Abstract
OBJECTIVE
To evaluate a multimodal strategy aimed at treating all sites of disease that provides a rapid readout of success or failure in men presenting with non-castrate metastatic prostate cancers that are incurable with single modality therapy.
MATERIALS AND METHODS
Twenty selected men with oligometastatic M1a (extrapelvic nodal disease) or M1b (bone disease) at diagnosis were treated using a multimodal approach that included androgen deprivation, radical prostatectomy plus pelvic lymphadenectomy (retroperitoneal lymphadenectomy in the presence of clinically positive retroperitoneal nodes), and stereotactic body radiotherapy to osseous disease or the primary site. Outcomes of each treatment were assessed sequentially. Androgen deprivation was discontinued in responding patients. The primary end point was an undetectable prostate-specific antigen (PSA) after testosterone recovery. The goal was to eliminate all detectable disease.
RESULTS
Each treatment modality contributed to the outcome: 95% of the cohort achieved an undetectable PSA with multimodal treatment, including 25% of patients after androgen deprivation alone and an additional 50% and 20% after surgery and radiotherapy, respectively. Overall, 20% of patients (95% confidence interval: 3%–38%) achieved the primary end point, which persisted for 5, 6, 27+, and 46+ months. All patients meeting the primary end point had been classified with M1b disease at presentation.
CONCLUSION
A sequentially applied multimodal treatment strategy can eliminate detectable disease in selected patients with metastatic spread at diagnosis. The end point of undetectable PSA after testosterone recovery should be considered when evaluating new approaches to rapidly set priorities for large-scale testing in early metastatic disease states and to shift the paradigm from palliation to cure.
A typical approach to developing a systemic treatment for cancer follows a paradigm in which studies begin in patients with advanced disease where unmet needs are greatest and survival times short. Therapies that are active are then studied in earlier disease states, culminating in large-scale trials that evaluate whether the systemic approach in combination with local therapy prevents or delays the time-to-event measures of disease progression or death in men with “high-risk” tumors predicted to recur after local therapy alone. Examples are the trials in localized disease that ultimately showed a survival benefit for radiation therapy to the prostate in combination with androgen deprivation therapy (ADT) versus either one alone.1–4 Together, these trials enrolled 3000 patients who were followed for 6–12 years to demonstrate the survival difference. Studies of radical surgery combined with ADT have shown that biochemical recurrence can be delayed, but no definitive effect on metastatic progression or survival has been shown.5,6 Now, with 6 life-prolonging systemic therapies available for metastatic castration-resistant prostate cancer (mCRPC) and olaparib given a breakthrough designation by the United States Food and Drug Administration and on a fast-track for approval,7 there are too many possible combinations to test sequentially. New paradigms are needed so that more regimens can be evaluated rapidly and prioritized for large-scale phase 3 testing.
Here, we describe a multimodal treatment strategy of limited duration for men who present with low-volume metastatic disease that shifts the end point from time-to-event measures that occur late to measures of response that occur early. Because these patients are incurable with any form of monotherapy, the approach combines treatment directed at each manifestation of disease: (1) systemic therapy that includes ADT, (2) radical prostatectomy (RP) with pelvic lymph node (LN) dissection (+/− retroperitoneal dissection) to resect the primary and all detectable nodal disease, and (3) radiation therapy to visible osseous lesions or the prostate bed and LNs as clinically indicated. Systemic therapy is stopped in men who achieve an undetectable prostate-specific antigen (PSA) level to enable testosterone recovery. The primary end point, an undetectable PSA with non-castrate levels of testosterone at 20 months, is a response indicator that occurs early, is due solely to treatment, and is a requisite first step to show that the disease may have been completely eliminated. Once achieved and maintained over longer periods, this binary readout of a “no evidence of disease” (NED) state begins to establish the possibility of cure.
This end point was first used in a trial of rapid androgen cycling in a state of rising PSA in patients with biochemical recurrence after local therapy,8 and in a more recent randomized trial of complete androgen suppression with abiraterone and degarelix (NCT01751451). It is the same principle used to show that a clinically localized tumor has been completely removed (eliminated) surgically, and it aligns with the pathologic complete response end point used in neoadjuvant breast cancer trials to support regulatory approvals.9
A 20-month landmark time to define “success or failure” is significantly shorter than the 42-month median survival time in this population10 (which may be prolonged further as more agents become available), reducing both study duration and the sample size needed to show efficacy, and enabling more approaches to be evaluated rapidly and prioritized for large-scale testing. We report our initial experience using the approach that has generated the information needed to guide the development of future protocols.
