Abstract
Copper-resistant strains of Xanthomonas axonopodis pv. vesicatoria were previously shown to carry plasmid-borne copper resistance genes related to the cop and pco operons of Pseudomonas syringae and Escherichia coli, respectively. However, instead of the two-component (copRS and pcoRS) systems determining copper-inducible expression of the operons in P. syringae and E. coli, a novel open reading frame, copL, was found to be required for copper-inducible expression of the downstream multicopper oxidase copA in X. axonopodis. copL encodes a predicted protein product of 122 amino acids that is rich in histidine and cysteine residues, suggesting a possible direct interaction with copper. Deletions or frameshift mutations within copL, as well as an amino acid substitution generated at the putative start codon of copL, caused a loss of copper-inducible transcriptional activation of copA. A nonpolar insertion of a kanamycin resistance gene in copL resulted in copper sensitivity in the wild-type strain. However, repeated attempts to complement copL mutations in trans failed. Analysis of the genomic sequence databases shows that there are copL homologs upstream of copAB genes in X. axonopodis pv. citri, X. campestris pv. campestris, and Xylella fastidiosa. The cloned promoter area upstream of copA in X. axonopodis pv. vesicatoria did not function in Pseudomonas syringae or in E. coli, nor did the P. syringae cop promoter function in Xanthomonas. However, a transcriptional fusion of the Xanthomonas cop promoter with the Pseudomonas copABCDRS was able to confer resistance to copper in Xanthomonas, showing divergence in the mechanisms of regulation of the resistance to copper in phytopathogenic bacteria.
Both eukaryotic and prokaryotic cells require copper for normal growth. Copper is an essential cofactor of a number of enzymes involved in respiration, such as oxygenases and electron transport proteins (17). However, above a certain concentration, copper is toxic to the cell. Therefore, its intracellular levels must be tightly controlled (16). Copper has the ability to generate free radicals able to damage DNA and lipid membranes (22, 36). As a consequence, bacteria developed detoxification systems to protect themselves from toxic concentration of copper and still ensure they met their nutritional requirements. These systems have been found to be plasmid-, or chromosomally borne (for reviews, see references 38, 39, and 42).
For years, copper-containing compounds have been sprayed on vegetable and fruit crops to limit the spread of plant pathogenic bacteria and fungi. This continuous and popular use of copper-based antimicrobial compounds favored the spread of copper resistance genes among saprophytic and plant pathogenic bacteria (2, 10, 41). Xanthomonas campestris pv. vesicatoria, renamed X. axonopodis pv. vesicatoria (53), is a common plant pathogen of tomato and peppers. There have been several reports of copper-resistant X. axonopodis strains (5, 8, 10, 18, 19, 26, 30). Copper-resistant strains of other plant pathogenic bacteria have also been identified, including Pseudomonas syringae (2, 4, 12, 20, 41, 44, 45). Cloning and characterization of both plasmid and chromosomal copper resistance (cop) genes from these pathogens has shown that most are related to each other (26, 27, 41, 55), and they are also related to the pco genes from enteric bacteria (6, 9, 46, 51; for a review, see reference 40). However, in spite of the considerable sequence similarities, there appear to be functional and regulatory differences between the Pseudomonas cop and enteric pco systems (7, for a review, see reference 40).
The copABCD operon of P. syringae (31) is specifically induced by copper (32). Copper-inducible expression requires a two-component regulatory system (copRS), which immediately follows the copABCD operon (34). CopR was purified and shown to bind to a conserved motif (cop box) at the −35 region of cop promoters from P. syringae (35). No sequences upstream from the cop box are required for copper-inducible expression (35). Chromosomal genes in some pseudomonads may substitute for the plasmid-borne copRS system (34), and expression of the cop promoter from P. syringae has only been observed in pseudomonads. In Escherichia coli, mutations in pcoR can also be complemented by a chromosomal gene (43). Expression of resistance to toxic levels of copper therefore appears to involve complex interactions between both plasmid and chromosomal genes. Cloned copper resistance genes from two species of Xanthomonas were also shown to be related to the cop operon from P. syringae, namely, homology to copA (26, 55).
We are interested in determining what natural barriers might exist to the dissemination of plasmid-borne copper resistance genes among different bacterial pathogens, possibly due to different features of the host background that may be required for expression of plasmid-borne resistance genes. In addition to the lack of expression of the cloned cop promoter from P. syringae in Xanthomonas (35), cloned copper resistance genes from X. axonopodis did not confer copper resistance to P. syringae or E. coli (10, 55). It was not known, however, whether the genes from Xanthomonas were not expressed or whether the mechanism of resistance was not functional in the other genera. The objective of this study was to investigate the regulation of copper resistance in X. axonopodis in comparison to the related cop system in P. syringae.
The copper-resistant strain of X. axonopodis used for this study was isolated from a tomato field in California and found to harbor copper resistance genes on a nonconjugative 100-kb plasmid (10). The smallest fragment able to confer substantial resistance to copper to a copper-sensitive X. axonopodis strain was a 6.8-kb clone (55). Transposon mutagenesis with a promoterless lacZ gene was carried out by using Tn1737 (52). A particular Tn1737 insertion in the cloned copper resistance genes inactivated resistance to copper but did not show elevated β-galactosidase activity, suggesting the insertion occurred in the promoter area. The characterization of this promoter area and its ability to function in plant pathogens related to X. axonopodis pv. vesicatoria are the object of this report. Since copper is an important bactericide in agriculture, the dissemination of plasmid-borne copper resistance among strains of a pathovar or between different pathovars, species, or genera of plant pathogenic bacteria has important implications for disease control efforts.
MATERIALS AND METHODS
Bacterial strains, culture conditions, and plasmids.
The bacterial strains and plasmids developed in the present study are listed in Table 1. Nutrient Agar (Difco) supplemented with yeast extract (1g/liter; NY) was used to maintain xanthomonads, whereas pseudomonads were grown on mannitol-glutamate agar (24) supplemented with yeast extract at 0.25 g/liter (MGY) at 28°C. Cultures of E. coli were grown in Luria-Bertani (LB) (33) broth at 37°C. Antibiotics were used at the following concentrations: rifampin, 50 μg/ml; chloramphenicol, 20 μg/ml; ampicillin, 50 μg/ml; tetracycline, 10 μg/ml; kanamycin, 25 μg/ml; and streptomycin 50 μg/ml. Nutrient broth (NY), MGY, and LB were used as liquid media to grow xanthomonads, pseudomonads, and E. coli, respectively. The antibiotic levels were decreased to half when bacteria were grown in liquid media. Constructed plasmids were introduced into Pseudomonas or Xanthomonas from E. coli strain S17-1 (47) by triparental matings on yeast-glucose-calcium carbonate agar with pRK2013 as the helper plasmid (15) or by electroporation. Mating mixtures or transformed cells were plated on media containing the appropriate antibiotics. Strain 78518 (Cus [10]) was transformed with a CsCl-purified plasmid preparation from 7882 (10) by electroporation. Electroporated cells were plated onto NY agar containing CuSO4 at 1.2 mM. A copper-resistant derivative of 78518 that had acquired the desired plasmid was designated 78518.2. The presence of the 100-kb copper-resistance plasmid was verified by plasmid isolation and comparing the plasmid size to the plasmid from the parent strain 7882.
