Abstract
The human ability to synchronize with other individuals is critical for the development of social behavior. Recent research has shown that physiological inter-personal synchronization may underlie behavioral synchrony. Nevertheless, the factors that modulate physiological coupling are still largely unknown. Here we suggest that social touch and empathy for pain may enhance interpersonal physiological coupling. Twenty-two romantic couples were assigned the roles of target (pain receiver) and observer (pain observer) under pain/no-pain and touch/no-touch conditions, and their ECG and respiration rates were recorded. The results indicate that the partner touch increased interpersonal respiration coupling under both pain and no-pain conditions and increased heart rate coupling under pain conditions. In addition, physiological coupling was diminished by pain in the absence of the partner’s touch. Critically, we found that high partner’s empathy and high levels of analgesia enhanced coupling during the partner’s touch. Collectively, the evidence indicates that social touch increases interpersonal physiological coupling during pain. Furthermore, the effects of touch on cardio-respiratory inter-partner coupling may contribute to the analgesic effects of touch via the autonomic nervous system.
Introduction
The human capacity for generating events in synchrony1 with other individuals has important evolutional significance. Behavioral synchrony is evident in the animal kingdom in various forms. Among them are synchronized periodic movements to create acoustic signals2–4, synchronous flashing among fireflies5, synchronized collective movements among predators while hunting6 and synchronized reactions to stressful and dangerous situations6–8. Humans also tend to coordinate their actions and imitate the postures or actions of others whether they are aware of this or not1, 9, 10. This ability develops early in life11, 12 and is crucial for social communication in general13 and for the development of infant and mother bonding in particular14. Furthermore, synchronized coordinated behaviors have also been noted in other social behavioral contexts, such as speech understanding15 or psychotherapy16. These studies indicate that social synchrony plays a major role in affiliative behaviors and in the development of social behavior.
Recently, an increasing number of studies have explored the physiological mechanisms that underlie social synchrony. These studies have shown that group synchrony is accompanied by cardiac rhythms that are synchronized between active participants and bystanders during collective rituals17 and people collectively watching emotional movies18. In addition, cardiac and respiratory synchronization was found to underlie interpersonal action coordination during choir singing19. Similarly, dual synchrony between romantic dyads was associated with cardiac and respiratory coupling during gazing and imitation tasks20 and even simply when the two members of the couple are together20, suggesting that the mere presence of one’s partner may trigger heart rate synchrony. Nonetheless, although synchrony has been reported in an abundance of social contexts, the conditions that facilitate synchrony remain unclear.
One condition that may increase synchrony is empathy for pain, a concept that describes our tendency to experience distress automatically when confronted with someone else’s pain21. Empathy for pain is associated with activity in pain neural networks22, 23, along with physiological responses such as increased skin conductance24 and increased heart rate17, 25. Since sharing the sufferer’s pain constitutes empathy for pain, inflicting pain to a target may increase the coupling between sufferer (target) and observer. In line with this speculation, Levenson and Gottman (1983) showed that distress situations enhance physiological coupling in romantic dyads26. Therefore, we hypothesized that empathy for pain would increase synchrony between the physiological responses of the target and those of the observer.
Another condition that may promote synchrony is touch. Interpersonal touch has important social and affective values27–29. Specifically, skin-to-skin touch contributes to the development of premature infants30, regulates their stress responses31, provides comfort and emotional well-being32–34 and has an analgesic effect34. Physiologically, interpersonal touch increases the coupling of electrodermal activity and pulse rate variability35 and modulates blood pressure reactivity to stress36 as well as reactivity to distress37. Therefore, our second hypothesis was that interpersonal touch would increase interactional physiological coupling.
Furthermore, it has been shown that touch moderates (1) the relationship between the observer’s trait empathy and the target’s analgesia; (2) inter-partner synchrony of pain rating; and (3) touch-related analgesia38. Moreover, it has been found that empathic accuracy, i.e., the extent to which the supporting partner accurately estimates the pain of the suffering person, is related to the sufferer’s pain perception39. Accordingly, we predicted that inter-dyad variability in level of physiological coupling while experiencing pain would be moderated by levels of trait empathy and empathic accuracy and by the analgesic effect of touch.
To examine these predictions, we designed an experiment consisting of six conditions in which romantic partners were instructed to hold hands or sit with no physical contact or in separated rooms during the pain vs. no pain conditions (Fig. 1). Throughout the experiment the electrocardiogram and respiration of both partners were simultaneously recorded.
Figure 1.
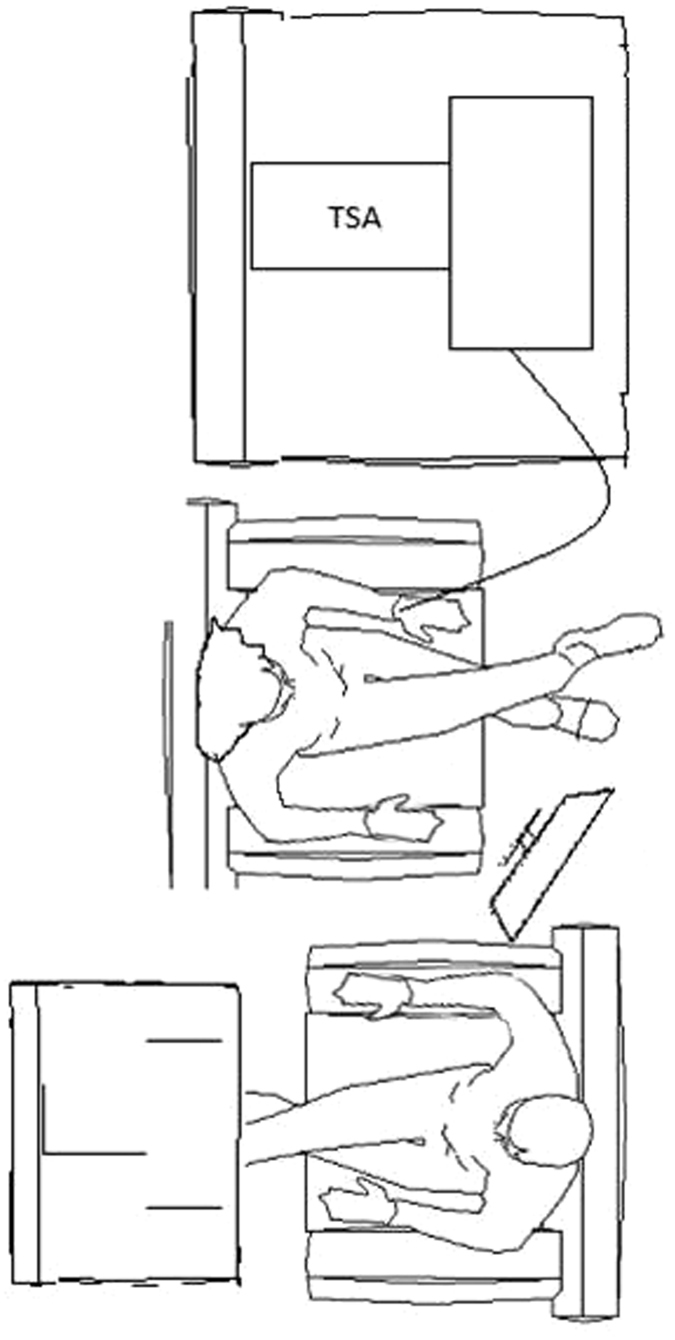
No touch-pain condition.
Results
The sample mean and standard deviations of the pain ratings of both partners appear in Table 1 (women rated their own pain and the men evaluated their partner’s pain). As was reported in the initial report of this data (Goldstein et al., 2016), the pain ratings in the partner-touch condition were lower than in the partner-no touch condition (Mdiff = −0.36, p = 0.029) and the pain-alone condition (Mdiff = −0.66, p < 0.001), confirming that touch had an analgesic effect. In addition, during the pain-alone condition, the women’s pain ratings were marginally higher than in the partner-no touch condition (Mdiff = 0.29, p = 0.093).
Table 1.
Average (standard deviation) pain ratings in each condition.
| Condition | Woman | Partner* |
|---|---|---|
| pain-alone | 52.41(29.41) | — |
| partner no-touch | 37.74(24.82) | 43.52(22.71) |
| partner touch | 25.03(20.32) | 38.51(17.32) |
*The male partner guessed the woman’s pain.
