SUMMARY
The activation of quiescent stem cells into the cell cycle is a key step in initiating the process of tissue repair. We recently reported that quiescent stem cells can transition into GAlert, a cellular state in which they have an increased functional ability to activate and participate in tissue repair. However, the precise molecular signals which induce GAlert in stem cells have remained elusive. Here, we show that the injury-induced regulation of Hepatocyte Growth Factor (HGF) proteolytic processing via the systemic protease, Hepatocyte Growth Factor Activator (HGFA), stimulates GAlert in skeletal muscle stem cells (MuSCs) and fibro-adipogenic progenitors (FAPs). We demonstrate that administering active HGFA to animals is sufficient to induce GAlert in stem cells throughout the body and to significantly accelerate the processes of stem cell activation and tissue repair. Our data suggest that factors that induce GAlert will have broad therapeutic applications for regenerative medicine and wound healing.
Keywords: Muscle stem cells, satellite cells, muscle regeneration, stem cell activation, quiescence, mTORC1, HGF, HGFA, tissue repair, systemic signals
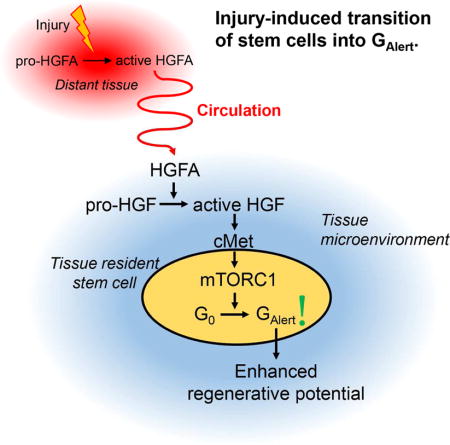
Graphical Abstract
Rodgers et al show that HGFA is a systemic protease that is activated by tissue injury and relays a signal to stem cells in non-injured tissues that induces their transition into a primed, “Alert”, state in which they possess an enhanced potential to activate and repair tissue damage.
INTRODUCTION
Tissue damage induces the activation of quiescent stem cells, initiating a cascade in which stem cells enter the cell cycle, divide, and proliferate to generate the cells required to repair or regenerate damaged tissue (Cheung and Rando, 2013; Li and Clevers, 2010; Weissman, 2000). Stem cell activation is a limiting step in the process of tissue repair (McClain et al., 1996; Sugiura et al., 2016). In many stem cell pools, the first cell division following activation is slow and can take many days to complete whereas subsequent cell divisions are much more rapid (Coller, 2007; Laurenti et al., 2015; Siegel et al., 2011). Defects in stem cell activation, such as a lengthening in the time of first cell division or a failure in stem cells to activate can result in significant impairments in the healing process (Jones and Rando, 2011). Little is known about the biologic regulation of stem cell quiescence and activation. Approaches to accelerate the rate limiting step of stem cell activation could have broad therapeutic applications in regenerative medicine.
We previously reported an acceleration of the activation properties of quiescent stem cells in response to a prior injury, distant from the tissue in which the stem cells were residing (Rodgers et al., 2014). We described this regulation as a transitioning of stem cells from the G0 to the GAlert state of quiescence, where GAlert stem cells are poised to activate quickly in response to injury and to repair tissue damage more effectively. Because of the enhanced functional properties of GAlert stem cells, there may be clinical applications for factors that induce the GAlert state. However, the endogenous signals that stimulate the G0-to-GAlert transition of stem cells in response to distant injuries have not been previously described. Here we show that a single systemic factor, HGFA, is sufficient to induce the transition of multiple pools of stem cells into GAlert and that administration of HGFA to animals, prior to an injury, improves the subsequent kinetics tissue repair.
RESULTS
Systemic factors mediate the injury-induced transition of stem cells into GAlert
The global response to injury that we observed, that injury to one part of the body resulted in the GAlert transition of many different types of stem cells in uninjured areas throughout the body (Rodgers et al., 2014), suggested that a systemic factor is involved. To test this hypothesis, we injected animals with serum isolated from mice that had been subjected to a muscle injury 2.5 days prior to serum isolation (we term this “injured serum”), and we compared this to serum obtained from mice which were not injured (“non-injured serum”) (Figure 1A). We sacrificed the animals 2.5 days after serum injection, FACS purified MuSCs, and assayed the MuSCs for characteristics of stem cells in GAlert. Using time lapse microscopy to analyze the kinetics of cell division, we found that MuSCs isolated from animals injected with injured serum required less time to complete the first cell division after isolation than did the MuSCs isolated from animals injected with non-injured serum (Figure 1B). Similarly, using incorporation of EdU nucleotide to identify cells that had entered S-phase of the cell cycle, we found that a higher percentage of MuSCs isolated from animals injected with injured serum had entered the cell cycle 24 hours after isolation (Figure 1C). MuSCs from animals injected with injured serum were also slightly larger than MuSCs from animals injected with non-injured serum, as measured by an increase in forward scatter (Figure 1D and S1A). We also observed a similar set of functional and phenotypic changes in FAPs isolated from animals injected with injured serum (Figures 1E, F, and S1B). Activation of mTORC1 signaling is the key molecular change in GAlert stem cells and, using genetic models, we demonstrated that it is necessary and sufficient for the transition of MuSCs into GAlert (Rodgers et al., 2014). Using Pax7 antibodies to identify sub-laminar quiescent MuSCs in TA muscle sections (Seale et al., 2000), we found that a higher frequency of MuSCs were positive for phosphorylated S6 (pS6), a marker of mTORC1 signaling, in muscles from animals injected with injured serum (Figure 1G). Collectively, these data show that injecting animals with injured serum is sufficient to induce a set of molecular, phenotypic, and functional changes in stem cells that are similar to the injury-induced GAlert response and suggest that the factor(s) that induce the transition of stem cells into GAlert are systemic in nature.