TABLE 1.
Bacterial strains and plasmids developed in this study
| Xanthomonas strain or plasmid | Descriptiona |
|---|---|
| X. axonopodis pv. vesicatoria | |
| 7882.3 | Cus, Rifr, Kanr; kanamycin cassette was inserted into the SalI site of copL in 7882 (10) |
| 78518.2 | Cur Rifr; the Cur plasmid of 7882 was electroporated into strain 78518 (10) |
| Plasmids | |
| pCOP151 | Smr Cmr; 1.9-kb BamHI fragment of pCOP120 (55) cloned in the BglII site of pMP190 (49) in the correct orientation to express lacZ |
| pCOP153B | Apr; 1.9-kb BamHI fragment of pCOP116 (55) cloned in pUC128 (25) in the anti-lac orientation |
| pCOP156 | Apr; pCOP153B was digested with KpnI to result in a deletion of the 500-bp KpnI-BamHI fragment and then relegated |
| pCOP157 | Apr; pCOP153B was digested with ClaI to result in a deletion of the 1.4-kb ClaI-BamHI fragment and then relegated |
| pCOP161 | Smr Cmr; 1.5-kb XbaI-KpnI-fragment of pCOP156 cloned in pMP190 |
| pCOP162 | Smr Cmr; 510-bp XbaI-KpnI fragment of pCOP157 cloned in pMP190 |
| pCOP166 | Apr; pCOP153B was digested with SphI to result in a deletion of 0.6-kb SphI-BamHI fragment and then religated |
| pCOP168 | Apr; pCOP156 was digested with SacII to result in a deletion of the 440-bp BamHI-SacII fragment and then religated |
| pCOP169 | Smr Cmr; 1.0-kb (SacII)b-Xbal fragment of pCOP168 cloned in (SalI)-XbaI sites of pMP190 |
| pCOP170 | Smr Cmr; 1.0-kb (XbaI)-SphI fragment of pCOP166 cloned in (XbaI)-KpnI sites of pMP190 |
| pCOP187 | Tcr; 510-bp BamHI-HindIII fragment of pCOP157 cloned in pRK415 (25) |
| pCOP189 | Tcr; 8.8-kb HindIII fragment of pDAC102 (34) cloned in the HindIII site of pCOP187, resulting in transcriptional fusion of the constitutive promoter with copABCDRS. |
| pCOP191 | Apr; pCOP153B was digested with ApaI to result in a deletion of 900-bp ApaI-BamHI fragment and then religated |
| pCOP192 | Apr; pCOP191 digested with BamHI and StuI, blunt ended, and religated |
| pCOP193 | Apr; pCOP191 digested with SalI and ClaI, blunt ended, and religated |
| pCOP196 | Apr; pCOP191 digested with StuI and ApaI, blunt ended and relegated |
| pCOP197 | Smr Cmr; the 985-bp XbaI-KpnI fragment of PCOP191 cloned in pMP190 |
| pCOP198 | Smr Cmr; the 735-bp XbaI-KpnI fragment of pCOP192 cloned in pMP190 |
| pCOP199 | Smr Cmr; the 935-bp Xbal-KpnI fragment of pCOP193 cloned in pMP190 |
| pCOP202 | Smr; Cmr; the 250-bp Xbal-KpnI fragment of pCOP196 cloned in pMP190 |
| pCOP203 | Apr; the 470-bp ClaI-ApaI fragment of pCOP191 cloned in pUC128 |
| pCOP204 | Apr; the 200 bp StuI-SalI fragment of pCOP191 cloned into the SmaI-SalI sites of pUC128 |
| pCOP205 | Smr Cmr; the 470-bp XbaI-KpnI fragment of pCOP203 cloned in pMP190 |
| pCOP206 | Smr Cmr; the 200-bp XbaI-KpnI fragment of pCOP204 cloned in pMP190 |
| pCOP210 | Apr; pCOP192 was digested with ClaI blunt ended with Klenow and religated |
| pCOP214 | Smr; Cmr; the 725-bp XbaI-KpnI fragment of pCOP210 was cloned in pMP190 |
| pCOPM1 | Smr; Cmr; substitution of first Met codon in copL (in pCOP192) to Val and subcloned into pMP190 |
| pCOPM2 | Smr; Cmr; substitution of second Met codon in copL (in pCOP192) to Leu and subcloned into pMP190 |
| pCOPM3 | Smr; Cmr; substitution of third Met codon in copL (in pCOP192) to Leu and subcloned into pMP190 |
Cur, copper resistance; Cus, copper sensitivity; Rifr, rifampin resistance; Kmr, kanamycin resistance; Smr, streptomycin-resistance; Apr, ampicillin resistance; Tcr, tetracycline resistance; Cmr, chloramphenicol resistance.
Restriction sites in parentheses describe sites that have been blunt ended before ligation.
General DNA manipulations, DNA sequencing, and sequence analysis.
Molecular biology techniques were performed by using standard protocols described by Maniatis et al. (29). Transposon insertion mutagenesis (55) narrowed down the promoter area for the copper resistance determinants to a 1.9-kb BamHI fragment (pCOP153B) that was subcloned further into a BamHI-ApaI fragment (Table 1), which was sequenced. The resulting DNA and deduced protein sequences were analyzed with the University of Wisconsin Genetics Computer Group (14) and the BLAST programs provided by the National Center for Biotechnology Information. The alignment of the deduced amino acid sequences of CopL homologs were made with the Multalin software version 5.4.1 (11).
Isolation of RNA and primer extension.
Bacterial cells of X. axonopodis pv. vesicatoria strain 7882 were grown overnight in LB broth without or with 7 mM CuSO4 at 28°C. A total of 25 ml of the culture was pelleted, and the rapid RNA isolation procedure was followed (3). The integrity of the RNA was determined by visualization of the rRNA bands on a denaturing 1.2% agarose gel in the presence of formaldehyde. The primer extension procedure was done according to the method of Ausubel et al. (3). Briefly, 20 μg of RNA was annealed in 1× hybridization solution with 5 × 105 cpm of 32P-end-labeled 18-mer oligonucleotides. To determine the 5′ end of the RNA for copL, the oligonucleotide 5′-GCCTGCTCTTCCATCACC-3′ (hybridizing to nucleotides 409 to 426, Fig. 1) was used, and the products were analyzed by fractionation on a 6% polyacrylamide gel. The primer extension reactions were run side-by-side with dideoxy-sequencing reactions, primed with the same primer, as size markers.
