Abstract
The function of dimethylsulphoniopropionate (DMSP) in plants is unclear. It has been proposed as an antioxidant, osmolyte and overflow for excess energy under stress conditions. The formation of DMSP is part of the methionine (MET) pathway that is involved in plant stress responses. We used a new analytical approach to accurately quantify the changes in DMSP concentration that occurred in two ecotypes of the biomass crop Arundo donax subject to moderate drought stress under field conditions. The ecotypes of A. donax were from a hot semi-arid habitat in Morocco and a warm-humid environment in Central Italy. The Moroccan ecotype showed more pronounced reductions in photosynthesis, stomatal conductance and photochemical electron transport than the Italian ecotype. An increase in isoprene emission occurred in both ecotypes alongside enhanced foliar concentrations of DMSP, indicative of a protective function of these two metabolites in the amelioration of the deleterious effects of excess energy and oxidative stress. This is consistent with the modification of carbon within the methyl-erythritol and MET pathways responsible for increased synthesis of isoprene and DMSP under moderate drought. The results of this study indicate that DMSP is an important adaptive component of the stress response regulated via the MET pathway in A. donax. DMSP is likely a multifunctional molecule playing a number of roles in the response of A. donax to reduced water availability.
Keywords: photosynthesis, stomatal conductance, methionine pathway, chlorophyll fluorescence, dimethylsulphide, giant reed, biomass crop
Introduction
Dimethylsulphoniopropionate (DMSP) is an important part of sulfur metabolism in photosynthetic organisms (Giovanelli, 1987; Stefels, 2000) and the precursor of the volatile organic compound (VOC) dimethyl sulphide (DMS) (Bentley and Chasteen, 2004). Approximately half of the global flux of sulfur to the atmosphere occurs in the form of DMS (Malin et al., 1992), and DMS plays a key role in the climate system by acting as a condensation nucleus (Keller et al., 2012). Despite the central role played by DMSP in the global sulfur cycle, comparatively little is known about its role in plant growth or potential dynamics in response to plant stress. Previous analyses of DMSP have focused on marine phytoplankton and plants where it is found in higher concentrations. This has led to a hypothesis that DMSP formation competes with the emission of isoprene during stress (Dani and Loreto, 2017). If replicated in vascular plants, this would have major implications for our understanding of the regulation VOC emissions during stress. However, analysis of DMSP in higher plants, particularly under drought stress, has been neglected, and as a consequence the role of DMSP is unclear.
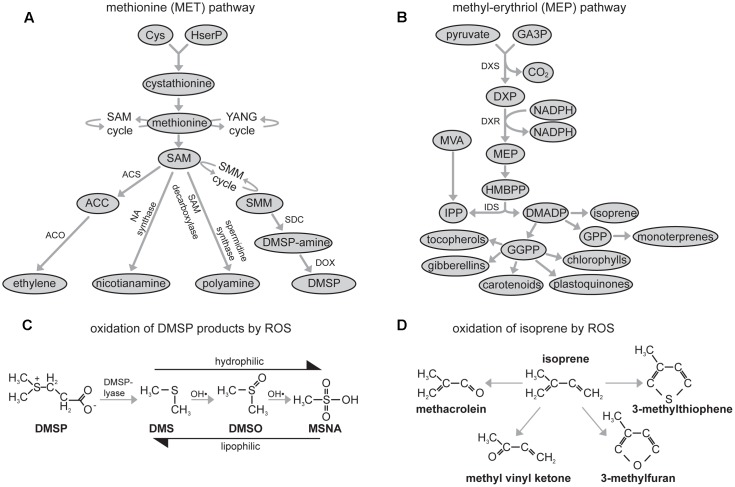
The function of DMSP is not well defined (Stefels, 2000), but it may serve as a metabolically compatible osmolyte (Kirst, 1996; Van Bergeijk et al., 2003), an antioxidant (Sunda et al., 2002; Husband and Kiene, 2007; Husband et al., 2012), a defensive compound against herbivores (Van Alstyne and Houser, 2003), a sink for excess sulfur (Stefels, 2000), a methyl donor in transmethylation reactions (Challenger et al., 1957; Giovanelli, 1987) or an overflow for excess energy (Groene, 1995; Stefels, 2000). DMSP is part of the methionine (MET) pathway (Figure 1A; James et al., 1995; Trossat et al., 1996). The MET pathway is an important component in the regulation of plant metabolism linked to the production of the antioxidant glutathione (Leustek and Saito, 1999), regulation of iron metabolism via the action of the amino acid nicotianamine (Curie et al., 2009; Klatte et al., 2009), the influence of polyamine on cellular signaling of abiotic stress (Alcázar et al., 2010) and the production of the stress hormone ethylene (Sato and Theologis, 1989). Given the importance of the MET pathway to metabolic adaptation to environmental stress, improved analysis of the dynamics of DMSP would contribute to our understanding of this critical component of plant metabolism.
FIGURE 1.
Schematic diagrams of: (A) the methionine (MET) pathway (DMSP, dimethylsulphoniopropionate; SAM, S-adenosylmethionine; SMM, S-methylmethionine; NA, nicotianamine; PA, polyamine; ACS, Aminocyclopropane-1-carboxylic acid synthase (ACC synthase); ACO, ACC oxidase; SDC, SMM decarboxylase; DOX, DMSP-amine oxidase; HserP, O-phosphohomoserine), the stages involving synthesis of SMM and DMSP-amine occur outside the chloroplast envelope before DMSP-amine is transported into the chloroplast envelope where DMSP is formed (Amir et al., 2002); (B) oxidation of DMSP products by ROS (DMS, dimethylsulphide; DMSO, dimethylsuphoxide; MSNA, methane sulphinic acid) (Stefels, 2000; Sunda et al., 2002; Bentley and Chasteen, 2004); (C) the methyl-erythritol (MEP) pathway (GA3P, glyceraldehyde 3-phosphate; DMADP, dimethylallyl diphosphate; DXR, 1-deoxy-D-xylulose 5-phosphate reductoisomerase; GPP, geranyl diphosphate; GGPP, geranylgeranyl diphosphate; DXP, 1-deoxy-D-xylulose 5-phosphate; DXS, 1-deoxyxylulose 5-phosphate synthase; HMBPP, (E)-4-hydroxy-3-methylbutyl-2-enyl pyrophosphate; MVA, mevalonate synthesized via the mevalonate pathway contributing to IPP) (Lichtenthaler et al., 1997), all stages other than MVA and the formation of gibberellins occur within the chloroplast, and; (D) oxidation of isoprene by ROS (Jardine et al., 2012).
