by inhibiting plasmin. During the reaction, the particles dissolve, the organic acid is buffered and CO2 is produced, which is highly soluble in blood. The rapid production of gas bubbles made particles transport through blood in all directions. Propulsion occurred from a combination of particles rising buoyantly, propelling laterally, and the large convection generated by the release of gas. Propulsion of particles greatly increased their delivery and accumulation in wounds and local microvasculature in mice with transected tails and in mice with lacerated livers. Together, our findings and those of Gao et al. demonstrate that propulsion can be achieved in vivo using simple, non-catalytic, gas-generating particles and without the need for external stimuli, such as ultrasound, or exogenous fuel sources, such as hydrogen peroxide.
Halting hemorrhage with self-propelling particles
Our self-propelling, carbonate-based particles were easily adapted to function as an effective hemostatic agent. In two mouse models, it was highly effective in halting hemorrhage compared to non-propelling particles and to a solution of recombinant thrombin that is used clinically. In a pig model of fatal junctional hemorrhage from the femoral artery, propulsion of thrombin-loaded particles significantly increased survival of pigs without the need to apply pressure (Figure 1D). Particles were well-tolerated in all animal models, and no evidence of thrombosis or local or systemic toxicity has been observed. These findings suggest that self-propelling particles are a promising vehicle to overcome transport limitations during severe bleeding and to deliver hemostatic agents to sites of vascular damage to halt hemorrhage. These particles may also be useful for delivering other hemostatic agents, such as TXA alone, which could be suitable for point-of-care scenarios and low-resource settings.
Potential applications in treating bleeding with advanced delivery mechanisms
There are many bleeding scenarios that could benefit from enhanced delivery of hemostatic agents. In some situations, the source of bleeding cannot be easily identified and visualized, such as during endoscopic surgery, or when major bleeding originates within a cavity [47]. In these situations, interventions such as catheter-directed embolization or hemostatic dressings cannot be easily used. In severe trauma, bleeding is often managed using hemostatic agents in combination with compression, but this is not practical in cases where bleeding originates from anatomical junctions, inside the abdomen, or during combat care under fire [5, 48, 49]. On the battlefield, incompressible hemorrhage comprises the majority of potentially survivable deaths [50]. In situations of severe and massive hemorrhage, catheter-based interventions or intravenous agents cannot always be administered in a timely fashion and patients are at risk of progression to hypovolemic shock and death by exsanguination [4]. A fast-acting topical agent that actively delivers therapeutics deep within difficult-to-reach, incompressible or massively bleeding wounds has potential to reduce fatal hemorrhage.
In summary, transporting therapeutics to sites of vascular damage is likely a major hurdle when managing bleeding. Many, diverse technologies are currently being developed which aim to enhance delivery of hemostatic agents to sites of bleeding and overcome the transport barriers that can render agents with no active delivery or transport mechanisms ineffective. These technologies are at various stages of discovery and validation in vivo, and may address a wide range of clinical scenarios. Self-propulsion, and its ability to move therapeutics upstream against blood flow, is one of these promising technologies.
Acknowledgments
This work was funded by Canadian Institutes of Health Research (PPP-136718, MOP-119426, and MSH-130166). We thank M. Lee for his helpful suggestions.
Footnotes
Conflicts of interest
J.R.B. and C.J.K. have filed a patent application describing self-propelling particles for hemorrhage and are involved in commercialization activities associated with this.
