Abstract
Background
The identification of anaerobic metabolism in critically ill patients is a challenging task. Observational studies have suggested that the ratio of venoarterial PCO2 (Pv–aCO2) to arteriovenous oxygen content difference (Ca–vO2) might be a good surrogate for respiratory quotient (RQ). Yet Pv–aCO2/Ca–vO2 might be increased by other factors, regardless of anaerobic metabolism. At present, comparisons between Pv–aCO2/Ca–vO2 and RQ have not been performed. We sought to compare these variables during stepwise hemorrhage and hemodilution. Since anemia predictably produces augmented Pv–aCO2 and decreased Ca–vO2, our hypothesis was that Pv–aCO2/Ca–vO2 might be an inadequate surrogate for RQ.
Methods
This is a subanalysis of a previously published study. In anesthetized and mechanically ventilated sheep (n = 16), we compared the effects of progressive hemodilution and hemorrhage by means of expired gases analysis.
Results
There were comparable reductions in oxygen consumption and increases in RQ in the last step of hemodilution and hemorrhage. The increase in Pv–aCO2/Ca–vO2 was higher in hemodilution than in hemorrhage (1.9 ± 0.2 to 10.0 ± 0.9 vs. 1.7 ± 0.2 to 2.5 ± 0.1, P < 0.0001). The increase in Pv–aCO2 was lower in hemodilution (6 ± 0 to 10 ± 1 vs. 6 ± 0 to 17 ± 1 mmHg, P < 0.0001). Venoarterial CO2 content difference and Ca–vO2 decreased in hemodilution and increased in hemorrhage (2.6 ± 0.3 to 1.2 ± 0.1 vs. 2.8 ± 0.2 to 6.9 ± 0.5, and 3.4 ± 0.3 to 1.0 ± 0.3 vs. 3.6 ± 0.3 to 6.8 ± 0.3 mL/dL, P < 0.0001 for both). In hemodilution, Pv–aCO2/Ca–vO2 increased before the fall in oxygen consumption and the increase in RQ. Pv–aCO2/Ca–vO2 was strongly correlated with Hb (R 2 = 0.79, P < 0.00001) and moderately with RQ (R 2 = 0.41, P < 0.0001). A multiple linear regression model found Hb, RQ, base excess, and mixed venous oxygen saturation and PCO2 as Pv–aCO2/Ca–vO2 determinants (adjusted R 2 = 0.86, P < 0.000001).
Conclusions
In hemodilution, Pv–aCO2/Ca–vO2 was considerably increased, irrespective of the presence of anaerobic metabolism. Pv–aCO2/Ca–vO2 is a complex variable, which depends on several factors. As such, it was a misleading indicator of anaerobic metabolism in hemodilution.
Keywords: Hemodilution, Hemorrhage, Anaerobic metabolism, Oxygen, Carbon dioxide, Respiratory quotient
Background
The identification of anaerobic metabolism in critically ill patients can be elusive. Hyperlactatemia, central venous oxygen saturation, or isolated values of oxygen transport and consumption (DO2 and VO2) are frequently misleading indicators of tissue hypoxia. In contrast, the acute increase in respiratory quotient (RQ) is an excellent marker of ongoing anaerobic metabolism in exercise [1] and oxygen supply dependency conditions [2, 3]. In both circumstances, there is an excess of carbon dioxide production (VCO2) compared to VO2. This is the result of anaerobic VCO2, which arises from the bicarbonate buffering of anaerobically generated protons [1]. The proper measurement of RQ, however, requires analysis of expired gases. This monitoring is not usually available in the critical care setting. Recently, some observational studies have suggested that the ratio of venoarterial PCO2 (Pv–aCO2) to arteriovenous oxygen content difference (Ca–vO2) might be a good surrogate for RQ. Accordingly, high Pv–aCO2/Ca–vO2 has been associated with hyperlactatemia [4], decreased lactate clearance [5, 6], oxygen supply dependency [7, 8], and worse outcome of critically ill patients [4]. Nevertheless, Pv–aCO2/Ca–vO2 might theoretically be increased by several other factors irrespective of the presence of anaerobic metabolism. Moreover, comparisons between Pv–aCO2/Ca–vO2 and RQ have not been performed yet.