Respiration analysis
We analyzed the data using the coupled linear oscillator (CLO) model (see Methods section), estimating the inter-partner relationship between one partner’s inhalation (predictor) and the other partner’s exchange between inhalation and exhalation (outcome) in six combinations of pain (no pain/pain) and touch (alone/no-touch/touch) factors. The CLO analysis indicated that touch and pain moderated the partners’ velocity effect both in men and in women (F(4,55000) = 14.44, p < 0.0001, ΔBIC = −47.8, ΔR2 = 0.18), indicating that the partner’s velocity effect differed across experimental conditions. We further carried out separate post-hoc analyses for men and women to examine differential effects in targets (women) and observers (men).
Model for male participants: How changes in respiration in females predict shifts in changes of males
The post-hoc analysis revealed a significant effect of cross-partner velocity for male participants during the touch-no pain (ηt(np) = −0.012, p < 0.001, 95% CI [−0.06, −0.18]), no touch-no pain (ζnt(np) = −0.006, p = 0.012, 95% CI [−0.001, −0.010]) and touch-pain (ζt(p) = −0.005, p = 0.017, 95% CI [−0.001, −0.010]) conditions (Fig. 2a). This pattern of effects describes a consistent pattern of inhalation among women while men shift from inhalation to exhalation. However, the woman’s cross-partner velocity during the no touch-pain condition (ζnt(p) < 0.001, p = 0.96) was not related to the man’s acceleration. No significant cross-partner effects were detected for the pain alone (ζa(p) = −0.001, p = 0.78) and the no pain alone (ζa(np) = 0.001, p = 0.86) conditions. In line with our hypotheses, the coupling during the touch conditions was higher than in the no touch conditions, whether without pain (Δζt/nt(np) = 0.006, p < 0.001, 95% CI [0.003, 0.008]) or with pain (Δζt/nt(p) = 0.012, p < 0.001, 95% CI [0.007, 0.016]). However, the pain vs. no pain comparison was associated with decreased respiration synchronization in both the touch (Δζt(p/np) = −0.007, p < 0.001, 95% CI [−0.004, 0.010]) and the no touch (Δζnt(p/np) = −0.006, p < 0.001, 95% CI [−0.004, 0.008]) conditions. Figure 3 depicts these findings.
Figure 2.
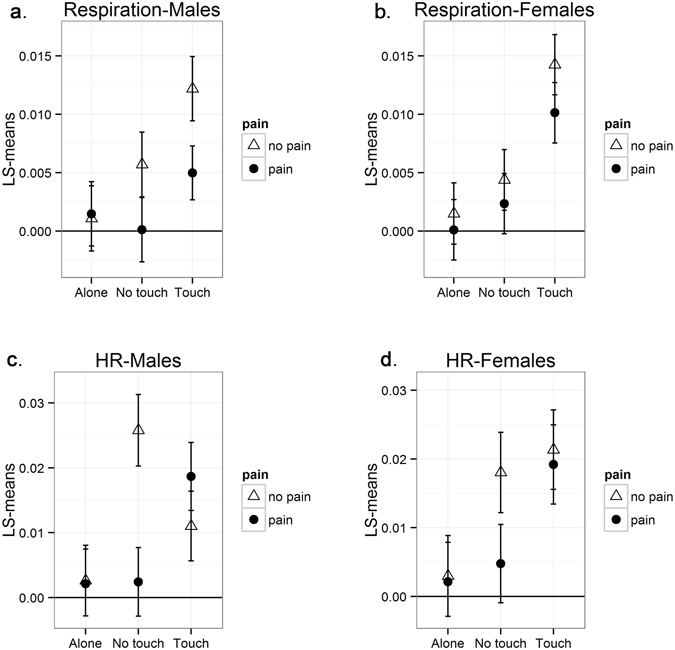
Results of the Coupled Linear Oscillator (CLO) Model for heart rate and respiration. For the sake of simplicity, results are presented as absolute values. The Y-axis presents models based on the least squares (LS) means of each experimental condition, expressing the level of physiological coupling in different experimental conditions. Zero represents a case without interpersonal coupling, while scores that differ from zero indicate interpersonal coupling.
Figure 3.
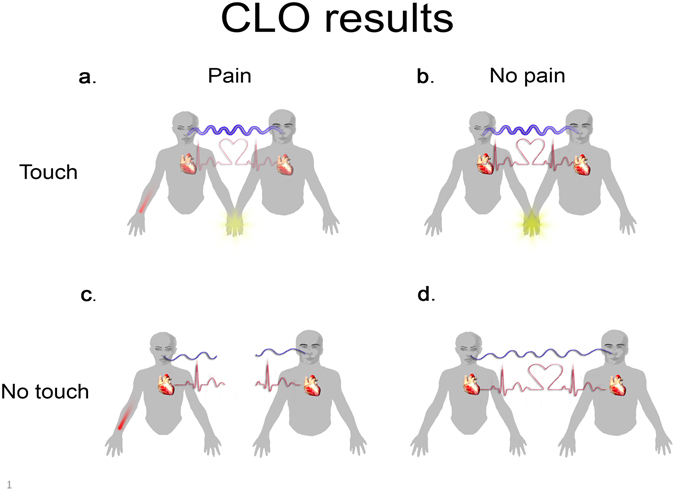
Graphical representation of Coupled Linear Oscillator (CLO) model findings for heart rate and respiration (Fig. 2). Blue lines represent respiration inter-partner coupling and red lines represent coupling in heart-rate. The line’s thickness represents the strength of the coupling, with broken lines denoting a total lack of the coupling. (a) Coupling of respiration and heart rate during touch-pain condition. (b) Coupling of respiration and heart rate during touch-no pain condition. (c) No coupling of respiration and heart rate during no touch-pain condition. (d) Coupling of respiration and heart rate during no touch-no pain condition.
Model for female participants: How changes in respiration in males predict shifts in changes of females
In line with the male model, a significant effect of cross-partner velocity was found for women during the touch-no pain (ζt(np) = 0.014, p < 0.001, 95% CI [0.009, 0.019]) and the touch-pain (ζt(p) = 0.010, p < 0.001, 95% CI [0.006, 0.014]) conditions, while a marginal effect was found in the no touch-no pain condition (ζnt(np) = 0.004, p = 0.092, 95% CI [−0.001, 0.008]) (Fig. 2b). These effects indicated that women tend to shift from exhalation to inhalation when men inhale. However, women’s cross-partner velocity during the no touch-pain condition was not significant (ζnt(p) = 0.002, p = 0.54). No significant cross-partner effects were detected for the pain alone (ζa(p) = −0.001, p = 0.64) or the no pain alone (ζa(np) < 0.001, p = 0.92) conditions.
In line with our hypotheses, the coupling during touch increased compared to during no touch in both the no pain (Δζt/nt(np) = 0.010, p < 0.001, 95% CI [0.006, 0.014]) and the pain (Δζt/nt(p) = 0.008, p < 0.001, 95% CI [0.003, 0.012]) conditions. However, there was no difference between pain and no pain during both touch (Δζt(p/np) = 0.004, p = 0.115) and no touch (Δζnt(p/np) = 0.002, p = 0.637) conditions.
Heart rate analysis
For heart rate we carried out a similar analysis based on the CLO model (see Methods section), estimating the inter-partner relationship between an increase in heart rate of one partner and the exchange between increase and decrease of heart rate in the second partner as a function of pain and touch factors. As in the case of respiratory rate, touch and pain moderated the partners’ velocity effect both in women and in men (F(4,25000) = 19.40, p < 0.0001, −ΔBIC = 7.7, ΔR2 = 0.12), indicating that the partner velocity effect differed across experiment conditions. We further carried out separate post-hoc analyses for male and female participants.
