Abstract
Background
Androgen deprivation therapy (ADT) increases the risk for fractures in patients with prostate cancer.
Objective
To assess the cost-effectiveness of measuring bone mineral density (BMD) before initiating ADT followed by alendronate therapy in men with localized prostate cancer.
Design
Markov state-transition model simulating the progression of prostate cancer and the incidence of hip fracture.
Data Sources
Published literature.
Target Population
A hypothetical cohort of men aged 70 years with locally advanced or high-risk localized prostate cancer starting a 2-year course of ADT after radiation therapy.
Time Horizon
Lifetime.
Perspective
Societal.
Intervention
No BMD test or alendronate therapy, a BMD test followed by selective alendronate therapy for patients with osteoporosis, or universal alendronate therapy without a BMD test.
Outcome Measures
Incremental cost-effectiveness ratio (ICER), measured by cost per quality-adjusted life-year (QALY) gained.
Results of Base-Case Analysis
The ICERs for the strategy of a BMD test and selective alendronate therapy for patients with osteoporosis and universal alendronate therapy without a BMD test were $66 800 per QALY gained and $178 700 per QALY gained, respectively.
Results of Sensitivity Analyses
The ICER for universal alendronate therapy without a BMD test decreased to $100 000 per QALY gained, assuming older age, a history of fractures, lower mean BMD before ADT, or a lower cost of alendronate.
Limitations
No evidence shows that alendronate reduces actual fracture rates in patients with prostate cancer who receive ADT. The model predicted fracture rates by using data on the surrogate BMD end point.
Conclusion
In patients starting adjuvant ADT for locally advanced or high-risk localized prostate cancer, a BMD test followed by selective alendronate for those with osteoporosis is a cost-effective use of resources. Routine use of alendronate without a BMD test is justifiable in patients at higher risk for hip fractures.
Primary Funding Source
None.
Androgen deprivation therapy (ADT) comprises orchiectomy or gonadotropin-releasing hormone agonists with or without an antiandrogen. Once used primarily to treat metastatic prostate cancer, ADT is now used as adjuvant therapy for locally advanced or high-risk localized prostate cancer and as treatment of biochemical failure after primary therapy (1–3). Because most men with prostate cancer receive the diagnosis at an older age and because androgen deficiency is associated with low bone mineral density (BMD), men with prostate cancer who receive treatment with ADT are at particularly increased risk for osteoporosis and related fractures (4–9).
A physician survey and several descriptive studies done at single centers suggest that most patients with prostate cancer who receive ADT do not receive screening or treatment for bone loss (10–13). In the absence of consensus guidelines about fracture prevention in these patients, many experts have recommended a case-finding approach: measuring BMD by dual-energy x-ray absorptiometry before ADT and administering antiresorptive agents to patients who are at high risk for fractures (14–17). Others have advocated routine use of antiresorptive agents regardless of baseline BMD (18, 19). These recommendations go beyond available evidence because only oral alendronate and risedronate have been shown to reduce fracture rates in healthy men with osteoporosis (20, 21) and because none of the several antiresorptive agents shown to prevent bone loss from ADT has been shown to prevent fractures and none has been approved by the U.S. Food and Drug Administration for this indication (22–31). Furthermore, the cost-effectiveness of various screening and treatment strategies has not been determined.
We sought to estimate the cost-effectiveness of no BMD test or alendronate therapy, a BMD test followed by selective alendronate therapy for patients with osteoporosis, and universal alendronate therapy without a BMD test for men starting adjuvant ADT for locally advanced or high-risk localized prostate cancer.
Methods
We developed a Markov state-transition model simulating the progression of prostate cancer and the incidence of hip fractures. We assumed a societal perspective, a lifetime horizon, and a discount rate of 3% per year for both health benefits and costs (32). The analysis was done by using TreeAge Pro Suite 2008 software (TreeAge Software, Williamstown, Massachusetts).
Population
The model simulated a hypothetical cohort of men aged 70 years with locally advanced or high-risk localized prostate cancer (T2c to T4N0) starting a 2-year course of ADT after radiation therapy (33). We did not target patients who received ADT as monotherapy for low- or intermediate-risk localized prostate cancer (1, 2, 34). We assumed that no patients in the base-case cohort had a history of fragility fractures (for example, hip, vertebral, or wrist fractures). In sensitivity analyses, we varied assumptions about patient age and history of fractures.
Strategies
We compared 3 strategies: no BMD test and no alendronate therapy; a one-time BMD test before initiating ADT, followed by selective alendronate therapy for patients with osteoporosis; and universal alendronate therapy without a BMD test (Figure, top). In the test strategy, all patients had femoral neck BMD measurement by dual-energy x-ray absorptiometry before starting ADT. Bone mineral density was quantified by a T-score—the number of SDs above or below the mean for non-Hispanic white men aged 20 to 29 years (35). A T-score of −2.5 or less indicated osteoporosis. We assumed that alendronate therapy was continued for 5 years (36).
Figure. Clinical strategies and progression model for prostate cancer.
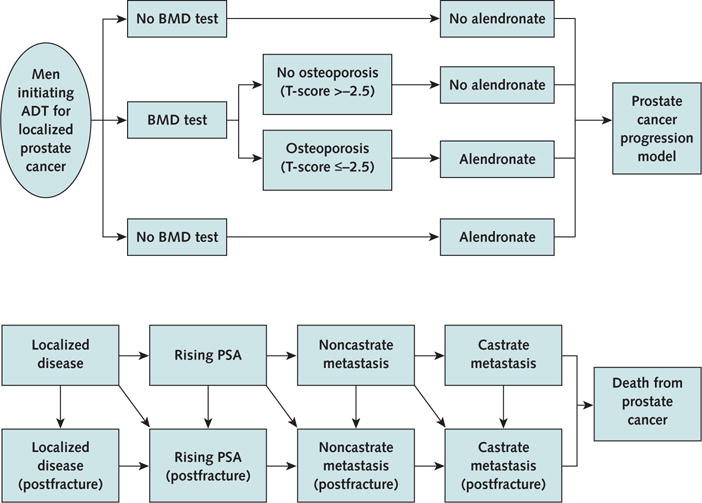
ADT = androgen deprivation therapy; BMD = bone mineral density; PSA = prostate-specific antigen. Top. Algorithm showing a decision made at the onset of ADT for localized prostate cancer. Patients assigned to the alendronate group received alendronate for 5 years after proceeding to the prostate cancer progression model. Bottom. Prostate cancer progression model. Patients enter the model with localized disease. Each year, patients are at risk for the progression of prostate cancer, the occurrence of a hip fracture, or both. Throughout the patients’ lifetime, all patients are at risk for death from causes unrelated to prostate cancer (not shown by the state or arrows). Squares represent the health states in the model. Arrows represent transitions between health states.
Model
The progression of prostate cancer was modeled through a sequence of health states: localized disease, rising prostate-specific antigen, noncastrate metastasis, castrate metastasis, and death from prostate cancer (Figure, bottom) (37). We assumed that if ADT had been discontinued after 2 years, it was resumed if patients developed noncastrate metastasis and was continued until death. Patients could die of other causes or experience hip fracture at any time and from any health state. We restricted analysis to hip fractures because the relationship between femoral neck BMD and fracture rates seems the most robust (38, 39). We assumed that the progression of prostate cancer was not altered by alendronate or hip fractures. We also assumed that recommended doses of supplemental calcium and vitamin D were administered in all patients and intravenous zoledronic acid was administered as a cancer-directed therapy in patients who developed castrate metastasis. All patients made annual transitions between the health states until they died or reached 100 years of age. Table 1 summarizes the model variables.