Given the increasing number of publications about the Pv–aCO2/Ca–vO2 and the lack of an adequate validation, further research is needed. This study was derived from a secondary subanalysis of a previous publication that sought to determine the relationship among oxygen transport, microvascular perfusion, and tissue CO2 in ischemic and anemic hypoxia [9]. The present investigation was focused on the behavior of Pv–aCO2/Ca–vO2 and its determinants, as well as its relationship with RQ, during stepwise hemorrhage and hemodilution. Since a progressive hemodilution, which does not compromise aerobic metabolism, will predictably result in increased Pv–aCO2 [10] and decreased Ca–vO2 [11], our hypothesis was that Pv–aCO2/Ca–vO2 might be an inadequate surrogate for RQ in isovolemic anemia.
Methods
Anesthesia and ventilation
Sixteen sheep (20 ± 2 kg, mean ± SEM) were anesthetized with 30 mg/kg of sodium pentobarbital, intubated, and mechanically ventilated with a Servo Ventilator 900C (Siemens-Elema AB, Solna, Sweden) with a tidal volume of 15 mL/kg, a FiO2 of 0.21 and a positive end-expiratory pressure of 6 cm H2O. The initial respiratory rate was set to keep the arterial PCO2 between 35 and 40 mmHg. This respiratory setting was maintained during the rest of the experiment. Neuromuscular blockade was performed with pancuronium bromide (0.06 mg/kg). Additional pentobarbital boluses (1 mg/kg) were administered hourly and when clinical signs of inadequate depth of anesthesia were evident. Analgesia was provided by fentanyl as a bolus of 2 µg/kg, followed by 1 µg/h/kg. These drugs were administered intravenously.
Surgical preparation
A 7.5-French Swan-Ganz standard thermodilution pulmonary artery catheter (Edwards Life Sciences, Irvine, CA, USA) was inserted through an introducer in the right external jugular vein to obtain mixed venous samples; its side port was used to administer fluids and drugs. Catheters were placed in the descending aorta via the left femoral artery to measure blood pressure, perform the bleeding, and obtain blood samples, and in the inferior vena cava to infuse fluids during isovolemic hemodilution.
Measurements and derived calculations
VO2, VCO2, and RQ were measured by analysis of expired gases (MedGraphics CPX Ultima, Medical Graphics Corporation, St. Paul, MN). VO2 and VCO2 were adjusted to body weight.
Arterial and mixed venous PO2, PCO2, pH, Hb, and O2 saturation were measured with a blood gas analyzer and a co-oximeter (ABL 5 and OSM 3, Radiometer, Copenhagen, Denmark). Ca–vO2 was calculated by standard formulae.
Cardiac output was calculated as VO2 divided by Ca–vO2. DO2 was calculated as cardiac output multiplied by arterial O2 content.
We also calculated Pv–aCO2 and Pv–aCO2/Ca–vO2. According to Fick’s principle, venoarterial CO2 content difference (Cv–aCO2) was calculated as VCO2 divided by cardiac output.
Experimental procedure
Basal measurements were taken after a period of no less than 30 min after systemic VO2 and VCO2 became stable. Animals were then assigned to hemodilution (n = 8) and hemorrhage (n = 8) groups. In the hemodilution group, we performed a stepwise hemodilution through isovolemic exchange of blood with 6% hydroxyethyl starch 130/0.4 in 0.9% NaCl (Voluven, Fresenius Kabi, Bad Homburg, Germany). The amount of blood exchanged to reach desired levels of hematocrit of about 0.15, 0.10, and 0.05 in each step was estimated as previously referred [12]. In the hemorrhage group, consecutive bleedings of 5–10 mL/kg were performed. Similar reductions in systemic VO2 were pursued in both groups in order to reach comparable degrees of anaerobic metabolism. Measurements were taken at 30, 60, and 90 min. Blood temperature was kept constant throughout the study with a heating lamp.