MATERIALS AND METHODS
Patients
Patients with histologically confirmed prostate cancer who presented with extrapelvic nodal disease (American Joint Committee on Cancer [AJCC] M1a) or bone disease (M1b) evaluated at Memorial Sloan Kettering Cancer Center within 6 months of diagnosis were considered. All underwent a complete history and physical examination, with laboratory studies that included a complete blood count, chemistry profile, and creatinine, serum testosterone, and PSA levels. Imaging included a prostate and pelvis magnetic resonance imaging, transaxial imaging of the chest, abdomen, and pelvis, and Tc-99 radionuclide bone scan. Up to 10 bone metastases based on bone scintigraphy or non-regional LN involvement were allowed. Patients were excluded if they had evidence of pelvic sidewall invasion, visceral disease, or metastases to the pelvic LN only (AJCC N1). Before undergoing treatment, all patients provided written informed consent to proceed with RP according to the principles outlined in the Declaration of Helsinki, with the understanding that RP plus pelvic and retroperitoneal lymphadenectomy (pelvic lymph node dissection [PLND] and retroperitoneal lymph node dissection [RPLND], respectively) had not been proven for M1 patients to prolong survival but could possibly provide locoregional control and symptomatic relief.
Interventions
ADT
All patients were started on ADT followed by surgery and radiation, or radiation followed by surgery. ADT was stopped after a minimum duration of 6 months if an undetectable PSA was achieved after combined modality therapy. Other patients were treated continuously.
Surgery
The surgical procedure was designed to resect all gross disease in a single stage and included bilateral PLND (external iliac, obturator, and hypogastric nodes) and open or robot-assisted laparoscopic RP. Nerve sparing was performed in some patients at the discretion of the surgeon. Radiographically evident nodal metastases (>2 cm) above the bifurcation of the common iliac vein were resected through an open approach by a second surgical team performing template-directed retroperitoneal RPLND that included the left para-aortic, preaortic, retroaortic, inter-aortocaval, paracaval, precaval, retrocaval, common iliac, and pre-sacral LNs.
Radiation Therapy
Hypofractionated stereotactic body radiotherapy (SBRT) was considered for patients with metastatic bone lesions. The lesions were treated to total doses ranging from 2000 to 3000 cGy delivered over 1 to 5 fractions encompassing the primary lesion, with a small (3 mm) margin for setup error. Based on the pathologic findings at surgery, postoperative radiation to the prostate bed +/− pelvic LNs or para-aortic LNs was given with conventional fractionation schedules using intensity-modulated radiotherapy at doses ranging from 6600 to 7200 cGy after RP.
Outcomes of Therapy
Outcomes were reported separately by modality and disease manifestation for patients with M1a (extrapelvic nodal disease) and M1b (bone disease with or without soft tissue disease). PSA changes were reported as whether or not an undetectable level (<.05 ng/mL) was achieved following each sequential therapy. Imaging changes were classified per the Prostate Cancer Clinical Trials Working Group 2 criteria.11 Later tumor-related events included time to PSA recurrence, local recurrence, or objective progression by imaging in a preexisting lesion or a new site of disease, or the development of symptoms.
Surgery
Surgical outcomes included pathologic TNM stage, margin status, number of LNs removed and the number with cancer, and postoperative PSA levels. Safety measures included operative time, blood loss, and length of hospital stay. Continence rates (defined as dry without use of pads) at 12 months were also assessed.
Radiation Therapy
Changes in PSA were reported as outlined, along with relief of symptoms if present.
Statistical Analysis
Categorical variables were presented as frequencies and proportions, and continuous variables as medians and ranges.
RESULTS
Patient Characteristics and Extent of Disease
The baseline characteristics of the 20 patients treated (5 with extrapelvic nodal disease [M1a] and 15 with bone disease [M1b]) are summarized in Table 1. Overall, 14 (70%) primary tumors were classified as T3a or greater, and clinically positive pelvic nodes (N1) were seen in 5 M1a patients (100%) and 6 M1b patients (40%). The median number of bone metastases was 3 (range: 1–7).
Table 1.
Baseline clinical characteristics
| Total | M1a | M1b | |
|---|---|---|---|
| Number of patients | 20 | 5 | 15 |
| Age (y) median (range) | 59 (44–74) | 59 (49–74) | 57 (44–69) |
| Extent of disease | |||
| T1c | 1 (5%) | 0 (0%) | 1 (7%) |
| T2 | 5 (25%) | 1 (20%) | 4 (27%) |
| T3a | 4 (20%) | 1 (20%) | 3 (20%) |
| T3b | 7 (35%) | 1 (20%) | 6 (40%) |
| T4 | 3 (15%) | 2 (40%) | 1 (7%) |
| N1 (pelvic LN) | 11 (55%) | 5 (100%) | 6 (40%) |
| M1a (non-regional LN) | 7 (35%) | 5 (100%) | 2 (13%) |
| M1b | 15 (100%) | N/A | 15 (100%) |
| Number of bone metastases | |||
| 1–3 | 11 (55%) | N/A | 11 (73%*) |
| 4–5 | 2 (10%) | N/A | 2 (13%) |
| 6–7 | 2 (10%) | N/A | 2 (13%) |
| Gleason score | |||
| ≤7 | 3 (15%) | 0 (0%) | 3 (20%) |
| 8 | 4 (20%) | 0 (0%) | 4 (27%) |
| 9 or 10 | 13 (65%) | 5 (100%) | 8 (53%) |
| PSA at diagnosis | |||
| <20 | 8 (40%) | 0 (0%) | 8 (53%) |
| >20 | 12 (60%) | 5 (100%) | 7 (47%) |
| Primary ADT | |||
| LHRH agonist/antagonist | 20 (100%) | 5 (100%) | 15 (100%) |
| +Abiraterone | 9 (45%) | 2 (40%) | 7 (47%) |
| Duration of pre-RP ADT, months, median (range) | 4 (2–9) | 5 (2–8) | 3 (2–9) |
| Duration of primary ADT, months, median (range) | 11 (6–16) | 10 (7–12) | 11 (6–16) |
| 0–6 months | 1 (5%) | 0 (0%) | 1 (7%) |
| 7–12 months | 11 (55%) | 4 (80%) | 7 (47%) |
| 13–16 months | 4 (20%) | 0 (0%) | 4 (27%) |
| Continuous | 4 (20%) | 1 (20%) | 3 (20%) |
ADT, androgen deprivation therapy; LHRH, luteinizing hormone-releasing hormone; LN, lymph node; PSA, prostate-specific antigen; RP, radical prostatectomy. M1a = extrapelvic nodal disease; M1b = bone metastasis.