Figure 1. HGFA is a factor in injured serum that stimulates the GAlert transition of MuSCs and FAPs.
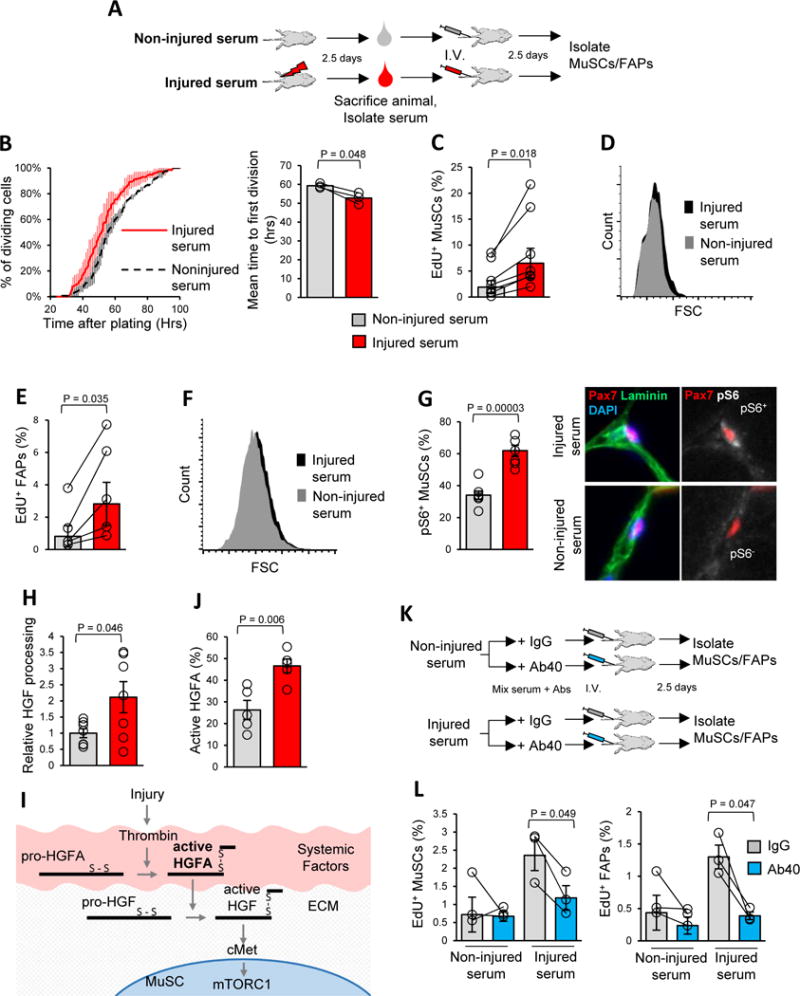
A) Schematic representation of the experimental design testing the effects of injured serum on MuSC and FAP function.
B) Injecting mice with injured serum reduces the time to first cell division of MuSCs. Data are displayed as a cumulative histogram (left) and bar graph (right) of the time required to complete the first cell division after isolation and plating, measured by time lapse microscopy, and presented as mean ± s.e.m. (n = 3) C) MuSCs isolated from mice injected with injured serum have shorter cell cycle entry time. Data are presented as the percentage of MuSCs that incorporated EdU in the first 24 hours after isolation (geometric mean ± s.e.m., n = 7).
D) Injured serum increases MuSC size. Data are presented as a representative FACS histogram of forward scatter (FSC) signal.
E) Injured serum reduces cell cycle entry time of FAPs. Data from EdU incorporation assays are displayed as geometric mean ± s.e.m. (n = 5) of the percentage of FAPs that incorporated EdU in the first 24 hours after isolation.
F) Injured serum increases FAP size. Data are displayed as a representative FACS plot of FAP FSC signal.
G) Injured serum activates mTORC1 signaling in MuSCs. Data are presented as mean ± s.e.m. of the percentage of Pax7+ cells in TA muscles that were pS6+ (n = 7). On the right are examples of pS6+ and pS6− MuSCs; the scale bar is 10 μm.
H) Injured serum has greater HGF-processing activity than non-injured serum. HGF processing data are presented as the mean ± s.e.m. of the proportion active HGF versus total HGF, normalized to that from non-injured serum (n = 7). Data are quantifications of Western blot analysis of the HGF-processing reactions.
I) Schematic depiction of the pathway leading to the processing and activation of HGF. In normal conditions, HGFA circulates in the blood in an enzymatically inactive form (pro-HGFA). Upon injury, activated thrombin catalyzes the cleavage of pro-HGFA into the disulfide linked two-chain form (active HGFA). Active HGFA circulates in the blood and catalyzes the proteolytic processing of pro-HGF into active HGF in tissues throughout the body. Tissue-localized active HGF then activates signaling through cMet.
J) Injured serum contains a higher proportion of active HGFA. Data are presented as mean ± s.e.m. of the proportion active HGFA versus total HGFA (n = 5). The data were obtained by quantification of Western blots.