At the end of the experiment, the animals were killed with an additional dose of pentobarbital and a KCl bolus.
Data analysis
Data were assessed for normality and expressed as mean ± SEM. Groups were compared with two-way repeated measures of ANOVA. After a P < 0.05 for time × group interaction, a post hoc Student’s t test with Bonferroni correction was used for pairwise comparisons. Simple linear regression analysis with Pv–aCO2/Ca–vO2 as the outcome variable was conducted, and variables showing a P value <0.20 or physiologically plausible were entered in a multiple linear regression model. The final model was tested for the presence of collinearity (VIF test). All analyses were done with Stata statistical software (Stata Corporation, Release 12, College Station, TX, USA).
Results
In both groups, DO2 fell progressively. In the hemorrhage group, the decrease in DO2 was primarily related to the reduction in cardiac output from 166 ± 13 to 54 ± 6 mL/min/kg (P < 0.0001). In addition, Hb fell from 8.4 ± 0.5 to 6.6 ± 0.4 g/dL (P < 0.0001). In hemodilution group, the drop in DO2 was completely explained by the reduction in Hb from 8.3 ± 0.4 to 1.2 ± 0.1 g/dL (P < 0.0001). Cardiac output concurrently increased from 165 ± 16 to 373 ± 41 mL/min/kg (P < 0.0001).
In the last stage, there were similar decreases in VO2 and increases in RQ in both groups. Pv–aCO2/Ca–vO2 also increased in the last stage in the hemorrhage group. Pv–aCO2/Ca–vO2 increased after the second step in the hemodilution group, and the increases were higher than in hemorrhage group (Fig. 1).
Fig. 1.
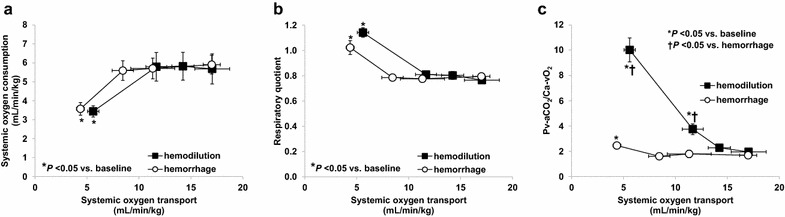
Relationship between oxygen transport to oxygen consumption (a), respiratory quotient (b), and venoarterial PCO2 difference-to-arteriovenous O2 content difference ratio (Pv–aCO2/Ca–vO2) (c). Oxygen consumption fell and respiratory quotient increased only in the last step of hemodilution and hemorrhage. In hemodilution, the increase in Pv–aCO2/Ca–vO2 was higher than in hemorrhage and appeared before the development of oxygen supply dependency
Pv–aCO2/Ca–vO2 was strongly correlated with Hb levels and moderately with RQ (Fig. 2). A similar behavior was observed in hemorrhage group (R 2 = 0.23, P < 0.002 and R 2 = 0.12, P < 0.03). A multiple linear regression model, developed with data from both groups, found Hb, RQ, base excess, and mixed venous oxygen saturation and PCO2 as Pv–aCO2/Ca–vO2 determinants (adjusted R 2 = 0.86, P < 0.000001). Hb was the explanatory variable with the highest independent contribution to the prediction (highest t ratio) (Table 1). The model did not exhibit collinearity.
Fig. 2.