FIG. 1.
RT-PCR analysis of copL and copA genes of X. axonopodis pv. vesicatoria 7882. Lanes 1 to 3, RT-PCR analysis for copL; lanes 4 to 6, RT-PCR analysis for copA. RT-PCR was performed on RNA isolated from cells grown in the absence (lanes 1 and 4) or presence (lanes 2 and 5) of 1 mM CuSO4. Lanes 3 and 6 show the results of the negative controls.
RT-PCR analysis of copL and copA.
X. axonopodis pv. vesicatoria strain 7882 cells were grown in NY until they reached an optical density at 600 nm of 0.5. The copper resistance genes were induced for 20 min by the addition of CuSO4 to a final concentration of 1 mM. Control cultures were left untreated. Total RNA was isolated by using TRIzol (Invitrogen) and RNA samples were treated with RNase-free DNase I (Promega) for 1 h at 37°C. Reverse transcription-PCR (RT-PCR) analysis was performed by using the Access RT-PCR kit (Promega) according to the manufacturer's instructions. RT-PCRs were carried out in 25-μl reactions using either the L1-L2 primer pair or the L3-AUP primer pair for copL and copA gene, respectively. The sequences of the primers used were as follows: L1, 5′-CGGAATTCATGCTCGTGCTTAACGGGG-3′ (hybridizes to nucleotides 355 to 374, Fig. 1); L2, 5′-TAACTGCAGCTCGAGACGCTTAGCCGATCGGTG-3′ (hybridizes to nucleotides 729 to 711); L3, 5′-CGCCTGCCTTGCCTCATCTG-3′ (hybridizes to nucleotides 684 to 703); and AUP, 5′-GGCCCGGCATCTTCTTCAAAC-3′ (hybridizes to nucleotides 622 to 642) of the copA gene. The full nucleotide sequence of copA and deduced amino acid sequences can be found in the GenBank entry AY536748. The RT reaction was performed at 48°C for 45 min, followed by a 2-min denaturation step at 94°C. For the amplification reactions, the conditions used were as follows: 40 cycles of 94°C for 30 s, 60°C for 1 min, and 68°C for 1 min, with a final extension step at 68°C for 5 min. The negative control comprised of samples subjected to the same reaction conditions, with the reverse transcriptase step being omitted. The amplified DNA fragments were separated on a 1.3% agarose gel.
Deletions and point mutations in copL.
To test the effect of mutations and deletions in copL on the induction of copA transcription in response to copper, several subclones of the 1.9-kb BamHI fragment from pCOP120 (55) were introduced into the promoterless β-galactosidase vector pMP190 (49). Similarly, amino acid substitutions at the first three methionines of CopL were carried out by PCR mutagenesis by using the “mega primer” method modified for improved amplification of the final product (1). The primers utilized for the mutagenesis were as follows (with introduced restriction endonuclease sites underlined and mutations in the methionine codon and changes to introduce endonuclease sites indicated in boldface): 5′-GATCGCCGTGCTCGTGCTTAAC-3′ for a valine substitution at the first methionine of CopL, 5′-TGCGTCGGTAAGCTTGAATCCGGT-3′ for a leucine substitution at the second methionine, and 5′-TATGAATCCGAAGCTTGAAGAGCA-3′ for a leucine substitution at the third methionine of CopL. Three separate PCR amplification reactions were carried out by using pCOP192 as a template. The first PCR used one of the above primers and the pUC19 universal forward primer. The second reaction used the pUC19 universal reverse primer and a primer complementary to downstream copL sequences. For the third PCR, the products from the first two steps were mixed in a 5:1 molar ratio, and the final product was amplified with the pUC19 universal reverse and forward primers. The amplified fragments were gel purified, digested with XbaI and KpnI, and cloned into pUC128 (25). The clones bearing mutations were confirmed by restriction endonuclease digestion and sequencing. The resulting mutated versions of copL were cloned directionally upstream of the promoterless lacZ gene in pMP190 for assessment of the copper-inducible expression of copA.
β-Galactosidase assays.
Strains of Xanthomonas containing the pMP190-based constructs obtained as described above were grown in NY to log phase and subcultured into NY or NY with 0.1 mM CuSO4 for 16 to 20 h at 28°C. Strains of Pseudomonas and E. coli containing recombinant plasmids were grown in MGY amended with streptomycin at 5 μg/ml and LB amended with chloramphenicol at 20 μg/ml. The inducing concentrations of CuSO4 were 0.1 and 0.7 mM for Pseudomonas and E. coli, respectively. The β-galactosidase activities were assayed as described by Miller (33) with o-nitrophenyl-β-d-galactopyranoside as the substrate
Marker-exchange mutagenesis of copL.
A kanamycin resistance gene cassette from pMKm (37) was inserted into the SalI site of copL in pCOP156. The kanamycin resistance gene is transcribed in the opposite direction relative to that of copL. The mutated copL was subcloned into the broad-host-range plasmid pRK415 (25) and electroporated into the wild-type strain 7882. Marker exchange was carried out as described previously (28), with selection for kanamycin resistance and screening for loss of the plasmid-determined tetracycline resistance. The resulting mutant strain was designated 7882.3. The presence of the kanamycin cassette in copL on the indigenous copper resistance plasmid in 7882.3 was confirmed by Southern blot hybridization.
Test for induction of copA transcription by other metals.
The metal salts used in the present study were: CuSO4, NiSO4, CrCl2, CoCl2, K2CrO4, MnCl2, HgCl2, Y(NO3)3, NaCl, CsCl, LiCl2, AgNO3, Th(NO3)3, ZnSO4, CaCl2, Al(NO3)3, Pb(C2H3O2), FeCl3, LaCl3, NdCl3, ErNO3, and CeCl3. The MIC (10) was calculated for each metal salt. One-tenth of the MIC concentration of each metal was used for induction, and in cases where the MIC was not accurately determined, the maximum concentration of the metals used in the test experiment was used for induction purposes (16 to 20 h). The test was performed in liquid cultures of NY at 28°C amended with different concentrations of the metals. Strain 7882(pCOP198) containing the intact copL and the 5′ end of copA fused to the β-galactosidase reporter gene was tested for the ability of these metals to induce transcription of copA. Uninduced cultures of 7882(pCOP198) were used as a negative control, and the same strain grown in the presence of CuSO4 was used as the positive control. β-Galactosidase activity was measured as described above.