Table 1.
Model Variables
| Variable | Value | Range | Data Source |
|---|---|---|---|
| Age at onset of ADT, y | 70 | 60–80 | 33 |
|
| |||
| Progression of prostate cancer (per year), % | 14 | 11–17* | 33 |
| Localized disease to rising PSA | |||
|
| |||
| Rising PSA to noncastrate metastasis | 18 | 14–22* | 40 |
|
| |||
| Noncastrate metastasis to castrate metastasis | 36 | 24–52 | 41 |
|
| |||
| Castrate metastasis to prostate cancer death | 50 | 43–58 | 41 |
|
| |||
| Mean BMD before ADT, g/cm2 | 0.7540 | 0.5915–0.8069 | 35 |
| Rate of BMD loss (per year), g/cm2 | |||
|
| |||
| No ADT | 0.0035 | 0.0026–0.0044 | 42 |
|
| |||
| During ADT | 0.0188 | 0.0141–0.0235 | 43 |
| Incidence of hip fractures per patient-year in patients with mean BMD, by age, % | 44 | ||
|
| |||
| 60–64 y | 0.055 | 0.028–0.083 | – |
|
| |||
| 65–69 y | 0.094 | 0.047–0.141 | – |
|
| |||
| 70–74 y | 0.195 | 0.098–0.293 | – |
|
| |||
| 75–79 y | 0.402 | 0.201–0.603 | – |
|
| |||
| 80–84 y | 0.922 | 0.461–1.383 | – |
|
| |||
| 85–100 y | 2.357 | 1.179–3.536 | – |
| Relative risk for hip fractures per Z score, by age | 39 | ||
|
| |||
| 60–64 y | 3.07 | 2.42–3.89* | – |
|
| |||
| 65–69 y | 2.89 | 2.39–3.50* | – |
|
| |||
| 70–74 y | 2.78 | 2.39–3.23* | – |
|
| |||
| 75–79 y | 2.58 | 2.30–2.90* | – |
|
| |||
| 80–84 y | 2.28 | 2.09–2.50* | – |
|
| |||
| 85–100 y | 1.93 | 1.76–2.10* | – |
| Relative risk for hip fractures due to a previous fracture, by age | 45 | ||
|
| |||
| 60–64 y | 3.16 | 1.88–5.32* | – |
|
| |||
| 65–69 y | 2.28 | 1.52–3.41* | – |
|
| |||
| 70–74 y | 1.90 | 1.37–2.65* | – |
|
| |||
| 75–79 y | 1.64 | 1.24–2.17* | – |
|
| |||
| 80–84 y | 1.41 | 1.12–1.78* | – |
|
| |||
| 85–100 y | 1.32 | 1.04–1.68* | – |
|
| |||
| Bone loss prevented by alendronate, % | 100 | 50–100 | Assumed |
|
| |||
| Adherence rate to alendronate therapy, % | 100 | 50–100 | 46 |
|
| |||
| Incidence of upper gastrointestinal side effects of alendronate, % | 0.8 | 0–2 | 47 |
|
| |||
| Background mortality per year, %† | 2.72 | 2.04–3.40 | 48 |
|
| |||
| Relative risk for death within the first year after a hip fracture | 1.375 | 1–2 | 49, 50 |
|
| |||
| Health state utility of prostate cancer | |||
| Localized disease | 0.840 | 0.630–1.000 | 51 |
|
| |||
| Rising PSA | 0.800 | 0.600–1.000 | Assumed |
|
| |||
| Noncastrate metastasis | 0.440 | 0.330–0.550 | 51 |
|
| |||
| Castrate metastasis | 0.130 | 0.098–0.163 | 51 |
|
| |||
| Utility multiplier | |||
| Hip fracture | |||
| First year | 0.792 | 0.594–0.990 | 52–54 |
|
| |||
| Subsequent years | 0.813 | 0.610–1.000 | 52–54 |
|
| |||
| Upper gastrointestinal side effects of alendronate | 0.980 | 0.735–1.000 | 55 |
| Cost, $ | |||
|
| |||
| BMD test | 131 | 98–164 | 56 |
|
| |||
| Alendronate (per year) | 600 | 300–900 | 57 |
|
| |||
| Hip fracture, first year | 33 200 | 24 900–41 500 | 52, 54, 58 |
|
| |||
| Hip fracture, subsequent years (per year) | 8100 | 6450–10 750 | 52, 54, 58 |
|
| |||
| Upper gastrointestinal side effects of alendronate | 3000 | 2250–3750 | 56, 57 |
|
| |||
| Discount rate, % | 3 | 0–6 | 32 |
ADT = androgen deprivation therapy; BMD = bone mineral density; PSA = prostate-specific antigen.
95% CI.
Variable was age-specific. Values shown are for persons aged 70 years.
Progression of Prostate Cancer
Base-case estimates of disease progression were from the 10-year follow-up analysis of Radiation Therapy Oncology Group protocol 92-02 (33), a natural history study of patients with rising prostate-specific antigen after ADT, and a previous cost-effectiveness model for localized prostate cancer (40, 41).
BMD and Incidence of Hip Fracture
We simulated changes in BMD over time and predicted the incidence of hip fractures as a function of age and BMD (59, 60). As patients aged, the model calculated an updated BMD on the basis of baseline BMD at the onset of ADT and the number of years since model entry. We assumed that no difference was found in baseline BMD between patients with prostate cancer who did not receive ADT and the white male population from the Third National Health and Nutrition Examination Survey (35). The estimated prevalence of osteoporosis in the base-case cohort was 11%. The rate of BMD loss in the absence of ADT was assumed to follow the rate reported in the Framingham Osteoporosis Study (42). The rate of BMD loss during ADT was calculated by fitting a linear regression to cross-sectional data of total hip BMD over a broad spectrum of therapy durations up to 10 years (43). We assumed that the rate of BMD loss was constant during the course of ADT and returned to the baseline rate of BMD loss in the year after completion of ADT. We converted the updated BMD to an equivalent Z score and then calculated the incidence of hip fractures specific for age and BMD (iage, BMD) by using the following relationship (38):
in which “iage” denotes the hip fracture incidence in men with mean BMD for that age (Z score of 0), “a” is the relative risk per each decrease in Z score, and “Z” is the Z score. We obtained iage from fracture data for white men from the 2001 Nationwide Inpatient Sample database (44). A history of fractures confers an increased risk for subsequent fractures (7, 45). We assumed that the prevalence of osteoporosis was 1.91 times higher in patients with a previous fracture than in those without fracture (53).
Treatment Effect
The effect of treatment on fracture incidence was modeled under the assumption that patients had no BMD loss throughout the course of alendronate therapy (22, 23, 59, 60). In the base case, we assumed 100% adherence to alendronate therapy and tested lower adherence in a sensitivity analysis (46). We assumed that alendronate did not affect BMD in patients who stopped taking alendronate and that zoledronic acid reduced the risk for hip fracture by 24% in patients with castrate metastasis (61).
Side Effects
We assumed that 0.8% of patients had serious upper gastrointestinal side effects (such as perforation, ulcer, or bleeding) in the first year of alendronate therapy (47). We assumed that each episode required a hospitalization, 2 additional physician visits, and treatment with a proton-pump inhibitor for 1 year. Alendronate therapy was stopped and never restarted after these events.