During drought events the availability of soil water declines and plants close stomatal pores to reduce transpirative water-loss. The decrease in stomatal conductance (Gs) reduces the availability of CO2 at the active site of carboxylation in the chloroplast envelope and lowers rates of photosynthesis (PN) (Centritto et al., 2011b; Lauteri et al., 2014; Dbara et al., 2016; Killi et al., 2017). Plant stress during drought is often the result of excess of energy as the amount of intercepted radiation utilized in photochemistry declines, and increasing amounts of energy instead induce oxygen photoreduction forming dangerous reactive oxygen species (ROS) (Pinheiro and Chaves, 2011; Zivcak et al., 2013). During the initial stages of drought, plants may utilize the emission of isoprene derived from the plastidic 2-C-methyl-D-erythritol 4-phosphate pathway (MEP) (Figure 1B) to neutralize ROS (Figure 1C). Isoprene can strengthen thylakoid membranes (Velikova et al., 2011) and act directly as an antioxidant (Figure 1D) quenching biochemical reactions with ROS and reactive nitrogen species (Vickers et al., 2009a). The carotenoid xanthophylls are also part of the MEP pathway and are involved in the dissipation of excess energy via non-photochemical quenching (NPQ) (Cousins et al., 2002). DMSP may also exert an antioxidant function (Sunda et al., 2002; Husband and Kiene, 2007; Husband et al., 2012). Solanum lycopersicum leaves subject to severe drought exhibited a 75% increase in foliar concentrations of DMSP (Catola et al., 2016), consistent with antioxidant (Sunda et al., 2002; Husband and Kiene, 2007) or overflow (Stefels, 2000) functions for DMSP. Dimethlysulphide has been proposed to act as a delocalised antioxidant, stabilizing the lipid phase of photosynthetic and cellular membranes during oxidative stress (Catola et al., 2016) in a manner similar to isoprene (Velikova and Loreto, 2005). The subsequent oxidation products of DMS by ROS, dimethylsuphoxide (DMSO) and methane sulphinic acid (MSNA), are increasingly hydrophilic. Therefore, the degradation products of DMSP have differential partitioning between lipid and aqueous phases, and may consequently protect different cellular compartments from oxidative stress (Sunda et al., 2002). Acrylate, a by-product of DMSP cleavage by DMSP-lyase (Figure 1B), is also a precursor for ethylene, and increased concentrations of acrylate stimulate ethylene release (Plettner et al., 2005). During drought emissions of ethylene generally decline (Habben et al., 2014), possibly indicating reduced availability of DMSP due to oxidation by ROS (Figure 1B).
A new analytical technique developed by Catola et al. (2016) now allows rapid and accurate analysis of low concentrations of DMSP. We utilized this approach to investigate the response of DMSP in the giant reed (Arundo donax) subject to moderate drought stress in field conditions. Arundo donax is a fast growing isoprene emitting (Haworth et al., 2017b) member of the Poaceae family that shows potential as a biomass crop (Mantineo et al., 2009). The rapid biomass accumulation of A. donax is sustained by high rates of CO2 assimilation (Haworth et al., 2017b). However, these levels of PN are accompanied by high rates of stomatal conductance (Gs) and transpirative water-loss that require a high level of water availability (Haworth et al., 2017b). In comparison to other biomass crops such as Populus (e.g., Kauter et al., 2003; Centritto et al., 2011a), little is known regarding the physiological and metabolic response of A. donax to drought, and the potential for varietal differences in this response that may be exploited in the commercial development of A. donax as a biomass crop. Information regarding the dynamics of DMSP may allow the modification of the MET pathway in A. donax to optimize the physiological response to drought stress and maximize growth. We selected two A. donax ecotypes from a temperate and a more arid habitat (Sesto Fiorentino, Central Italy, and Marrakesh, Morocco, respectively) that had shown contrasting responses to severe drought in a previous study (Haworth et al., 2017a). These A. donax ecotypes were planted in a common garden field trial and subjected to moderate drought to: (i) investigate the impact on leaf gas exchange characteristics; (ii) quantify the effect on photochemical and non-photochemical energy usage using chlorophyll fluorescence; (iii) examine any change in the emission of the VOC isoprene, (iv) study the response of DMSP to drought, and; (v) propose a hypothesis regarding the possible role of DMSP in the drought response of A. donax and the potential to utilize this response, and any varietal differences found in this study, to optimize biomass production of A. donax in drought prone semi-arid marginal lands. We hypothesize that the MET pathway plays a key role in the drought response of A. donax via increased production of DMSP (e.g., Catola et al., 2016).
