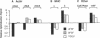Abstract
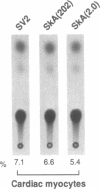
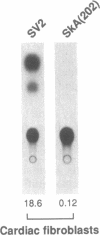
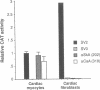
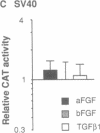
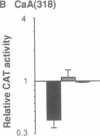
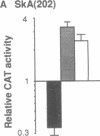
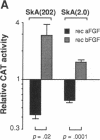
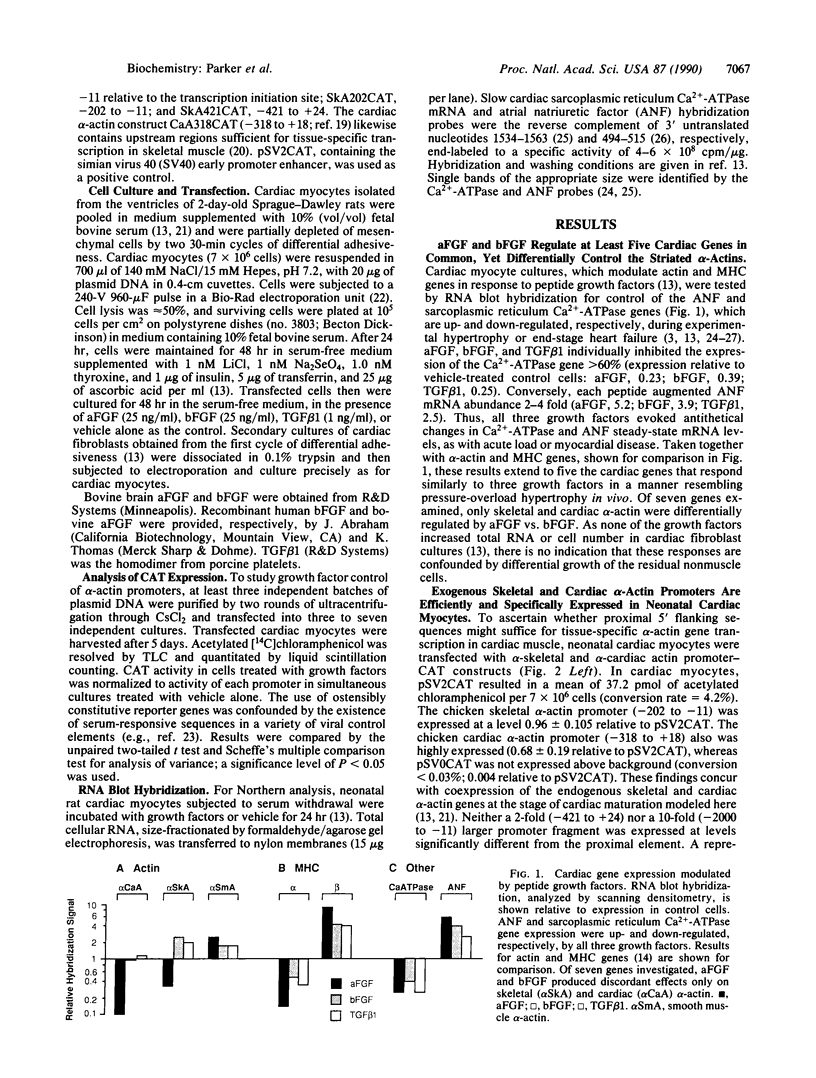
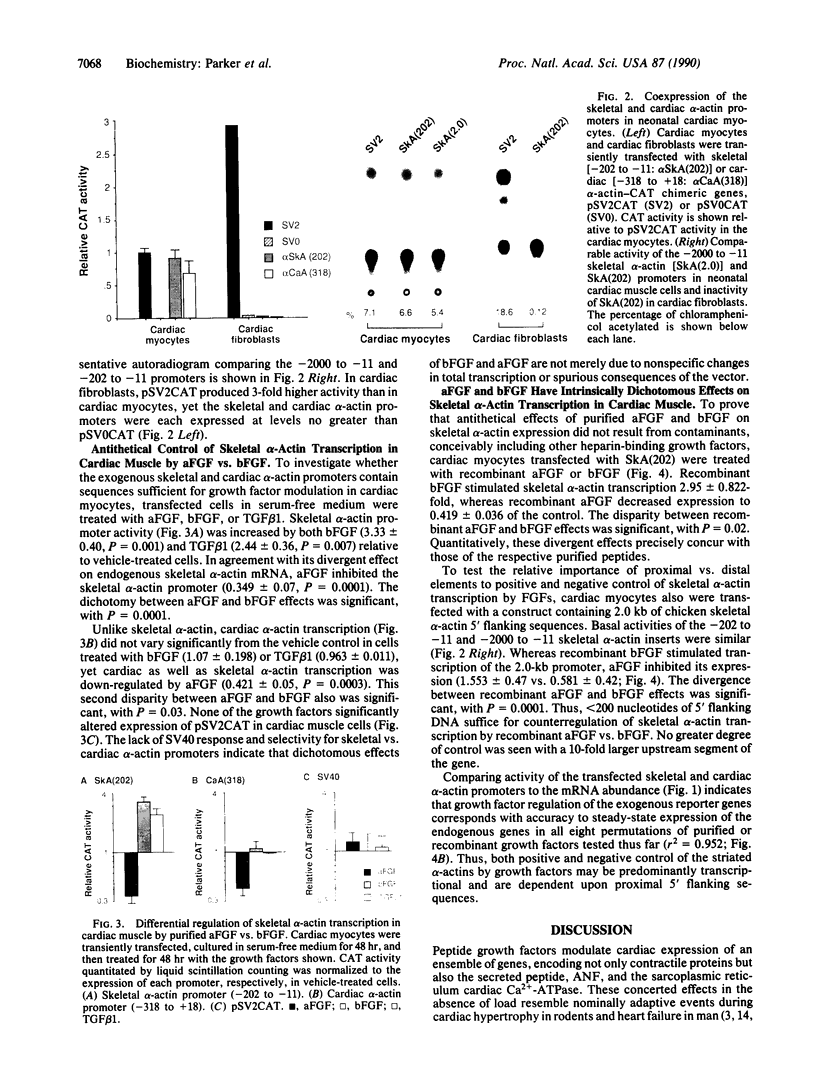
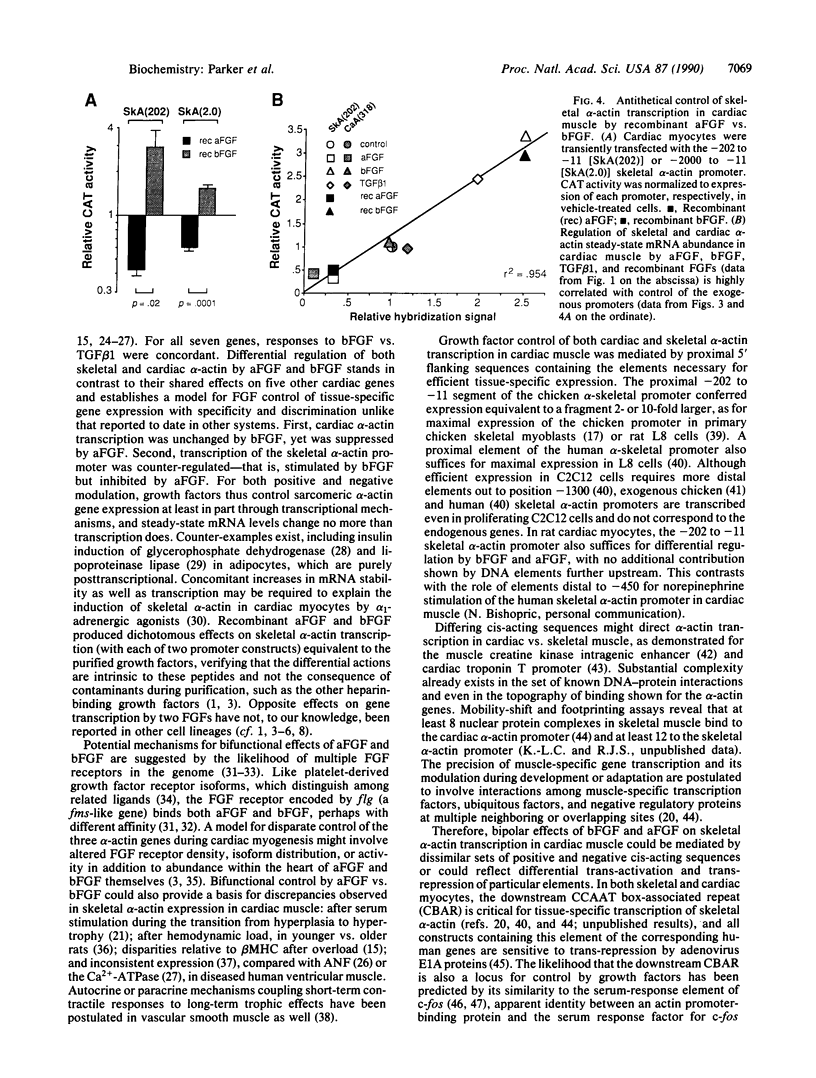
In cardiac muscle, acidic and basic fibroblast growth factors (aFGF and bFGF) regulate at least five genes in common (including alpha and beta myosin heavy chains, atrial natriuretic factor, and the sarcoplasmic reticulum calcium ATPase), provoking a generalized "fetal" phenotype similar to events in pressure-overload hypertrophy; however, aFGF and bFGF differentially control the striated alpha-actins. bFGF stimulates and aFGF inhibits skeletal alpha-actin transcripts associated with the embryonic heart, whereas cardiac alpha-actin mRNA is inhibited by aFGF but not bFGF. To elucidate mechanisms for these selective and discordant actions of aFGF and bFGF on cardiac muscle, chicken skeletal and cardiac alpha-actin promoter-driven reporter genes were introduced into neonatal rat cardiac myocytes by electroporation. Skeletal alpha-actin transcription was selectively stimulated by bFGF, whereas the cardiac alpha-actin promoter was unaffected. In contrast, aFGF suppressed both transfected alpha-actin genes. The differential regulation of skeletal alpha-actin transcription was equivalent with either purified or recombinant FGFs and was observed with 5' flanking sequences from either nucleotide -202 or -2000 to nucleotide -11. Positive and negative modulation of alpha-actin transcription by growth factors corresponded accurately to the endogenous genes in all permutations studied. These investigations provide a model for reciprocal control of gene transcription by aFGF vs. bFGF.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bergsma D. J., Grichnik J. M., Gossett L. M., Schwartz R. J. Delimitation and characterization of cis-acting DNA sequences required for the regulated expression and transcriptional control of the chicken skeletal alpha-actin gene. Mol Cell Biol. 1986 Jul;6(7):2462–2475. doi: 10.1128/mcb.6.7.2462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boxer L. M., Prywes R., Roeder R. G., Kedes L. The sarcomeric actin CArG-binding factor is indistinguishable from the c-fos serum response factor. Mol Cell Biol. 1989 Feb;9(2):515–522. doi: 10.1128/mcb.9.2.