References
- 1.Groenewold MD, Gribnau AJ, Ubbink DT. Topical haemostatic agents for skin wounds: a systematic review. BMC Surg. 2011;11:11–15. doi: 10.1186/1471-2482-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baker SE, Sawvel AM, Zheng N, Stucky GD. Controlling bioprocesses with inorganic surfaces: Layered clay hemostatic agents. Chem Mater. 2007;19:4390–4392. [Google Scholar]
- 3.Tengborn L, Blomback M, Berntorp E. Tranexamic acid - an old drug still going strong and making a revival. Thromb Res. 2015;135:231–242. doi: 10.1016/j.thromres.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 4.Samudrala S. Topical hemostatic agents in surgery: a surgeon’s perspective. AORN J. 2011;88:S2–11. doi: 10.1016/S0001-2092(08)00586-3. [DOI] [PubMed] [Google Scholar]
- 5.Kauvar DS, Lefering R, Wade CE. Impact of hemorrhage on trauma outcome: An overview of epidemiology, clinical presentations, and therapeutic considerations. J Trauma. 2006;60:S3–S9. doi: 10.1097/01.ta.0000199961.02677.19. [DOI] [PubMed] [Google Scholar]
- 6.Sondeen JL, Coppes VG, Holcomb JB. Blood pressure at which rehleeding occurs after resuscitation in swine with aortic injury. J Trauma. 2003;54:S110–S117. doi: 10.1097/01.TA.0000047220.81795.3D. [DOI] [PubMed] [Google Scholar]
- 7.Brass LF, Zhu L, Stalker TJ. Minding the gaps to promote thrombus growth and stability. J Clin Invest. 2005;115:3385–3392. doi: 10.1172/JCI26869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lord ST. Molecular mechanisms affecting fibrin structure and stability. Arterioscler Thromb Vasc Biol. 2011;31:494–499. doi: 10.1161/ATVBAHA.110.213389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldoni F, Di Saverio S, Antonacci N, Coniglio C, Giugni A, Montanari N, Biscardi A, Villani S, Gordini G, Tugnoli G. Refinement in the technique of perihepatic packing: a safe and effective surgical hemostasis and multidisciplinary approach can improve the outcome in severe liver trauma. Am J Surg. 2011;201:E5–E14. doi: 10.1016/j.amjsurg.2010.05.015. [DOI] [PubMed] [Google Scholar]
- 10.Morgenstern LB, Demchuk AM, Kim DH, Frankowski RF, Grotta JC. Rebleeding leads to poor outcome in ultra-early craniotomy for intracerebral hemorrhage. Neurology. 2001;56:1294–1299. doi: 10.1212/wnl.56.10.1294. [DOI] [PubMed] [Google Scholar]
- 11.Roberts I, Shakur H, Coats T, Hunt B, Balogun E, Barnetson L, Cook L, Kawahara T, Perel P, Prieto-Merino D, Ramos M, Cairns J, Guerriero C. The CRASH-2 trial: a randomised controlled trial and economic evaluation of the effects of tranexamic acid on death, vascular occlusive events and transfusion requirement in bleeding trauma patients. Health Technol Assess. 2013;17:1–79. doi: 10.3310/hta17100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benharash P, Bongard F, Putnam B. Use of recombinant factor VIIa for adjunctive hemorrhage control in trauma and surgical patients. Am Surg. 2005;71:776–780. doi: 10.1177/000313480507100917. [DOI] [PubMed] [Google Scholar]
- 13.Chan LW, White NJ, Pun SH. Synthetic strategies for engineering intravenous hemostats. Bioconjugate Chem. 2015;26:1224–1236. doi: 10.1021/acs.bioconjchem.5b00070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Roberts I, Shakur H, Afolabi A, Brohi K, Coats T, Dewan Y, Gando S, Guyatt G, Hunt J, Morales C, Perel P, Prieto-Merino D, Woolley T Crash Collaborators. The importance of early treatment with tranexamic acid in bleeding trauma patients: an exploratory analysis of the CRASH-2 randomised controlled trial. Lancet. 2011;377:1096–1101. doi: 10.1016/S0140-6736(11)60278-X. [DOI] [PubMed] [Google Scholar]
- 15.Bertram JP, Williams CA, Robinson R, Segal SS, Flynn NT, Lavik EB. Intravenous hemostat: nanotechnology to halt bleeding. Sci Transl Med. 2009;1 doi: 10.1126/scitranslmed.3000397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lashof-Sullivan MM, Shoffstall E, Atkins KT, Keane N, Bir C, VandeVord P, VandeVord P, Lavik EB. Intravenously administered nanoparticles increase survival following blast trauma. Proc Natl Acad Sci USA. 2014;111:10293–10298. doi: 10.1073/pnas.1406979111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Modery-Pawlowski CL, Tian LL, Pan V, McCrae KR, Mitragotri S, Sen Gupta A. Approaches to synthetic platelet analogs. Biomaterials. 2013;34:526–541. doi: 10.1016/j.biomaterials.2012.09.074. [DOI] [PubMed] [Google Scholar]