Expression of the Pseudomonas cop operon in Xanthomonas under the control of the Xanthomonas cop promoter.
To determine whether the lack of expression of the Pseudomonas cop operon in X. axonopodis is due to a transcriptional difference, a transcriptional fusion of the copper-inducible promoter from X. axonopodis with the cop operon of P. syringae was constructed (pDAC102 [34]). The resulting construct was introduced in a copper-sensitive (Cus) X. axonopodis pv. vesicatoria strain 78518, and its resistance to CuSO4 was determined.
Nucleotide sequence accession number.
The 987-bp sequence containing copL and the 5′ end of copA was deposited in GenBank (accession number AY380578).
RESULTS
Sequence analysis of the promoter region of the copper resistance determinants from X. axonopodis.
Plasmid pCOP151 (Table 1), containing a 1.9-kb fragment cloned in the lacZ reporter vector pMP190, was further subcloned. A region of about 1 kb in the 5′ portion of this fragment that was shown to be sufficient for the copper-dependent induction of the β-galactosidase reporter gene. This region was sequenced, revealing an open reading frame (ORF) of 369 bp, designated copL, located at the 5′ end of the fragment. The 3′ end of this ORF is also present in the published sequence of the copper resistance operon from X. arboricola pv. juglandis (26), but no sequences further upstream were included in that study.
Putative −10 and −35 regions were identified for copL, but no easily identifiable ribosome-binding site (RBS) was found. The lack of a strong RBS could suggest that CopL, a protein of 122 amino acids, is translated at very low levels. Although the putative −10 region (TAAAGT) is close to the conventional E. coli promoters, the putative −35 region (TTGTTC) matches the consensus motif for Xanthomonas (TTGTNN [23]).
Downstream of copL, there is a homolog to the multicopper oxidase copA starting at nucleotide 838. A putative RBS (TGGAG) can be found −12 to −8 from the ATG of CopA. It is worth noting that the spacing of 113 bases between the stop codon of copL and the start codon of copA is larger than what is commonly found in bacteria for two genes part of an operon. The intergenic region could allow the binding of regulatory factors (A.E. Voloudakis and D. A. Cooksey, unpublished data). There is also an inverted repeat GGCGCC-N4-GGCGCC starting nine bases downstream of the stop codon for copL.
The deduced amino acid sequence for CopL revealed the presence of potential metal-binding sites, such as C37-X2-H and C73-X-C-X-C-H, a well as pairs of cysteines (C67-C) and histidines (H40-H and H45-H). However, based on DNA or amino acid sequence data, copL was not related to any copper resistance systems known thus far. Careful examination of the genomic sequences of X. axonopodis pv. citri, X. campestris pv. campestris (13), and Xylella fastidiosa (48) revealed the presence of copL-like ORFs. A point of similarity between all four bacteria is the location of CopL upstream of copAB, which are homologous to the first two structural copper resistance genes of X. campestris pv. juglandis and P. syringae pv. tomato.
X. axonopodis pv. vesicatoria CopL was aligned with CopLXAC (accession number NP_643936; 26% identity, 37% similarity) from X. axonopodis pv. citri, CopL XCC (accession number AAM39894; 26% identity, 32% similarity) from X. campestris pv. campestris, and CopLXF (accession number AAO27998; 27% identity, 39% similarity) from Xylella fastidiosa. The alignment revealed the conservation of several cysteine and histidine residues, which suggests they may have a functional role in the proteins.
There were no identifiable similarities in the region 5′ to copA with the promoter region of the cop operon from P. syringae or already-characterized Xanthomonas promoters. Most notably, no similarity was observed to the cop box sequence, where the CopR regulatory protein was shown to bind in P. syringae (35). Sequence data indicated the presence of a strong RBS (TGGAG) located −11 to −8 bp upstream from the translation start codon of copA. Over this N-terminal region of CopA, the amino acid identity and similarity were 66 and 70%, respectively, with the CopA homolog of copper-resistant X. arboricola pv. juglandis (26).
Primer extension analysis of copL.
An extension product was observed, corresponding to a 5′ end of the RNA for copL. The extension products were observed with RNA isolated from induced and uninduced cells. However, uninduced cells gave a signal that was approximately three times weaker than the extension product from induced cells. There were no additional extension products observed when another primer, complementary to nucleotides 258 to 275, was used to detect the 5′ end of mRNAs further upstream (data not shown).
RT-PCR analysis of copL and copA.
To determine the presence of transcripts for copL and copA in the presence or absence of Cu2+ ions, an RT-PCR analysis was used. The results indicated the presence of a transcript for copL, regardless of the presence of copper. The expected size of the amplified product is 396 bp (Fig. 1, lanes 1 and 2). In contrast, a transcript for copA was only detected in the presence of Cu2+ (Fig. 1, lanes 4 and 5), as shown by the presence of an amplified product of 797 bp. This suggests that copL is transcribed from a constitutive promoter, but the expression of copA is copper dependent.
Deletion, insertion, and site-specific mutations in copL.
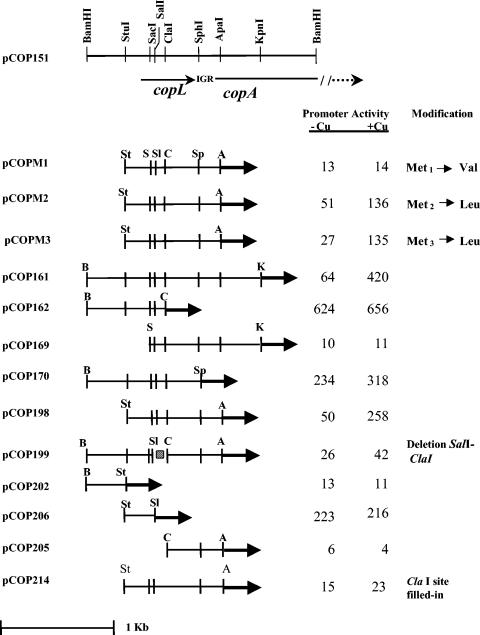
A 740-bp StuI-ApaI fragment, which contained all of copL and the 5′ end of copA, was the smallest subcloned fragment that gave full copper-inducible activity of copA in the lacZ reporter vector pMP190. Subclones containing only the 5′ part of this fragment (the 5′ end of copL and about 100 bp upstream from copL) had constitutive promoter activity (pCOP162, pCOP206, and pCOP170) (Fig. 2), probably corresponding to the transcriptional start site that was detected with or without copper exposure in this upstream region. However, a subclone of the 3′ region (pCOP205), which included the last half of copL through the beginning of copA, did not have any promoter activity.