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun T., Buschhausen-Denker G., Bober E., Tannich E., Arnold H. H. A novel human muscle factor related to but distinct from MyoD1 induces myogenic conversion in 10T1/2 fibroblasts. EMBO J. 1989 Mar;8(3):701–709. doi: 10.1002/j.1460-2075.1989.tb03429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caffrey J. M., Brown A. M., Schneider M. D. Mitogens and oncogenes can block the induction of specific voltage-gated ion channels. Science. 1987 May 1;236(4801):570–573. doi: 10.1126/science.2437651. [DOI] [PubMed] [Google Scholar]
- Chow K. L., Schwartz R. J. A combination of closely associated positive and negative cis-acting promoter elements regulates transcription of the skeletal alpha-actin gene. Mol Cell Biol. 1990 Feb;10(2):528–538. doi: 10.1128/mcb.10.2.528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg C. H., Hauschka S. D. Heterokaryon analysis of muscle differentiation: regulation of the postmitotic state. J Cell Biol. 1987 Aug;105(2):937–947. doi: 10.1083/jcb.105.2.937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clegg C. H., Linkhart T. A., Olwin B. B., Hauschka S. D. Growth factor control of skeletal muscle differentiation: commitment to terminal differentiation occurs in G1 phase and is repressed by fibroblast growth factor. J Cell Biol. 1987 Aug;105(2):949–956. doi: 10.1083/jcb.105.2.949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmondson D. G., Olson E. N. A gene with homology to the myc similarity region of MyoD1 is expressed during myogenesis and is sufficient to activate the muscle differentiation program. Genes Dev. 1989 May;3(5):628–640. doi: 10.1101/gad.3.5.628. [DOI] [PubMed] [Google Scholar]
- Ewton D. Z., Spizz G., Olson E. N., Florini J. R. Decrease in transforming growth factor-beta binding and action during differentiation in muscle cells. J Biol Chem. 1988 Mar 15;263(8):4029–4032. [PubMed] [Google Scholar]
- Gustafson T. A., Kedes L. Identification of multiple proteins that interact with functional regions of the human cardiac alpha-actin promoter. Mol Cell Biol. 1989 Aug;9(8):3269–3283. doi: 10.1128/mcb.9.8.3269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart C. E., Forstrom J. W., Kelly J. D., Seifert R. A., Smith R. A., Ross R., Murray M. J., Bowen-Pope D. F. Two classes of PDGF receptor recognize different isoforms of PDGF. Science. 1988 Jun 10;240(4858):1529–1531. doi: 10.1126/science.2836952. [DOI] [PubMed] [Google Scholar]
- Imbra R. J., Karin M. Phorbol ester induces the transcriptional stimulatory activity of the SV40 enhancer. Nature. 1986 Oct 9;323(6088):555–558. doi: 10.1038/323555a0. [DOI] [PubMed] [Google Scholar]