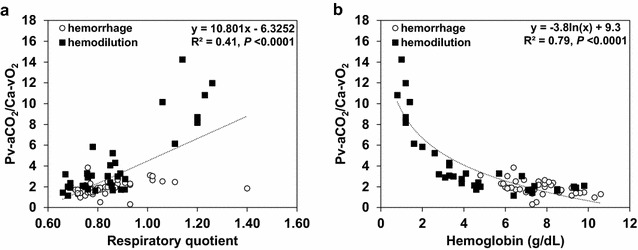
Correlation of venoarterial PCO2 difference-to-arteriovenous O2 content difference ratio (Pv–aCO2/Ca–vO2) with respiratory quotient (a) and Hb levels (b). The correlation between Pv–aCO2/Ca–vO2 and RQ was statistically significant but moderate. In contrast, Pv–aCO2/Ca–vO2 and Hb levels were strongly correlated
Table 1.
Multiple linear regression model for the ratio of venoarterial PCO2 to arteriovenous oxygen content difference (Pv–aCO2/Ca–vO2)
| Pv–aCO2/Ca–vO2 | Coefficient | Standard error | t ratio | P value | [95% confidence interval] |
|---|---|---|---|---|---|
| Ln hemoglobin (g/dL) | −3.60 | 0.26 | −13.62 | <0.000001 | −4.13 −3.08 |
| Respiratory quotient | 2.43 | 1.03 | 2.35 | <0.03 | 0.37 4.49 |
| Base excess (mEq/L) | −0.06 | 0.03 | −2.42 | <0.02 | −0.12 −0.01 |
| Mixed venous O2 saturation (fraction) | 0.03 | 0.01 | 3.90 | <0.0003 | 0.01 0.04 |
| Mixed venous PCO2 (mmHg) | 0.15 | 0.03 | 4.58 | <0.00003 | 0.08 0.21 |
| Intercept | −0.98 | 1.72 | −0.57 | 0.57 | −4.40 2.44 |
Pv–aCO2 increased in the hemorrhage group from the first stage and in hemodilution group only in the last phase. The increases in Pv–aCO2 were higher in hemorrhage than in hemodilution, while Cv–aCO2 increased in hemorrhage and decreased in hemodilution (Fig. 3). In the hemodilution group, there was a right shift in the relationship between CO2 pressures and contents (Fig. 4). During reductions in DO2, Ca–vO2 increased in the hemorrhage group and fell in the hemodilution group (Fig. 3).
Fig. 3.
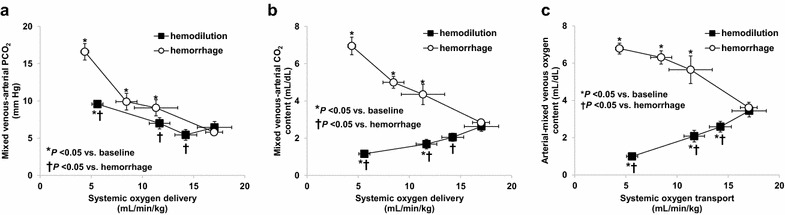
Relationship between oxygen transport to venoarterial PCO2 difference (Pv–aCO2) (a), venoarterial CO2 content difference (Cv–aCO2) (b), and arteriovenous O2 content difference (Ca–vO2) (c). Hemodilution produced opposite effects on Pv–aCO2 and Cv–aCO2. Cv–aCO2 decreased in hemodilution and increased in hemorrhage. These changes are the underlying explanation for different behavior of Pv–aCO2/Ca–vO2 in both groups
Fig. 4.