Model for male participants: How changes in respiration in females predict shifts in changes of males
The post-hoc analysis revealed a significant effect of cross-partner velocity for men during the no touch-no pain (ζnt(np) = 0.026, p < 0.001, 95% CI [0.016, 0.036]), touch-pain (ζt(p) = 0.019, p < 0.001, 95% CI [0.010, 0.027]) and touch-no pain (ζt(np) = 0.011, p < 0.001, 95% CI [0.005, 0.016]) conditions (Fig. 2c). Thus, an increase in the woman’s heart rate was related to a shift from a decrease to an increase in the man’s heart rate under the above-mentioned conditions. However, cross-partner female velocity during the no touch-pain condition (ζnt(p) = 0.002, p = 0.57) was not related to acceleration in the men’s heart rate. No significant cross-partner effects were detected for the pain alone (ζa(p) = −0.002, p = 0.62) and no pain alone (ζa(np) = 0.002, p = 0.56) conditions. In line with our hypotheses, the synchronization during touch-pain was higher than in the no touch-pain (Δζt/nt(p) = 0.017, p < 0.001, 95% CI [0.009, 0.024]) and the touch-no pain (Δζt(p/np) = 0.007, p < 0.001, 95% CI [0.003, 0.010]) conditions. However, during the no touch-no pain condition, there was increased synchronization compared to the touch-no pain (Δζt/nt(np) = 0.015, p < 0.001, 95% CI [0.008, 0.021]) and no touch-pain (Δζnt(p/np) = 0.024, p < 0.001, 95% CI [0.014, 0.034]) conditions.
Model for female participants: How changes in respiration in males predict shifts in changes of females
In line with the results of the model for male participants, a significant effect of cross-partner velocity was found for female participants during the touch-no pain (ζt(np) = 0.021, p < 0.001, 95% CI [0.012, 0.029]), touch-pain (ζt(p) = 0.019, p < 0.001, 95% CI [0.011, 0.027]) and no touch-no pain (ζnt(np) = 0.018, p < 0.001, 95% CI [0.010, 0.026]) conditions (Fig. 2d). These effects indicate that the increase in the men’s heart rate was associated with the change in the women’s heart rate from decreasing to increasing. In addition, the women’s cross-partner velocity was not related to the men’s acceleration in heart rate (ζnt(p) = −0.005, p = 0.32) in the no touch-pain (ζnt(p) = 0.004, p = 0.183), pain alone (ζa(p) = 0.002, p = 0.59) or the no pain alone (ζa(np) = 0.002, p = 0.71) conditions. The increased synchronization during the touch-pain condition compared to the no touch-pain (Δζt/nt(p) = 0.014, p < 0.001, 95% CI [0.007, 0.021]) and touch-no pain (Δζt(p/np) = 0.007, p < 0.001, 95% CI [0.003, 0.010]) conditions is in line with our hypothesis. However, the heart rate synchronization during the touch-no pain condition did not differ from the touch-pain (Δζt (np/p) = 0.002, p = 0.368) or the no touch-no pain (Δζnt/nt(np) = 0.003, p = 0.274) conditions.
In summary, all four analyses (men/women X respiration/heart rate) followed a common pattern—touch increased synchronization during pain.
Moderation analysis
We applied Confirmatory Factor Analysis (CFA) to test the structure of the Interpersonal Reactivity Index (IRI) questionnaire, assuming the same unique latent empathy content for both partners. The analysis revealed a good fit between the measurement model and the data (χ2/df = 1.69, CFI = 0.94, RMSEA = 0.071). Thus, in the following analysis we treated trait empathy as a single factor. For the empathy trait measure we used the average of all questions from the IRI questionnaire. Empathic accuracy and trait empathy measurements demonstrated high correlation (r = 0.62, p < 0.001). We tested the moderation effect of empathic accuracy, trait empathy and women’s analgesia on across-partner coupling of heart rate and respiration fluctuations in the touch-pain and no touch pain conditions. This analysis tested the hypothesis that the observer’s level of empathy, his empathic accuracy and the levels of pain analgesia moderate touch-related physiological coupling.
In line with our hypothesis, the male partner’s empathic accuracy significantly moderated the effect of touch on synchronization for respiration fluctuations (F(4,28000) = 27.87, p < 0.0001, ΔBIC = 419439.8, ΔR2 = 0.23). More specifically, a high level of empathic accuracy (one standard deviation above the mean) compared to a low level (one standard deviation below the mean) was associated with increased coupling between female velocity and male acceleration in respiration during the touch condition (Δζt(p) = 0.028, p < 0.001, 95% CI [0.016, 0.040]). Correspondently, high levels of empathic accuracy between partners compared to low levels indicated increased coupling between male velocity and female acceleration in the touch condition (Δζt(p) = 0.029, p < 0.001, 95% CI [0.016, 0.041]) (Fig. 4a,b). The corresponding contrasts were not significant in the condition without partner touch (Δζnt(p) = 0.002, p = 0.56, men), (Δζnt(p) = 0.001, p < 0.73, women) (Fig. 4c,d).
Figure 4.
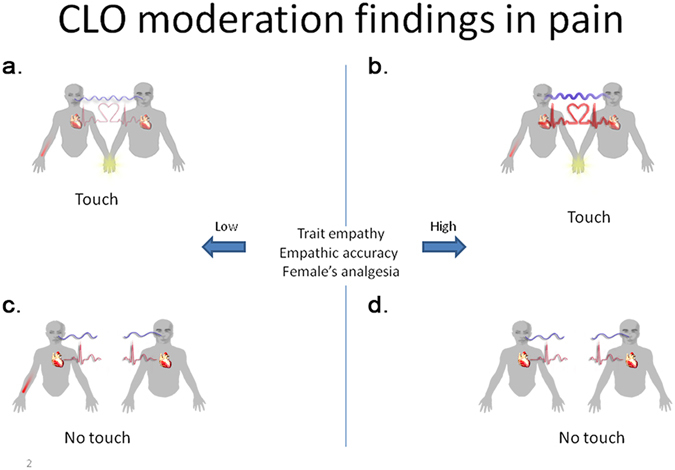
Graphical representation of moderation analysis of trait empathy, empathic accuracy and women’s analgesia on across-partner synchronization in HR and RR fluctuations. Empathic accuracy = man’s accuracy in estimating woman’s pain, trait empathy = IRI questionnaire, woman’s analgesia = reduction in woman’s pain as a result of man’s presence or touch. Blue and red lines mark respiration and heart rate inter-partner coupling, respectively. The line’s thickness represents the strength of the synchronization, and a broken line indicates a total lack of the coupling. (a) Coupling of respiration and heart rate during touch-pain condition for dyads with low (−1 SD) trait empathy, low empathic accuracy and low women’s analgesia. (b) Coupling of respiration and heart rate during touch-pain condition for dyads with high (+1 SD) trait empathy, high empathic accuracy and high women’s analgesia. (c) No coupling of respiration and heart rate during no touch-pain condition for dyads with low (−1 SD) trait empathy, low empathic accuracy and low women’s analgesia. (d) No coupling of respiration and heart rate during no touch-pain condition for dyads with high (+1 SD) trait empathy, high empathic accuracy and high women’s analgesia.
We found significant moderation of the effect of women’s analgesia and touch on the cross-partner synchronization in velocity of respiration fluctuations (F(4,28000) = 26.59, p < 0.0001, ΔBIC = −418367.9, ΔR2 = 0.19). Higher levels of women’s analgesia predicted increased coupling between female velocity and male acceleration (Δζt(p) = 0.027, p < 0.001, 95% CI [0.015, 0.039]) and increased associations between male velocity and female acceleration (Δζt(p) = 0.068, p < 0.001, 95% CI [0.042, 0.093]) in the touch condition. However, the corresponding contrasts were not significant in the absence of partner touch (Δζnt(p) = 0.005, p = 0.32, men; Δζnt(p) < 0.001, p = 0.87, women). It is important to note that trait empathy showed a pattern of moderation similar to that of empathic accuracy. However, the effect of trait empathy was redundant in the model that included empathic accuracy and women’s analgesia as moderators (most likely because of the high correlation between them).