Initial bone scan not available for 1 patient; subsequent scan with 3 documented metastases.
Details of Treatment
Systemic Therapy
All patients received ADT as initial therapy, with a median of 4 months (range: 2–9) prior to RP. All those in whom primary ADT was discontinued (n = 16) received it for a median of 11 months (range: 6–16), as shown in Table 1.
Surgery
M1a (Extrapelvic Nodal Disease)
All 5 patients had an RP and bilateral PLND, and 4 also had RPLND at a median of 5 months (range: 3–9) after diagnosis. At surgery, a median of 17 pelvic LNs (range: 11–25) and 40 retroperitoneal LNs (range: 22–48) per patient were removed (Table 2). Pathologic pelvic LN involvement was documented in 4 cases (80%); M1a status was confirmed in 3, with a median of 21 (range: 0–30) positive nodes. The median operative time was 390 minutes (range: 236–618). There was one grade 3 complication (Clavien-Dindo classification): a suspected intraoperative ureteral injury requiring stent placement. All 5 patients were continent by 12 months after surgery.
Table 2.
Surgical pathology and staging
| Total N = 20 |
M1a N = 5 |
M1b N = 15 |
|
|---|---|---|---|
| Time to surgery (mo) median (range) | 5 (3–11) | 5 (3–9) | 5 (3–11) |
| Procedure | |||
| RP + PLND | 14 (70%) | 1 (20%) | 13 (87%) |
| RP + PLND + RPLND | 6 (30%) | 4 (80%) | 2 (13%) |
| Primary tumor | |||
| ≤T2b | 3 (15%) | 2 (40%) | 1 (7%) |
| T3a | 5 (25%) | 0 (0%) | 5 (33%) |
| T3b | 9 (45%) | 1 (20%) | 8 (53%) |
| T4 | 3 (15%) | 2 (40%) | 1 (6%) |
| Vascular invasion | 14 (70%) | 5 (100%) | 9 (60%) |
| Positive surgical margin | 5 (25%) | 3 (60%) | 2 (13%) |
| Pathologic T stage* | |||
| Upstaged | 9 (45%) | 2 (40%) | 7 (46%) |
| Downstaged | 6 (30%) | 2 (40%) | 4 (27%) |
| No change | 5 (25%) | 1 (20%) | 4 (27%) |
| PLND | |||
| Pelvic LN removed, median (range) | 17 (9–25) | 17 (11–25) | 17 (9–25) |
| Number of positive LN, median (range) | 1 (0–13) | 3 (0–13) | 1 (0–9) |
| Number of patients with positive LN | 13 (65%) | 4 (80%) | 9 (60%) |
| Pathologic N stage* | |||
| Upstaged | 4 (20%) | 0 (0%) | 4 (27%) |
| Downstaged | 2 (10%) | 1 (20%) | 1 (7%) |
| No change | 14 (70%) | 4 (80%) | 10 (66%) |
| RPLND | N = 6 | N = 4 | N = 2 |
| Retroperitoneal LN removed, median (range) | 31 (22–48) | 40 (22–48) | 25 (23–27) |
| Number of positive LN, median (range) | 15 (0–30) | 21 (0–30) | 7 (0–14) |
| Number of patients with positive LN | 4 (67%) | 3 (75%) | 1 (50%) |
PLND, pelvic lymph node dissection; RPLND, retroperitoneal lymph node dissection.
Compared with initial clinical staging.
M1b (Bone Disease)
All 15 patients had an RP and bilateral PLND at a median of 5 months (range: 3–11) after diagnosis. All were resectable, although 2 (13%) had positive margins. A median of 17 pelvic nodes (range: 9–25) were removed, of which a median of 1 (range: 0–9) was found to be malignant. In the 2 patients who had RPLND, 23 and 27 retroperitoneal nodes were removed, of which 0 and 14, respectively, had tumor. Clinical and pathologic staging of the primary tumor was discordant in 11 patients (73%), whereas N staging was discordant in 5 (33%) (Table 2). The median operative time was 252 minutes (range: 150–323 for RP + PLND and 386–500 minutes for RP + PLND + RPLND). There were two grade 3 complications: 1 pelvic abscess requiring drain placement and 1 anastomotic leak. Thirteen of 14 patients (93%) with follow-up of at least 12 months were continent.