Materials and Methods
Field Site and Experimental Design
Rhizomes were collected from clonal populations from a warm sub-Mediterranean humid region, Sesto Fiorentino, Florence, in Central Italy, that receives 800 mm of precipitation each year and has a mean summer (June to August) temperature of 23°C, and from an arid pre-desert area of Marrakesh, Morocco, that receives 200 mm of precipitation each year and has a mean summer temperature of 30°C. The same ecotypes were exposed to severe drought in another study and exhibited differences in xylem morphology associated with the response to soil drying (Haworth et al., 2017a). The rhizomes were cut into equal portions of 20 cm in length (at least one bud was visible on each portion) and planted in a common garden experiment at the experimental farm of the University of Catania, Sicily (37° 25′N 15° 03′E), in March 2015. The rhizomes were planted at a depth of 15 cm every 0.5 m in rows spaced 0.8 m apart in 4.0 m × 3.0 m sized plots. Six plots of each of the Italian and Moroccan A. donax ecotypes were planted (12 plots in total), and to avoid edge effects a 2.4 m border of three rows of A. donax was placed around the field. The field was unfertilised and all plants were irrigated equally to field capacity until July 2015 when the rhizomes had established and the stems were roughly 1.5 m in height. On the 7th July irrigation was ceased to half of the field; three plots each of the Italian and Moroccan ecotypes were rain-fed, and the remaining three plots for each ecotype continued to receive irrigation. Supplementary irrigation was equivalent to 100% of potential evapotranspiration (ETc) during July to August, calculated each day as:
Where Eo is the evaporation of water from a class-A pan (mm); Kp is the pan coefficient, and; Kc, the crop growth stage (between 0.4 and 1.9) (Triana et al., 2015). Daily rain-fall was subtracted from the daily calculation of water to be supplied as irrigation (Allen et al., 1998). Soil samples were collected on the same day as the leaf samples were collected from 0 to 90 cm depth and the soil water content determined gravimetrically (Klute, 1986; Killi et al., 2014). Full details of the site and soil conditions at the experimental farm are given in Cosentino et al. (2006).
Leaf Gas Exchange and Chlorophyll Fluorescence
Six weeks after the cessation of supplementary irrigation to half of the A. donax plants, analyses of leaf gas exchange and chlorophyll fluorescence parameters were performed. Measurements of PN, Gs, the sub-stomatal concentration of [CO2] (Ci) and electron transport rate (ETR) were performed on the mid-section of the second newest fully expanded leaf using a LiCor Li6400XT fitted with a 2 cm2 leaf cuvette (Li-Cor, Inc., Lincoln, NE, United States). Two replicate plants from the centre of three plots were analyzed for each ecotype and treatment (n = 6). Environmental parameters were controlled and the following settings were used: 400 ppm [CO2], 2000 μmol m-2 s-1 of photosynthetically active radiation (PAR, 10% blue and 90% red light), and leaf temperature of 30°C. To reduce diffusive leaks through the chamber gasket, a supplementary gasket was added and the IRGA exhaust air was fed into the interspace between the chamber and the supplementary gaskets. The ETR was calculated as:
where: PPFD is the photosynthetic photon flux density (μmol m-2 s-1); ΦPSII is the actual quantum yield of photosystem II; α is the leaf absorbance (a standard value of 0.85 was used), and; β is the partitioning of electrons between photosystem I and II (assumed to be 0.5) (Genty et al., 1989). The respiration in the dark (RN) was estimated 10 min after switching off the light unit in the cuvette, when CO2 emission from the leaf had stabilized (e.g., Shi et al., 2015). Gas-exchange measurements were performed between 10.00 and 12.00 each day, when the plants exhibited the highest levels of PN and Gs. The maximum (Fv/Fm) and the actual quantum yield of photosystem II (ΦPSII: ΔF/F’m), and the dissipation of light energy as NPQ were recorded using a Hansatech FMS-2 (Hansatech, King’s Lynn, United Kingdom) after 30 min of dark adaptation (Genty et al., 1989).
Isoprene Emission
The emission of isoprene was measured in the field from the same leaves of A. donax used for gas-exchange analysis, under the same environmental settings, but a LiCor Li6400 fitted with a 6 cm2 cuvette and LED light unit was used. When monitoring isoprene emission, air from the cuvette with the enclosed leaf passed through a biphasic adsorbent trap containing 30 mg of Tenax and 20 mg of Carboxen (GERSTEL GmbH & Co.KG, Germany). A pump (Elite 5, A.P. Buck, Orlando, FL, United States) was used to pass 2 L of air through each trap at a rate of 200 ml min-1. Measurements of the concentration of isoprene in the ambient air (blanks) were performed using an empty leaf cuvette before and after each measurement. The traps were then stored at 4°C prior to analysis in the laboratory. Isoprene was first desorbed from traps at high temperature and then measured using a gas chromatograph – mass spectrometer (GC-MS) with an Agilent HP-INNOWAX (30 m × 0.32 mm × 0.15 μm) GC column. A 5977A mass selective detector with electron ionization operating at 70eV was used for analysis. Isoprene was identified by matching the spectrum peak with a library spectral database (NIST 11.L) and through comparison of the retention time and mass spectrum with an isoprene analytical standard (Sigma Aldrich, St. Louis, MO, United States) injected into the GC-MS at different concentrations. The isoprene analytical standard was also used to construct a calibration curve by injecting known concentrations of isoprene into the GC-MS. The data was analyzed using Agilent MassHunter Workstation software (Agilent 7890A, Agilent Technologies, Santa Clara, CA, United States). The concentration of isoprene within the leaf was calculated using the approach of Singsaas et al. (1997).