The same pattern of moderation effects emerged in the heart rate analysis. Empathic accuracy and touch moderated the effect of synchronization in heart rate (F(4,13000) = 20.73, p < 0.0001, ΔBIC = −265162.9, ΔR2 = 0.24). High as opposed to low levels of empathic accuracy predicted greater coupling between female velocity and male acceleration (Δζt(p) = 0.046, p < 0.001, 95% CI [0.026, 0.066]) and larger associations between male velocity and female acceleration (Δζt(p) = 0.049, p < 0.001, 95% CI [0.028, 0.070]) in the touch condition. The corresponding contrasts were not significant in the condition without partner touch (Δζnt(p) = 0.008, p = 0.28, males), (Δζnt(p) = 0.008, p = 0.34, females). As in the case of respiration fluctuations, women’s analgesia and touch significantly moderated the effect of cross-partner velocity for heart rate (F(4,13000) = 5.03, p < 0.0001, ΔBIC = −267895.6, ΔR2 = 0.25). Specifically, high women’s analgesia as opposed to low analgesia was related to increased coupling between female velocity and male acceleration (Δζt(p) = 0.015, p < 0.001, 95% CI [0.008, 0.022]) and greater association between male velocity and female acceleration (Δζt(p) = 0.019, p < 0.001, 95% CI [0.010, 0.028]) in the touch condition. However, these contrasts were not significant in the no-touch conditions (Δζnt(p) = 0.003, p = 0.67, males), (Δζnt(p) = 0.022, p = 0.17, females). Similar to respiration fluctuations, trait empathy showed a pattern of moderation similar to that of empathic accuracy. However, the moderation effect of trait empathy did not contribute beyond empathic accuracy and women’s analgesia. In addition, the females’ feeling of comfort during the touch did not moderate coupling in respiration fluctuations (F(4,28000) = 1.37, p = 0.24) nor heart rate (F(4,13000) = 1.47, p = 0.21).
Discussion
The current study sought to examine the role of touch and pain in inter-partner heart rate and respiration coupling.
The statistical analysis was based on the CLO model20, which allows estimating interpersonal bi-directional coupling between one partner’s signal exchange and a shift in the signal exchange in the interacting partner. Our findings confirm that interpersonal touch as compared to no-touch is associated with increased respiration coupling, during both pain and no-pain conditions. In line with these findings, interpersonal touch has been reported to increase coupling of electrodermal activity between partners35. Moreover, researchers have shown that touch can communicate emotions in a way that the receiver is able to recognize the emotional states communicated by the toucher40–42. Thus, in the current study the partners may have communicated their emotions via touch, as evidenced by an increase in physiological coupling.
The finding of an increased pattern of heart rate coupling during the pain and touch condition indicates that touch may allow communication between the participants but only during pain. This indicates that coupling in heart rate is evident only when the target experience pain and empathy in the observer is possibly provoked. Indeed, the powerful effect of social touch has been shown to affect our emotional well-being and diminish distress or pain in various settings28, 37, 43–48. For example, it has been reported that skin-to-skin touch may have an analgesic effect on human babies undergoing minor medical procedures45 and may even therapeutically reduce pain in cancer patients44, 49 and those with chronic pain50. Previously, Coan et al. (2006) reported that greater pain reduction is observed following the touch of a partner as compared to a stranger37. Thus, it could be suggested that inter-personal physiological coupling underlie touch-related analgesia.
In contrast to our original hypothesis, pain did not increase physiological coupling in the absence of the partner’s touch. This effect was consistent across both physiological signals and for both model directions (female-male, male-female). The decrease in coupling when the women experienced pain and their partners observed their pain partially contradicts the findings of Konvalinka et al.17. This study reports that during a fire-walking ritual, the cardiac rhythms of active participants were synchronized with those of related bystanders. However, one should take into account major differences between this study and the current study. While the sample studied by Konvalinka et al., 2011 consisted of experienced fire-walkers who were well trained to manage their pain and did not feel the pain51, our participants, who represented a normal population, did not have such experience. As a result they probably experienced higher levels of pain and distress during the pain condition, which may disrupt the physiological coupling. In addition, a possible explanation for these contradictory findings may rely on the different definitions of synchronization used in the two studies. Konvalinka (2011) defines synchronization as a similarity between the heart rate of a firewalker and that of a related spectator, while we defined interpersonal coupling as “one partner’s signal changing from increasing to decreasing while the other partner’s signal decreases”.
Here we show that in the absence of touch, the experience of pain disrupted the physiological coupling as the targets probably were focusing on their own pain experience. Indeed, previous research showed that pain interrupts the attention, that the suffering person pays to the external world52. It is thus possible that the women may have focused on her own pain and engaged in self-based strategies to cope with pain53, 54, which may explain their physiological “disconnection” from the male partners. In line with this idea, it was shown that social touch increases our attention to social stimuli55. However, self-based strategies seem to be less effective than those involving physical touch between partners, as we and others found that touch is associated with a greater analgesic effect than no-touch36, 38.
Indeed, the moderation analysis provides additional evidence for the notion that touch-related analgesia is related to the empathy of the toucher. The results show that both empathic accuracy and trait empathy moderated inter-partner physiological coupling, so that dyads with highly empathic male partners demonstrated increased coupling. That is, we observed a shift from exhalation to inhalation in a participant when their partner inhales (the heart rate shifts from a decrease to an increase when the partner exhibited an increase in heart rate).Consistent with this, researchers have shown that greater empathy is associated with better physiological linkage between romantic partners56 and greater touch-related coupling of electrodermal activity35. Moreover, in the couples where the female partners reported greater touch-related analgesia, we observed enhanced physiological coupling. Thus, the effects of touch on cardio-respiratory inter-partner coupling may be associated with pain analgesia. It is thus possible that cardio-respiratory changes, where the observer of pain affect’s the target of pain, is associated with the level of empathy of the observer. That is, the empathic response of the observer is communicated to the target of pain. This idea is in line with Hertestein et al. (2006) who demonstrated that people can identify various emotions including love and sympathy from the experience of being touched41.
Similarly, it is possible that the target of pain communicates back the analgesic effect of touch to the observer. Thus, the use of touch may improve the quality of non-verbal physiological communication between partners, especially when one of them feels pain, enabling the toucher to better project his empathy to the female partner and consequently have an analgesic effect.
It is important to note that the CLO model enables the identification of associations in both directions (i.e. the male partner signal change predicts the shift in signal change of the female partner and vice versa). However, in this study, both directions showed the same pattern of results supporting the idea of physiological bi-directional communication. These findings of physiological coupling may also explain recent findings of partners being able to influence women during labor. For example, it was reported that massage and breathing coaching from partners can decrease negative affect, as expressed by a depressed mood, anxiety, and pain as well as enhance positive affect, shorten labors and hospital stays, and decrease postpartum depression57.
Apparently, skin to skin touch is important for pain reduction, which may explain people’s preference for social touch58. Moreover, touch activates reward circuits in the brain59, 60. Indeed, skin-to-skin touch has been shown to activate the reward system, which results in pain reduction both in animals and in humans47. It seems that this phenomenon has evolutionary roots. For example, non-human primates devote much more time to grooming than they actually need for hygiene reasons, resulting in endogenous opioid release61, 62, as well as pain and stress reduction63, 64.
It is still not clear exactly how inter-personal physiological resonance is related to touch-related analgesia. Observing the pain of others can trigger emotional resonance in the observer, activating brain mechanisms similar to those of the suffering person (e.g., anterior cingulate and insula cortices) and areas that are classified as the “mirror neuron system” (e.g., inferior parietal cortex)23. As the parietal lobe integrates sensory information among various modalities, the assumption is that multisensory integration of both visual and tactile stimuli may facilitate the emotion resonance with the observed target, as also expressed in associated autonomic and somatic responses65. Moreover, tactile-induced analgesia66 correlates with activations in brain areas related to multimodal neural activity67 and emotional processes68–72. The partner’s touch may also enhance inter-partner brain synchronization in areas related to the pain matrix, a hypothesis that should be tested in future research using novel hyperscanning techniques73. In addition, the anterior cingulate cortex (ACC) has also been found to be relevant in the context of pain perception, empathy for pain22, touch60 and reward systems74. The ACC appears to play a role in a variety of autonomic functions, such as regulating heart rate and blood pressure75, 76. This complicated physiological mechanism may underlie the observed coupling between the partners.
Recent research shows that the neuropeptide oxytocin may play a key role in synchrony77 as well as in touch analgesia via physiological coupling. It was demonstrated that warm touch can increase the levels of plasma oxytocin, and reduce stress78 and depression79. Moreover, recent studies have highlighted the role of oxytocin in inter-personal coupling, increasing touch interaction synchronization while reducing stress80, 81 and enhancing brain-to brain coupling in alpha rhythms82. Thus, future research should test the role of oxytocin in touch-related analgesia.