Radiation Therapy
M1a (Extrapelvic Nodal Disease)
One patient who did not undergo RPLND received radiation to his para-aortic LN in addition to radiation to the prostate bed for positive surgical margins.
M1b (Bone Disease)
Twelve patients (80%) received hypofractionated SBRT to osseous metastases, which encompassed all visible disease on bone scan in 10. Most patients were treated at 1–3 sites of bone metastasis, and 3 patients were treated at 4 or 5 sites. Of these, 10 were treated postoperatively after a median of 4 months (range: 2–15) of ADT, and 2 patients with symptoms were treated preoperatively. Three patients (20%) were not treated initially: 1 had a negative biopsy, which later proved positive and was subsequently treated, 1 was denied insurance coverage and treated later for symptoms, and the third was not treated because of disease extent.
Two patients (13%) received postoperative radiation to the prostate bed and pelvic LN basins based on surgical findings of grossly positive margins or nodal disease with extranodal extension. There were no significant treatment-related adverse events related to radiation.
Outcomes: Post-therapy PSA Changes and Clinical Response
M1a (Extrapelvic Nodal Disease)
None of the 5 patients achieved an undetectable PSA after ADT alone, but 4 (80%) did so after surgery. The fifth patient, who did not have an RPLND, had a persistently elevated PSA post-RP that became undetectable after radiation to the para-aortic LNs (Figs. 1, 2). ADT was discontinued in 4 (80%) but restarted for PSA progression after a median time of 6 months (range: 0–15). None achieved the primary end point of undetectable PSA with testosterone recovery at 20 months after initiation of therapy (Table 3), although 1 patient had a PSA of <.05 ng/mL with a testosterone level of 47 ng/dL at 39 months. With a median follow-up of 34 months, castration-resistant disease developed in 2 patients (40%) at 18 and 32 months. Two (40%) progressed radiographically: in the prostate bed and retroperitoneum in 1 patient, and bone and lung in the other. There was 1 death from prostate cancer at 24 months from the start of therapy.
Figure 1.
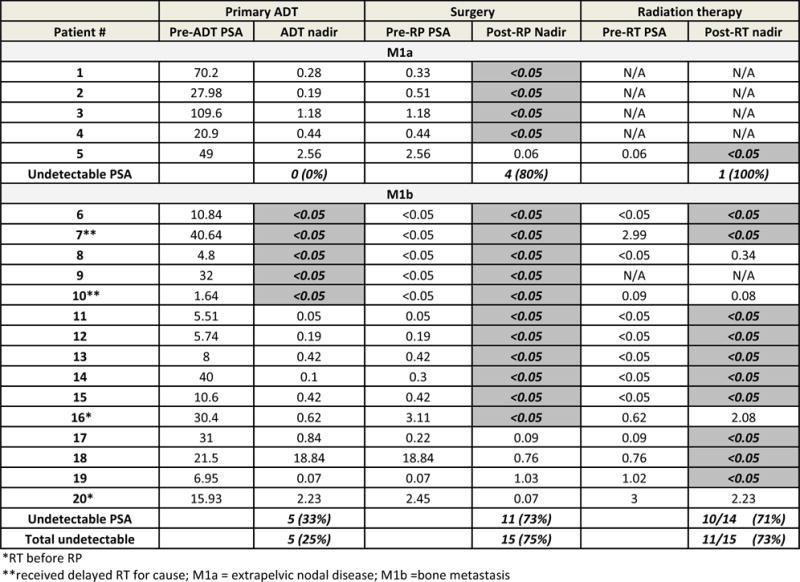
Individual PSA best absolute response by treatment modality (primary ADT, surgery, and radiation therapy) with best PSA response before next treatment is shown. Undetectable PSA values are highlighted. ADT, androgen deprivation therapy; PSA, prostate-specific antigen; RP, radical prostatectomy; RT, radiation therapy.
Figure 2.
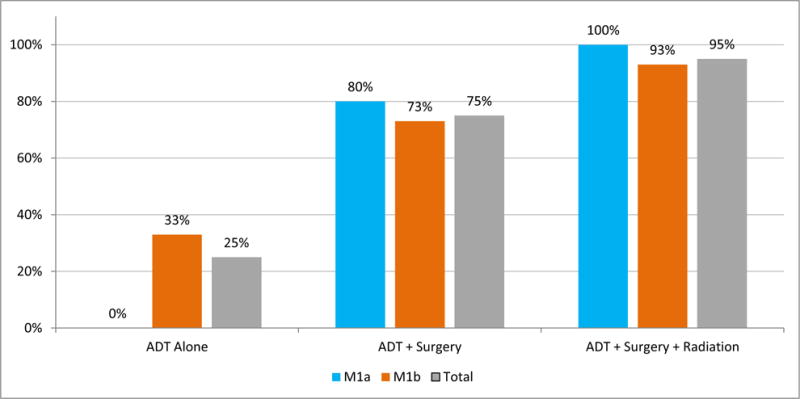
Percentage of patients who achieved undetectable PSA during the treatment phase increased with each component of multimodal therapy. Response to treatment was assessed with serum PSA measurements, and the frequency of patients who achieved undetectable PSA after ADT alone, ADT + surgery, and ADT + surgery + radiation is shown. ADT, androgen deprivation therapy; M1a, extrapelvic nodal disease; M1b, bone metastasis; PSA, prostate-specific antigen.