Analysis of Dimethylsulphoniopropionate (DMSP) and Relative Water Content (RWC)
Leaf samples were collected after completion of the leaf gas exchange and chlorophyll fluorescence measurements. The first fully most expanded leaf was collected from the same two plants in each plot (n = 6 for each ecotype and treatment) adjacent to the leaf that had been used for physiological analysis. The lower 4–5 cm of each leaf was removed to be used for determination of foliar relative water content (RWC) following the protocol of Diaz-Pérez et al. (1995). The remainder of the leaf was flash frozen in liquid nitrogen before being stored at -80°C prior to analysis of DMSP by solid phase micro extraction from head-space (HS-SPME). An in-depth description of the method is provided by Catola et al. (2016). Briefly, the leaf samples were ground in liquid nitrogen. An aliquot (0.2 g) of each sample was then placed inside a 20 ml screw-cap head-space vial (Agilent Technologies, Santa Clara, CA, United States), together with 250 μl 0.5 M NaOH, 2 g of NaCl and sufficient distilled water to make up a total 5 ml volume. Teflon coated silicon septa (Agilent Technologies) were used to seal the head-space vials, which were then incubated at 60°C for 12 h, to allow complete hydrolysis of DMSP to DMS. A three-phase divinylbenzene/carboxen/polydimethylsiloxane (Supelco, Bellafonte, PA, United States) 75 μm width, 2 cm long, solid phase micro-extraction fiber was placed in the head-space of the vials for 10 min at 40°C. To ensure consistent sampling and mixing of DMS in the head-space of the vials, a Gerstel MPS2 XL auto-sampler (Gerstel GmbH & Co. KG, Mülheim an der Ruhr, Germany) was used. The VOCs adsorbed by the fiber were analyzed using an Agilent 7820 GC-chromatograph with a 5977A M5D with electron ionization running at 70 eV and a HP-Innowax column (50 m, 0.2 mm, ID 0.4 μm DF). Dimethyl sulfide was identified via comparison with a spectral database library (NIST11.L) and injection of a known standard of DMS into the GC-MS (Sigma Aldrich). An example chromatograph of DMS is given in the Supplementary Data. A calibration curve was constructed by injecting increasing concentrations of DMS into the GC-MS and the amount of DMS used to infer the concentration of DMSP within the leaves.
Statistical Analysis
Statistical analyses were performed using SPSS 20 (IBM, Armonk, New York, NY, United States). To test the effect of water deficit on the Moroccan and Italian A. donax ecotypes we used a two-way ANOVA (Supplementary Information) and a one-way ANOVA with an LSD post hoc test to assess differences in variance between samples associated with either ecotype or treatment effects.
Results
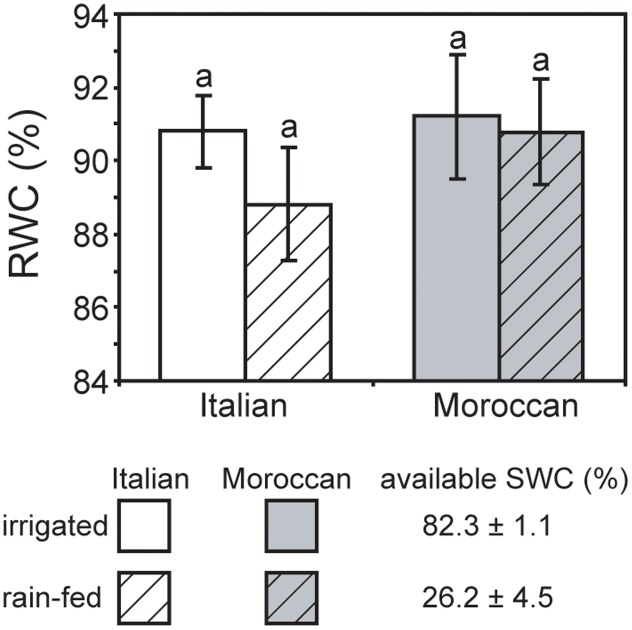
Growth under rain-fed field conditions resulted in a reduced RWC of A. donax leaves (particularly in the Italian ecotype), but the difference in comparison with leaves of irrigated plots was not statistically significant (Figure 2). The maximum (Fv/Fm) (Figure 3A) and actual (ΦPSII) (Figure 3B) quantum yields of PSII were also not significantly affected by the cessation of supplementary irrigation, although the two parameters were more reduced again in the Italian than in the Moroccan ecotype. Under rain-fed conditions, the Italian ecotype exhibited a significant 90.0% increase in the dissipation of energy as heat (as shown by the parameter NPQ), while the Moroccan ecotype showed no response (Figure 3C). Photosynthesis (Figure 4A) and Gs (Figure 4B) showed no difference between the A. donax ecotypes under irrigated conditions. However, under moderate drought stress the Moroccan ecotype exhibited more pronounced reductions in PN and Gs (52.0 and 75.5%), in comparison to the Italian ecotype (33.0 and 64.5%). Reductions in Gs resulted in lower Ci in both A. donax ecotypes, especially in the Moroccan (Figure 4C). The drought stressed Moroccan ecotype also exhibited an ETR/PN ratio higher than the Italian. However, the ETR/PN ratio was not different in the ecotypes under irrigated conditions. No ecotype or treatment effect was observed in RN (Figure 4D).
FIGURE 2.
Relative water content of leaves of Moroccan (gray) and Italian (white) Arundo donax ecotypes grown in the field under irrigated control (open) and rain-fed drought (hatched) conditions. Error bars indicate one standard error either side of the mean (n = 6). Letters indicate homogenous groups determined using a one-way ANOVA and LSD post hoc test.
FIGURE 3.