- 18.Modery-Pawlowski CL, Tian LL, Ravikumar M, Wong TL, Sen Gupta A. In vitro and in vivo hemostatic capabilities of a functionally integrated platelet-mimetic liposomal nanoconstruct. Biomaterials. 2013;34:3031–3041. doi: 10.1016/j.biomaterials.2012.12.045. [DOI] [PubMed] [Google Scholar]
- 19.Maitz MF, Freudenberg U, Tsurkan MV, Fischer M, Beyrich T, Werner C. Bio-responsive polymer hydrogels homeostatically regulate blood coagulation. Nat Commun. 2013;4 doi: 10.1038/ncomms3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Korin N, Kanapathipillai M, Matthews BD, Crescente M, Brill A, Mammoto T, Ghosh K, Jurek S, Bencherif SA, Bhatt D, Coskun AU, Feldman CL, Wagner DD, Ingber DE. Shear-activated nanotherapeutics for drug targeting to obstructed blood vessels. Science. 2012;337:738–742. doi: 10.1126/science.1217815. [DOI] [PubMed] [Google Scholar]
- 21.Chien S, Usami S, Taylor HM, Lundberg JL, Gregerse MI. Effects of hematocrit and plasma proteins on human blood rheology at low shear rates. J Appl Physiol. 1966;21:81. doi: 10.1152/jappl.1966.21.1.81. [DOI] [PubMed] [Google Scholar]
- 22.Vaidya S, Tozer KR, Chen J. An overview of embolic agents. Semin Interven Radiol. 2008:204–215. doi: 10.1055/s-0028-1085930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lanzino G, Kanaan Y, Perrini P, Dayoub H, Fraser K. Emerging concepts in the treatment of intracranial aneurysms: Stents, coated coils, and liquid embolic agents. Neurosurgery. 2005;57:449–458. doi: 10.1227/01.neu.0000170538.74899.7f. [DOI] [PubMed] [Google Scholar]
- 24.Dowling MB, Smith W, Balogh P, Duggan MJ, MacIntire IC, Harris E, Mesar T, Raghavan SR, King DR. Hydrophobically-modified chitosan foam: description and hemostatic efficacy. J Surg Res. 2015;193:316–323. doi: 10.1016/j.jss.2014.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12:991–1003. doi: 10.1038/nmat3776. [DOI] [PubMed] [Google Scholar]
- 26.Bajpai AK, Shukla SK, Bhanu S, Kankane S. Responsive polymers in controlled drug delivery. Prog Polym Sci. 2008;33:1088–1118. [Google Scholar]
- 27.Ganta S, Devalapally H, Shahiwala A, Amiji M. A review of stimuli-responsive nanocarriers for drug and gene delivery. J Control Release. 2008;126:187–204. doi: 10.1016/j.jconrel.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 28.Mitragotri S, Burke PA, Langer R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov. 2014;13:655–672. doi: 10.1038/nrd4363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patra D, Sengupta S, Duan W, Zhang H, Pavlick R, Sen A. Intelligent, self-powered, drug delivery systems. Nanoscale. 2013;5:1273–1283. doi: 10.1039/c2nr32600k. [DOI] [PubMed] [Google Scholar]
- 30.Paxton WF, Sundararajan S, Mallouk TE, Sen A. Chemical locomotion. Angew Chem Int Ed. 2006;45:5420–5429. doi: 10.1002/anie.200600060. [DOI] [PubMed] [Google Scholar]
- 31.Abdelmohsen LKEA, Peng F, Tu Y, Wilson DA. Micro- and nano-motors for biomedical applications. J Mat Chem B. 2014;2:2395–2408. doi: 10.1039/c3tb21451f. [DOI] [PubMed] [Google Scholar]
- 32.Ismagilov RF, Schwartz A, Bowden N, Whitesides GM. Autonomous movement and self-assembly. Angew Chem Int Ed. 2001;41:652–654. [Google Scholar]
- 33.Wilson DA, Nolte RJM, van Hest JCM. Autonomous movement of platinum-loaded stomatocytes. Nat Chem. 2012;4:268–274. doi: 10.1038/nchem.1281. [DOI] [PubMed] [Google Scholar]
- 34.Lee TC, Alarcon-Correa M, Miksch C, Hahn K, Gibbs JG, Fischer P. Self-propelling nanomotors in the presence of strong Brownian forces. Nano Letters. 2014;14:2407–2412. doi: 10.1021/nl500068n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Garcia-Gradilla V, Orozco J, Sattayasamitsathit S, Soto F, Kuralay F, Pourazary A, Katzenberg A, Gao W, Shen Y, Wang J. Functionalized ultrasound-propelled magnetically guided nanomotors: toward practical biomedical applications. ACS Nano. 2013;7:9232–9240. doi: 10.1021/nn403851v. [DOI] [PubMed] [Google Scholar]