Table 3.
Clinical response and primary end point
| Total N = 20 |
M1a N = 5 |
M1b N = 15 |
|
|---|---|---|---|
| Time of follow-up (mo) median (range) | 40 (17–89) | 34 (18–40) | 47 (17–89) |
| Time off ADT (mo) median (range) | 9 (0–54) | 6 (0–15) | 9 (0–54) |
| PSA ≤.05 ng/mL at 12 months | |||
| Castrate | 10 (50%) | 3 (60%) | 7 (47%) |
| Non-castrate* | 2 (10%) | 0 (0%) | 2 (13%)† |
| PSA ≤.05 ng/mL at 20 months | |||
| Castrate | 6 (30%) | 2 (50%)‡ | 4 (27%)‡ |
| Non-castrate* | 4 (20%) | 0 (0%)‡ | 4 (27%)‡ |
Non-castrate defined as >150 mg/dL serum testosterone level.
One patient did not have a serum testosterone assessment at the 12-month mark.
One patient has not met the 20-month mark.
M1b (Bone Disease)
Five (33%) achieved an undetectable PSA with ADT alone, whereas 9 (60%) reached a nadir of <0.2 ng/mL and 13 (87%) a nadir of <1.0 ng/mL. After surgery, PSA in an additional 6 (total: 73%) became undetectable (Figs. 1, 2). Seven patients had a PSA of <.05 ng/mL before radiation therapy, which was maintained in 6 after radiation. Another 7 had elevated PSA levels, which became undetectable in 4 after radiation. When considering the best overall PSA response during the treatment phase, 14 of 15 M1b patients (93%) reached an undetectable PSA when ADT, surgery, and radiation were used (Fig. 2). Ultimately, 4 (27%) achieved the proposed end point, a PSA of <.05 ng/mL and serum testosterone of >150 ng/dL at 20 months after the start of ADT (Table 3), which remained undetectable in 2 patients for 27 and 46 months, respectively. Overall, with a median follow-up of 47 months, castration-resistant disease has been documented in only 8 patients (53%; 95% confidence interval: 24%–81%) at a median of 23 months (range: 14–54). There was 1 death from prostate cancer at 59 months from the start of therapy.
DISCUSSION
The results of this pilot study show that a multimodal treatment strategy can eliminate all detectable disease in patients with metastatic disease at presentation who are considered incurable with any single form of therapy. The approach includes treatment of all visible disease, building on the ADT standard for metastatic disease by adding radical surgery to resect the primary tumor and LNs, SBRT to treat visible lesions, and intensity-modulated radiotherapy to prostate bed and nodal basins in patients at high risk of recurrence in these locations. Overall, 20% of the patients treated had an undetectable PSA and non-castrate levels of testosterone at 20 months, an end point that could not have been achieved with any single therapy alone. Although longer follow-up is needed to assess durability, this binary end point represents a first step toward establishing a paradigm of cure in patients with low-volume metastatic disease.
The sequential use of the 3 different modalities helped illustrate the role and importance of each in achieving the undetectable PSA end point, which represents an NED status, in patients with soft tissue only (M1a) and those with osseous +/− soft tissue disease (M1b). It is notable that none of the 5 M1a patients achieved an undetectable PSA following ADT alone, but 4 (80%) achieved an undetectable PSA after RP + PLND + RPLND (Figs. 1, 2). In contrast, 14 of the 15 M1b patients (93%; 95% confidence interval: 77%–100%) achieved an undetectable PSA: 5 after ADT alone, 6 more after RP + PLND, and 3 more after SBRT to metastatic lesions. This is consistent with reports demonstrating the inability of systemic therapy alone to eliminate disease in either the primary or metastatic sites,12–14 and highlights the need to treat each site of disease in order to reach the NED end point. Failure to achieve an undetectable PSA was associated with early relapse, consistent with the Southwest Oncology Group (SWOG) 9346 trial, which showed that the failure to achieve a PSA nadir of <0.2 ng/mL after ADT was associated with inferior survival times.15 Consistent with the multimodal approach of treating all visible disease, bone metastases were treated with SBRT. Recent data show that at least short-term local tumor control can be achieved by this approach in 79%–99% of patients with limited bone metastasis, depending on treatment dose.16,17
Whether treatment of the primary tumor in the setting of metastatic disease provides long-term benefit remains an open question in prostate cancer research.18,19 It is, however, the standard of care in the management of other urologic cancers, including urothelial,20 testicular,21 and renal cell carcinoma,22 where surgical resection of disease to achieve a complete remission status provides incremental cures. It is also becoming more prevalent in non-genitourinary malignancies, likely due to multiple factors including better systemic therapies and decreased perioperative morbidity as a result of improved patient selection.23 Men with N1 disease treated with RP have a reduced risk of death from prostate cancer compared to those treated with non-curative therapies.24,25 A reduction in prostate cancer-specific mortality in men with M1 disease whose primary tumor is treated definitively has also been shown in population-based studies,26–28 and the feasibility of RP in this population has been established.29,30 Prospective trials to test this question are combining surgery or radiation with “best systemic therapy” (NCT01751438) or radiation to the primary tumor with systemic therapy (NCT00268476, NCT01957436, and NTR271). Over time, these trials may ultimately demonstrate a survival benefit from local therapy in metastatic disease, but as the end points are time-to-event measures that occur late, the readouts of success or failure will take years, and the number of regimens that can be tested are limited.