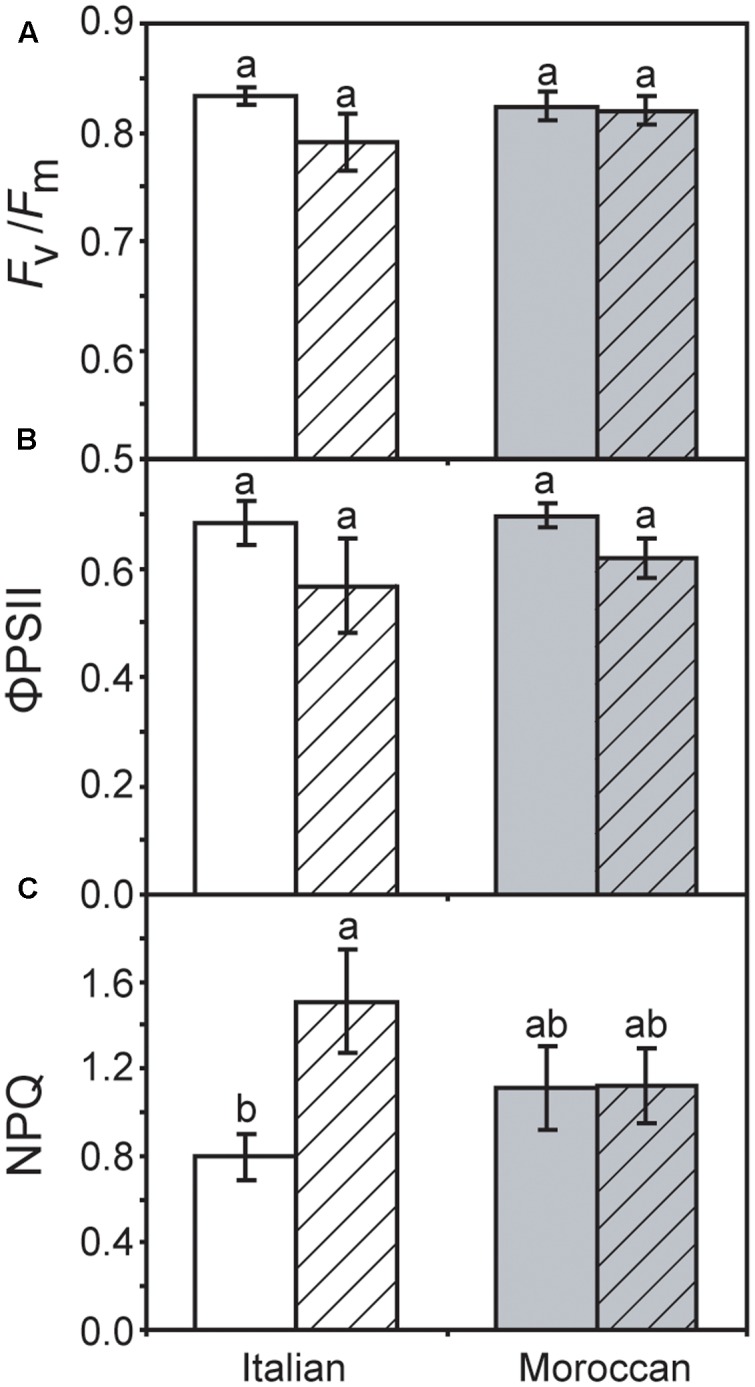
Chlorophyll fluorescence measurements of Moroccan (gray) and Italian (white) A. donax ecotypes grown in the field under irrigated control (open) and rain-fed drought (hatched) conditions: (A) maximum quantum efficiency of PSII (Fv/Fm); (B) actual quantum efficiency of PSII (ΦPSII), and; (C) non-photochemical quenching (NPQ). Error bars indicate one standard error either side of the mean (n = 6). Letters indicate homogenous groups determined using a one-way ANOVA and LSD post hoc test.
FIGURE 4.
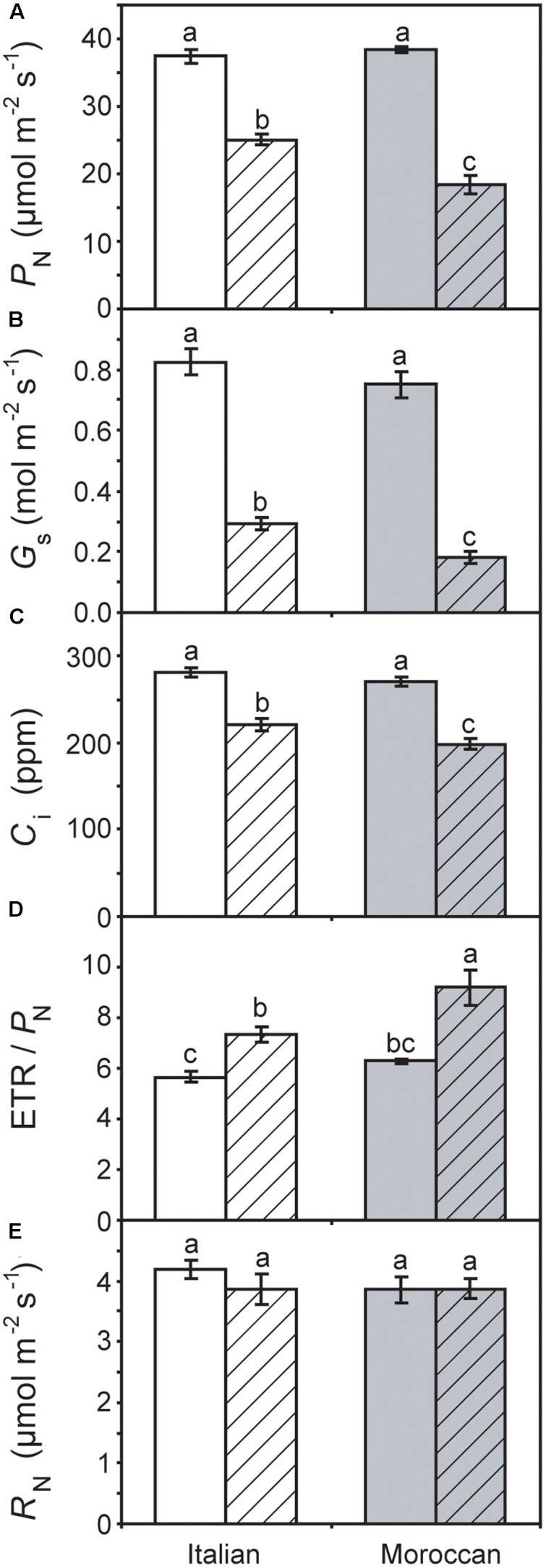
Leaf gas exchange and simultaneous chlorophyll fluorescence parameters of Moroccan (gray) and Italian (white) A. donax ecotypes grown in the field under irrigated control (open) and rain-fed drought (hatched) conditions: (A) photosynthesis (PN); (B) stomatal conductance (Gs); (C) internal sub-stomatal concentration of CO2 (Ci); (D) the ratio of electron transport rate (ETR) to PN, and; (E) respiration in the dark (RN). Error bars indicate one standard error either side of the mean (n = 6). Letters indicate homogenous groups determined using a one-way ANOVA and LSD post hoc test.