- Izumo S., Nadal-Ginard B., Mahdavi V. Protooncogene induction and reprogramming of cardiac gene expression produced by pressure overload. Proc Natl Acad Sci U S A. 1988 Jan;85(2):339–343. doi: 10.1073/pnas.85.2.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson J. E., Wold B. J., Hauschka S. D. Muscle creatine kinase sequence elements regulating skeletal and cardiac muscle expression in transgenic mice. Mol Cell Biol. 1989 Aug;9(8):3393–3399. doi: 10.1128/mcb.9.8.3393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Komuro I., Kurabayashi M., Shibazaki Y., Takaku F., Yazaki Y. Molecular cloning and characterization of a Ca2+ + Mg2+-dependent adenosine triphosphatase from rat cardiac sarcoplasmic reticulum. Regulation of its expression by pressure overload and developmental stage. J Clin Invest. 1989 Apr;83(4):1102–1108. doi: 10.1172/JCI113989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassar A. B., Thayer M. J., Overell R. W., Weintraub H. Transformation by activated ras or fos prevents myogenesis by inhibiting expression of MyoD1. Cell. 1989 Aug 25;58(4):659–667. doi: 10.1016/0092-8674(89)90101-3. [DOI] [PubMed] [Google Scholar]
- Lathrop B., Olson E., Glaser L. Control by fibroblast growth factor of differentiation in the BC3H1 muscle cell line. J Cell Biol. 1985 May;100(5):1540–1547. doi: 10.1083/jcb.100.5.1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee P. L., Johnson D. E., Cousens L. S., Fried V. A., Williams L. T. Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor. Science. 1989 Jul 7;245(4913):57–60. doi: 10.1126/science.2544996. [DOI] [PubMed] [Google Scholar]
- Long C. S., Ordahl C. P., Simpson P. C. Alpha 1-adrenergic receptor stimulation of sarcomeric actin isogene transcription in hypertrophy of cultured rat heart muscle cells. J Clin Invest. 1989 Mar;83(3):1078–1082. doi: 10.1172/JCI113951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida C. M., Rodland K. D., Matrisian L., Magun B. E., Ciment G. NGF induction of the gene encoding the protease transin accompanies neuronal differentiation in PC12 cells. Neuron. 1989 Jun;2(6):1587–1596. doi: 10.1016/0896-6273(89)90047-0. [DOI] [PubMed] [Google Scholar]
- Majesky M. W., Daemen M. J., Schwartz S. M. Alpha 1-adrenergic stimulation of platelet-derived growth factor A-chain gene expression in rat aorta. J Biol Chem. 1990 Jan 15;265(2):1082–1088. [PubMed] [Google Scholar]