FIG. 2.
Localization and deletion analysis of the 1.9-kb BamHI fragment containing copL and the 5′ end of copA from X. axonopodis pv. vesicatoria strain 7882. All constructs were fused to lacZ in pMP190 and introduced into strain 7882. Vertical lines indicate important restriction sites. Promoter activity was measured as β-galactosidase activity (Miller units). β-Galactosidase assays for the three methionine replacement mutants—pCOPM1, pCOPM2, and pCOPM3—were carried out at a different time than the assays for the other mutants. The copper-inducible control for the methionine replacement mutants was pCOP197, which, in this assay, gave 42 Miller units for uninduced cells and 152 Miller units for induced cells. Alterations to restriction sites, deletions, and point mutations are indicated in the right-hand column. Abbreviations: St, StuI; S, SacII; Sl, SalI; C, ClaI; Sp, SphI; A, ApaI; K, KpnI. IGR, intergenic region.
Specific mutations in copL were also made, including a frameshift mutation at the ClaI site (pCOP214; Fig. 2), a deletion between the SalI and ClaI sites (pCOP199; Fig. 2), and amino acid substitutions at the first three methionine codons of the copL ORF (pCOPM1, pCOPM2, and pCOPM3; Fig. 2), The frameshift mutation at the ClaI site and the SalI-ClaI deletion resulted in a complete loss copper-dependent induction of copA, again measured by expression of lacZ transcriptionally fused to these constructs (Fig. 2). Substitution of the first methionine codon of CopL with that of valine also abolished copper-dependent β-galactosidase expression, but substitution of the second and third methionines of copL with leucine did not. These results suggested that the translation of an intact message for copL is required for the copper-dependent transcription of copA.
A nonpolar copL insertional mutation was also constructed in the wild-type strain 7882 to determine its effect on the expression of the copper resistance phenotype. The wild-type copL gene in the indigenous copper resistance plasmid of 7882 was replaced by marker exchange mutagenesis with a derivative of copL containing a kanamycin resistance gene insertion at the SalI site with the direction of transcription of the kanamycin resistance gene in the opposite direction relative to copL and the downstream cop genes. The kanamycin cassette also did not have transcriptional terminators. The MIC of CuSO4 was reduced from 2.0 mM in 7882 to 1.4 mM in the marker exchange mutant 7882.3, an MIC level that was higher than the MIC level of 0.6 mM obtained for the Cus strains 78518 and 7882.1.
Specific induction of the copper resistance determinants by copper.
Of 22 different metal salts tested for induction of the copper resistance promoter-lacZ reporter construct (pCOP198) in X. axonopodis, only the copper salt (CuSO4) resulted in a large increase in β-galactosidase production (data not shown). This indicated that copA transcription is induced specifically by copper ions.
Complementation analysis of mutations in copL.
Plasmids containing an intact, copper-inducible copL-copA region transcriptionally fused to lacZ (pCOP198; Fig. 2) or several derivatives with deletions (pCOP199 and pCOP205; Fig. 2) or a frameshift mutation (pCOP214; Fig. 2) in copL were introduced individually into strain 7882, 78518.2, or 78518(pCOP138). All three recipient strains contained intact copL and structural copper resistance genes, and thus the copL mutations were expected to be complemented in trans. However, none of the copL mutants was complemented in any of these backgrounds. The presence of the expected plasmids of these constructs was confirmed by plasmid isolation, electrophoresis, and Southern blotting. The integrity of the deletion and other mutations was confirmed by sequencing. In addition, introduction of copL on pCOP161 into the mutant strain 7882.3 containing a kanamycin resistance gene cassette inserted in copL failed to restore copper resistance to this strain (data not shown).
Specificity of promoter expression in Xanthomonas.
The StuI-ApaI fragment of pCOP198 (Fig. 2), containing copL and the 5′ end of copA transcriptionally fused to lacZ, showed copper-dependent expression of copA in both copper-resistant and copper-sensitive strains of X. axonopodis pv. vesicatoria (data not shown). A similar result was obtained when the construct was introduced in X. axonopodis pv. vignicola, X. arboricola pv. pruni, X. axonopodis pv. phaseoli, and a pectolytic xanthomonad (data not shown). However, no copA expression could be detected when this fragment or the subclone pCOP162 (Fig. 2) was introduced into E. coli strain DH5α or into P. syringae pv. syringae, although pCOP162 showed constitutive copA expression in Xanthomonas. Similarly, when pCOP198 was introduced in strains PT23.2 (4) and PT12.2 (4) of P. syringae pv. tomato, which are known to contain trans-acting factors for the expression of the Pseudomonas cop promoter, there was no copper-dependent expression of copA.
In addition, we have shown previously (34) that the copper-inducible cop promoter from P. syringae was not expressed in Xanthomonas. In the present study, when we introduced plasmids containing copL (pCOP138) or copRS from P. syringae (pDAC102) into X. axonopodis strain 78518 that also carried pCOP38, a transcriptional fusion of the P. syringae cop promoter with lacZ, no β-galactosidase activity was detected, showing that the P. syringae cop promoter was still not active.
Expression of the Pseudomonas cop operon in Xanthomonas under the control of the Xanthomonas cop promoter.
We constructed a transcriptional fusion of the constitutive copL promoter (included in the StuI-SalI fragment of pCOP206, Fig. 2) from X. axonopodis with the P. syringae cop operon. As a result, the recombinant DNA conferred copper resistance in Xanthomonas due to the expression of the Pseudomonas cop operon. The MIC of 78518(pCOP189) was 1.8 mM compared to 0.6 mM for the Cus 78518 (55).
DISCUSSION
Transposon mutagenesis of cloned copper resistance determinants in X. axonopodis pv. vesicatoria identified an area responsible for their transcriptional control (55). Sequencing of this region revealed an ORF of 369 bases coding for a protein of 122 amino acids, which was named CopL. Downstream of copL was found the 5′ end of another ORF coding for a gene homolog to the multicopper oxidase copA. ORFs similar to copL could be found upstream of copAB in X. campestris pv. campestris, X. axonopodis pv. citri, and Xylella fastidiosa. The deduced amino acid sequence of CopL and other CopL-like sequences showed they have numerous cysteine and histidine residues, suggesting they may have a role in binding copper ions. However, no other recognizable motif identified thus far in proteins were present. CopL does not appear to be a negative regulator since its inactivation by marker exchange mutagenesis and point mutations did not result in a constitutive expression of copA. The size of CopL (122 amino acids) is similar to that of response regulators of many other bacterial regulatory systems (for a review, see reference 21). Other similarities to these regulators include a pair of aspartate residues near the N terminus (positions 34 and 36) and an aspartate residue at position 100 that is within a motif (LALGLDVMPLG) with some similarity to conserved motifs surrounding the aspartate phosphorylation site normally found near position 55. However, CopL is lacking the lysine (K) that is commonly present near the C terminus of response regulators (21).