- 36.Dreyfus R, Baudry J, Roper ML, Fermigier M, Stone HA, Bibette J. Microscopic artificial swimmers. Nature. 2005;437:862–865. doi: 10.1038/nature04090. [DOI] [PubMed] [Google Scholar]
- 37.Fischer P, Ghosh A. Magnetically actuated propulsion at low Reynolds numbers: towards nanoscale control. Nanoscale. 2011;3:557–563. doi: 10.1039/c0nr00566e. [DOI] [PubMed] [Google Scholar]
- 38.Kuralay F, Sattayasamitsathit S, Gao W, Uygun A, Katzenberg A, Wang J. Self-propelled carbohydrate-sensitive microtransporters with built-in boronic acid recognition for isolating sugars and cells. J Am Chem Soc. 2012;134:15217–15220. doi: 10.1021/ja306080t. [DOI] [PubMed] [Google Scholar]
- 39.Banerjee SS, Jalota-Badhwar A, Zope KR, Todkar KJ, Mascarenhas RR, Chate GP, Khutale GV, Bharde A, calderon M, Khandare JJ. Self-propelled carbon nanotube based microrockets for rapid capture and isolation of circulating tumor cells. Nanoscale. 2015;7:8684–8688. doi: 10.1039/c5nr01797a. [DOI] [PubMed] [Google Scholar]
- 40.Wu Z, Wu Y, He W, Lin X, Sun J, He Q. Self-propelled polymer-based multilayer nanorockets for transportation and drug release. Angew Chem Int Ed. 2013;52:7000–7003. doi: 10.1002/anie.201301643. [DOI] [PubMed] [Google Scholar]
- 41.Wu Y, Lin X, Wu Z, Moehwald H, He Q. Self-propelled polymer multilayer Janus capsules for effective drug delivery and light-triggered release. ACS Appl Mat Interfaces. 2014;6:10476–10481. doi: 10.1021/am502458h. [DOI] [PubMed] [Google Scholar]
- 42.Li J, Shklyaev OE, Li T, Liu W, Shum H, Rozen I, Balasz AC, Wang J. Self-propelled nanomotors autonomously seek and repair cracks. Nano Letters. 2015;15:7077–7085. doi: 10.1021/acs.nanolett.5b03140. [DOI] [PubMed] [Google Scholar]
- 43.Zhao G, Viehrig M, Pumera M. Challenges of the movement of catalytic micromotors in blood. Lab Chip. 2013;13:1930–1936. doi: 10.1039/c3lc41423j. [DOI] [PubMed] [Google Scholar]
- 44.Gao W, Dong R, Thamphiwatana S, Li J, Gao W, Zhang L, Wang J. Artificial micromotors in the mouse’s stomach: a step toward in vivo use of synthetic motors. ACS Nano. 2015;9:117–123. doi: 10.1021/nn507097k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mou F, Chen C, Ma H, Yin Y, Wu Q, Guan J. Self-propelled micromotors driven by the magnesium-water reaction and their hemolytic properties. Angew Chem Int Ed. 2013;52:7208–7212. doi: 10.1002/anie.201300913. [DOI] [PubMed] [Google Scholar]
- 46.Baylis JR, Yeon JH, Thomson MH, Kazerooni A, Wang X, St John AE, Lim EB, Chien D, Lee A, Zhang JQ, Piret JM, Machan LS, Burke TF, Burke NJ, White NJ, Kastrup CJ. Self-propelled particles that transport cargo through flowing blood and halt hemorrhage. Sci Adv. 2015;1:e1500379. doi: 10.1126/sciadv.1500379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sieskiewicz A, Olszewska E, Rogowski M, Grycz E. Preoperative corticosteroid oral therapy and intraoperative bleeding during functional endoscopic sinus surgery in patients with severe nasal polyposis: a preliminary investigation. Ann Otol Rhinol Laryngol. 2006;115:490–494. doi: 10.1177/000348940611500702. [DOI] [PubMed] [Google Scholar]
- 48.Eastridge BJ, Mabry RL, Seguin P, Cantrell J, Tops T, Uribe P, Mallett O, Zubko T, Oetjen-Gerdes L, Rasmussen TE, Butler FK, Kotwal RS, Holcomb JB, Wade C, Champion H, Lawnick M, Moores L, Blackbourne LH. Death on the battlefield (2001–2011): implications for the future of combat casualty care. J Trauma. 2012;73:S431–S437. doi: 10.1097/TA.0b013e3182755dcc. [DOI] [PubMed] [Google Scholar]
- 49.Pannell D, Brisebois R, Talbot M, Trottier V, Clement J, Garraway N, McAlister V, Tien HC. Causes of Death in Canadian Forces Members Deployed to Afghanistan and Implications on Tactical Combat Casualty Care Provision. J Trauma. 2011;71:S401–S407. doi: 10.1097/TA.0b013e318232e53f. [DOI] [PubMed] [Google Scholar]
- 50.Holcomb JB, McMullin NR, Pearse L, Caruso J, Wade CE, Oetyen-Gerdes L, Champion HR, Lawnick M, Farr W, Sam, Rodriguez, Butler FK. Causes of death in US Special Operations Forces in the global war on terrorism - 2001–2004. Ann Surg. 2007;245:986–991. doi: 10.1097/01.sla.0000259433.03754.98. [DOI] [PMC free article] [PubMed] [Google Scholar]