- Mar J. H., Antin P. B., Cooper T. A., Ordahl C. P. Analysis of the upstream regions governing expression of the chicken cardiac troponin T gene in embryonic cardiac and skeletal muscle cells. J Cell Biol. 1988 Aug;107(2):573–585. doi: 10.1083/jcb.107.2.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Massagué J., Cheifetz S., Endo T., Nadal-Ginard B. Type beta transforming growth factor is an inhibitor of myogenic differentiation. Proc Natl Acad Sci U S A. 1986 Nov;83(21):8206–8210. doi: 10.1073/pnas.83.21.8206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercadier J. J., Lompré A. M., Duc P., Boheler K. R., Fraysse J. B., Wisnewsky C., Allen P. D., Komajda M., Schwartz K. Altered sarcoplasmic reticulum Ca2(+)-ATPase gene expression in the human ventricle during end-stage heart failure. J Clin Invest. 1990 Jan;85(1):305–309. doi: 10.1172/JCI114429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muscat G. E., Kedes L. Multiple 5'-flanking regions of the human alpha-skeletal actin gene synergistically modulate muscle-specific expression. Mol Cell Biol. 1987 Nov;7(11):4089–4099. doi: 10.1128/mcb.7.11.4089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nudel U., Zuk D., Einat P., Zeelon E., Levy Z., Neuman S., Yaffe D. Duchenne muscular dystrophy gene product is not identical in muscle and brain. Nature. 1989 Jan 5;337(6202):76–78. doi: 10.1038/337076a0. [DOI] [PubMed] [Google Scholar]
- Olson E. N., Sternberg E., Hu J. S., Spizz G., Wilcox C. Regulation of myogenic differentiation by type beta transforming growth factor. J Cell Biol. 1986 Nov;103(5):1799–1805. doi: 10.1083/jcb.103.5.1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olwin B. B., Hauschka S. D. Cell surface fibroblast growth factor and epidermal growth factor receptors are permanently lost during skeletal muscle terminal differentiation in culture. J Cell Biol. 1988 Aug;107(2):761–769. doi: 10.1083/jcb.107.2.761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parker T. G., Packer S. E., Schneider M. D. Peptide growth factors can provoke "fetal" contractile protein gene expression in rat cardiac myocytes. J Clin Invest. 1990 Feb;85(2):507–514. doi: 10.1172/JCI114466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne P. A., Olson E. N., Hsiau P., Roberts R., Perryman M. B., Schneider M. D. An activated c-Ha-ras allele blocks the induction of muscle-specific genes whose expression is contingent on mitogen withdrawal. Proc Natl Acad Sci U S A. 1987 Dec;84(24):8956–8960. doi: 10.1073/pnas.84.24.8956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penttinen R. P., Kobayashi S., Bornstein P. Transforming growth factor beta increases mRNA for matrix proteins both in the presence and in the absence of changes in mRNA stability. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1105–1108. doi: 10.1073/pnas.85.