No difference was observed in the rate of isoprene emission between the two A. donax ecotypes under irrigated conditions. However, the emission of isoprene was significantly enhanced under rain-fed conditions, by 236.4% in the Moroccan ecotype, and to a lesser extent (76.4%) in the Italian ecotype (Figure 5A). A similar pattern was also observed in the concentration of isoprene within the leaf (Figure 5B). The amount of DMSP when determined on a leaf area basis was significantly greater in rain-fed than irrigated plants. This increase was 98.4 and 67.3% in the Italian and Moroccan ecotypes, respectively (Figure 5C). A similar increase in the concentration of DMSP was also recorded in both A. donax ecotypes under rain-fed conditions, when a dry weight basis was used (Figure 5D). No significant difference in the response of DMSP was observed between the two A. donax ecotypes, both when irrigated and rain-fed. The results of a two-way ANOVA of gas exchange, chlorophyll fluorescence, DMSP and isoprene parameters are presented in the Supplementary Information.
FIGURE 5.
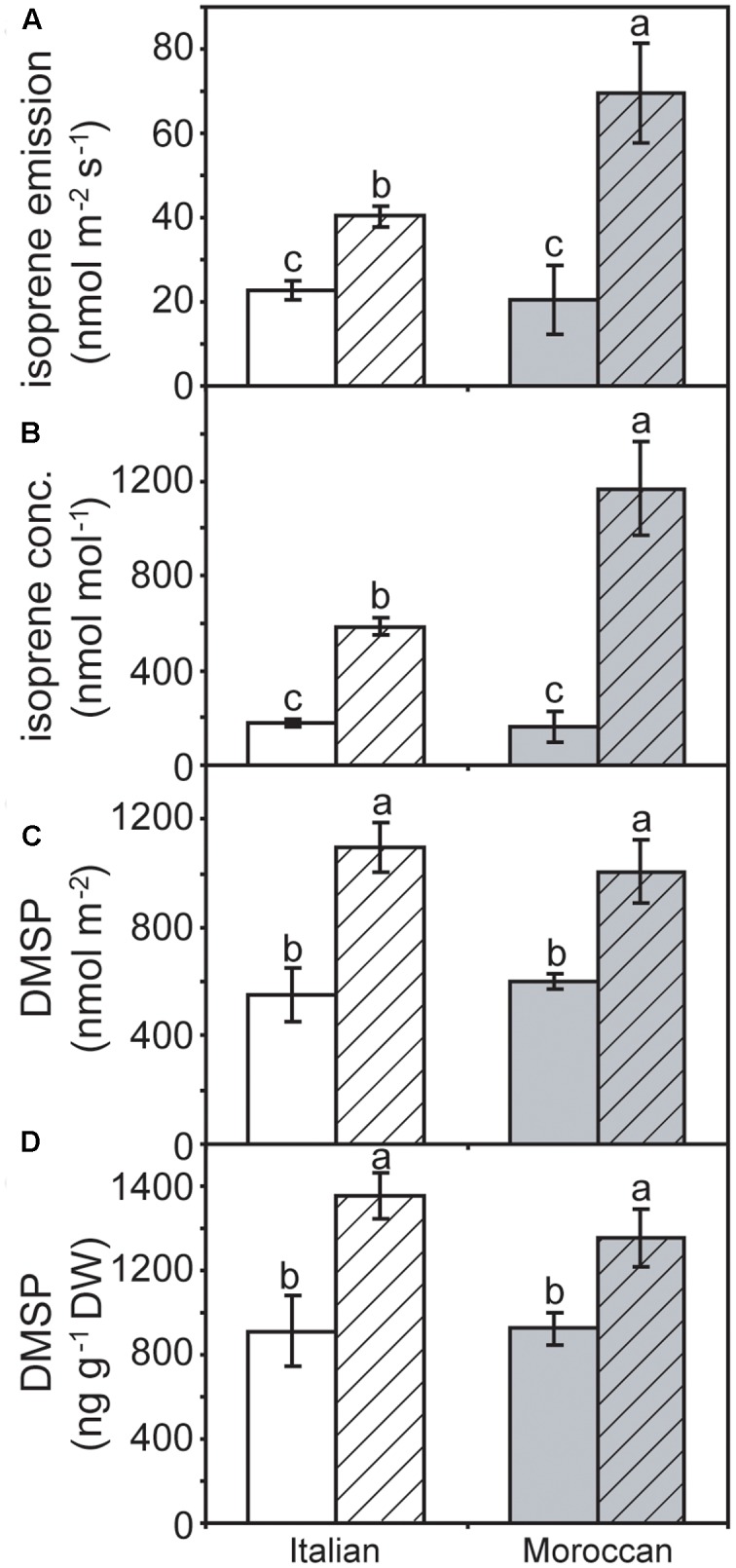
(A) Isoprene emission, (B) the concentration of isoprene within the sub-stomatal air-spaces inside the leaf, (C) dimethylsulphoniopropionate (DMSP) as a function of leaf area, and (D) concentration of DMSP per unit dry mass of leaf matter in Moroccan (gray) and Italian (white) A. donax ecotypes grown in the field under irrigated control (open) and rain-fed drought (hatched) conditions. Error bars indicate one standard error either side of the mean (n = 6). Letters indicate homogenous groups determined using a one-way ANOVA and LSD post hoc test.