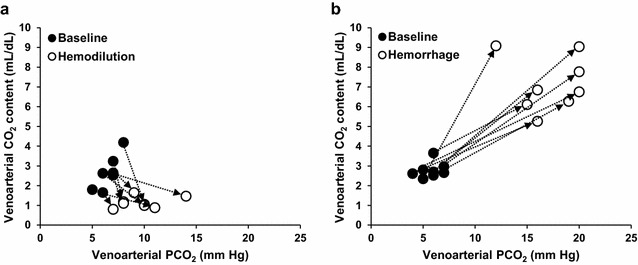
Changes in the relationship between venoarterial CO2 pressure and content differences in hemodilution (a) and hemorrhage (b). Hemodilution shifted CO2Hb dissociation curve to the right
Discussion
The main finding of this study was that Pv–aCO2/Ca–vO2 failed to properly reflect RQ in hemodilution. It increased before the appearance of the dependency of VO2 on DO2. Its correlation with RQ was moderate, but it showed a strong association with Hb levels. Indeed, Pv–aCO2/Ca–vO2 was more explained by Hb levels than by anaerobic metabolism. Changes in the dissociation of CO2 from Hb mostly account for these results.
Several studies have tried to link Pv–aCO2/Ca–vO2 with some events suggestive of anaerobic metabolism such as hyperlactatemia [4], decreased lactate clearance [5, 6], increased VO2 in response to fluid challenge [7, 8], and worse outcome [4]. Since RQ was not measured in those studies, it was not clear whether Pv–aCO2/Ca–vO2 effectively reflected the presence of anaerobic metabolism or was only the result of factors that could increase that ratio in the absence of anaerobic metabolism. In fact, Pv–aCO2/Ca–vO2 is not a straightforward variable. Although related to RQ, it might be hypothetically increased by several factors beyond anaerobic metabolism. Many of the changes in Pv–aCO2/Ca–vO2 might be ascribed to modifications of the CO2-Hb dissociation curve. Haldane effect, metabolic acidosis, and anemia can increase PCO2 for a given CCO2 [13]. In addition, taking into account the curvilinear characteristics of the dissociation curve, the effects are even greater at higher PCO2. When the slope of the dissociation curve flattens, substantial increases in Pv–aCO2 may actually represent negligible increases in Cv–aCO2. Therefore, high oxygen venous saturation [14], hyperlactatemia [15], and hemodilution [16] can increase Pv–aCO2 even though Cv–aCO2 remains unchanged.
In line with the previous discussion, our results showed that isovolemic anemia disproportionally increased Pv–aCO2/Ca–vO2, compared to hemorrhage. Furthermore, this ratio was elevated before the beginning of oxygen supply dependency. Progressive hemodilution was associated with opposing effects on Pv–aCO2 and Cv–aCO2: Pv–aCO2 increased and Cv–aCO2 decreased. Previous studies showed that decreasing hemoglobin levels results in widened Pv–aCO2 for a given Cv–aCO2 [16]. In a similar model of progressive hemodilution, the contrasting effects of low Hb levels on Pv–aCO2 and Cv–aCO2 were also noticed [10]. Therefore, increased Pv–aCO2 is a predictable consequence of anemia.
Another expected consequence from hemodilution is the decrease in Ca–vO2 [11]. Increases in oxygen extraction always occur in response to reductions in DO2, irrespective of the mechanism of oxygen supply limitation. The impact of the increase in oxygen extraction on Ca–vO2, however, depends on cardiac output. According to Fick’s principle, Ca–vO2 should widen in conditions of low cardiac output and decreased in states of reduced DO2 with increased cardiac output, if VO2 remains constant. Our study also confirmed this assumption.
As a result of the opposite effects of hemodilution on Pv–aCO2 and Ca–vO2, the ratio between both variables markedly augmented in the absence of anaerobic metabolism. The increase in Pv–aCO2/Ca–vO2 was even higher during the oxygen supply dependency, due to the interplay of the aforementioned factors and the ongoing anaerobic CO2 production.
Considering the coefficient of determination of the regression (R 2 = 0.41), RQ only explains a minor part of the Pv–aCO2/Ca–vO2 variability. As supported by the results of the multiple linear regression model, Pv–aCO2/Ca–vO2 is a complex variable that has several determinants. Although Hb was the main contributor to the prediction of Pv–aCO2/Ca–vO2, it was also influenced by RQ and by the changes in the dissociation of CO2 from hemoglobin induced by metabolic acidosis and Haldane effect. These effects were magnified at the flattened portion of the CO2Hb dissociation curve as shown by the impact of mixed venous PCO2 in the model.