It is important to note that our findings of interpersonal coupling during touch can also be explained by the phenomenon of Huygens synchronization of two connected pendulum clocks83. According to this explanation, the mere connection between objects creates synchrony between these objects. Nevertheless, the moderation effects of empathic accuracy and pain analgesia reduce the probability of this explanation. Interestingly, the difference in pain between the conditions with and without touch was higher in females than in males, who estimated their partners’ pain. These findings can be explained by the fact that female participants experienced the real pain and their male partners only guessed the females’ pain level. It is thus possible that males did not realize the magnitude of the effectiveness of their touch and therefore their pain ratings did not decrease in the touch condition.
Although the current study used a controlled design with several balanced conditions, it has several limitations that need to be acknowledged. First, only the female participants underwent pain stimuli while the male participants did not, so that the generalizability of our results is restricted to the male-to-female direction. Therefore, future research should test the effect of touch and pain in both men and women as well as in homosexual and heterosexual participants. This could also include parent-child, sibling, and best-friend interactions, in comparison to the interactions between strangers. Second, this study used a single subjectively adjusted degree of heat pain. Future research should test physiological coupling on varying pain intensities and also cold pain stimuli. Third, the subjects were asked for a static handholding, without squeezing, stroking or rubbing following the paradigm proposed by Goldstein et al. (2016). However, comparing between these different types of touch is of high interest and would benefit from future investigation. Fourth, partners could use visual information in all interacting conditions. The visual and tactile sources of information may interact in the brain and therefore future research should estimate these effects. Finally, reporting pain for both emotional and intensity components may shed more light on our findings.
To conclude, we show here that touch regulates physiological coupling during pain, suggesting that interpersonal coupling is affected by various contextual social cues. Yet the prevalent approach in testing perception and behavior is to split one complex system (e.g., the delivery of pain) into several subparts and to explore each of them independently. Although this simplified approach allows analyses of human responses, it lacks the sensitivity to capture elements involved in real-life social interactions. Considering that human behavior is fundamentally different when we are interacting with others rather than merely observing ourselves, here we investigate physiological response using a paradigm that also consider social contexts. Since physiological resonance has important evolutionary significance for animals and humans84–86, investigation of inter-personal coupling provides an interesting opportunity to understand our behavior in the natural social environment.
Methods
Participants
Twenty-two couples (44 participants) completed the study. Participants ranged in age from 23–32 years old (mean and SD for men: 26.4 ± 2.27 years; mean and SD for women: 25.6 ± 1.9 years), had no children, were in long-term relationships (mean and SD: 3.46 ± 2.25 years) and had around 13 years of education (mean and SD of years of education for men: 13.3 ± 1.5, and for women: 13.6 ± 1.3). Only 9% of the couples were married.
Since previous research indicated that women elicit more empathy and compassion87, men were always assigned the role of the observer, while women were the targets. Two of the couples were dropped from the analysis because of unsuccessful physiological recording.
The couples were screened by a phone interview based on the following criteria: (1) right-handed and between the ages of 22 and 40; (2) no chronic or acute pain of any sort; (3) no medication use (except for oral contraceptives); (4) no history of neurological disorders, psychiatric problems or other problems relevant to the study; (5) not pregnant; (6) in a romantic relationship (defined as couples who reported being in a serious relationship, living together for at least one year and having significant feelings of love for each other). The study was approved by the Faculty of Social Welfare and Health Sciences Ethics Committee, University of Haifa and informed consent was obtained from all participants. All methods were carried out in accordance with relevant guidelines and regulations.
Assessment of empathy
Interpersonal Reactivity Index (IRI). The IRI is a 28-item questionnaire measuring empathic capacity on four separate subscales: (1) perspective taking; (2) empathic concern; (3) personal distress; (4) fantasy88. The reliability of all scales is 0.84. After the experiment terminated female participants were asked to rate: “How comfortable did you feel during the partner’s touch”? on a 1–10 scale.
Pain familiarization and pain-60 determination
All contact heat stimuli in this experiment were applied to the left volar forearm using a 3 cm2 computer-controlled Peltier-type thermode (TSA-2001, Medoc, Ramat-Yishai, Israel) (see Fig. 1). During the procedure of pain familiarization, female participants were exposed to three short contact-heat stimuli (43, 45, and 47 °C), each for 7 seconds, administered in a semi-randomized order with a break of 10 seconds. Participants were asked to report pain intensity using the numerical pain score (NPS), ranging from 0, denoting “no pain” to 100, denoting “the worst pain imaginable.” Thereafter, the stimulus intensity was adjusted to each participant to evoke a peak pain magnitude of 60/100 (pain-60) on NPS, using the algorithm described by ref. 89.
Experimental conditions
The experiment consisted of six experimental conditions within one session. The pain-alone condition included 120 seconds of pain stimulation applied to the woman’s left forearm at a temperature individually tailored to induce an NPS score of 60, while the partner sat in an adjacent room. During the touch-no pain condition, the participants sat facing each other holding their dominant hands, while during the no touch-no pain condition, the partner was only present without any physical contact. During the touch-pain condition, the pain stimulus was administered to the female participant while her partner held her dominant hand. In the no touch-pain condition, the female participant was administered the same pain stimulus, but her partner was only present without any physical contact. During the no pain-alone condition the partner sat in the adjacent rooms. In the no-touch conditions, participants were instructed to hold the handles of their armchair (Fig. 1). A 10 min break was kept between successive conditions.
Dual-ECG and respiration data acquisition
Standard electrocardiogram ECG readings were recorded simultaneously for both participants via MindWare MW1000A recorder (MindWare Technologies, Gahanna, OH) at a 256 Hz sampling rate, using Wi-Fi local network for the signal sync. Three ECG electrodes were placed on the right shoulder, and on the right and left lower quadrant correspondently. Respiration was recorded with respiration belts from MindWare Technologies (Gahanna, OH). The belt was placed around the waist just above the trousers. As a calibration procedure, participants were instructed to breathe at a normal rate into a bag of fixed volume (600 ml) for several cycles. This technology provides synchronous recording of all physiological signals from two participants.
Procedure
Upon arrival, the partners were sent to different rooms and asked not to communicate verbally with each other until the experiment was over. After completing the IRI, participants underwent pain familiarization and pain-60 determination. This was followed by six counterbalanced conditions: no pain-alone, pain-alone, partner touch-no pain, partner no touch-no pain, partner touch-pain and partner no touch-pain. The women were asked to rate their pain intensity two seconds before the end of each condition using the NPS. Simultaneously, the male partners were instructed to rate their female partners’ level of pain. Both partners wrote the number on a small piece of paper not visible to the other member of the couple. A 10-minute break separated successive conditions.
Pre-processing
Raw respiration data were cleaned from the clipping artifacts using an algorithm described by Helm et al.20. R-R intervals were calculated from raw ECG data by HRV 2.0 software (MindWare Technologies) and were then interpolated, as proposed by90, in order to merge the data between the partners.
Statistical analysis
Our statistical framework was based on an entirely dyadic perspective that requires continuously measuring both individuals in the relationship at the same time. Examining physiological coupling between couple partners requires statistical models that can capture cross-partner dynamics. The model should take into account the trajectory of a given physiological signal and to estimate the association between the couple partners. Since respiration and heart rate follow oscillating patterns, the model should refer to the sinusoidal fluctuating pattern of the signal, testing the relationship between the partners’ signals. Linear oscillator (CLO) model20, which is an extension of the Damped Linear Oscillator model (DLO)91 suits these requirements, providing a complex framework for the given type of the data. The DLO model uses estimates of the first derivative (signal change or velocity) and the second derivative (shift in signal change or acceleration) of a dynamic signal to model data that fluctuate around a constant point over time. The parameter η in the DLO describes the relationship between the position (signal itself) and the acceleration, while the parameter ζ quantifies the relationship between the velocity and the acceleration. The CLO model is the bivariate extension of the DLO and provides an estimation of linkage between two members of a system (for all details see ref. 20). The model can be characterized by the following equations:
where x and y are physiological signals from the two members of the dyad. The first two terms of each equation characterize within-person linkage between velocity and position, with acceleration as defined in DLO model. The next two terms describe corresponding cross-partner associations that can be interpreted as inter-personal coupling. In this study, we refer to inter-partner velocity-acceleration coupling in heart rate and respiration as an indicator of interpersonal synchronization. Positive can be interpreted as changes in one partner’s signal from decreasing to increasing while the other partner’s signal increases.