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quitschke W. W., DePonti-Zilli L., Lin Z. Y., Paterson B. M. Identification of two nuclear factor-binding domains on the chicken cardiac actin promoter: implications for regulation of the gene. Mol Cell Biol. 1989 Aug;9(8):3218–3230. doi: 10.1128/mcb.9.8.3218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid H. H., Wilks A. F., Bernard O. Two forms of the basic fibroblast growth factor receptor-like mRNA are expressed in the developing mouse brain. Proc Natl Acad Sci U S A. 1990 Feb;87(4):1596–1600. doi: 10.1073/pnas.87.4.1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhodes S. J., Konieczny S. F. Identification of MRF4: a new member of the muscle regulatory factor gene family. Genes Dev. 1989 Dec;3(12B):2050–2061. doi: 10.1101/gad.3.12b.2050. [DOI] [PubMed] [Google Scholar]
- Rivera V. M., Sheng M., Greenberg M. E. The inner core of the serum response element mediates both the rapid induction and subsequent repression of c-fos transcription following serum stimulation. Genes Dev. 1990 Feb;4(2):255–268. doi: 10.1101/gad.4.2.255. [DOI] [PubMed] [Google Scholar]
- Ruta M., Burgess W., Givol D., Epstein J., Neiger N., Kaplow J., Crumley G., Dionne C., Jaye M., Schlessinger J. Receptor for acidic fibroblast growth factor is related to the tyrosine kinase encoded by the fms-like gene (FLG). Proc Natl Acad Sci U S A. 1989 Nov;86(22):8722–8726. doi: 10.1073/pnas.86.22.8722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruzicka D. L., Schwartz R. J. Sequential activation of alpha-actin genes during avian cardiogenesis: vascular smooth muscle alpha-actin gene transcripts mark the onset of cardiomyocyte differentiation. J Cell Biol. 1988 Dec;107(6 Pt 2):2575–2586. doi: 10.1083/jcb.107.6.2575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito Y., Nakao K., Arai H., Nishimura K., Okumura K., Obata K., Takemura G., Fujiwara H., Sugawara A., Yamada T. Augmented expression of atrial natriuretic polypeptide gene in ventricle of human failing heart. J Clin Invest. 1989 Jan;83(1):298–305. doi: 10.1172/JCI113872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sassoon D., Lyons G., Wright W. E., Lin V., Lassar A., Weintraub H., Buckingham M. Expression of two myogenic regulatory factors myogenin and MyoD1 during mouse embryogenesis. Nature. 1989 Sep 28;341(6240):303–307. doi: 10.1038/341303a0. [DOI] [PubMed] [Google Scholar]
- Schiaffino S., Samuel J. L., Sassoon D., Lompré A. M., Garner I., Marotte F., Buckingham M., Rappaport L., Schwartz K. Nonsynchronous accumulation of alpha-skeletal actin and beta-myosin heavy chain mRNAs during early stages of pressure-overload--induced cardiac hypertrophy demonstrated by in situ hybridization. Circ Res. 1989 May;64(5):937–948. doi: 10.1161/01.res.64.5.937. [DOI] [PubMed] [Google Scholar]