A study has proposed a Pv–aCO2/Ca–vO2 cutoff of 1.4 for the identification of anaerobic metabolism [4]. This suggestion, however, should be carefully interpreted. The development of anaerobic metabolism is identified by acute increases in RQ, not by isolated values [1–3]. Actually, the normal range of RQ is 0.67–1.30 [17] depending also on other factors such as energy source [18] and overfeeding [19]. In our experiments, values of Pv–aCO2/Ca–vO2 during oxygen supply dependency were considerably higher (10.0 ± 2.7 and 2.5 ± 0.4 in hemodilution and hemorrhage groups, respectively).
Our findings do not challenge the value of Pv–aCO2/Ca–vO2 as an outcome predictor of critically ill patients, which was previously described [4]. The composite characteristics of Pv–aCO2/Ca–vO2, however, suggest that the prognostic ability might be mainly related to the interaction of several mechanisms, not only to anaerobic metabolism.
Our study has certain drawbacks. Secondary analyses pose inherent limitations that have been subject to critiques [20]. In addition, part of our analysis was based on calculations of CCO2, not in actual measurements [21]. This last procedure is complex and cumbersome and is not available in our laboratory. Accordingly, we calculated CCO2 from Fick’s principle. We prefer this method, because the different algorithms for computing CCO2 from blood gases and Hb are frequently misleading and can produce negative Cv–aCO2 values. Finally, the experimental model of hemorrhage and hemodilution does not address the applicability of our results to septic conditions.
Conclusions
Hemodilution produced higher increases in Pv–aCO2/Ca–vO2, compared to hemorrhage, and this ratio was widened even in the absence of oxygen supply dependency. These findings were related to the effects of anemia on CO2Hb dissociation curve and Ca–vO2. Our results suggest that Pv–aCO2/Ca–vO2 is a multifactorial variable, which results from interactions among anaerobic metabolism, anemia, metabolic acidosis, and Haldane effect. Since it is not an accurate surrogate for RQ, values of Pv–aCO2/Ca–vO2 should be cautiously interpreted. Further studies in septic models are needed to confirm the limitations of Pv–aCO2/Ca–vO2 in such condition.
Authors’ contributions
AD, GF, VSKE, EM, HSC, CC, GM, and MOP carried out the animal experiments and participated in the design of the study. AD performed the statistical analysis and drafted the manuscript. EE participated in the study design, statistical analysis, and interpretation of the data. All authors discussed the results and participated in the writing. All authors read and approved the final manuscript.
Acknowledgements
None.
Competing interests
The authors declare that they have no competing interests.
Availability of data and materials
The data set supporting the conclusions of this article is available from the corresponding author on reasonable request.
Ethics approval
The study was approved by the local Animal Research Committee [0800-009634/11-000]. Care of animals was in accordance with National Institutes of Health (USA).
Funding
This study was supported by the Grant PICT 2010-00495, Agencia Nacional de Promoción Científica y Tecnológica, Argentina.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Abbreviations
- DO2
oxygen transport
- VO2
oxygen consumption
- RQ
respiratory quotient
- VCO2
carbon dioxide production
- Pv–aCO2
venoarterial PCO2
- Ca–vO2
arteriovenous oxygen content difference
- Pv–aCO2/Ca–vO2
ratio of venoarterial PCO2 to arteriovenous oxygen content difference
- Cv–aCO2
venoarterial CO2 content difference
Contributor Information
Arnaldo Dubin, Phone: +5491150102431, Email: arnaldodubin@gmail.com.
Gonzalo Ferrara, Email: gonzaloferrara@gmail.com.
Vanina Siham Kanoore Edul, Email: vaninaedul@gmail.com.