To analyze the data, we used Linear Mixed Models (LMM) via MIXED procedure in SAS92. LMM allows taking the hierarchical structure of data into account93. The Bayesian Information Criterion (BIC) was used for model selection. The model with the smaller BIC shows a better fit. Since a slope representing signal trends is absent in the CLO model, all data were linearly detrended94.
To estimate the partners’ velocity effect in each condition, we applied post-hoc contrast analysis using simulation-based multiple test correction proposed by95. Moderation analysis of empathy and pain reduction on cross-partner coupling was conducted in the same statistical framework, including corresponding interaction terms.
In order to systemize reporting of the findings, we used the following condition notations: “p” = pain, “np” = no pain, “t” = touch, “nt” = no touch, “a’ = alone. Thus, ζnt(np) means coupling during the no touch-no pain condition.
Empathic Accuracy: The measure of empathic accuracy was defined as the absolute difference between the partners’ pain ratings (each male partner’s pain ratings minus his female partner’s pain ratings) divided by the female partner’s pain ratings. Touch related analgesia: We also referred to reduction in the female partner’s pain (i.e., women’s analgesia) and calculated the percentage difference between each woman’s pain rating in the no touch-pain and the touch-pain conditions and her rating in the pain-alone condition. In the moderation analysis the three-way interaction term of partner’s velocity X touch X moderator was tested and then corresponding post-hoc contrast analysis was applied to interpret the findings.
Acknowledgements
This research was supported by the Binational Science Foundation (BSF) grant number 2015068.
Author Contributions
P.G., S.S.T. and I.W.F. designed research; P.G. performed research; P.G. analyzed data; P.G., S.S.T. and I.W.F. wrote the paper.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Bernieri, F. J. & Rosenthal, R. 11. Interpersonal coordination: Behavior matching and interactional synchrony. Fundam. Nonverbal Behav. 401 (1991).
- 2.GREENFIELD MD. Synchronous and Alternating Choruses in Insects and Anurans: Common Mechanisms and Diverse Functions. Integr. Comp. Biol. 1994;34:605–615. [Google Scholar]
- 3.Kotiaho JS, Alatalo RV, Mappes J, Parri S. Adaptive significance of synchronous chorusing in an acoustically signalling wolf spider. Proceedings. Biol. Sci. /R. Soc. 2004;271:1847–1850. doi: 10.1098/rspb.2004.2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gibson G, Russell I. Flying in tune: sexual recognition in mosquitoes. Curr. Biol. 2006;16:1311–1316. doi: 10.1016/j.cub.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 5.Mirollo RE, Strogatz SH. Synchronization of Pulse-Coupled Biological Oscillators. SIAM J. Appl. Math. 1990;50:1645–1662. doi: 10.1137/0150098. [DOI] [Google Scholar]
- 6.Handegard NO, et al. The dynamics of coordinated group hunting and collective information transfer among schooling prey. Curr. Biol. 2012;22:1213–1217. doi: 10.1016/j.cub.2012.04.050. [DOI] [PubMed] [Google Scholar]
- 7.Procaccini A, et al. Propagating waves in starling, Sturnus vulgaris, flocks under predation. Anim. Behav. 2011;82:759–765. doi: 10.1016/j.anbehav.2011.07.006. [DOI] [Google Scholar]
- 8.Couzin, I. D. & Krause, J. Self-Organization and Collective Behavior in Vertebrates. Advances in the Study of Behavior 1–75, doi:10.1016/s0065-3454(03)01001-5 (2002).
- 9.Noy L, Dekel E, Alon U. The mirror game as a paradigm for studying the dynamics of two people improvising motion together. Proc. Natl. Acad. Sci. 2011;108:20947–20952. doi: 10.1073/pnas.1108155108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sebanz N, Bekkering H, Knoblich G. Joint action: bodies and minds moving together. Trends Cogn. Sci. 2006;10:70–76. doi: 10.1016/j.tics.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 11.Leclère C, et al. Why synchrony matters during mother-child interactions: a systematic review. PloS one. 2014;9:e113571. doi: 10.1371/journal.pone.0113571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Feldman R, Magori-Cohen R, Galili G, Singer M, Louzoun Y. Mother and infant coordinate heart rhythms through episodes of interaction synchrony. Infant Behav. Dev. 2011;34:569–577. doi: 10.1016/j.infbeh.2011.06.008. [DOI] [PubMed] [Google Scholar]
- 13.Siller M, Sigman M. The behaviors of parents of children with autism predict the subsequent development of their children’s communication. J. Autism Dev. Disord. 2002;32:77–89. doi: 10.1023/A:1014884404276. [DOI] [PubMed] [Google Scholar]
- 14.Dumas G, Lachat F, Martinerie J, Nadel J, George N. From social behaviour to brain synchronization: Review and perspectives in hyperscanning. IRBM. 2011;32:48–53. doi: 10.1016/j.irbm.2011.01.002. [DOI] [Google Scholar]
- 15.Bavelas JB, Coates L, Johnson T. Listener Responses as a Collaborative Process: The Role of Gaze. J. Commun. 2002;52:941–580. doi: 10.1111/j.1460-2466.2002.tb02562.x. [DOI] [Google Scholar]
- 16.Ramseyer F, Tschacher W. Nonverbal synchrony in psychotherapy: coordinated body movement reflects relationship quality and outcome. J. Consult. Clin. Psychol. 2011;79:284–295. doi: 10.1037/a0023419. [DOI] [PubMed] [Google Scholar]
- 17.Konvalinka I, et al. Synchronized arousal between performers and related spectators in a fire-walking ritual. Proc. Natl. Acad. Sci. 2011;108:8514–8519. doi: 10.1073/pnas.1016955108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Golland Y, Arzouan Y, Levit-Binnun N. The Mere Co-Presence: Synchronization of Autonomic Signals and Emotional Responses across Co-Present Individuals Not Engaged in Direct Interaction. PloS one. 2014;10:e0125804–e0125804. doi: 10.1371/journal.pone.0125804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Müller V, Lindenberger U. Cardiac and respiratory patterns synchronize between persons during choir singing. PloS one. 2011;6:e24893. doi: 10.1371/journal.pone.0024893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Helm JL, Sbarra D, Ferrer E. Assessing cross-partner associations in physiological responses via coupled oscillator models. Emot. 2012;12:748–762. doi: 10.1037/a0025036. [DOI] [PubMed] [Google Scholar]
- 21.Singer T, et al. Empathy for pain involves the affective but not sensory components of pain. Science. 2004;303:1157–1162. doi: 10.1126/science.1093535. [DOI] [PubMed] [Google Scholar]