Death
Background mortality rates were based on 2004 U.S. life tables published by the National Center for Health Statistics (48). Excess mortality from a hip fracture was modeled only in the same year that the hip fracture occurred (49, 50).
Quality of Life
We assigned a utility to each health state that reflected the preference for, or desirability of, that state. Health state utilities were taken from studies that used standardized methods (the time-tradeoff or standard gamble technique) to elicit preferences. Because no utility has been reported for the rising prostate-specific antigen state, we assigned a slightly lower utility than that for localized disease. The utility multiplier of hip fractures was obtained from the Swedish prospective study of fracture patients (52). The utility for serious upper gastrointestinal side effects of alendronate was a value for complicated peptic ulcer that required hospitalization (55). All health state utilities were varied in sensitivity analyses.
Costs
The costs of dual-energy x-ray absorptiometry, a physician visit, and a hospitalization for serious upper gastrointestinal side effects of alendronate (diagnosis-related group code 183) were based on average Medicare reimbursement for these services (56). We used retail prices of alendronate and a proton-pump inhibitor (omeprazole) reported by the New York State Board of Pharmacy (57). Patients who did not adhere to alendronate therapy accrued the medication cost for only 6 months (46). Fracture costs were taken from a population-based cost analysis in Olmsted County, Minnesota (53, 54, 58). We assumed that the cost of treating prostate cancer was independent of BMD and fracture status. All costs were inflated to 2008 dollars by using the Consumer Price Index for Medical Care for All Urban Consumers (62).
Outcomes
We measured health benefits in quality-adjusted life-years (QALYs) gained. Incremental cost-effectiveness analysis was done by first ranking the strategies in order of increasing cost. Then, after eliminating strategies that were more or equally costly and less effective than a competing strategy (that is, ruled out by simple dominance), we calculated the incremental cost-effectiveness ratio (ICER) of each strategy as the additional cost of that strategy divided by its additional benefit compared with the next most costly strategy. If a strategy was less effective and had a higher ICER than another strategy, it was ruled out by extended dominance. We eliminated strategies exhibiting extended dominance from the rank-ordered list, and we recalculated ICERs of the remaining strategies. After these standard methods, each nondominated strategy was compared with the next most costly strategy. The incremental cost-effectiveness of the least costly, viable (nondominated) strategy was not calculated (32) because there was no comparator.
Model Validation
Ten-year overall survival was 51%, and disease-free survival was 15% in the simulated cohort, which approximated estimates of 54% (95% CI, 50% to 58%) and 23% (CI, 19% to 26%) found in Radiation Therapy Oncology Group protocol 92-02 (33). The estimated mean overall survival was 11.0 years. The cumulative lifetime probability of hip fracture, assuming no BMD test or alendronate therapy, was 12.6% (1.15% per patient-year), slightly lower than claim-based data (1.26% to 1.36% per patient-year) (8, 9).
Role of the Funding Source
We received no funding for this study.
Results
Base-Case Analysis
Table 2 shows the cumulative lifetime probability of hip fracture and mortality due to hip fractures, cost, undiscounted life-years, QALYs, and ICER for each strategy. Among all strategies, the no test–no alendronate strategy became the reference strategy because it was the least costly, viable (nondominated) option. Compared with the no test–no alendronate strategy, the strategy of a BMD test and selective alendronate therapy for patients with osteoporosis was more costly and more effective and had an ICER of $66 800 per QALY gained. Compared with the strategy of a BMD test and selective alendronate therapy for patients with osteoporosis, universal alendronate therapy without a BMD test was even more costly and more effective but had an ICER of $178 700 per QALY gained.
Table 2.
Base-Case Analysis*
| Strategy | Cumulative Lifetime Probability, % | Cost, $ | Life-Years† | QALYs | Incremental Cost, $ | Incremental QALYs | ICER, $/QALY‡ | |
|---|---|---|---|---|---|---|---|---|
| Hip Fracture | Death Due to Hip Fracture | |||||||
| No test and no alendronate therapy | 12.6 | 0.43 | 75 474 | 10.9965 | 6.5930 | Reference§ | Reference§ | Reference§ |
| Test and selective alendronate therapy | 12.0 | 0.40 | 75 652 | 10.9971 | 6.5957 | 178 | 0.0027 | 66 800 |
| No test and universal alendronate therapy | 9.9 | 0.33 | 77 153 | 10.9991 | 6.6041 | 1501 | 0.0084 | 178 700 |
ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year.
The strategy was considered cost-effective if its ICER was less than $100 000 per QALY gained (32, 63).
Undiscounted results.
ICER was measured by cost per QALY gained.
The no test–no alendronate strategy was the reference strategy because it was the least costly, viable (nondominated) option.
Sensitivity Analyses
The ICER for each strategy improved with older age at the onset of ADT and was substantially better for patients with a previous fracture (Table 3). If society would be willing to pay $100 000 per QALY gained, universal alendronate therapy without a BMD test would be preferred for patients 75 years or older without a previous fracture, as well as patients 65 years or older with a previous fracture. Universal alendronate therapy without a BMD test would become more effective and less costly than the strategy of a BMD test and selective alendronate therapy for patients aged 80 years with a previous fracture.
Table 3.
ICERs for Each Strategy, by Age and Previous Fracture Status*
| Age, y | ICER, by Previous Fracture, $/QALY | |||
|---|---|---|---|---|
| No Previous Fracture† | Previous Fracture | |||
| Test and Selective Alendronate Therapy | No Test and Universal Alendronate Therapy | Test and Selective Alendronate Therapy | No Test and Universal Alendronate Therapy | |
| 60 | 156 900 | 470 300 | 19 600 | 119 000 |
| 65 | 95 500 | 283 000 | 8500 | 72 300 |
| 70† | 66 800 | 178 700 | 6300 | 44 500 |
| 75 | 46 900 | 103 000 | 5700 | 17 300 |
| 80 | 37 200 | 61 500 | Dominated‡ | 2300 |
ICER = incremental cost-effectiveness ratio; QALY = quality-adjusted life-year.
ICER was measured by cost per QALY gained. The no test–no alendronate strategy was the reference strategy because it was the least costly, viable (nondominated) option. The strategy was considered cost-effective if its ICER was less than $100 000 per QALY gained (32, 63).
Base-case assumptions.
Universal alendronate therapy without a bone mineral density test dominated this strategy by simple dominance because it was less effective and more costly.
Our results were sensitive to assumptions about the cost of alendronate. If society would be willing to pay $100 000 per QALY gained, universal alendronate therapy without a BMD test would be preferred if the cost of alendronate decreased to $430 per year. If society would be willing to pay $50 000 per QALY gained, universal alendronate therapy without a BMD test would be preferred if the cost of alendronate decreased to $320 per year.
Our results were also sensitive to assumptions about the mean BMD in the base-case population and the effectiveness of alendronate in preventing bone loss (Table 4). If society would be willing to pay $100 000 per QALY gained, universal alendronate therapy without a BMD test would be preferred if the mean BMD was lower than 0.6970 g/cm2 (that is, prevalence of osteoporosis was higher than 21%), assuming no bone loss during alendronate therapy. If society would be willing to pay $50 000 per QALY gained, universal alendronate therapy without a BMD test would be preferred if the mean BMD was lower than 0.6490 g/cm2 (that is, the prevalence of osteoporosis was higher than 33%), assuming no bone loss during alendronate therapy. The ICER for each strategy remained greater than $100 000 per QALY gained assuming a 50% reduction in bone loss from alendronate therapy.