Discussion
Arundo donax has potential to be a productive biomass crop in many warm to hot regions (Angelini et al., 2009; Mantineo et al., 2009), which are, however, often exposed to recurrent drought. The A. donax plants examined in this study showed no effect of drought on foliar RWC, and comparatively minor reductions in leaf gas exchange parameters in comparison to other studies (e.g., Cosentino et al., 2016; Haworth et al., 2017b); therefore the drought stress experienced by the plants in this study was considered to be moderate for A. donax. A number of studies have examined the effect of severe drought on A. donax (e.g., Sánchez et al., 2015; Haworth et al., 2017b), but comparatively few have investigated the response of this species to moderate drought over a sustained period, which may be more representative of the majority of situations where water availability limits growth in the field (Farooq et al., 2009; Yan et al., 2016). As such, the results of this study provide valuable physiological and biochemical insights into the response of A. donax to moderate drought under field conditions.
Impact of Moderate Drought Stress on Photosynthesis
Previous studies have reported comparatively little ecotypic variation in the leaf gas exchange and chlorophyll fluorescence parameters of contrasting A. donax ecotypes when subjected to severe drought (i.e., where Gs falls below 10% of control values) (Sánchez et al., 2015; Haworth et al., 2017a). In contrast, we have observed statistically significant ecotypic differences, albeit comparatively minor, in PN, Gs, Ci and ETR/PN responses of the Italian and Moroccan A. donax ecotypes to moderate drought (Figure 4). Despite both ecotypes showing no significant differences in RWC (Figure 2), stem height or stem number per rhizome (data not shown), the Moroccan ecotype generally exhibited more pronounced reductions in leaf gas exchange parameters under the moderate drought stress in the rain-fed treatment. The Moroccan ecotype has been shown to increase xylem vessel size during drought, while the Italian ecotype reduced xylem vessel size (Haworth et al., 2017a). This contrast in the morphological response of the water transport systems could be related to the adaptation of the A. donax ecotypes to their respective environments. The increase in xylem vessel size found in the Moroccan A. donax may promote the loss of above ground photosynthetic tissues via enhanced xylem embolism (e.g., Cochard, 2002; Cochard et al., 2002) to preserve the viability of the rhizome in an environment where drought is more severe and its onset more rapid. In contrast, the reduction in the xylem vessel diameter in the Italian ecotype would favor increased resistance to xylem embolism and the retention of leaves and stems (Tyree and Sperry, 1989). The lower Gs observed in the Moroccan ecotype under moderate drought stress (Figure 4B) may be due to a higher rate of xylem embolism promoting stomatal closure than in the Italian A. donax (Cochard, 2002). The Moroccan ecotype also exhibited a higher ETR/PN ratio under drought, indicative of a greater proportion of electrons being utilized in photorespiration (Sun et al., 2014). However, no difference was observed in Fv/Fm values of both ecotypes under irrigated and rain-fed conditions (Figure 3A) and alongside reduced Gs (Figure 4B) suggest that the limitations to PN under moderate drought were mostly diffusive (Centritto et al., 2003; Aganchich et al., 2009). However, the Italian ecotype did exhibit increased dissipation of heat as NPQ alongside a lower increase in isoprene emission under rain-fed conditions, in comparison to the Moroccan ecotype. Increased isoprene formation during drought might have helped maintain PN limitations only at the diffusion level (limited by stomatal closure and low Ci; Figure 4), avoiding the increase of NPQ and the probable resultant photochemical damage. Indeed, isoprene emitters show lower NPQ than non-emitters under physiological and stress conditions, especially under drought (Beckett et al., 2012; Pollastri et al., 2014). This possibly indicates lower oxidative stress at the chloroplast thylakoid level and enhanced protection of photochemistry of photosynthesis (Velikova et al., 2011) associated with high isoprene emission in the Moroccan ecotype. Severe drought stress has been shown to induce an increase in the proportion of photorespiration and RN relative to PN, with absolute values of photorespiration and RN declining as drought progresses (Centritto et al., 2011a; Sun et al., 2014; Killi et al., 2017). The moderate drought stress imposed on the A. donax did not appear sufficient to induce any alteration in RN values (Figure 4E).
Dynamics of Dimethylsulphoniopropionate and Isoprene Concentrations under Moderate Drought Stress
Under severe drought stress in the field the rate of isoprene emission in A. donax did not increase (Haworth et al., 2017b). However, in many other experiments including this study, isoprene emission and concentration are stimulated by moderate stress conditions, which uncouple isoprene from photosynthesis, its main source of carbon (Figure 1A). Isoprene has been proposed to act as a mobile diffusive antioxidant stabilizing chloroplast membranes under stress conditions (Velikova and Loreto, 2005; Vickers et al., 2009b). As already mentioned, the higher synthesis and emission of isoprene in the drought-stressed Moroccan ecotype, in comparison to the Italian ecotype, might be related to avoidance of photochemical damage in leaves where photosynthesis is impaired by diffusive limitations, or due to enhanced xanthophyll function in the Italian ecotype to promote NPQ. The increased emission of isoprene might be more suitable for ecotypes that frequently endure prolonged drought in their natural habitats. Exploitation of differences in isoprene emission in commercial A. donax clones would require consideration of the benefits of enhanced protective capacity balanced against increased losses of assimilated carbon.