Enrique Martins, Email: enriqueflmartins@gmail.com.
Héctor Saúl Canales, Email: canaleshector@hotmail.com.
Carlos Canullán, Email: carloscanullan@yahoo.com.ar.
Gastón Murias, Email: gmurias@gmail.com.
Mario Omar Pozo, Email: pozomario@gmail.com.
Elisa Estenssoro, Email: estenssoro.elisa@gmail.com.
References
- 1.Wasserman K, Whipp BJ, Koyl SN, Beaver WL. Anaerobic threshold and respiratory gas exchange during exercise. J Appl Physiol. 1973;35:236–243. doi: 10.1152/jappl.1973.35.2.236. [DOI] [PubMed] [Google Scholar]
- 2.Cohen IL, Sheikh FM, Perkins RJ, Feustel PJ, Foster ED. Effect of hemorrhagic shock and reperfusion on the respiratory quotient in swine. Crit Care Med. 1995;23:545–552. doi: 10.1097/00003246-199503000-00021. [DOI] [PubMed] [Google Scholar]
- 3.Dubin A, Murias G, Estenssoro E, Canales H, Sottile P, Badie J, Barán M, Rossi S, Laporte M, Pálizas F, Giampieri J, Mediavilla D, Vacca E, Botta D. End-tidal CO2 pressure determinants during hemorrhagic shock. Intensive Care Med. 2000;26:1619–1623. doi: 10.1007/s001340000669. [DOI] [PubMed] [Google Scholar]
- 4.Mekontso-Dessap A, Castelain V, Anguel N, Bahloul M, Schauvliege F, Richard C, Teboul JL. Combination of venoarterial PCO2 difference with arteriovenous O2 content difference to detect anaerobic metabolism in patients. Intensive Care Med. 2002;28:272–277. doi: 10.1007/s00134-002-1215-8. [DOI] [PubMed] [Google Scholar]
- 5.Mesquida J, Saludes P, Gruartmoner G, Espinal C, Torrents E, Baigorri F, Artigas A. Central venous-to-arterial carbon dioxide difference combined with arterial-to-venous oxygen content difference is associated with lactate evolution in the hemodynamic resuscitation process in early septic shock. Crit Care. 2015;19:126. doi: 10.1186/s13054-015-0858-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.He HW, Liu DW, Long Y, Wang XT. High central venous-to-arterial CO2 difference/arterial–central venous O2 difference ratio is associated with poor lactate clearance in septic patients after resuscitation. J Crit Care. 2016;31:76–81. doi: 10.1016/j.jcrc.2015.10.017. [DOI] [PubMed] [Google Scholar]
- 7.Monnet X, Julien F, Ait-Hamou N, Lequoy M, Gosset C, Jozwiak M, Persichini R, Anguel N, Richard C, Teboul JL. Lactate and venoarterial carbon dioxide difference/arterial–venous oxygen difference ratio, but not central venous oxygen saturation, predict increase in oxygen consumption in fluid responders. Crit Care Med. 2013;41:1412–1420. doi: 10.1097/CCM.0b013e318275cece. [DOI] [PubMed] [Google Scholar]
- 8.Mallat J, Lemyze M, Meddour M, Pepy F, Gasan G, Barrailler S, Durville E, Temime J, Vangrunderbeeck N, Tronchon L, Vallet B, Thevenin D. Ratios of central venous-to-arterial carbon dioxide content or tension to arteriovenous oxygen content are better markers of global anaerobic metabolism than lactate in septic shock patients. Ann Intensive Care. 2016;6:10. doi: 10.1186/s13613-016-0110-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ferrara G, Kanoore Edul VS, Martins E, Canales HS, Canullán C, Murias G, Pozo MO, Estenssoro E, Ince C, Dubin A. Intestinal and sublingual microcirculation are more severely compromised in hemodilution than in hemorrhage. J Appl Physiol. 1985;2016(120):1132–1140. doi: 10.1152/japplphysiol.00007.2016. [DOI] [PubMed] [Google Scholar]