Table 4.
ICERs for Each Strategy, by Mean BMD Before ADT and Effectiveness of Alendronate*
| Mean BMD, g/cm2 | Prevalence of Osteoporosis, %† | ICER, by Bone Loss Prevented by Alendronate, $/QALY | ||||||
|---|---|---|---|---|---|---|---|---|
| 100%‡ | 75% | 50% | ||||||
| Test and Selective Alendronate Therapy | No Test and Universal Alendronate Therapy | Test and Selective Alendronate Therapy | No Test and Universal Alendronate Therapy | Test and Selective Alendronate Therapy | No Test and Universal Alendronate Therapy | |||
| 0.8069 | 5 | 123 300 | 271 700 | 185 900 | 391 500 | 312 100 | 615 600 | |
| 0.7540‡ | 11 | 66 800 | 178 700 | 112 700 | 257 400 | 205 400 | 417 200 | |
| 0.7017 | 20 | 44 000 | 104 800 | 83 200 | 161 500 | 162 200 | 276 400 | |
| 0.6601 | 30 | 35 200 | 60 500 | 71 700 | 104 200 | 145 600 | 192 600 | |
| 0.6246 | 40 | Dominated§ | 30 500 | Dominated§ | 65 600 | Dominated§ | 136 400 | |
| 0.5915 | 50 | Dominated§ | 17 500 | Dominated§ | 48 900 | Dominated§ | 112 300 | |
BMD = bone mineral density; ICER = incremental cost-effectiveness ratio; QALY quality-adjusted life-year.
ICER was measured by cost per QALY gained. The no test–no alendronate strategy was the reference strategy because it was the least costly, viable (nondominated) option. The strategy was considered cost-effective if its ICER was less than $100 000 per QALY gained (32, 63).
Assumed a normal distribution of BMD and 0.5915 g/cm2 as a BMD cut-off value for the diagnosis of osteoporosis.
Base-case assumptions.
Universal alendronate therapy without a BMD test dominated this strategy by simple dominance because it was less effective and more costly.
The ICER for each strategy did not substantially change across a wide range of assumptions evaluated in all other sensitivity analyses (Appendix Table, available at www.annals.org).
Discussion
The American College of Physicians recently concluded that osteoporosis screening would not be cost-effective in U.S. men younger than 80 years and recommended screening only for “men who are at increased risk for osteoporosis” and candidates for drug therapy, with ADT identified as an important risk factor for low BMD–mediated fractures (64). The results of our analysis support that recommendation. In men aged 70 years with locally advanced or high-risk localized prostate cancer, a BMD test before adjuvant ADT followed by selective alendronate therapy for those who received a diagnosis of osteoporosis was reasonably cost-effective. Although universal alendronate therapy without a BMD test yielded the greatest average health benefit, its estimated ICER was higher than generally accepted cost-effectiveness thresholds in the United States (32, 63). Our analysis suggested that universal alendronate therapy without a BMD test had a potential to become reasonably cost-effective if the target population was older, had a history of fractures, or had lower mean BMD before ADT or if the cost of alendronate was lower than our base-case estimates.
The National Osteoporosis Foundation recommends shifting the treatment approach from one based on BMD to one based on absolute fracture risk calculated by the World Health Organization Fracture Risk Assessment Tool (FRAX) (65, 66). The FRAX is designed to help physicians decide when to initiate antiresorptive therapy by providing a person’s 10-year absolute fracture probability based on clinical risk factors with or without femoral neck BMD. The concept of treating patients regardless of BMD status is intuitively appealing, although it depends on an unproven assumption that antiresorptive therapy reduces the incidence of fractures across all levels of BMD (67, 68). Even though the FRAX is derived and validated for population-based cohorts across the world, the algorithm does not take into account accelerated bone loss during the course of ADT or excess mortality due to prostate cancer and has yet to be validated for patients with prostate cancer who receive ADT. Therefore, we used the presence of osteoporosis, as defined by T-score of BMD, as a treatment threshold.
The most frequently cited barriers for osteoporosis screening include uncertainty about effectiveness, costs, and potential side effects of treatment (69). Relative to the strategy of no BMD test and no alendronate therapy, the estimated health benefits of more active strategies were modest: an added 4.1 days of quality-adjusted life for universal alendronate therapy without a BMD test and even fewer for the strategy of a BMD test and selective alendronate therapy. Compared with the recently published cost-effectiveness analyses for U.S. men, our base-case assumptions related to the effectiveness of alendronate are conservative (53, 54). By excluding the effect of non-hip fractures, we may have underestimated the total health benefit of alendronate therapy. Also, the retail price of alendronate has decreased substantially since the loss of patent protection in February 2008, and our sensitivity analysis suggested that universal alendronate therapy without testing is increasingly more cost-effective with a progressive reduction in cost of alendronate. We chose alendronate as a therapeutic intervention because other, particularly intravenous, bisphosphonates are associated with substantially higher direct costs (70). Although the long-term safety of oral alendronate has not been formally evaluated in patients with prostate cancer, a pooled analysis of clinical trials showed no difference in upper gastrointestinal events between alendronate and placebo (71). Our conclusions were robust to a reasonable range of assumptions about the incidence, cost, and quality-of-life effects of upper gastrointestinal adverse events of alendronate. Recently, osteonecrosis of the jaw has been recognized as an important complication of bisphosphonate therapy, with a large effect on quality of life (72). The reported incidence is low (from 1 in 10 000 to 1 in 100 000 patient-treatment-years) in patients who receive oral bisphosphonates for osteoporosis (73, 74), and we therefore did not model it explicitly.
The main limitation of our analysis is that BMD is a surrogate measure of risk for hip fractures and fracture risk reduction by alendronate. Whether the beneficial effect of alendronate on BMD correlates with a decreased fracture incidence has yet to be determined in patients with prostate cancer who receive ADT. Evidence of a statistically significant reduction of nonvertebral fractures in men is currently insufficient, but clinical trials of newer agents have been emerging. For example, a clinical trial of denosumab, a human monoclonal antibody against receptor activator of nuclear factor-κB ligand, showed a statistically significant reduction of new vertebral fractures and a trend toward a reduction of nonvertebral fractures in patients who receive ADT for prostate cancer (31).
As ADT is used with increasing frequency in men with localized prostate cancer, maintenance of their bone health is a growing public health challenge. Our results suggest that in patients with locally advanced or high-risk localized prostate cancer starting a 2-year course of ADT after radiation therapy, the strategy of a BMD test and alendronate therapy in those with osteoporosis for 5 years is a cost-effective use of resources. Routine use of alendronate is not justifiable unless patients are older, have a history of fractures, or have lower mean BMD before ADT. These results are encouraging and suggest that prevention of bone loss with alendronate is cost-effective when treatment is targeted to patients at high risk for fractures. Our results also suggest that Medicare coverage of a BMD test could be expanded to this patient population (75). Future research should assess whether the effect of alendronate on BMD correlates with a reduction in fracture rates in this patient population.
Context
Androgen deprivation therapy increases fracture risk in men with prostate cancer.
Contribution
This analysis suggests that in a population of men with prostate cancer who receive androgen deprivation therapy, dual-energy x-ray absorptiometry screening followed by treatment of those with osteoporosis is more cost-effective than no screening and no treatment, and more cost-effective than treating all men.