Using undetectable PSA with non-castrate testosterone, or NED, as the primary measure of treatment efficacy contrasts with the more traditional time-to-event measures used in early-state trials—including time to PSA progression or recurrence, metastatic progression, or death—which typically require large numbers of patients to be followed for long periods of time and often provide uncertain results that do not impact practice. For example, docetaxel was approved for treatment of mCRPC in 2004, and was shown to prolong survival in combination with ADT (vs ADT alone) for men with metastatic non-castrate disease in the CHAARTED and STAMPEDE trials in 2014 and 2015, respectively.31,32 Establishing the role of ADT + docetaxel in men with high-risk localized disease has proven to be more challenging. GETUG 12 explored this question using a similar regimen but required 13 years from activation to reporting to show an absolute 8% difference in progression-free survival events, most of which were PSA recurrences of uncertain clinical significance.33 The first report of STAMPEDE showing a clear survival benefit for patients with metastatic disease did not show the same benefit for patients with “low-volume” disease.34 In both trials, there were too few events to show a difference in overall survival, an end point that in itself is becoming even more difficult to demonstrate because of the availability of multiple life-prolonging treatments for patients after they have completed treatment on a protocol.
Limitations to the general application of the results of this pilot study include the highly selected nature of the patients treated and small sample size in this pilot experience. Nevertheless, that 4 patients achieved the end point of an undetectable PSA with non-castrate levels of testosterone at 20 months demonstrates that achieving an NED state with a multimodal approach is possible, and suggests a threshold on which to power larger trials evaluating recently approved life-prolonging agents in this context.
The NED state we describe here is a binary response indicator resulting solely from treatment that occurs in under 2 years and is a requisite first step toward establishing a cure paradigm. It is justified by the level of activity seen with the approved and generally available life-prolonging systemic therapies for men with mCRPC, which are comparable with those seen in other tumor types that can result in cure when used earlier in the disease course. It is necessary because of the large number of potential combinations in need of testing and the need for an objective methodology to rank and prioritize them for large-scale testing. Because of the novelty of the end point—an undetectable PSA with non-castrate testosterone levels—and the recognition that the benchmark for what is considered sufficient activity is evolving, a multi-arm randomized (phase 2) clinical trial framework with a concurrent control will enable new experimental approaches to be added at any time. Whether the end point translates into durable oncological benefit is an open question and will require validation in phase 3 clinical trials, but combined with the proposed multimodal treatment strategy it will ensure the timely evaluation of multiple strategies so that only the most promising ones are moved forward. Regimens that do not result in an NED state are unlikely to translate into durable responses and can be de-prioritized.
CONCLUSION
A multimodal treatment strategy for patients who present with disease that is beyond the limits of curability by any single modality enables the evaluation of new approaches in order to prioritize large-scale testing in early stages of advanced disease. The end point, an undetectable PSA with non-castrate testosterone levels, also shifts the paradigm from palliation to cure.
Acknowledgments
Funding Support: The study was supported by The Sidney Kimmel Center for Prostate and Urologic Cancers, NIH/NCI Cancer Center Support Grant P30 CA008748, NIH/NCI Cancer Center Support Grant P50 CA092629, The Prostate Cancer Foundation, and the David H. Koch Fund for Prostate Cancer Research.
Footnotes
Financial Disclosure: The authors declare that they have no relevant financial interests.