- 10.Dubin A, Estenssoro E, Murias G, Pozo MO, Sottile JP, Barán M, Piacentini E, Canales HS, Etcheverry G. Intramucosal–arterial PCO2 gradient does not reflect intestinal dysoxia in anemic hypoxia. J Trauma. 2004;57:1211–1217. doi: 10.1097/01.TA.0000107182.43213.4B. [DOI] [PubMed] [Google Scholar]
- 11.Laks J, Pilon RN, Klovekorn WP, Anderson W, MacCallum JR, O’Connor NE. Acute hemodilution: its effect of hemodynamics and oxygen transport in anesthetized man. Ann Surg. 1974;180:103–109. doi: 10.1097/00000658-197407000-00016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bourke DL, Smith TC. Estimating allowable hemodilution. Anesthesiology. 1974;41:609–612. doi: 10.1097/00000542-197412000-00015. [DOI] [PubMed] [Google Scholar]
- 13.Teboul JL, Scheeren T. Understanding the Haldane effect. Intensive Care Med. 2016;43:91–93. doi: 10.1007/s00134-016-4261-3. [DOI] [PubMed] [Google Scholar]
- 14.Jakob SM, Kosonen P, Ruokonen E, Parviainen I, Takala J. The Haldane effect—an alternative explanation for increasing gastric mucosal PCO2 gradients? Br J Anaesth. 1999;83:740–746. doi: 10.1093/bja/83.5.740. [DOI] [PubMed] [Google Scholar]
- 15.Sun XG, Hansen JE, Stringer WW, Ting H, Wasserman K. Carbon dioxide pressure–concentration relationship in arterial and mixed venous blood during exercise. J Appl Physiol. 1985;2001(90):1798–1810. doi: 10.1152/jappl.2001.90.5.1798. [DOI] [PubMed] [Google Scholar]
- 16.Chiarla C, Giovannini I, Giuliante F, Vellone M, Ardito F, Tenhunen J, Nuzzo G. Significance of hemoglobin concentration in determining blood CO2 binding capacity in critical illness. Respir Physiol Neurobiol. 2010;172:32–36. doi: 10.1016/j.resp.2010.04.012. [DOI] [PubMed] [Google Scholar]
- 17.McClave SA, Lowen CC, Kleber MJ, McConnell JW, Jung LY, Goldsmith LJ. Clinical use of the respiratory quotient obtained from indirect calorimetry. J Parenter Enter Nutr. 2003;27:21–26. doi: 10.1177/014860710302700121. [DOI] [PubMed] [Google Scholar]
- 18.MacFie J, Holmfield JH, King RF, Hill GL. Effect of the energy source on changes in energy expenditure and respiratory quotient during total parenteral nutrition. J Parenter Enter Nutr. 1983;7:1–5. doi: 10.1177/014860718300700101. [DOI] [PubMed] [Google Scholar]
- 19.Hulst JM, van Goudoever JB, Zimmermann LJ, Hop WC, Büller HA, Tibboel D, Joosten KF. Adequate feeding and the usefulness of the respiratory quotient in critically ill children. Nutrition. 2005;21:192–198. doi: 10.1016/j.nut.2004.05.020. [DOI] [PubMed] [Google Scholar]
- 20.Mariano ER, Ilfeld BM, Neal JM. ‘‘Going fishing’’—the practice of reporting secondary outcomes as separate studies. Reg Anesth Pain Med. 2007;32:183–185. doi: 10.1097/00115550-200705000-00002. [DOI] [PubMed] [Google Scholar]
- 21.Van Slyke DD, Neill JM. The determination of gases in blood and other solutions by vacuum extraction and manometric measurement. J Biol Chem. 1924;61:523–573. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data set supporting the conclusions of this article is available from the corresponding author on reasonable request.