Caution
No data show that bisphosphonates decrease fractures in men with prostate cancer. The estimates apply only to men older than 70 years.
Implication
In men with prostate cancer who receive androgen deprivation therapy, dual-energy x-ray absorptiometry screening followed by treatment of selective alendronate for those with osteoporosis might be a cost-effective way to prevent fractures.
—The Editors
Acknowledgments
This study was presented at the 2009 American Society for Clinical Oncology Annual Meeting, Orlando, Florida, 29 May–2 June 2009.
Appendix Table.
ICERs for Each Strategy in Additional 1-Way Sensitivity Analyses*
| Variable | ICER, $/QALY
|
|
|---|---|---|
| Test and Selective Alendronate Therapy | No Test and Universal Alendronate Therapy | |
| Base case | 66 800 | 178 700 |
|
| ||
| ADT for 5 y | 47 800 | 160 000 |
| Progression of prostate cancer (per year) | ||
| Localized disease to rising PSA | ||
|
| ||
| 11% | 45 200 | 135 500 |
|
| ||
| 17% | 87 300 | 220 300 |
| Rising PSA to noncastrate metastasis | ||
|
| ||
| 14% | 48 800 | 143 400 |
|
| ||
| 22% | 82 000 | 208 500 |
| Noncastrate metastasis to castrate metastasis | ||
|
| ||
| 24% | 67 200 | 179 200 |
|
| ||
| 52% | 66 400 | 178 000 |
| Castrate metastasis to prostate cancer death | ||
|
| ||
| 43% | 66 900 | 178 800 |
|
| ||
| 58% | 66 600 | 178 500 |
| Rate of BMD loss (per year) | ||
| No ADT | ||
|
| ||
| 0.0026 g/cm2 | 77 100 | 198 100 |
|
| ||
| 0.0044 g/cm2 | 57 300 | 160 800 |
| During ADT | ||
|
| ||
| 0.0141 g/cm2 | 112 000 | 256 300 |
|
| ||
| 0.0235 g/cm2 | 37 200 | 127 700 |
| Incidence of hip fractures per patient-year in patients with mean BMD† | ||
|
| ||
| 50% lower (0.098%) | 202 300 | 429 200 |
|
| ||
| 50% higher (0.293%) | 19 600 | 94 600 |
| Relative risk for hip fractures per Z score† | ||
|
| ||
| 2.39 | 107 900 | 211 700 |
|
| ||
| 3.23 | 35 400 | 151 600 |
| Relative risk for hip fractures due to a previous fracture† | ||
|
| ||
| 1.37 | 72 500 | 183 300 |
|
| ||
| 2.65 | 60 600 | 173 800 |
| Adherence rate to alendronate therapy | ||
|
| ||
| 75% | 87 300 | 194 700 |
|
| ||
| 50% | 128 200 | 226 800 |
| Incidence of upper gastrointestinal adverse events | ||
|
| ||
| 0% | 64 900 | 173 300 |
|
| ||
| 2% | 69 800 | 187 300 |
| Background mortality per year† | ||
|
| ||
| 25% lower (2.04%) | 48 300 | 141 700 |
|
| ||
| 25% higher (3.40%) | 86 100 | 217 200 |
| Relative risk for death within the first year after a hip fracture | ||
|
| ||
| 1.00 | 68 200 | 190 100 |
|
| ||
| 2.00 | 64 400 | 163 500 |
| Health state utility of prostate cancer | ||
| Localized disease | ||
|
| ||
| 0.630 | 75 400 | 200 400 |
|
| ||
| 1.000 | 61 500 | 165 100 |
| Rising PSA | ||
|
| ||
| 0.600 | 76 400 | 205 700 |
|
| ||
| 1.000 | 59 300 | 158 000 |
| Noncastrate metastasis | ||
|
| ||
| 0.330 | 67 500 | 180 500 |
|
| ||
| 0.550 | 66 200 | 176 900 |
| Castrate metastasis | ||
|
| ||
| 0.098 | 66 800 | 178 800 |
|
| ||
| 0.163 | 66 800 | 178 600 |
| Utility multiplier | ||
| Hip fracture (first year) | ||
|
| ||
| 0.594 | 53 800 | 144 600 |
|
| ||
| 0.990 | 88 200 | 233 900 |
| Hip fracture (subsequent years) | ||
|
| ||
| 0.610 | 40 000 | 105 200 |
|
| ||
| 1.000 | 174 200 | 501 800 |
| Upper gastrointestinal side effects of alendronate | ||
|
| ||
| 0.735 | 71 600 | 216 600 |
|
| ||
| 1.000 | 66 500 | 176 200 |
| Cost | ||
| BMD test | ||
|
| ||
| $98 | 54 400 | 182 600 |
|
| ||
| $164 | 79 200 | 174 800 |
| Hip fracture (first year) | ||
|
| ||
| $24 900 | 80 300 | 191 500 |
|
| ||
| $41 500 | 53 300 | 165 900 |
| Hip fracture (subsequent years) | ||
|
| ||
| $6450 | 74 100 | 186 200 |
|
| ||
| $10 750 | 55 100 | 166 600 |
| Upper gastrointestinal side effects of alendronate | ||
|
| ||
| $2250 | 66 600 | 178 000 |
|
| ||
| $3750 | 67 100 | 179 300 |
| Discount rate | ||
|
| ||
| 0% | 45 800 | 134 200 |
|
| ||
| 6% | 87 700 | 222 300 |
ADT = androgen deprivation therapy; BMD = bone mineral density; ICER = incremental cost-effectiveness ratio; PSA = prostate-specific antigen; QALY = quality-adjusted life-year.
ICER was measured by cost per QALY gained. The no test–no alendronate strategy was the reference strategy because it was the least costly, viable (nondominated) option. The strategy was considered cost-effective if its ICER was less than $100 000 per QALY gained (32, 63). None of the strategies was excluded by simple or extended dominance.
Variables were age-specific. Values shown were for persons aged 70 years.
Footnotes
Potential Conflicts of Interest: None disclosed. Forms can be viewed at www.acponline.org/authors/icmje/ConflictOfInterestForms.do?msNum=M09-1089.
Reproducible Research Statement: Study protocol: Available from Dr. Ito (e-mail, itok1@mskcc.org). Statistical code and data set: Not available.
Current author addresses and author contributions are available at www.annals.org.
Author Contributions: Conception and design: K. Ito, M. Girotra.
Analysis and interpretation of the data: K. Ito, E.B. Elkin, M. Girotra, M.J. Morris.
Drafting of the article: K. Ito, E.B. Elkin, M.J. Morris.
Critical revision of the article for important intellectual content: K. Ito, E.B. Elkin, M. Girotra.
Final approval of the article: K. Ito, E.B. Elkin, M. Girotra.
Provision of study materials or patients: K. Ito.
Statistical expertise: K. Ito.
Administrative, technical, or logistic support: K. Ito.
Collection and assembly of data: K. Ito.