References
- 1.Bolla M, Van Tienhoven G, Warde P, et al. External irradiation with or without long-term androgen suppression for prostate cancer with high metastatic risk: 10-year results of an EORTC randomised study. Lancet Oncol. 2010;11:1066–1073. doi: 10.1016/S1470-2045(10)70223-0. [DOI] [PubMed] [Google Scholar]
- 2.Roach M, 3rd, Bae K, Speight J, et al. Short-term neoadjuvant androgen deprivation therapy and external-beam radiotherapy for locally advanced prostate cancer: long-term results of RTOG 8610. J Clin Oncol. 2008;26:585–591. doi: 10.1200/JCO.2007.13.9881. [DOI] [PubMed] [Google Scholar]
- 3.Widmark A, Klepp O, Solberg A, et al. Endocrine treatment, with or without radiotherapy, in locally advanced prostate cancer (SPCG-7/SFUO-3): an open randomised phase III trial. Lancet. 2009;373:301–308. doi: 10.1016/S0140-6736(08)61815-2. [DOI] [PubMed] [Google Scholar]
- 4.Mason MD, Parulekar WR, Sydes MR, et al. Final report of the intergroup randomized study of combined androgen-deprivation therapy plus radiotherapy versus androgen-deprivation therapy alone in locally advanced prostate cancer. J Clin Oncol. 2015;33:2143–2150. doi: 10.1200/JCO.2014.57.7510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klotz LH, Goldenberg SL, Jewett MA, et al. Long-term followup of a randomized trial of 0 versus 3 months of neoadjuvant androgen ablation before radical prostatectomy. J Urol. 2003;170:791–794. doi: 10.1097/01.ju.0000081404.98273.fd. [DOI] [PubMed] [Google Scholar]
- 6.Schulman CC, Debruyne FM, Forster G, Selvaggi FP, Zlotta AR, Witjes WP. 4-year follow-up results of a European prospective randomized study on neoadjuvant hormonal therapy prior to radical prostatectomy in T2-3N0M0 prostate cancer. European Study Group on Neoadjuvant Treatment of Prostate Cancer. Eur Urol. 2000;38:706–713. doi: 10.1159/000020366. [DOI] [PubMed] [Google Scholar]
- 7.AstraZeneca. Lynparza (olaparib) granted Breakthrough Therapy designation by US FDA for treatment of BRCA1/2 or ATM gene mutated metastatic castration resistant prostate cancer [press release] Available at: https://www.astrazeneca.com/media-centre/press-releases/2016/Lynparza-Olaparib-granted-Breakthrough-Therapy-Designation-by-US-FDA-for-treatment-of-BRCA1-2-or-ATM-gene-mutated-metastatic-Castration-Resistant-Prostate-Cancer-28012016.html. Accessed January 28, 2016.
- 8.Rathkopf D, Carducci MA, Morris MJ, et al. Phase II trial of docetaxel with rapid androgen cycling for progressive noncastrate prostate cancer. J Clin Oncol. 2008;26:2959–2965. doi: 10.1200/JCO.2007.15.1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER) Guidance for industry: pathological complete response in neoadjuvant treatment of high-risk early-stage breast cancer: use as an endpoint to support accelerated approval. 2014 Oct; Available at: http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm305501.pdf. Accessed September 29, 2016.
- 10.James ND, Spears MR, Clarke NW, et al. Survival with newly diagnosed metastatic prostate cancer in the “docetaxel era”: data from 917 patients in the control arm of the STAMPEDE trial (MRC PR08, CRUK/06/019) Eur Urol. 2015;67:1028–1038. doi: 10.1016/j.eururo.2014.09.032. [DOI] [PubMed] [Google Scholar]
- 11.Scher HI, Halabi S, Tannock I, et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J Clin Oncol. 2008;26:1148–1159. doi: 10.1200/JCO.2007.12.4487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tzelepi V, Efstathiou E, Wen S, et al. Persistent, biologically meaningful prostate cancer after 1 year of androgen ablation and docetaxel treatment. J Clin Oncol. 2011;29:2574–2581. doi: 10.1200/JCO.2010.33.2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gleave ME, Goldenberg SL, Chin JL, et al. Randomized comparative study of 3 versus 8-month neoadjuvant hormonal therapy before radical prostatectomy: biochemical and pathological effects. J Urol. 2001;166:500–506. discussion 506–507. [PubMed] [Google Scholar]
- 14.Taplin ME, Montgomery B, Logothetis CJ, et al. Intense androgen-deprivation therapy with abiraterone acetate plus leuprolide acetate in patients with localized high-risk prostate cancer: results of a randomized phase II neoadjuvant study. J Clin Oncol. 2014;32:3705–3715. doi: 10.1200/JCO.2013.53.4578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hussain M, Tangen CM, Higano C, et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162) J Clin Oncol. 2006;24:3984–3990. doi: 10.1200/JCO.2006.06.4246. [DOI] [PubMed] [Google Scholar]
- 16.Muacevic A, Kufeld M, Rist C, Wowra B, Stief C, Staehler M. Safety and feasibility of image-guided robotic radiosurgery for patients with limited bone metastases of prostate cancer. Urol Oncol. 2013;31:455–460. doi: 10.1016/j.urolonc.2011.02.023. [DOI] [PubMed] [Google Scholar]