K) Schematic depiction of the experiments using HGFA blocking antibody (Ab40).
L) Ab40 suppresses the injured serum-mediated acceleration of cell cycle entry kinetics of MuSCs and FAPs. Data from EdU incorporation assays are presented as geometric mean ± s.e.m. (n = 3).
(See also Figures S1 and S2)
Our previous work identified that cMet was required for the injury-induced transition of MuSCs into GAlert (Rodgers et al., 2014). To test if injured serum signaled through the same pathway, we utilized the Pax7CreER driver and a cMetflox/flox mouse strain to specifically ablate cMet expression, conditional upon Tamoxifen administration, in MuSCs. Using a similar experimental design as above (Figure 1A), we found that MuSCs from these cMet conditional KO (cKO) mice displayed no phenotypic or functional response to injured serum (Figures S1C–E). As a control, we analyzed FAPs that were isolated in parallel with MuSCs from cMet cKO animals and found that they displayed a similar response to injured serum as did FAPs from control mice (Figure 1E and S1F). As FAPs do not express Pax7 (Joe et al., 2010), they remain wild-type (in terms of cMet expression) in cMet cKO animals. These data suggest that signaling through cMet is required for the response of MuSCs to injured serum.
HGFA is an injury-regulated systemic factor
cMet is a receptor tyrosine kinase that is activated by the binding of HGF (Trusolino et al., 2010). A plausible mechanism for injured serum-mediated induction of GAlert is that injured serum contains higher levels of HGF. To test this, we measured serum HGF levels by ELISA. Surprisingly, we found no differences in serum HGF levels when comparing serum samples from injured and non-injured mice (Figure S1G). Also surprising was that we were unable to induce the transition of MuSCs into GAlert in vivo by administering exogenous, purified, active HGF to animals (not shown). However and importantly, both MuSCs and FAPs responded to direct HGF stimulation, ex vivo, by activating mTORC1 signaling (Figure S1H). Even though both MuSCs and FAPs can respond to HGF, we obtained no evidence that circulating HGF accounted for the in vivo induction of GAlert by injured serum. These results suggest that a different systemic factor is responsible for activating signaling through the HGF-cMet pathway.
HGF is resident in the ECM of most tissues in a latent, biologically inactive form (Fajardo-Puerta et al., 2016; Stoker et al., 1987). In order to possess biologic activity (i.e., bind to and activate cMet), HGF must undergo proteolytic processing from a unprocessed single chain form (pro-HGF) into a processed two-chain form (active HGF) (Gak et al., 1992). Western blot analysis revealed a clear increase in levels of processed, active HGF in muscles contralateral to an injury (Figure S1I). Thus, the induction of active HGF in muscles contralateral to injury suggests that the pathways that regulate HGF processing are induced in response to injury.
To test if systemic factors could directly mediate the processing of HGF, we measured the ability of serum to catalyze the proteolytic cleavage of recombinant pro-HGF into active HGF in vitro. In HGF-processing assays, we found that injured serum possessed twice the HGF-processing activity as non-injured serum (Figure 1H and S2A). These results suggest a mechanism in which systemic factors regulate the processing of tissue-resident HGF and that local (now active) HGF signals through cMet-mTORC1 to induce the G0-to-GAlert transition of MuSCs. Moreover, these results suggest that an HGF-processing enzyme is the ‘alerting’ signal in injured serum.
HGFA is the primary HGF protease, is very abundant in the blood, and, like HGF, is also regulated by proteolytic cleavage (Miyazawa, 2010). HGFA is processed from an enzymatically inactive single-chain form (pro-HGFA) into a two-chain form (active HGFA) which possesses HGF-processing enzymatic activity (Kataoka and Kawaguchi, 2010) (Figure 1I). The site on which HGFA is processed to induce enzymatic activity is a consensus thrombin cleavage site and it is well established that both thrombin and HGFA activity are induced in response to injury (Coughlin, 2000; Miyazawa, 2010; O’Reilly et al., 2008; Shimomura et al., 1993). Consistent with this, we found that injured serum contained a higher proportion of HGFA in the active form (Figure 1J and S2B). These results suggest that active HGFA is the factor in injured serum that induces the transition of stem cells into GAlert.
To test if HGFA enzymatic activity is the crucial component of injured serum that induced GAlert, we utilized a monoclonal antibody (Ab40) that was developed to specifically inhibit HGFA enzymatic activity (Figure S2C) (Ganesan et al., 2009). We incubated injured and non-injured serum with Ab40 or control IgG prior to injection of the serum samples into mice (Figure 1K). Two and a half days after injection, we analyzed HGF processing in the TA muscles of these animals and found that Ab40 blocked the injured serum-mediated induction of active-HGF (Figure S2D). Consistent with the decrease in active HGF, we found that Ab40 significantly reduced, and in the case of FAPs completely abolished, the injured serum-mediated induction of EdU incorporation in MuSCs and FAPs (Figure 1L). By contrast, Ab40 did not have any significant effect on the EdU incorporation of MuSCs and FAPs when it was pre-incubated and injected with non-injured serum (Figure 1L). These results suggest that HGFA enzymatic activity is necessary for the ability of injured serum to induce the GAlert transition.