Transcriptional analysis revealed that copL was transcribed constitutively, whereas copA was expressed only in the presence of copper. No cop box similar to the sequence found in the promoter area of the copper resistance genes in P. syringae was present, and no sequence previously determined to function as a promoter in Xanthomonas could be found in the intergenic region between copL and copA. This intergenic region does not reveal the presence of either an intrinsic terminator or a Rho-dependent terminator. However, the implementation of full copper resistance is dependent on the presence of an intact copL gene, coupled with the ability to translate CopL. In addition, the transcriptional control of copA in the presence of copper is also dependent on the sequence downstream of the SphI site located between copL and copA. The precise role of the intergenic region in the copper-dependent response of the downstream genes and its relationship to CopL activity remain to be determined.
The newly sequenced genomes of X. axonopodis pv. citri and X. campestris pv. campestris show the presence of three ORFs similar to copL and copAB. It was proposed that these chromosomal copies may have other functions than copper resistance. Another remarkable feature uncovered by sequencing the genomes of X. axonopodis pv. citri and X. campestris pv. campestris is the abundance of insertion sequence elements. A total of 109 insertion sequence elements were identified in X. campestris pv. campestris, and 87 were identified in X. axonopodis pv. citri. Mobile genetic elements have shown to be involved in the dissemination of antibiotic and copper resistance genes in P. syringae pv. syringae (50). Likewise, these elements could facilitate the transfer of the chromosomal copies of copL- and copAB-like genes to a plasmid. The involvement of plasmids in disseminating certain genes in plant pathogens has been largely documented (54). Eventually, the plasmid-borne copies of the structural genes would evolve other modes of regulation of their expression. The transcriptional fusions of X. axonopodis copL and truncated copA to the reporter gene β-galactosidase showed copper-dependent transcription of copA in all of the xanthomonads tested but not in P. syringae or E. coli. In addition, we have shown previously (34) that the copper-inducible cop promoter from P. syringae was not expressed in Xanthomonas. In the present study, the cop promoter from P. syringae was still not expressed in Xanthomonas when the P. syringae copRS regulatory genes, or copL, were supplied in trans. The Pseudomonas cop operon was found to be functional in Xanthomonas, since a transcriptional fusion of the X. axonopodis cop promoter with the P. syringae cop operon provided resistance to the Cus strain 78518. This supports a functional conservation of the structural cop genes, but the mechanisms of regulation of cop genes have clearly diverged in these related genera of bacteria. One system has a copper-inducible two-component signal transduction mechanism, and the other is dependent on the copL regulatory gene.
Acknowledgments
We thank N. T. Keen, M. N. Schroth, B. Benett, H. R. Azad, S. D. Mills, and K. Dumenyo for helpful discussions and assistance.
This study was supported by National Science Foundation grant DEB-9306559 and a University of California Systemwide Biotechnology Research and Education Program grant, the UCR Graduate Program in Genetics, and the University of California Agricultural Experiment Station.
REFERENCES
- 1.Aiyar, A., and J. Leis. 1993. Modification of the megaprimer method of PCR mutagenesis: improved amplification of the final product. BioTechniques 14:366-368. [PubMed] [Google Scholar]
- 2.Andersen, G. L., O. Menkissoglou, and S. E. Lindow. 1991. Occurrence and properties of copper-tolerant strains of Pseudomonas syringae isolated from fruit trees in California. Phytopathology 81:648-656. [Google Scholar]
- 3.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1987. Current protocols in molecular biology, vol. 2, p. 102.1-108.6. Wiley Interscience, New York, N.Y.
- 4.Bender, C. L., and D. A. Cooksey. 1986. Indigenous plasmids in Pseudomonas syringae pv. tomato: conjugative transfer and role in copper resistance. J. Bacteriol. 165:534-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bender, C. L., D. K. Malvick, K. E. Conway, S. George, and P. Pratt. 1990. Characterization of pXV10A, a copper resistance plasmid in Xanthomonas campestris pv. vesicatoria. Appl. Environ. Microbiol. 56:170-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brown, N. L., S. R. Barrett, J. Camakaris, B. T. O. Lee, and D. A. Rouch. 1995. Molecular-genetics and transport analysis of the copper-resistance determinant (pco) from Escherichia coli. Mol. Microbiol. 17:1153-1166. [DOI] [PubMed] [Google Scholar]
- 7.Cha, J.-S., and D. A. Cooksey. 1991. Copper resistance in Pseudomonas syringae mediated by periplasmic and outer membrane proteins. Proc. Natl. Acad. Sci. USA 88:8915-8919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cooksey, D. A. 1990. Genetics of bactericide resistance in plant pathogenic bacteria. Annu. Rev. Phytopathol. 28:201-219. [Google Scholar]
- 9.Cooksey, D. A. 1996. Molecular genetics and evolution of copper resistance in bacterial plant pathogens, p. 79-88. In T. M. Brown (ed.), Molecular genetics and evolution of pesticide resistance. American Chemical Society, Washington, D.C.