References
- 1.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Prostate Cancer. Fort Washington, PA: National Comprehensive Cancer Network; 2010. v.l.2010. Accessed at www.nccn.org/professionals/physician_gls/PDF/prostate.pdf [registration required] on 1 February 2010. [DOI] [PubMed] [Google Scholar]
- 2.American Urological Association. Guideline for the management of clinically localized prostate cancer: 2007 Update. Baltimore: American Urological Assoc; 2007. Accessed at www.auanet.org/content/guidelines-and-quality-care/clinical-guidelines/main-reports/proscan07/content.pdf on 1 February 2010. [Google Scholar]
- 3.Wilt TJ, MacDonald R, Rutks I, Shamliyan TA, Taylor BC, Kane RL. Systematic review: comparative effectiveness and harms of treatments for clinically localized prostate cancer. Ann Intern Med. 2008;148:435–48. doi: 10.7326/0003-4819-148-6-200803180-00209. [DOI] [PubMed] [Google Scholar]
- 4.Maillefert JF, Sibilia J, Michel F, Saussine C, Javier RM, Tavernier C. Bone mineral density in men treated with synthetic gonadotropin-releasing hormone agonists for prostatic carcinoma. J Urol. 1999;161:1219–22. [PubMed] [Google Scholar]
- 5.Daniell HW, Dunn SR, Ferguson DW, Lomas G, Niazi Z, Stratte PT. Progressive osteoporosis during androgen deprivation therapy for prostate cancer. J Urol. 2000;163:181–6. [PubMed] [Google Scholar]
- 6.Berruti A, Dogliotti L, Terrone C, Cerutti S, Isaia G, Tarabuzzi R, et al. Gruppo Onco Urologico Piemontese (G.O.U.P.), Rete Oncologica Piemontese Changes in bone mineral density, lean body mass and fat content as measured by dual energy x-ray absorptiometry in patients with prostate cancer without apparent bone metastases given androgen deprivation therapy. J Urol. 2002;167:2361–7. [PubMed] [Google Scholar]
- 7.Shahinian VB, Kuo YF, Freeman JL, Goodwin JS. Risk of fracture after androgen deprivation for prostate cancer. N Engl J Med. 2005;352:154–64. doi: 10.1056/NEJMoa041943. [DOI] [PubMed] [Google Scholar]
- 8.Smith MR, Lee WC, Brandman J, Wang Q, Botteman M, Pashos CL. Gonadotropin-releasing hormone agonists and fracture risk: a claims-based cohort study of men with nonmetastatic prostate cancer. J Clin Oncol. 2005;23:7897–903. doi: 10.1200/JCO.2004.00.6908. [DOI] [PubMed] [Google Scholar]
- 9.Smith MR, Boyce SP, Moyneur E, Duh MS, Raut MK, Brandman J. Risk of clinical fractures after gonadotropin-releasing hormone agonist therapy for prostate cancer. J Urol. 2006;175:136–9. doi: 10.1016/S0022-5347(05)00033-9. [DOI] [PubMed] [Google Scholar]
- 10.Tanvetyanon T. Physician practices of bone density testing and drug prescribing to prevent or treat osteoporosis during androgen deprivation therapy. Cancer. 2005;103:237–41. doi: 10.1002/cncr.20766. [DOI] [PubMed] [Google Scholar]
- 11.Alibhai SM, Rahman S, Warde PR, Jewett MA, Jaffer T, Cheung AM. Prevention and management of osteoporosis in men receiving androgen deprivation therapy: a survey of urologists and radiation oncologists. Urology. 2006;68:126–31. doi: 10.1016/j.urology.2006.01.054. [DOI] [PubMed] [Google Scholar]
- 12.Wilcox A, Carnes ML, Moon TD, Tobias R, Baade H, Stamos E, et al. Androgen deprivation in veterans with prostate cancer: implications for skeletal health. Ann Pharmacother. 2006;40:2107–14. doi: 10.1345/aph.1H209. [DOI] [PubMed] [Google Scholar]
- 13.Yee EF, White RE, Murata GH, Handanos C, Hoffman RM. Osteoporosis management in prostate cancer patients treated with androgen deprivation therapy. J Gen Intern Med. 2007;22:1305–10. doi: 10.1007/s11606-007-0291-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diamond TH, Higano CS, Smith MR, Guise TA, Singer FR. Osteoporosis in men with prostate carcinoma receiving androgen-deprivation therapy: recommendations for diagnosis and therapies. Cancer. 2004;100:892–9. doi: 10.1002/cncr.20056. [DOI] [PubMed] [Google Scholar]
- 15.Eastham JA. Bone health in men receiving androgen deprivation therapy for prostate cancer. J Urol. 2007;177:17–24. doi: 10.1016/j.juro.2006.08.089. [DOI] [PubMed] [Google Scholar]
- 16.Greenspan SL. Approach to the prostate cancer patient with bone disease. J Clin Endocrinol Metab. 2008;93:2–7. doi: 10.1210/jc.2007-1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Saad F, Adachi JD, Brown JP, Canning LA, Gelmon KA, Josse RG, et al. Cancer treatment-induced bone loss in breast and prostate cancer. J Clin Oncol. 2008;26:5465–76. doi: 10.1200/JCO.2008.18.4184. [DOI] [PubMed] [Google Scholar]
- 18.Allain TJ. Prostate cancer, osteoporosis and fracture risk. Gerontology. 2006;52:107–10. doi: 10.1159/000090956. [DOI] [PubMed] [Google Scholar]
- 19.Polascik TJ. Bone health in prostate cancer patients receiving androgen-deprivation therapy: the role of bisphosphonates. Prostate Cancer Prostatic Dis. 2008;11:13–9. doi: 10.1038/sj.pcan.4501019. [DOI] [PubMed] [Google Scholar]
- 20.Orwoll E, Ettinger M, Weiss S, Miller P, Kendler D, Graham J, et al. Alendronate for the treatment of osteoporosis in men. N Engl J Med. 2000;343:604–10. doi: 10.1056/NEJM200008313430902. [DOI] [PubMed] [Google Scholar]
- 21.Ringe JD, Faber H, Farahmand P, Dorst A. Efficacy of risedronate in men with primary and secondary osteoporosis: results of a 1-year study. Rheumatol Int. 2006;26:427–31. doi: 10.1007/s00296-005-0004-4. [DOI] [PubMed] [Google Scholar]
- 22.Greenspan SL, Nelson JB, Trump DL, Resnick NM. Effect of once-weekly oral alendronate on bone loss in men receiving androgen deprivation therapy for prostate cancer: a randomized trial. Ann Intern Med. 2007;146:416–24. doi: 10.7326/0003-4819-146-6-200703200-00006. [DOI] [PubMed] [Google Scholar]
- 23.Greenspan SL, Nelson JB, Trump DL, Wagner JM, Miller ME, Perera S, et al. Skeletal health after continuation, withdrawal, or delay of alendronate in men with prostate cancer undergoing androgen-deprivation therapy. J Clin Oncol. 2008;26:4426–34. doi: 10.1200/JCO.2007.15.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Smith MR, McGovern FJ, Zietman AL, Fallon MA, Hayden DL, Schoenfeld DA, et al. Pamidronate to prevent bone loss during androgen-deprivation therapy for prostate cancer. N Engl J Med. 2001;345:948–55. doi: 10.1056/NEJMoa010845. [DOI] [PubMed] [Google Scholar]
- 25.Smith MR, Eastham J, Gleason DM, Shasha D, Tchekmedyian S, Zinner N. Randomized controlled trial of zoledronic acid to prevent bone loss in men receiving androgen deprivation therapy for nonmetastatic prostate cancer. J Urol. 2003;169:2008–12. doi: 10.1097/01.ju.0000063820.94994.95. [DOI] [PubMed] [Google Scholar]
- 26.Smith MR, Fallon MA, Lee H, Finkelstein JS. Raloxifene to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer: a randomized controlled trial. J Clin Endocrinol Metab. 2004;89:3841–6. doi: 10.1210/jc.2003-032058. [DOI] [PubMed] [Google Scholar]
- 27.Ryan CW, Huo D, Demers LM, Beer TM, Lacerna LV. Zoledronic acid initiated during the first year of androgen deprivation therapy increases bone mineral density in patients with prostate cancer. J Urol. 2006;176:972–8. doi: 10.1016/j.juro.2006.04.078. [DOI] [PubMed] [Google Scholar]