Active HGFA is sufficient to induce the GAlert transition of stem cells
Next, we tested if active HGFA itself is sufficient to recapitulate the effects of injection of injured serum. To do this, we obtained purified, recombinant, active HGFA (hereafter ‘HGFA’) and administered it to animals (Figure 2A). Strikingly, we found that a single intravenous dose of HGFA was sufficient to induce the GAlert transition of MuSCs and FAPs. Animals injected with HGFA had a higher frequency of pS6+ MuSCs (Figure 2B). Upon isolation and culturing, MuSCs from animals administered HGFA displayed faster kinetics of cell cycle entry and cell division (Figures 2C, D and S2E). MuSCs from HGFA-injected animals also displayed an increase in cell size (Figures 2E, S2F). Importantly, we observed a similar set of molecular, phenotypic, and functional changes in FAPs (Figures 2F–H and S3A–B). These data show that administering an active form of the serum HGF protease, HGFA, is sufficient to induce the GAlert transition of both MuSCs and FAPs.
Figure 2. HGFA is sufficient to induce the GAlert transition of stem cells.
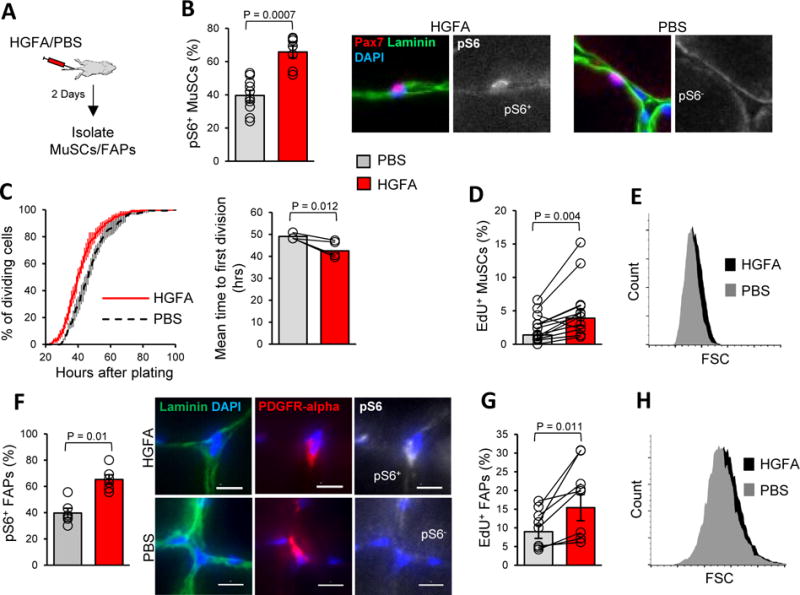
A) Schematic depiction of experiments testing the effect of HGFA on stem cell function.
B) Injecting mice with HGFA induces mTORC1 signaling in MuSCs. Data presented are the quantification of pS6 staining of Pax7+ cells by IF-IHC analysis of TA muscles, mean ± s.e.m. (PBS: n = 10; HGFA: n = 7). Displayed on the right are examples of pS6+ and pS6− MuSCs from HGFA-and PBS-injected mice, respectively. The scale bar is 10 μm.
C) Injecting mice with HGFA reduces the time to first division of MuSCs. Time to first division was measured by time-lapse microscopy and presented as a cumulative histogram on the left and as a bar graph of the geometric mean ± s.e.m. time to first division on the right (n = 4).
D) MuSCs isolated from animals administered HGFA enter the cell cycle more rapidly. Data from EdU incorporation assays are presented as the geometric mean ± s.e.m. of the percentage of MuSCs that incorporated EdU in first 24 hours after isolation (n = 13).
E) MuSCs isolated from animals injected with HGFA are larger. Data are presented as a representative FSC plot as measured by FACS.
F) HGFA injection increases the frequency of pS6+ FAPs. Data are presented as the percentage of PDGFR-alpha+ FAPS that are pS6+, mean ± s.e.m. (n = 5). Displayed on the right are examples of pS6+ and pS6− FAPs from animals injected with HGFA and PBS, respectively. The scale bar is 10 μm.
G) FAPs isolated from animals administered HGFA enter the cell cycle more rapidly. Data from EdU incorporation assays are presented a bar graph of the geometric mean ± s.e.m. of the percentage of FAPs that incorporated EdU in the first 40 hours after isolation (n = 8).
H) FAPs isolated from animals injected with HGFA are larger. Data are presented as a representative FSC plot as measured by FACS.
Pre-injury administration of HGFA improves the kinetics of tissue repair
A key physiological observation about the functional changes in GAlert stem cells is that they strongly correlate with enhancements in the process tissue repair (Rodgers et al., 2014). Therefore, we tested if administration of HGFA affects tissue repair. To do this, we administered a single dose of HGFA or vehicle (PBS) to animals via the tail vein two days before we subjected the animals to muscle injury (Figure 3A). One metric of injury-induced muscle regeneration is the size of nascent muscle fibers, which are small immediately following injury and gradually increase in size (Figure 3B). We found that HGFA stimulated a notable enhancement in the progression of muscle fiber size (Figure 3B, C). These data suggest that, compared to control, the injured TA muscles from HGFA-injected mice are at an advanced stage in muscle regeneration. HGFA did not have an effect on the number of regenerating muscle fibers in injured muscle (Figure S3C). Taking this a step further, we found that animals which were injected with HGFA displayed faster recovery in their wheel running behavior following injury to a gastrocnemius muscle (Figure 3D), suggesting an improvement in functional recovery from muscle injury (Carmichael et al., 2005; Knab et al., 2009).