- 10.Cooksey, D. A., H. R. Azad, J.-S. Cha, and C.-K. Lim. 1990. Copper resistance gene homologs in pathogenic and saprophytic bacterial species from tomato. Appl. Environ. Microbiol. 56:431-435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Corpet, F. 1988. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 16:10881-10890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cuppels, D. A., and J. Elmhirst. 1999. Disease development and changes in the natural Pseudomonas syringae pv. tomato populations on field tomato plants. Plant Dis. 83:759-764. [DOI] [PubMed] [Google Scholar]
- 13.da Silva, A. C. R., J. A. Ferro, F. C. Reinach, C. S. Farah, L. R. Furlan, R. B. Quaggio, C. B. Monteiro-Vitorello, M. A. Van Sluys, N. F. Almeida, L. M. C. Alves, A. M. do Amaral, M. C. Bertolini, L. E. A. Camargo, G. Camarotte, F. Cannavan, J. Cardozo, F. Chambergo, L. P. Ciapina, R. M. B. Cicarelli, L. L. Coutinho, J. R. Cursino-Santos, H. El-Dorry, J. B. Faria, A. J. S. Ferreira, R. C. C. Ferreira, M. I. T. Ferro, E. F. Formighieri, M. C. Franco, C. C. Greggio, A. Gruber, A. M. Katsuyama, L. T. Kishi, R. P. Leite, E. G. M. Lemos, M. V. F. Lemos, E. C. Locali, M. A. Machado, A. M. B. N. Madeira, N. M. Martinez-Rossi, E. C. Martins, J. Meidanis, C. F. M. Menck, C. Y. Miyaki, D. H. Moon, L. M. Moreira, M. T. M. Novo, V. K. Okura, M. C. Oliveira, V. R. Oliveira, H. A. Pereira, A. Rossi, J. A. D. Sena, C. Silva, R. F. de Souza, L. A. F. Spinola, M. A. Takita, R. E. Tamura, E. C. Teixeira, R. I. D. Tezza, M. Trindade dos Santos, D. Truffi, S. M. Tsai, F. F. White, J. C. Setubal, and J. P. Kitajima. 2002. Comparison of the genomes of two Xanthomonas pathogens with differing host specificities. Nature 417:459-462. [DOI] [PubMed] [Google Scholar]
- 14.Deveraux, J. P. Haeberli, and O. Smithies. 1984. A comprehensive set of sequence analysis programs for the Vax. Nucleic Acids Res. 12:387-395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Figurski, D. H., and D. R. Helinski. 1979. Replication of an origin-containing derivative of plasmid RK2 dependent on a plasmid function provided in trans. Proc. Natl. Acad. Sci. USA 76:1648-1652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Finney, L. A., and T. V. O'Halloran. 2003. Transition metal speciation in the cell: insights from the chemistry of metal ion receptors. Science 300:931-936. [DOI] [PubMed] [Google Scholar]
- 17.Garcia-Horsman, J. A., B. Barquera, R. Rumbley, J. Ma, and R. B. Gennis. 1994. The superfamily of heme-copper respiratory oxidases. J. Bacteriol. 176:5587-5600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gardan, L., T. Brault, and E. Germain. 1993. Copper resistance of Xanthomonas campestris pv. juglandis in French walnut orchards and its association with conjugative plasmids. Acta Horticult. 311:259-265. [Google Scholar]
- 19.Garde, S., and C. L. Bender. 1991. DNA probes for detection of copper resistance genes in Xanthomonas campestris pv. vesicatoria. Appl. Environ. Microbiol. 57:2435-2439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Garrett, K. A., and H. F. Schwartz. 1998. Epiphytic Pseudomonas syringae on dry beans treated with copper-based bactericides. Plant Dis. 82:30-35. [DOI] [PubMed] [Google Scholar]
- 21.Hoch, J. A., and K. I. Varughese. 2001. Keeping signals straight in phosphorelay signal transduction. J. Bacteriol. 183:4941-4949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hoshino, N., T. Kimura, A. Yamaji, and T. Ando. 1999. Damage to the cytoplasmic membrane of Escherichia coli by catechin-copper complexes. Free Radic. Biol. Med. 27:1245-1250. [DOI] [PubMed] [Google Scholar]
- 23.Katzen, F., A. Becker, A. Zorreguita, A. Puhler, and L. Ielpi. 1996. Promoter analysis of the Xanthomonas campestris pv. campestris gum operon directing biosynthesis of the xanthan polysaccharide. J. Bacteriol. 178:4313-4318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Keane, P. J., A. Kerr, and P. B. New. 1970. Crown gall of stone fruit. II. Identification and nomenclature of Agrobacterium isolates. Aust. J. Biol. Sci. 23:585-595. [Google Scholar]
- 25.Keen, N. T., S. Tamaki, D. Kobayashi, and D. Trollinger. 1988. Improved broad host-range plasmids for DNA cloning in gram-negative bacteria. Gene 70:191-197. [DOI] [PubMed] [Google Scholar]
- 26.Lee, Y. A., M. Hendson, N. J. Panopoulos, and M. N. Schroth. 1994. Molecular-cloning, chromosomal mapping, and sequence-analysis of copper-resistance genes from Xanthomonas campestris pv. juglandis: homology with small blue copper proteins and multicopper oxidase. J. Bacteriol. 176:173-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lim, C-. K., and D. A. Cooksey. 1993. Characterization of chromosomal homologs of the plasmid-borne copper resistance operon of Pseudomonas syringae. J. Bacteriol. 175:4492-4498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lorang, J. M., H. Shen, D. Kobayashi, D. A. Cooksey, and N. T. Keen. 1994. AvrA and avrB in Pseudomonas syringae pv. tomato PT23 play a role in virulence on tomato plants. Mol. Plant-Microbe Interact. 7:508-515. [Google Scholar]
- 29.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 30.Marco, G. M., and R. E. Stall. 1983. Control of bacterial spot of pepper initiated by strains of Xanthomonas campestris pv. vesicatoria that differ in sensitivity to copper. Plant Dis. 67:779-781. [Google Scholar]
- 31.Mellano, M. A., and D. A. Cooksey. 1988. Nucleotide sequence and organization of copper resistance genes from Pseudomonas syringae pv. tomato. J. Bacteriol. 170:2879-2883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mellano, M. A., and D. A. Cooksey. 1988. Induction of the copper resistance operon from Pseudomonas syringae. J. Bacteriol. 170:4399-4401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miller, J. H. 1972. Experiments in molecular genetics. Cold Spring Harbor Laboratory, Cold Spring Harbor, N.Y.