- Schneider M. D., Parker T. G. Cardiac myocytes as targets for the action of peptide growth factors. Circulation. 1990 May;81(5):1443–1456. doi: 10.1161/01.cir.81.5.1443. [DOI] [PubMed] [Google Scholar]
- Seidman C. E., Wong D. W., Jarcho J. A., Bloch K. D., Seidman J. G. Cis-acting sequences that modulate atrial natriuretic factor gene expression. Proc Natl Acad Sci U S A. 1988 Jun;85(11):4104–4108. doi: 10.1073/pnas.85.11.4104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler-Tuyns A., Eldridge J. D., Paterson B. M. Expression and regulation of chicken actin genes introduced into mouse myogenic and nonmyogenic cells. Proc Natl Acad Sci U S A. 1984 May;81(10):2980–2984. doi: 10.1073/pnas.81.10.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Peptide growth factors are multifunctional. Nature. 1988 Mar 17;332(6161):217–219. doi: 10.1038/332217a0. [DOI] [PubMed] [Google Scholar]
- Tsika R. W., Bahl J. J., Leinwand L. A., Morkin E. Thyroid hormone regulates expression of a transfected human alpha-myosin heavy-chain fusion gene in fetal rat heart cells. Proc Natl Acad Sci U S A. 1990 Jan;87(1):379–383. doi: 10.1073/pnas.87.1.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ueno H., Perryman M. B., Roberts R., Schneider M. D. Differentiation of cardiac myocytes after mitogen withdrawal exhibits three sequential states of the ventricular growth response. J Cell Biol. 1988 Nov;107(5):1911–1918. doi: 10.1083/jcb.107.5.1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaidya T. B., Rhodes S. J., Taparowsky E. J., Konieczny S. F. Fibroblast growth factor and transforming growth factor beta repress transcription of the myogenic regulatory gene MyoD1. Mol Cell Biol. 1989 Aug;9(8):3576–3579. doi: 10.1128/mcb.9.8.3576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walsh K. Cross-binding of factors to functionally different promoter elements in c-fos and skeletal actin genes. Mol Cell Biol. 1989 May;9(5):2191–2201. doi: 10.1128/mcb.9.5.2191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webster K. A., Muscat G. E., Kedes L. Adenovirus E1A products suppress myogenic differentiation and inhibit transcription from muscle-specific promoters. Nature. 1988 Apr 7;332(6164):553–557. doi: 10.1038/332553a0. [DOI] [PubMed] [Google Scholar]
- Weiner H. L., Swain J. L. Acidic fibroblast growth factor mRNA is expressed by cardiac myocytes in culture and the protein is localized to the extracellular matrix. Proc Natl Acad Sci U S A. 1989 Apr;86(8):2683–2687. doi: 10.1073/pnas.86.8.2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodland H. R. Mesoderm formation in Xenopus. Cell. 1989 Dec 1;59(5):767–770. doi: 10.1016/0092-8674(89)90596-5. [DOI] [PubMed] [Google Scholar]