Figure 3. Administering HGFA prior to an injury accelerates the process of tissue repair.
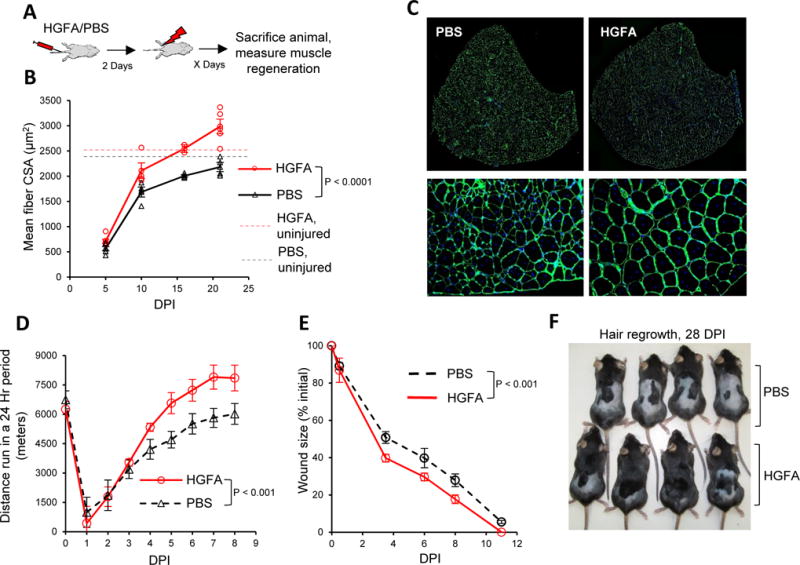
A) Schematic depicting experimental design testing the effects of a single dose of HGFA or vehicle (PBS) administered two days prior to subjecting animals to muscle injury.
B) HGFA accelerates muscle regeneration. Data are presented as the mean cross-sectional area (CSA) of nascent muscle fibers from TA muscles at indicated day post injury (DPI) as measured by IF-IHC analysis. Data are presented as mean ± s.e.m. Replicate values are presented as open circles (HGFA) or triangles (PBS) (HGFA 5 DPI, n = 6; 10 DPI, n = 4; 16 DPI, n = 2; 21 DPI, n = 5; PBS 5 DPI, n = 6; 10 DPI, n = 4; 16 DPI, n = 4; 21 DPI, n = 4). Dashed lines represent the mean CSA of muscle fibers in TA muscles contralateral to injury, measured 21 days after injury. Significance was calculated by two-way ANOVA.
C) Representative IF-IHC staining with Laminin (green) and DAPI (blue) of TA muscles at 21 DPI. The scale bar is 500 μm in the top row of images, 50 μm in the bottom row.
D) Animals injected with HGFA display improvements in running behavior following injury. Data are presented as the wheel running distance in the 24 hour period prior to the time point, mean ± s.e.m (n = 7). Significance was calculated by two-way ANOVA.
E) Animals injected with HGFA show improvements in skin wound healing. Quantification of wound closure is presented as the mean ± s.e.m. of the percent of initial wound size (n = 4). Significance was calculated by two-way ANOVA.
F) Animals administered HGFA prior to shaving and skin wounding display improvements in hair regrowth. Presented are representative images of animals 28 days post injury.
(See also Figure S3)
The improved recovery of HGFA injected animals from muscle injury suggests that HGFA may have a clinical application in contexts were muscle injury can be anticipated, such as surgery. Therefore, we tested if HGFA has an effect on another common aspect of surgery, skin wound healing. We found that animals that had been administered HGFA two days prior to being subjected to full-thickness skin wounds healed these wound more quickly (Figure 3E, S3D). Interestingly, at later time points following skin wounding, we observed that animals injected with HGFA had more complete regrowth of hair in the shaved area (Figure 3F). Aspects of both skin wound healing and the initiation of hair growth are dependent upon the activation of quiescent stem and progenitor cells (Arwert et al., 2012). The improvements that we observed suggest that HGFA induced the cells required for these processes into a GAlert state that is similar to that of the stem cells in the muscle compartment.
DISCUSSION
In most tissues, efficient and successful repair is dependent upon the coordinated activation of many different pools of stem and progenitor cells. We suspect the HGFA-mediated improvements in tissue repair that we observe are due to a coordinated GAlert transition of many cell types. Indeed, many populations of stem and progenitor cells, such as mesenchymal stem cells, epidermal stem cells, keratinocytes, and hematopoietic stem cells, have been shown to express cMet and could respond to HGFA via a similar mechanism as MuSCs (Allen et al., 1995; Chmielowiec et al., 2007; Neuss et al., 2004; Tesio et al., 2011). Another potential mechanism by which HGFA could induce a cellular response is through the proteolytic activation of the other known substrate of HGFA, Macrophage Stimulating Protein (MSP), and its signaling through RON receptor tyrosine kinase (Kataoka and Kawaguchi, 2010). The data we present here, as well as our previous work, provide evidence that several different stem cell pools adopt a functionally similar GAlert state in response to a common stimulus. Combined, this suggests that HGFA is a central and single factor that is capable of ‘alerting’ many types of stem cells throughout the body, priming them for an accelerated functional response to injury. We propose that HGFA could have applications as a treatment administered prior to situations where tissue damage is expected, such as surgery or combat, and that this constitutes a conceptually novel treatment strategy for regenerative medicine.