- 22.Jackson PL, Brunet E, Meltzoff AN, Decety J. Empathy examined through the neural mechanisms involved in imagining how I feel versus how you feel pain. Neuropsychologia. 2006;44:752–761. doi: 10.1016/j.neuropsychologia.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 23.Lamm C, Decety J, Singer T. Meta-analytic evidence for common and distinct neural networks associated with directly experienced pain and empathy for pain. NeuroImage. 2011;54:2492–2502. doi: 10.1016/j.neuroimage.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 24.Block AR. Investigation of the response of the spouse to chronic pain behavior. Psychosom. Med. 1981;43:415–422. doi: 10.1097/00006842-198110000-00004. [DOI] [PubMed] [Google Scholar]
- 25.Lamm C, Porges EC, Cacioppo JT, Decety J. Perspective taking is associated with specific facial responses during empathy for pain. Brain Res. 2008;1227:153–161. doi: 10.1016/j.brainres.2008.06.066. [DOI] [PubMed] [Google Scholar]
- 26.Levenson RW, Gottman JM. Marital interaction: physiological linkage and affective exchange. J. Personal. Soc. Psychol. 1983;45:587–597. doi: 10.1037/0022-3514.45.3.587. [DOI] [PubMed] [Google Scholar]
- 27.Löken LS, Olausson H. The skin as a social organ. Exp. brain Res. 2010;204:305–314. doi: 10.1007/s00221-009-2007-y. [DOI] [PubMed] [Google Scholar]
- 28.Gallace A, Spence C. The science of interpersonal touch: an overview. Neurosci. & Biobehav. Rev. 2010;34:246–259. doi: 10.1016/j.neubiorev.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 29.Fairhurst MT, Löken L, Grossmann T. Physiological and behavioral responses reveal 9-month-old infants’ sensitivity to pleasant touch. Psychol. Sci. 2014;25:1124–1131. doi: 10.1177/0956797614527114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Field T. Massage Therapy Facilitates Weight Gain in Preterm Infants. Curr. Dir. Psychol. Sci. 2001;10:51–54. doi: 10.1111/1467-8721.00113. [DOI] [Google Scholar]
- 31.Feldman R, Singer M, Zagoory O. Touch attenuates infants’ physiological reactivity to stress. Dev. Sci. 2010;13:271–278. doi: 10.1111/j.1467-7687.2009.00890.x. [DOI] [PubMed] [Google Scholar]
- 32.Meehan TC. Therapeutic touch as a nursing intervention. J. Adv. Nurs. 1998;28:117–125. doi: 10.1046/j.1365-2648.1998.00771.x. [DOI] [PubMed] [Google Scholar]
- 33.Miles P, True G. Reiki-review of a biofield therapy: history, theory, practice, and research. Altern. Ther. Health Med. 2003;9:62–73. [PubMed] [Google Scholar]
- 34.So PS, Jiang JY, Qin Y. Touch therapies for pain relief in adults. Cochrane Libr. 2008 [Google Scholar]
- 35.Chatel-Goldman, J., Congedo, M., Jutten, C. & Schwartz, J.-L. Touch increases autonomic coupling between romantic partners. Front. Behav. Neurosci. 8, doi:10.3389/fnbeh.2014.00095 (2014). [DOI] [PMC free article] [PubMed]
- 36.Grewen KM, Anderson BJ, Girdler SS, Light KC. Warm Partner Contact Is Related to Lower Cardiovascular Reactivity. Behav. Med. 2003;29:123–130. doi: 10.1080/08964280309596065. [DOI] [PubMed] [Google Scholar]
- 37.Coan JA, Schaefer HS, Davidson RJ. Lending a hand: social regulation of the neural response to threat. Psychol. Sci. 2006;17:1032–1039. doi: 10.1111/j.1467-9280.2006.01832.x. [DOI] [PubMed] [Google Scholar]
- 38.Goldstein P, Shamay-Tsoory SG, Yellinek S, Weissman-Fogel I. Empathy predicts an experimental pain reduction during touch. J. Pain. 2016;17:1049–57. doi: 10.1016/j.jpain.2016.06.007. [DOI] [PubMed] [Google Scholar]
- 39.Leonard MT, Issner JH, Cano A, Williams AM. Correlates of spousal empathic accuracy for pain-related thoughts and feelings. Clin. J. pain. 2013;29:324–333. doi: 10.1097/AJP.0b013e3182527bfd. [DOI] [PubMed] [Google Scholar]
- 40.Hertenstein MJ, Holmes R, McCullough M, Keltner D. The communication of emotion via touch. Emotion. 2009;9:566–73. doi: 10.1037/a0016108. [DOI] [PubMed] [Google Scholar]
- 41.Hertenstein MJ, Keltner D, App B, Bulleit BA, Jaskolka AR. Touch communicates distinct emotions. Emotion. 2005;6:528–533. doi: 10.1037/1528-3542.6.3.528. [DOI] [PubMed] [Google Scholar]
- 42.Thompson EH, Hampton JA. The effect of relationship status on communicating emotions through touch. Cogn. Emot. 2011;25:295–306. doi: 10.1080/02699931.2010.492957. [DOI] [PubMed] [Google Scholar]
- 43.Field T. Massage therapy for infants and children. Journal of developmental and behavioral pediatrics: JDBP. 1995;16:105–111. doi: 10.1097/00004703-199504000-00008. [DOI] [PubMed] [Google Scholar]
- 44.Fleisher KA, et al. Integrative Reiki for Cancer Patients A Program Evaluation. Integr. Cancer Ther. 2014;13:62–67. doi: 10.1177/1534735413503547. [DOI] [PubMed] [Google Scholar]
- 45.Gray L, Watt L, Blass EM. Skin-to-skin contact is analgesic in healthy newborns. Pediatrics. 2000;105:e14–e14. doi: 10.1542/peds.105.1.e14. [DOI] [PubMed] [Google Scholar]
- 46.Inui K, Tsuji T, Kakigi R. Temporal analysis of cortical mechanisms for pain relief by tactile stimuli in humans. Cereb. cortex. 2006;16:355–365. doi: 10.1093/cercor/bhi114. [DOI] [PubMed] [Google Scholar]
- 47.Younger J, Aron A, Parke S, Chatterjee N, Mackey S. Viewing pictures of a romantic partner reduces experimental pain: involvement of neural reward systems. PloS one. 2010;5:e13309. doi: 10.1371/journal.pone.0013309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tarr B, Launay J, Cohen E, Dunbar R. Synchrony and exertion during dance independently raise pain threshold and encourage social bonding. Biol. Lett. 2015;11:20150767. doi: 10.1098/rsbl.2015.0767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Post-White J, et al. Therapeutic massage and healing touch improve symptoms in cancer. Integr. Cancer Ther. 2003;2:332–344. doi: 10.1177/1534735403259064. [DOI] [PubMed] [Google Scholar]
- 50.Smith, A. A., Kimmel, S. R. & Milz, S. A. Effects of therapeutic touch on pain, function and well being in persons with osteo-arthritis of the knee: a pilot study. Internet J Adv Nurs Pr. 10 (2010).
- 51.Sansom J. Firewalking: Explanation and the Mind‐Body Relationship1. Aust. J. Anthropol. 1998;9:194–208. doi: 10.1111/j.1835-9310.1998.tb00208.x. [DOI] [Google Scholar]
- 52.Eccleston C, Crombez G. Pain demands attention: A cognitive–affective model of the interruptive function of pain. Psychol. Bull. 1999;125:356–66. doi: 10.1037/0033-2909.125.3.356. [DOI] [PubMed] [Google Scholar]
- 53.Turk, D. C., Meichenbaum, D. & Genest, M. Pain and behavioral medicine: A cognitive-behavioral perspective. 1, (Guilford Press, 1986).
- 54.Bradley, L. A. Cognitive-behavioral therapy for chronic pain (1996).
- 55.Ellingsen D-M, et al. In touch with your emotions: Oxytocin and touch change social impressions while others’ facial expressions can alter touch. Psychoneuroendocrinology. 2014;39:11–20. doi: 10.1016/j.psyneuen.2013.09.017. [DOI] [PubMed] [Google Scholar]
- 56.Ruef, A. M. Empathy in long-term marriage: Behavioral and physiological correlates (Doctoral dissertation, University of California, Berkeley, 2001). Diss. Abstr. Int. 62 (2001).