- 17.Ost P, Jereczek-Fossa BA, As NV, et al. Progression-free survival following stereotactic body radiotherapy for oligometastatic prostate cancer treatment-naive recurrence: a multi-institutional analysis. Eur Urol. 2015;69:9–12. doi: 10.1016/j.eururo.2015.07.004. [DOI] [PubMed] [Google Scholar]
- 18.Ristau BT, Cahn D, Uzzo RG, Chapin BF, Smaldone MC. The role of radical prostatectomy in high-risk localized, node-positive and metastatic prostate cancer. Future Oncol. 2016;12:687–699. doi: 10.2217/fon.15.355. [DOI] [PubMed] [Google Scholar]
- 19.Logothetis CJ, Aparicio AM. Is it time to re-examine the prostate cancer treatment paradigm by targeting the interaction between the prostate and metastases? J Clin Oncol. 2016;34:2810–2811. doi: 10.1200/JCO.2016.68.4738. [DOI] [PubMed] [Google Scholar]
- 20.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859–866. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 21.Donohue JP, Roth LM, Zachary JM, Rowland RG, Einhorn LH, Williams SG. Cytoreductive surgery for metastatic testis cancer: tissue analysis of retroperitoneal masses after chemotherapy. J Urol. 1982;127:1111–1114. doi: 10.1016/s0022-5347(17)54256-1. [DOI] [PubMed] [Google Scholar]
- 22.Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345:1655–1659. doi: 10.1056/NEJMoa003013. [DOI] [PubMed] [Google Scholar]
- 23.Bartlett EK, Simmons KD, Wachtel H, et al. The rise in metastasectomy across cancer types over the past decade. Cancer. 2015;121:747–757. doi: 10.1002/cncr.29134. [DOI] [PubMed] [Google Scholar]
- 24.Engel J, Bastian PJ, Baur H, et al. Survival benefit of radical prostatectomy in lymph node-positive patients with prostate cancer. Eur Urol. 2010;57:754–761. doi: 10.1016/j.eururo.2009.12.034. [DOI] [PubMed] [Google Scholar]
- 25.Steuber T, Budaus L, Walz J, et al. Radical prostatectomy improves progression-free and cancer-specific survival in men with lymph node positive prostate cancer in the prostate-specific antigen era: a confirmatory study. BJU Int. 2011;107:1755–1761. doi: 10.1111/j.1464-410X.2010.09730.x. [DOI] [PubMed] [Google Scholar]
- 26.Culp SH, Schellhammer PF, Williams MB. Might men diagnosed with metastatic prostate cancer benefit from definitive treatment of the primary tumor? A SEER-based study. Eur Urol. 2014;65:1058–1066. doi: 10.1016/j.eururo.2013.11.012. [DOI] [PubMed] [Google Scholar]
- 27.Satkunasivam R, Kim AE, Desai M, et al. Radical prostatectomy or external beam radiation therapy vs no local therapy for survival benefit in metastatic prostate cancer: a SEER-Medicare analysis. J Urol. 2015;194:378–385. doi: 10.1016/j.juro.2015.02.084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rusthoven CG, Jones BL, Flaig TW, et al. Improved survival with prostate radiation in addition to androgen deprivation therapy for men with newly diagnosed metastatic prostate cancer. J Clin Oncol. 2016;34:2835–2842. doi: 10.1200/JCO.2016.67.4788. [DOI] [PubMed] [Google Scholar]
- 29.Heidenreich A, Pfister D, Porres D. Cytoreductive radical prostatectomy in patients with prostate cancer and low volume skeletal metastases: results of a feasibility and case-control study. J Urol. 2015;193:832–838. doi: 10.1016/j.juro.2014.09.089. [DOI] [PubMed] [Google Scholar]
- 30.Sooriakumaran P, Karnes J, Stief C, et al. A multi-institutional analysis of perioperative outcomes in 106 men who underwent radical prostatectomy for distant metastatic prostate cancer at presentation. Eur Urol. 2016;69:788–794. doi: 10.1016/j.eururo.2015.05.023. [DOI] [PubMed] [Google Scholar]
- 31.Sweeney CJ, Chen YH, Carducci M, et al. Chemohormonal therapy in metastatic hormone-sensitive prostate cancer. N Engl J Med. 2015;373:737–746. doi: 10.1056/NEJMoa1503747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.James ND, Sydes MR, Mason MD, et al. Docetaxel and/or zoledronic acid for hormone-naive prostate cancer: first overall survival results from STAMPEDE ( NCT00268476) J Clin Oncol. 2015;33(Suppl) abstr 5001. [Google Scholar]
- 33.Fizazi K, Faivre L, Lesaunier F, et al. Androgen deprivation therapy plus docetaxel and estramustine versus androgen deprivation therapy alone for high-risk localised prostate cancer (GETUG 12): a phase 3 randomised controlled trial. Lancet Oncol. 2015;16:787–794. doi: 10.1016/S1470-2045(15)00011-X. [DOI] [PubMed] [Google Scholar]
- 34.James ND, Sydes MR, Clarke NW, et al. Addition of docetaxel, zoledronic acid, or both to first-line long-term hormone therapy in prostate cancer (STAMPEDE): survival results from an adaptive, multiarm, multistage, platform randomised controlled trial. Lancet. 2016;387:1163–1177. doi: 10.1016/S0140-6736(15)01037-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