EXPERIMENTAL PROCEDURES
Statistics
Data are presented as mean or geometric mean ± s.e.m. and overlaid with the individual biologic replicate measurements. In figures presented as bar graphs, the mean or geometric mean of the biologic replicates is represented as column and individual biologic replicates are represented as open circles. When experiments involved the isolation and analysis of primary cells, they were performed as paired measurements (i.e. control versus experimental, performed on the same day, in parallel, using the same instrumentation) and are presented as lines connecting the individual paired measurements. This is to control for day-to-day variability in the preparation of primary cells and subtle differences in FACS instrumentation, settings, and gating. Bar graphs summarizing paired measurements are presented as the geometric mean ± s.e.m. Time lapse analyses are presented in two ways, as cumulative histograms, binned per hour, and displayed as the mean ± s.e.m. of the percentage of the cells that had divided at each bin and as bar graphs displaying the mean time to cell division for each replicate sample. Analysis of FSC by FACS is presented as a representative FACS histogram plot and a bar graph displaying the geometric mean of the median FSC ± s.e.m. of all replicate experiments. All replicate data points represent individual biologic replicates (i.e. different mice). Except when stated otherwise, statistical significance (P values) was calculated using two-tailed Students t-test, unpaired or paired where appropriate. The statistical significance of the data presented in Figure 3 was calculated using two-way ANOVA (GraphPad, Prism).
Mice
Pax7CreER mice were provided by Dr. Charles Keller (OHSU). Rosa26EYFP and cMetFlox mice were obtained from Jackson Labs. All experiments were performed with 12–20 week old male C57BL6 mice (Jackson Labs), except for experiments using conditional cMet KO mice which had a mixed background of C57BL6 and FVB. Animals were genotyped by PCR of tail DNA; primer sequences are available upon request. Tamoxifen (Sigma) was prepared in 7% EtOH and corn oil and administered to 8–12 week old mice in 5 doses of 5 mg/mouse every 2–3 days by intraperitoneal injection. Mice were housed and maintained in the Veterinary Medical Unit at the Veterans Affairs Palo Alto Health Care System. All animal protocols were approved by the IACUC board of the VA Palo Alto and/or USC.
HGFA administration
Purified, recombinant-active HGFA (R&D systems #1200-SE) was administered via intravenous tail vein injection at a dose of 1 μg diluted into 200 μL of sterile PBS. Control injections were performed using 200 μL of PBS alone. Experiments using HGFA were performed over the course of two years utilizing HGFA obtained from several different lots (HGS07152A, HGS0714051, HGS0715011, HGS0614041, HGS0613101). The specific activity of each lot was validated prior to use.
Serum isolation and injection
Serum was isolated from blood samples collected via cardiac puncture immediately after CO2 euthanasia of the animals. Blood was left to clot for 1 hour, subject to centrifugation, and serum was isolated by removing the upper clear layer of the blood sample. Serum samples were stored at −80°C until use. Serum was administered to animals via intravenous tail vein injection. Injections were performed in a total volume of 200 μL, with serum diluted 1:1 with sterile PBS. In experiments utilizing serum, each replicate measurement represents a biologic replicate serum sample (i.e. serum from a different mouse).
Muscle Injury
Muscle injuries were induced by intramuscular injection of BaCl2. Animals were anesthetized using isoflurane and 30 μL of a 1.2% BaCl2 (w/v H2O) was injected along the length of a tibialis anterior (TA) or 70 μL was injected into the gastrocnemius muscle. Animals were then administered buprenorphine and Baytril and allowed to recover. Sham muscle injuries were performed by anesthetizing the mice followed by administration of buprenorphine and Baytril.
Cell Isolation, FACS purification, and cell culture
MuSC and FAP isolations were performed using FACS as previously described (Liu et al., 2015). MuSCs were purified as a population of YFP+ cells when utilizing mice containing the alleles Pax7CreER/+;Rosa26EYFP/+ or as a population of CD31neg; CD45neg; Sca-1neg; VCAM+ cells. FAPs were purified as a population of CD31neg; CD45neg; Sca-1+ cells. Antibodies used for FACS isolation are listed in Figure S3E. All experiments that involve the comparison of primary cells were performed in parallel using the same FACS instrumentation (BD FACS Aria II or III). Cells were sorted into plating medium (Hams F10 (Cellgro), 20% FBS (Invitrogen), 5 ng/ml bFGF (Invitrogen) and 1x Pen/Strep (Gibco)). After isolation, MuSCs and FAPs were plated and cultured on poly-D lysine (Millipore) and ECM (Sigma E1270) coated 8-well chamber slides (Lab-Tech II). Twelve hours after isolation, the medium was changed to culturing medium (Hams F10 (Cellgro), 10% FBS (Invitrogen), 10% Horse serum (Invitrogen), and 1x Pen/Strep (Gibco)). Cells were then used for EdU incorporation assays or time lapse microscopy.
EdU incorporation assay
Twelve hours after isolation, EdU was added to culturing medium at a final concentration of 10 μM and cells where then fixed at either 24 or 40 hours after plating. EdU was detected using the Click-It kit (Invitrogen) according to the manufacturer’s instructions. Data on EdU incorporation are presented as the percentage of total cells (measured by DAPI).