- 28.Michaelson MD, Kaufman DS, Lee H, McGovern FJ, Kantoff PW, Fallon MA, et al. Randomized controlled trial of annual zoledronic acid to prevent gonadotropin-releasing hormone agonist-induced bone loss in men with prostate cancer. J Clin Oncol. 2007;25:1038–42. doi: 10.1200/JCO.2006.07.3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Israeli RS, Rosenberg SJ, Saltzstein DR, Gottesman JE, Goldstein HR, Hull GW, et al. The effect of zoledronic acid on bone mineral density in patients undergoing androgen deprivation therapy. Clin Genitourin Cancer. 2007;5:271–7. doi: 10.3816/CGC.2007.n.003. [DOI] [PubMed] [Google Scholar]
- 30.Smith MR, Malkowicz SB, Chu F, Forrest J, Price D, Sieber P, et al. Toremifene increases bone mineral density in men receiving androgen deprivation therapy for prostate cancer: interim analysis of a multicenter phase 3 clinical study. J Urol. 2008;179:152–5. doi: 10.1016/j.juro.2007.08.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Smith MR, Egerdie B, Hernández Toriz N, Feldman R, Tammela TL, Saad F, et al. Denosumab HALT Prostate Cancer Study Group Denosumab in men receiving androgen-deprivation therapy for prostate cancer. N Engl J Med. 2009;361:745–55. doi: 10.1056/NEJMoa0809003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gold MR, Seigel JE, Russell LB, Weinstein MC, editors. Cost-Effectiveness in Health and Medicine. New York: Oxford Univ Pr; 1996. [Google Scholar]
- 33.Horwitz EM, Bae K, Hanks GE, Porter A, Grignon DJ, Brereton HD, et al. Ten-year follow-up of radiation therapy oncology group protocol 92-02: a phase III trial of the duration of elective androgen deprivation in locally advanced prostate cancer. J Clin Oncol. 2008;26:2497–504. doi: 10.1200/JCO.2007.14.9021. [DOI] [PubMed] [Google Scholar]
- 34.Lu-Yao GL, Albertsen PC, Moore DF, Shih W, Lin Y, DiPaola RS, et al. Survival following primary androgen deprivation therapy among men with localized prostate cancer. JAMA. 2008;300:173–81. doi: 10.1001/jama.300.2.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Looker AC, Wahner HW, Dunn WL, Calvo MS, Harris TB, Heyse SP, et al. Updated data on proximal femur bone mineral levels of US adults. Osteoporos Int. 1998;8:468–89. doi: 10.1007/s001980050093. [DOI] [PubMed] [Google Scholar]
- 36.Black DM, Schwartz AV, Ensrud KE, Cauley JA, Levis S, Quandt SA, et al. FLEX Research Group Effects of continuing or stopping alendronate after 5 years of treatment: the Fracture Intervention Trial Long-term Extension (FLEX): a randomized trial. JAMA. 2006;296:2927–38. doi: 10.1001/jama.296.24.2927. [DOI] [PubMed] [Google Scholar]
- 37.Scher HI, Heller G. Clinical states in prostate cancer: toward a dynamic model of disease progression. Urology. 2000;55:323–7. doi: 10.1016/s0090-4295(99)00471-9. [DOI] [PubMed] [Google Scholar]
- 38.De Laet CE, van Hout BA, Burger H, Hofman A, Pols HA. Bone density and risk of hip fracture in men and women: cross sectional analysis. BMJ. 1997;315:221–5. doi: 10.1136/bmj.315.7102.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Johnell O, Kanis JA, Oden A, Johansson H, De Laet C, Delmas P, et al. Predictive value of BMD for hip and other fractures. J Bone Miner Res. 2005;20:1185–94. doi: 10.1359/JBMR.050304. [DOI] [PubMed] [Google Scholar]
- 40.Smith MR, Kabbinavar F, Saad F, Hussain A, Gittelman MC, Bilhartz DL, et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J Clin Oncol. 2005;23:2918–25. doi: 10.1200/JCO.2005.01.529. [DOI] [PubMed] [Google Scholar]
- 41.Hummel S, Paisley S, Morgan A, Currie E, Brewer N. Clinical and cost-effectiveness of new and emerging technologies for early localised prostate cancer: a systematic review. Health Technol Assess. 2003;7:iii, ix–x, 1–157. doi: 10.3310/hta7330. [DOI] [PubMed] [Google Scholar]
- 42.Hannan MT, Felson DT, Dawson-Hughes B, Tucker KL, Cupples LA, Wilson PW, et al. Risk factors for longitudinal bone loss in elderly men and women: the Framingham Osteoporosis Study. J Bone Miner Res. 2000;15:710–20. doi: 10.1359/jbmr.2000.15.4.710. [DOI] [PubMed] [Google Scholar]
- 43.Kiratli BJ, Srinivas S, Perkash I, Terris MK. Progressive decrease in bone density over 10 years of androgen deprivation therapy in patients with prostate cancer. Urology. 2001;57:127–32. doi: 10.1016/s0090-4295(00)00895-5. [DOI] [PubMed] [Google Scholar]
- 44.Burge R, Dawson-Hughes B, Solomon DH, Wong JB, King A, Tosteson A. Incidence and economic burden of osteoporosis-related fractures in the United States, 2005–2025. J Bone Miner Res. 2007;22:465–75. doi: 10.1359/jbmr.061113. [DOI] [PubMed] [Google Scholar]
- 45.Kanis JA, Johnell O, De Laet C, Johansson H, Oden A, Delmas P, et al. A meta-analysis of previous fracture and subsequent fracture risk. Bone. 2004;35:375–82. doi: 10.1016/j.bone.2004.03.024. [DOI] [PubMed] [Google Scholar]
- 46.Kothawala P, Badamgarav E, Ryu S, Miller RM, Halbert RJ. Systematic review and meta-analysis of real-world adherence to drug therapy for osteoporosis. Mayo Clin Proc. 2007;82:1493–501. doi: 10.1016/S0025-6196(11)61093-8. [DOI] [PubMed] [Google Scholar]
- 47.Cadarette SM, Katz JN, Brookhart MA, Stürmer T, Stedman MR, Levin R, et al. Comparative gastrointestinal safety of weekly oral bisphosphonates. Osteoporos Int. 2009;20:1735–47. doi: 10.1007/s00198-009-0871-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Arias E, United States life tables . National vital statistics reports. 9. Vol. 56. Hyattsville, MD: National Center for Health Statistics; 2004. 2007. Accessed at www.cdc.gov/nchs/data/nvsr/nvsr56/nvsr56_09.pdf on 1 February 2010. [PubMed] [Google Scholar]
- 49.Dawson-Hughes B, Tosteson AN, Melton LJ, 3rd, Baim S, Favus MJ, Khosla S, et al. National Osteoporosis Foundation Guide Committee Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. Osteoporos Int. 2008;19:449–58. doi: 10.1007/s00198-008-0559-5. [DOI] [PubMed] [Google Scholar]
- 50.Kanis JA, Johansson H, Oden A, Jonsson B, Johnell O, De Laet C. How many deaths from hip fracture might be prevented [Abstract] J Bone Miner Res. 2002;17(Suppl 1):S146. [Google Scholar]
- 51.Kattan MW, Cowen ME, Miles BJ. A decision analysis for treatment of clinically localized prostate cancer. J Gen Intern Med. 1997;12:299–305. doi: 10.1046/j.1525-1497.1997.012005299.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kanis JA, Johnell O, Oden A, Borgstrom F, Zethraeus N, De Laet C, et al. The risk and burden of vertebral fractures in Sweden. Osteoporos Int. 2004;15:20–6. doi: 10.1007/s00198-003-1463-7. [DOI] [PubMed] [Google Scholar]
- 53.Schousboe JT, Taylor BC, Fink HA, Kane RL, Cummings SR, Orwoll ES, et al. Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA. 2007;298:629–37. doi: 10.1001/jama.298.6.629. [DOI] [PubMed] [Google Scholar]
- 54.Tosteson AN, Melton LJ, 3rd, Dawson-Hughes B, Baim S, Favus MJ, Khosla S, et al. National Osteoporosis Foundation Guide Committee Cost-effective osteoporosis treatment thresholds: the United States perspective. Osteoporos Int. 2008;19:437–47. doi: 10.1007/s00198-007-0550-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Winstead NS, Wilcox CM. Erythromycin prior to endoscopy for acute upper gastrointestinal haemorrhage: a cost-effectiveness analysis. Aliment Pharmacol Ther. 2007;26:1371–7. doi: 10.1111/j.1365-2036.2007.03516.x. [DOI] [PubMed] [Google Scholar]
- 56.Center for Medicare and Medicaid Services. Physician fee schedule search. 2007 Accessed at www.cms.hhs.gov/PFSlookup/02_PFSSearch.asp on 1 February 2010.