- 57.Field T, Hemandez-Reif M, Taylor S, Quintino O, Burman I. Labor pain is reduced by massage therapy. J. Psychosom. Obstet. & Gynecol. 1997;18:286–291. doi: 10.3109/01674829709080701. [DOI] [PubMed] [Google Scholar]
- 58.Gentsch A, Panagiotopoulou E, Fotopoulou A. Active interpersonal touch gives rise to the social softness illusion. Curr. Biol. 2015;25:2392–2397. doi: 10.1016/j.cub.2015.07.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kida T, Shinohara K. Gentle touch activates the anterior prefrontal cortex: an NIRS study. Neurosci. Res. 2013;76:76–82. doi: 10.1016/j.neures.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 60.Rolls ET, et al. Representations of pleasant and painful touch in the human orbitofrontal and cingulate cortices. Cereb. cortex. 2003;13:308–317. doi: 10.1093/cercor/13.3.308. [DOI] [PubMed] [Google Scholar]
- 61.Keverne EB, Martensz ND, Tuite B. Beta-endorphin concentrations in cerebrospinal fluid of monkeys are influenced by grooming relationships. Psychoneuroendocrinology. 1989;14:155–161. doi: 10.1016/0306-4530(89)90065-6. [DOI] [PubMed] [Google Scholar]
- 62.Martel FL, Nevison CM, Simpson MJ, Keverne EB. Effects of opioid receptor blockade on the social behavior of rhesus monkeys living in large family groups. Dev. Psychobiol. 1995;28:71–84. doi: 10.1002/dev.420280202. [DOI] [PubMed] [Google Scholar]
- 63.Wittig RM, et al. Focused grooming networks and stress alleviation in wild female baboons. Horm. Behav. 2008;54:170–177. doi: 10.1016/j.yhbeh.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dunbar RI. The social role of touch in humans and primates: behavioural function and neurobiological mechanisms. Neurosci. & Biobehav. Rev. 2010;34:260–268. doi: 10.1016/j.neubiorev.2008.07.001. [DOI] [PubMed] [Google Scholar]
- 65.Preston SD, De Waal F. Empathy: Its ultimate and proximate bases. Behav. brain Sci. 2002;25:1–20. doi: 10.1017/s0140525x02000018. [DOI] [PubMed] [Google Scholar]
- 66.Mancini F, Beaumont A-L, Hu L, Haggard P, Iannetti GD. Touch inhibits subcortical and cortical nociceptive responses. Pain. 2015;156:1936–44. doi: 10.1097/j.pain.0000000000000253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mouraux A, Iannetti GD. Nociceptive laser-evoked brain potentials do not reflect nociceptive-specific neural activity. J. Neurophysiol. 2009;101:3258–3269. doi: 10.1152/jn.91181.2008. [DOI] [PubMed] [Google Scholar]
- 68.Kanske P, Kotz SA. Concreteness in emotional words: ERP evidence from a hemifield study. Brain Res. 2007;1148:138–148. doi: 10.1016/j.brainres.2007.02.044. [DOI] [PubMed] [Google Scholar]
- 69.Kissler J, Assadollahi R, Herbert C. Emotional and semantic networks in visual word processing: insights from ERP studies. Prog. brain Res. 2006;156:147–183. doi: 10.1016/S0079-6123(06)56008-X. [DOI] [PubMed] [Google Scholar]
- 70.Schindler S, Wegrzyn M, Steppacher I, Kissler J. Perceived communicative context and emotional content amplify visual word processing in the fusiform gyrus. J. Neurosci. 2015;35:6010–6019. doi: 10.1523/JNEUROSCI.3346-14.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Raz S, Dan O, Zysberg L. Neural correlates of emotional intelligence in a visual emotional oddball task: An ERP study. Brain Cogn. 2014;91:79–86. doi: 10.1016/j.bandc.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 72.Ring C, Kavussanu M, Willoughby AR. Emotional modulation of pain-related evoked potentials. Biol. Psychol. 2013;93:373–376. doi: 10.1016/j.biopsycho.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Babiloni F, Astolfi L. Social neuroscience and hyperscanning techniques: past, present and future. Neurosci. & Biobehav. Rev. 2014;44:76–93. doi: 10.1016/j.neubiorev.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bush G, et al. Dorsal anterior cingulate cortex: a role in reward-based decision making. Proc. Natl. Acad. Sci. 2002;99:523–528. doi: 10.1073/pnas.012470999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Devinsky O, Morrell MJ, Vogt BA. Contributions of anterior cingulate cortex to behaviour. Brain. 1995;118:279–306. doi: 10.1093/brain/118.1.279. [DOI] [PubMed] [Google Scholar]
- 76.Medford N, Critchley HD. Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct. Funct. 2010;214:535–549. doi: 10.1007/s00429-010-0265-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Arueti M, Perach N. When two become one: the role of oxytocin in interpersonal coordination and cooperation. Journal of cognitive neuroscience. 2013;25:1418–27. doi: 10.1162/jocn_a_00400. [DOI] [PubMed] [Google Scholar]
- 78.Holt-Lunstad J, Birmingham WA, Light KC. Influence of a ‘Warm Touch’ Support Enhancement Intervention Among Married Couples on Ambulatory Blood Pressure, Oxytocin, Alpha Amylase, and Cortisol. Psychosom. Med. 2008;70:976–985. doi: 10.1097/PSY.0b013e318187aef7. [DOI] [PubMed] [Google Scholar]
- 79.Holt-Lunstad J, Birmingham W, Light KC. The influence of depressive symptomatology and perceived stress on plasma and salivary oxytocin before, during and after a support enhancement intervention. Psychoneuroendocrinology. 2011;36:1249–1256. doi: 10.1016/j.psyneuen.2011.03.007. [DOI] [PubMed] [Google Scholar]
- 80.Feldman R, Gordon I, Schneiderman I, Weisman O, Zagoory-Sharon O. Natural variations in maternal and paternal care are associated with systematic changes in oxytocin following parent–infant contact. Psychoneuroendocrinology. 2010;35:1133–1141. doi: 10.1016/j.psyneuen.2010.01.013. [DOI] [PubMed] [Google Scholar]
- 81.Gordon I, Zagoory-Sharon O, Leckman JF, Feldman R. Oxytocin, cortisol, and triadic family interactions. Physiol. & Behav. 2010;101:679–684. doi: 10.1016/j.physbeh.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 82.Mu, Y., Guo, C. & Han, S. Oxytocin enhances inter-brain synchrony during social coordination in male adults. Soc. Cogn. Affect. Neurosci. 106 doi:10.1093/scan/nsw106 (2016). [DOI] [PMC free article] [PubMed]
- 83.Oliveira, H. M. & Melo, L. V. Huygens synchronization of two clocks. Sci. reports5, doi:10.1038/srep11548 (2015). [DOI] [PMC free article] [PubMed]
- 84.Patel AD, Iversen JR, Bregman MR, Schulz I. Studying synchronization to a musical beat in nonhuman animals. Ann. New York Acad. Sci. 2009;1169:459–469. doi: 10.1111/j.1749-6632.2009.04581.x. [DOI] [PubMed] [Google Scholar]
- 85.Bode, N. W., Faria, J. J., Franks, D. W., Krause, J. & Wood, A. J. How perceived threat increases synchronization in collectively moving animal groups. Proc. R. Soc. Lond. B: Biol. Sci. rspb20100855-rspb20100855 (2010). [DOI] [PMC free article] [PubMed]
- 86.Senigaglia V, de Stephanis R, Verborgh P, Lusseau D. The role of synchronized swimming as affiliative and anti-predatory behavior in long-finned pilot whales. Behav. Process. 2012;91:8–14. doi: 10.1016/j.beproc.2012.04.011. [DOI] [PubMed] [Google Scholar]
- 87.Eagly AH, Steffen VJ. Gender and aggressive behavior: A meta-analytic review of the social psychological literature. Psychol. Bull. 1986;100:309–30. doi: 10.1037/0033-2909.100.3.309. [DOI] [PubMed] [Google Scholar]
- 88.Davis, M. H. A multidimensional approach to individual differences in empathy (1980).
- 89.Granot M, et al. Determinants of endogenous analgesia magnitude in a diffuse noxious inhibitory control (DNIC) paradigm: do conditioning stimulus painfulness, gender and personality variables matter? Pain. 2008;136:142–149. doi: 10.1016/j.pain.2007.06.029. [DOI] [PubMed] [Google Scholar]
- 90.Allen JJB, Chambers AS, Towers DN. The many metrics of cardiac chronotropy: a pragmatic primer and a brief comparison of metrics. Biol. Psychol. 2007;74:243–262. doi: 10.1016/j.biopsycho.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 91.Boker SM, Nesselroade JR. A Method for Modeling the Intrinsic Dynamics of Intraindividual Variability: Recovering the Parameters of Simulated Oscillators in Multi-Wave Panel Data. Multivar. Behav. Res. 2002;37:127–160. doi: 10.1207/S15327906MBR3701_06. [DOI] [PubMed] [Google Scholar]
- 92.Littell, R. C., Milliken, G. A., Stroup, W. W., Wolfinger, R. D. & Schabenberber, O. SAS system for linear mixed models. Cary, NC: SAS Inst. (1996).
- 93.Raudenbush, S. W. & Bryk, A. S. Hierarchical linear models: Applications and data analysis methods. 1, (Sage, 2002).
- 94.Boker, S. M. & Laurenceau, J.-P. Dynamical systems modeling: An application to the regulation of intimacy and disclosure in marriage. Model. Intensive Longitud. data 195–218, doi:10.1093/acprof:oso/9780195173444.001.0001 (2006).
- 95.Edwards D, Berry JJ. The efficiency of simulation-based multiple comparisons. Biometrics. 1987;43:913–928. doi: 10.2307/2531545. [DOI] [PubMed] [Google Scholar]