Time lapse microscopy
After changing to culturing medium, chamber slides were transferred to a temperature and CO2 controlled Zeiss Axio observer Z1. Time lapse acquisition was performed under 10X magnification, capturing images every 10 minutes, and controlled by Axiovision software. Time to division was recorded only for cells that stayed in the field of view during acquisition and presented as the time from plating. For each biologic replicate, the time to division was manually measured for at least 40 cells.
In situ analysis of muscle fiber size and pS6
Immediately after euthanasia, TA muscles were dissected, mounted onto tragacanth gum, and snap frozen in liquid N2-cooled isopentane. After cryosectioning, 8 μm muscle sections were fixed in 4% PFA for 5 minutes and washed with PBS 0.3% Triton. Muscle fiber size measurements were performed on TA muscle sections taken at 1/3 – 1/2 of the distance in from the distal end of the TA. Muscles sections were stained with laminin antibodies to outline muscle fibers and fiber area was measured by morphometric analysis of centrally nucleated muscle fibers (Image J). For each biologic replicate, at least 300 muscle fibers along the lateral/anterior border of the TA muscle were analyzed.
pS6 analysis
To identify MuSCs, muscle sections were stained with Pax7 antibodies using the M.O.M. kit (Vector) according to manufacturer’s instructions. Follow Pax7 staining, sections were stained with phospho-S6 and laminin antibodies overnight. FAPs were identified as PDGFR-alpha-positive cells in the interstitia between muscle fibers. Antibodies and concentrations used are listed in Figure S3E. Data on pS6 staining are presented as the percentage of sub-laminar Pax7-positive cells or PDGFR-alpha-positive cells that possessed detectable pS6 signal; at least 50 MuSCs or FAPs were analyzed per biologic replicate.
Supplementary Material
HIGHLIGHTS.
Systemic factors control the GAlert transition of stem cells.
HGFA is a circulating HGF protease that is activated in response to injury.
Active HGFA is sufficient to stimulate GAlert in MuSCs and FAPs.
Pre-injury HGFA administration accelerates stem cell activation and tissue repair.
Acknowledgments
We would like to acknowledge Jamie Brett, Ling Liu, Mike Wosczyna, and Antoine de Morree for helpful discussions during the course of this research. We would like to thank Lusijah Rott for her expertise and help with FACS. We thank Daniel Kirchhofer (Genentech) for providing the HGFA blocking antibody. This work has been funded by grants from the NIH (AG041764) and The Donald E. and Delia B. Baxter Foundation to J.T.R. and by grants from the Glenn Foundation for Medical Research, the NIH (P01 AG036695, R01 AR062185), the VA (Merit Reviews) to T.A.R.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Author Contributions
Conceptualization, J.T.R. and T.A.R.; Methodology, J.T.R. and T.A.R.; Investigation, J.T.R, M.D.S., and C.M.; Writing — Original Draft, J.T.R. and T.A.R.; Writing — Review & Editing, J.T.R. and T.A.R.; Visualization, J.T.R.; Supervision, J.T.R. and T.A.R.; Funding Acquisition, J.T.R. and T.A.R.
Conflict of interest.
We have no conflicts of interest to declare.
References
- Allen RE, Sheehan SM, Taylor RG, Kendall TL, Rice GM. Hepatocyte growth factor activates quiescent skeletal muscle satellite cells in vitro. J Cell Physiol. 1995;165:307–312. doi: 10.1002/jcp.1041650211. [DOI] [PubMed] [Google Scholar]
- Arwert EN, Hoste E, Watt FM. Epithelial stem cells, wound healing and cancer. Nat Rev Cancer. 2012;12:170–180. doi: 10.1038/nrc3217. [DOI] [PubMed] [Google Scholar]
- Carmichael MD, Davis JM, Murphy EA, Brown AS, Carson JA, Mayer E, Ghaffar A. Recovery of running performance following muscle-damaging exercise: relationship to brain IL-1beta. Brain Behav Immun. 2005;19:445–452. doi: 10.1016/j.bbi.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Cheung TH, Rando TA. Molecular regulation of stem cell quiescence. Nat Rev Mol Cell Biol. 2013;14:329–340. doi: 10.1038/nrm3591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chmielowiec J, Borowiak M, Morkel M, Stradal T, Munz B, Werner S, Wehland J, Birchmeier C, Birchmeier W. c-Met is essential for wound healing in the skin. J Cell Biol. 2007;177:151–162. doi: 10.1083/jcb.200701086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coller HA. What’s taking so long? S-phase entry from quiescence versus proliferation. Nat Rev Mol Cell Biol. 2007;8:667–670. doi: 10.1038/nrm2223. [DOI] [PubMed] [Google Scholar]
- Coughlin SR. Thrombin signalling and protease-activated receptors. Nature. 2000;407:258–264. doi: 10.1038/35025229. [DOI] [PubMed] [Google Scholar]
- Fajardo-Puerta AB, Mato Prado M, Frampton AE, Jiao LR. Gene of the month: HGF. J Clin Pathol. 2016 doi: 10.1136/jclinpath-2015-203575. [DOI] [PubMed] [Google Scholar]