- 34.Mills, S. D., C. A. Jasalavich, and D. A. Cooksey. 1993. A two-component regulatory system required for copper-inducible expression of the copper resistance operon of Pseudomonas syringae. J. Bacteriol. 175:1656-1664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mills, S. D., C.-K. Lim, and D. A. Cooksey. 1994. Purification and characterization of CopR, a transcriptional activator protein that binds to a conserved domain (cop box) in copper-inducible promoters of Pseudomonas syringae. Mol. Gen. Genet. 244:341-351. [DOI] [PubMed] [Google Scholar]
- 36.Muller, J., Sigel, R. K. O., and Lippert, B. 2000. Heavy metal mutagenesis: insights from bioinorganic model chemistry. J. Inorg. Biochem. 79:261-265. [DOI] [PubMed] [Google Scholar]
- 37.Murillo, J., H. Shen, D. Gerhold, A. Sharma, D. A. Cooksey, and N. T. Keen. 1994. Characterization of pPT23B, the plasmid involved in syringolide production by Pseudomonas syringae pv. tomato PT23. Plasmid 31:275-287. [DOI] [PubMed] [Google Scholar]
- 38.Nies, D. H. 1999. Microbial heavy-metal resistance. Appl. Microbiol. Biotechnol. 51:730-750. [DOI] [PubMed] [Google Scholar]
- 39.Nies, D. H.,. 2003. Efflux-mediated heavy-metal resistance in prokaryotes. FEMS Microbiol. Rev. 27:313-339. [DOI] [PubMed] [Google Scholar]
- 40.Rensing, C., and G. Grass. 2003. Escherichia coli mechanisms of copper homeostasis in a changing environment. FEMS Microbiol. Rev. 27:197-213. [DOI] [PubMed] [Google Scholar]
- 41.Rogers, J. S., E. Clark, G. Cirvilleri, and S. E. Lindow. 1994. Cloning and characterization of genes conferring copper resistance in epiphytic ice nucleation-active Pseudomonas syringae strains. Phytopathology 84:891-897. [Google Scholar]
- 42.Rosen, B. P. 2002. Transport and detoxification systems for transition metals, heavy metals and metalloids in eukaryotic and prokaryotic microbes. Comp. Biochem. Physiol. Part A 133:689-693. [DOI] [PubMed] [Google Scholar]
- 43.Rouch, D. A., and N. L. Brown. 1997. Copper-inducible transcriptional regulation at two promoters in the Escherichia coli copper resistance determinant pco. Microbiology 143:1191-1202. [DOI] [PubMed] [Google Scholar]
- 44.Scheck, H. J., J. W. Pscheidt, and L. W. Moore. 1996. Copper and streptomycin resistance in strains of Pseudomonas syringae from Pacific Northwest nurseries. Plant Dis. 80:1034-1039. [Google Scholar]
- 45.Scheck, H. J., and J. W. Pscheidt. 1998. Effect of copper bactericides on copper-resistant and -sensitive strains of Pseudomonas syringae pv. syringae. Plant Dis. 82:397-406. [DOI] [PubMed] [Google Scholar]
- 46.Silver, S., B. T. O. Lee, N. L. Brown, and D. A. Cooksey. 1993. Bacterial plasmid resistances to copper, cadmium and zinc, p. 38-53. In A. J. Welch and S. U. Chapman (ed.), The chemistry of copper and zinc triads. The Royal Society of Chemistry, London, United Kingdom.
- 47.Simon, R., Priefer, U., and Pühler, A. 1983. A broad host range mobilization system for in vivo genetic engineering: transposon mutagenesis in Gram negative bacteria. Bio/Technology 1:784-791. [Google Scholar]
- 48.Simpson, A. J. G., F. Reinach, P. Arruda, F. A. Abreu, M. Acencio, R. Alvarenga, L. M. C Alves, J. E. Araya, G. S. Baia, C. S. Baptista, M. H. Barros, E. D. Bonaccorsi, S. Bordin, J. M. Bové, M. R. S Briones, M. R. P Bueno, A. A. Camargo, L. E. A. Camargo, D. M. Carraro, H. Carrer, N. B. Colauto, C. Colombo, F. F. Costa, M. C. R. Costa, C. M. Costa-Neto, L. L. Coutinho, M. Cristofani, E. Dias-Neto, C. Docena, H. El-Dorry, A. P. Facincani, A. J. S. Ferreira, V. C. A. Ferreira, J. A. Ferro, J. S. Fraga, S. C. França, M. C. Franco, M. Frohme, L. R. Furlan, M. Garnier, G. H. Goldman, M. H. S. Goldman, S. L. Gomes, A. Gruber, P. L. Ho, J. D. Hoheisel, M. L. Junqueira, E. L. Kemper, J. P. Kitajima, J. E. Krieger, E. E. Kuramae, F. Laigret, M. R. Lambais, L. C. C. Leite, E. G. M. Lemos, M. V. F. Lemos, S. A. Lopes, C. R. Lopes, J. A. Machado, M. A. Machado, A. M. B. N. Madeira, H. M. F. Madeira, C. L. Marino, M. V. Marques, E. A. L. Martins, E. M. F. Martins, A. Y. Matsukuma, C. F. M. Menck, E. C. Miracca, C. Y. Miyaki, C. B. Monteiro-Vitorello, D. H. Moon, M. A. Nagai, A. L. T. O. Nascimento, L. E. S. Netto, A. Nhani, F. G. Nobrega, L. R. Nunes, M. A. Oliveira, M. C. de Oliveira, R. C. de Oliveira, D. A. Palmieri, A. Paris, B. R. Peixoto, G. A. G. Pereira, H. A. Pereira, J. B. Pesquero, R. B. Quaggio, P. G. Roberto, V. Rodrigues, A. J. de M. Rosa, V. E. de Rosa, R. G. de Sá, R. V. Santelli, H. E. Sawasaki, A. C. R. da Silva, A. M. da Silva, F. R. da Silva, W. A. Silva, J. F. da Silveira, M. L. Z. Silvestri, W. J. Siqueira, A. A. de Souza, A. P. de Souza, M. F. Terenzi, D. Truffi, S. M. Tsai, M. H. Tsuhako, H. Vallada, M. A. Van Sluys, S. Verjovski-Almeida, A. L. Vettore, M. A. Zago, M. Zatz, J. Meidanis, and J. C. Setubal. 2000. The genome sequence of the plant pathogen Xylella fastidiosa. 406:151-157.
- 49.Spaink, H. P., R. J. H. Okker, C. A. Wijffelman, E. Pees, and B. Lugtenberg. 1986. Promoters and operon structure of the nodulation region of the Rhizobium leguminosarum symbiosis plasmid pRL1JI. NATO ASI Ser. Ser. H Cell Biol. 4:55-68. [Google Scholar]
- 50.Sundin, G. W., and C. L. Bender. 1996. Molecular analysis of closely related copper- and streptomycin-resistance plasmids in Pseudomonas syringae pv. syringae. Plasmid 35:98-107. [DOI] [PubMed] [Google Scholar]
- 51.Tetaz, T. J., and R. K. J. Luke. 1983. Plasmid controlled resistance to copper in Escherichia coli. J. Bacteriol. 154:1263-1268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ubben, D., and R. Schmitt. 1987. A transposable promoter and transposable promoter probes derived from Tn1721. Gene 53:127-134. [DOI] [PubMed] [Google Scholar]
- 53.Vauterin, L., J. Rademaker, and J. Swings. 2000. Synopsis on the taxonomy of the genus Xanthomonas. Phytopathology 90:677-682. [DOI] [PubMed] [Google Scholar]
- 54.Vivian, A., J. Murillo, and R. W. Jackson. 2001. The roles of plasmids in phytopathogenic bacteria: mobile arsenals? Microbiology 147:763-780. [DOI] [PubMed] [Google Scholar]
- 55.Voloudakis, A. E., C. L. Bender, and D. A. Cooksey. 1993. Similarity between copper resistance genes from Xanthomonas campestris and Pseudomonas syringae. Appl. Environ. Microbiol. 59:1627-1634. [DOI] [PMC free article] [PubMed] [Google Scholar]