- 57.New York State Department of Health. Prescription drug prices in New York State. Accessed at http://rx.nyhealth.gov/pdpw/ on 1 February 2010.
- 58.Gabriel SE, Tosteson AN, Leibson CL, Crowson CS, Pond GR, Hammond CS, et al. Direct medical costs attributable to osteoporotic fractures. Osteoporos Int. 2002;13:323–30. doi: 10.1007/s001980200033. [DOI] [PubMed] [Google Scholar]
- 59.Tosteson AN, Rosenthal DI, Melton LJ, 3rd, Weinstein MC. Cost effectiveness of screening perimenopausal white women for osteoporosis: bone densitometry and hormone replacement therapy. Ann Intern Med. 1990;113:594–603. doi: 10.7326/0003-4819-113-8-594. [DOI] [PubMed] [Google Scholar]
- 60.Office US of Technology Assessment. Evidence on Benefits, Risks, and Costs. Vol. 2. Washington, DC: Congress Office of Technology Assessment, Congress of the United States; 1995. Effectiveness and Costs of Osteoporosis Screening and Hormone Replacement Therapy. [Google Scholar]
- 61.Ross JR, Saunders Y, Edmonds PM, Patel S, Broadley KE, Johnston SR. Systematic review of role of bisphosphonates on skeletal morbidity in metastatic cancer. BMJ. 2003;327:469. doi: 10.1136/bmj.327.7413.469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Department US of Labor. US Consumer Price Index for Medical Care for All Urban Consumers. Accessed at www.bls.gov/cpi/ on 1 February 2010.
- 63.Ubel PA, Hirth RA, Chernew ME, Fendrick AM. What is the price of life and why doesn’t it increase at the rate of inflation? Arch Intern Med. 2003;163:1637–41. doi: 10.1001/archinte.163.14.1637. [DOI] [PubMed] [Google Scholar]
- 64.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK, Clinical Efficacy Assessment Subcommittee of the American College of Physicians Screening for osteoporosis in men: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;148:680–4. doi: 10.7326/0003-4819-148-9-200805060-00008. [DOI] [PubMed] [Google Scholar]
- 65.National Osteoporosis Foundation. Clinician’s guide to prevention and treatment of osteoporosis. Accessed at www.nof.org/professionals/NOF_Clinicians_Guide.pdf on 1 February 2010.
- 66.WHO fracture risk assessment tool. Accessed at www.shef.ac.uk/FRAX/ on 1 February 2010.
- 67.Cummings SR, Black DM, Thompson DE, Applegate WB, Barrett-Connor E, Musliner TA, et al. Effect of alendronate on risk of fracture in women with low bone density but without vertebral fractures: results from the Fracture Intervention Trial. JAMA. 1998;280:2077–82. doi: 10.1001/jama.280.24.2077. [DOI] [PubMed] [Google Scholar]
- 68.McClung MR, Geusens P, Miller PD, Zippel H, Bensen WG, Roux C, et al. Hip Intervention Program Study Group Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med. 2001;344:333–40. doi: 10.1056/NEJM200102013440503. [DOI] [PubMed] [Google Scholar]
- 69.Simonelli C, Killeen K, Mehle S, Swanson L. Barriers to osteoporosis identification and treatment among primary care physicians and orthopedic surgeons. Mayo Clin Proc. 2002;77:334–8. doi: 10.4065/77.4.334. [DOI] [PubMed] [Google Scholar]
- 70.DesHarnais Castel L, Bajwa K, Markle JP, Timbie JW, Zacker C, Schulman KA. A microcosting analysis of zoledronic acid and pamidronate therapy in patients with metastatic bone disease. Support Care Cancer. 2001;9:545–51. doi: 10.1007/s005200100249. [DOI] [PubMed] [Google Scholar]
- 71.Qaseem A, Snow V, Shekelle P, Hopkins R, Jr, Forciea MA, Owens DK, Clinical Efficacy Assessment Subcommittee of the American College of Physicians Pharmacologic treatment of low bone density or osteoporosis to prevent fractures: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2008;149:404–15. [PubMed] [Google Scholar]
- 72.Miksad RA, Come S, Weinstein M. The quality-of-life impact of osteonecrosis of the jaw: implications for bisphosphonate use in metastatic breast cancer. J Clin Oncol. 2007;25(Suppl 18):6620. [Google Scholar]
- 73.Woo SB, Hellstein JW, Kalmar JR. Narrative [corrected] review: bisphosphonates and osteonecrosis of the jaws. Ann Intern Med. 2006;144:753–61. doi: 10.7326/0003-4819-144-10-200605160-00009. [DOI] [PubMed] [Google Scholar]
- 74.Edwards BJ, Gounder M, McKoy JM, Boyd I, Farrugia M, Migliorati C, et al. Pharmacovigilance and reporting oversight in US FDA fast-track process: bisphosphonates and osteonecrosis of the jaw. Lancet Oncol. 2008;9:1166–72. doi: 10.1016/S1470-2045(08)70305-X. [DOI] [PubMed] [Google Scholar]
- 75.The Endocrine Society. Position statement: Medicare coverage of DXA bone density testing in men. 2009 Jun; Accessed at www.endo-society.org/advocacy/policy/upload/Medicare-Coverage-of-DXA-Bone-Density-Testing-in-Men-Position-Statement-2.pdf on 1 February 2010.