- Gak E, Taylor WG, Chan AM, Rubin JS. Processing of hepatocyte growth factor to the heterodimeric form is required for biological activity. FEBS Lett. 1992;311:17–21. doi: 10.1016/0014-5793(92)81356-q. [DOI] [PubMed] [Google Scholar]
- Ganesan R, Eigenbrot C, Wu Y, Liang WC, Shia S, Lipari MT, Kirchhofer D. Unraveling the allosteric mechanism of serine protease inhibition by an antibody. Structure. 2009;17:1614–1624. doi: 10.1016/j.str.2009.09.014. [DOI] [PubMed] [Google Scholar]
- Joe AW, Yi L, Natarajan A, Le Grand F, So L, Wang J, Rudnicki MA, Rossi FM. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones DL, Rando TA. Emerging models and paradigms for stem cell ageing. Nat Cell Biol. 2011;13:506–512. doi: 10.1038/ncb0511-506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka H, Kawaguchi M. Hepatocyte growth factor activator (HGFA): pathophysiological functions in vivo. FEBS J. 2010;277:2230–2237. doi: 10.1111/j.1742-4658.2010.07640.x. [DOI] [PubMed] [Google Scholar]
- Knab AM, Bowen RS, Moore-Harrison T, Hamilton AT, Turner MJ, Lightfoot JT. Repeatability of exercise behaviors in mice. Physiol Behav. 2009;98:433–440. doi: 10.1016/j.physbeh.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laurenti E, Frelin C, Xie S, Ferrari R, Dunant CF, Zandi S, Neumann A, Plumb I, Doulatov S, Chen J, et al. CDK6 levels regulate quiescence exit in human hematopoietic stem cells. Cell Stem Cell. 2015;16:302–313. doi: 10.1016/j.stem.2015.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Cheung TH, Charville GW, Rando TA. Isolation of skeletal muscle stem cells by fluorescence-activated cell sorting. Nat Protoc. 2015;10:1612–1624. doi: 10.1038/nprot.2015.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClain SA, Simon M, Jones E, Nandi A, Gailit JO, Tonnesen MG, Newman D, Clark RA. Mesenchymal cell activation is the rate-limiting step of granulation tissue induction. Am J Pathol. 1996;149:1257–1270. [PMC free article] [PubMed] [Google Scholar]
- Miyazawa K. Hepatocyte growth factor activator (HGFA): a serine protease that links tissue injury to activation of hepatocyte growth factor. FEBS J. 2010;277:2208–2214. doi: 10.1111/j.1742-4658.2010.07637.x. [DOI] [PubMed] [Google Scholar]
- Neuss S, Becher E, Woltje M, Tietze L, Jahnen-Dechent W. Functional expression of HGF and HGF receptor/c-met in adult human mesenchymal stem cells suggests a role in cell mobilization, tissue repair, and wound healing. Stem Cells. 2004;22:405–414. doi: 10.1634/stemcells.22-3-405. [DOI] [PubMed] [Google Scholar]
- O’Reilly C, McKay B, Phillips S, Tarnopolsky M, Parise G. Hepatocyte growth factor (HGF) and the satellite cell response following muscle lengthening contractions in humans. Muscle Nerve. 2008;38:1434–1442. doi: 10.1002/mus.21146. [DOI] [PubMed] [Google Scholar]
- Rodgers JT, King KY, Brett JO, Cromie MJ, Charville GW, Maguire KK, Brunson C, Mastey N, Liu L, Tsai CR, et al. mTORC1 controls the adaptive transition of quiescent stem cells from G0 to G(Alert) Nature. 2014;510:393–396. doi: 10.1038/nature13255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seale P, Sabourin LA, Girgis-Gabardo A, Mansouri A, Gruss P, Rudnicki MA. Pax7 is required for the specification of myogenic satellite cells. Cell. 2000;102:777–786. doi: 10.1016/s0092-8674(00)00066-0. [DOI] [PubMed] [Google Scholar]
- Shimomura T, Kondo J, Ochiai M, Naka D, Miyazawa K, Morimoto Y, Kitamura N. Activation of the zymogen of hepatocyte growth factor activator by thrombin. J Biol Chem. 1993;268:22927–22932. [PubMed] [Google Scholar]
- Siegel AL, Kuhlmann PK, Cornelison DD. Muscle satellite cell proliferation and association: new insights from myofiber time-lapse imaging. Skelet Muscle. 2011;1:7. doi: 10.1186/2044-5040-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoker M, Gherardi E, Perryman M, Gray J. Scatter factor is a fibroblast-derived modulator of epithelial cell mobility. Nature. 1987;327:239–242. doi: 10.1038/327239a0. [DOI] [PubMed] [Google Scholar]
- Sugiura T, Wang H, Barsacchi R, Simon A, Tanaka EM. MARCKS-like protein is an initiating molecule in axolotl appendage regeneration. Nature. 2016;531:237–240. doi: 10.1038/nature16974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tesio M, Golan K, Corso S, Giordano S, Schajnovitz A, Vagima Y, Shivtiel S, Kalinkovich A, Caione L, Gammaitoni L, et al. Enhanced c-Met activity promotes G-CSF-induced mobilization of hematopoietic progenitor cells via ROS signaling. Blood. 2011;117:419–428. doi: 10.1182/blood-2009-06-230359. [DOI] [PubMed] [Google Scholar]
- Trusolino L, Bertotti A, Comoglio PM. MET signalling: principles and functions in development, organ regeneration and cancer. Nat Rev Mol Cell Biol. 2010;11:834–848. doi: 10.1038/nrm3012. [DOI] [PubMed] [Google Scholar]
- Weissman IL. Stem cells: units of development, units of regeneration, and units in evolution. Cell. 2000;100:157–168. doi: 10.1016/s0092-8674(00)81692-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