The results of this study are consistent with the observations of Catola et al. (2016) of stimulated DMSP formation under drought. This enhanced foliar concentrations of DMSP occurred alongside the increase in isoprene emission in the A. donax plants in the rain-fed treatment when compared to irrigated plants (Figure 5D). This is not consistent with competition between DMSP and isoprene synthesis within the chloroplast proposed to take place in marine phytoplankton (Dani and Loreto, 2017). A previous study at the same site found no difference in isoprene emission rates between irrigated and rain-fed A. donax plants (Haworth et al., 2017b). However, the previously analyzed A. donax experienced a longer more severe drought stress, while the A. donax in the present study experienced a shorter less severe drought that allowed the maintenance of PN (Figure 4A) and the likely existence of a pool of labile carbon sufficient to sustain enhanced isoprene emission (Barta and Loreto, 2006). The synthesis of isoprene (Banerjee et al., 2013) and DMSP (James et al., 1995; Trossat et al., 1996) reveal activation of two chloroplastic pathways (MEP and MET, respectively). As largely demonstrated for the MEP pathway (Loreto and Schnitzler, 2010), also the MET pathway possibly plays a protective role in moderate drought stress conditions. The increase in DMSP concentration would be consistent with a role as an overflow for excess energy (Stefels, 2000). The increased NPQ in the Italian A. donax (Figure 3C) is indicative of a reduction in the usage of energy for photochemistry (Haworth et al., 2017b). DMSP is considered to act more strongly as a protective overflow under conditions of low nitrogen availability, facilitating the redistribution of nitrogen to other amino acids via the MET pathway (Groene and Kirst, 1992). As the field where the A. donax rhizomes were planted was unfertilised prior to the field trial, this may have promoted the increase in DMSP levels observed during growth under moderate drought stress, when the energy partitioning to photochemistry became unbalanced (Stefels, 2000). The increase in foliar DMSP concentration found in the A. donax plants subject to moderate drought in this study may have also acted as an osmolyte reducing the leaf water potential and allowing the maintenance of RWC (Kirst, 1996; Van Bergeijk et al., 2003). This is consistent with observations in the salt-marsh grass Spartina alterniflora where the concentration of DMSP did not adjust in response to an increase in oxidative stress (Husband et al., 2012), indicating that the role of DMSP may not be protective.
The MET pathway leads to the formation of many compounds that have well-defined roles in abiotic tolerance. However, it is unclear whether DMSP in higher plants is a by-product of the synthesis of these other MET derived compounds. However, a protective role for DMSP is consistent with its synthesis within the chloroplast. Greater availability of DMSP may also promote enhanced emission of DMS (Husband and Kiene, 2007) to act as a mobile antioxidant protecting thylakoid membranes in a manner similar to isoprene (Velikova and Loreto, 2005), via the oxidation of DMS to DMSO (Husband and Kiene, 2007). The rate of DMS emission is often correlated to the availability of DMSP (Groene, 1995; Catola et al., 2016); however, the rate of release of DMS is also dependent upon the activity of DMSP-lyase (Stefels et al., 1995) and of bacterial degradation of DMS (Carini, 2016). It is not possible to estimate potential rates of DMS emission based upon the concentrations of DMSP observed in the present study due to the low affinity of DMSP-lyase for DMSP (Stefels et al., 2007; Reisch et al., 2011) Further analytical advances are required to directly determine accurate foliar DMS release at the low levels likely to occur in A. donax before more definitive conclusions may be drawn as to the role of DMS and its precursor DMSP as aqueous and gaseous antioxidants.
Conclusion
Arundo donax has typically shown little ecotypic variation in physiological responses to severe drought. However, our analysis of the responses to moderate drought stress under field conditions in A. donax ecotypes from warm sub-humid (Central Italy) and hot semi-arid (Morocco) habitats indicated a degree of ecotypic variation, with the Moroccan ecotype exhibiting more pronounced reductions in PN and Gs. This may reflect selective pressures experienced by the Moroccan ecotype to preserve the viability of the rhizome in a habitat where droughts develop more rapidly, are more severe and longer in duration. As synthesis of isoprene and DMSP increased significantly under moderate stress, we suggest that the underlying MEP and MET pathways, play an important role in adapting to moderate drought and preserving photosynthetic capacity once the stress is relieved. Modification of MEP and MET pathways may potentially assist in the development of stress resistant and climate-adapted A. donax biomass crops.
Author Contributions
MH, GM, ER, GA, SLC, CB, SC, and MM conducted the experiment. MH, MC, and FL wrote the manuscript.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We are grateful for the assistance of the staff of the Di3A Experimental Field (University of Catania), Alessandra Pellegrino and Salvatore La Rosa (CNR-IVALSA). Luca Calamai(University of Florence) and Gabriele Cencetti (CNR – IBBR) assisted with GC-MS measurements. We thank Said Wahbi (Université Cadi Ayyad, Marrakech) for providing rhizomes. The comments of two reviewers significantly improved this work.
Footnotes
Funding. This work was funded by the EU FP7 project WATBIO (Development of improved perennial non-food biomass and bioproduct crops for water stressed environments – no.311929).
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2017.01016/full#supplementary-material
References
- Aganchich B., Wahbi S., Loreto F., Centritto M. (2009). Partial root zone drying: regulation of photosynthetic limitations and antioxidant enzymatic activities in young olive (Olea europaea) saplings. Tree Physiol. 29 685–696. 10.1093/treephys/tpp012 [DOI] [PubMed] [Google Scholar]
- Alcázar R., Altabella T., Marco F., Bortolotti C., Reymond M., Koncz C., et al. (2010). Polyamines: molecules with regulatory functions in plant abiotic stress tolerance. Planta 231 1237–1249. 10.1007/s00425-010-1130-0 [DOI] [PubMed] [Google Scholar]
- Allen R. G., Pereira L. S., Raes D., Smith M. (1998). Crop Evapotranspiration-Guidelines for Computing Crop Water Requirements-FAO Irrigation and Drainage Paper 56, Vol. 300 Rome: FAO, D05109. [Google Scholar]
- Amir R., Hacham Y., Galili G. (2002). Cystathionine γ-synthase and threonine synthase operate in concert to regulate carbon flow towards methionine in plants. Trends Plant Sci. 7 153–156. 10.1016/S1360-1385(02)02227-6 [DOI] [PubMed] [Google Scholar]
- Angelini L. G., Ceccarini L., Di Nassa N. N., Bonari E. (2009). Comparison of Arundo donax L. and Miscanthus x giganteus in a long-term field experiment in Central Italy: analysis of productive characteristics and energy balance. Biomass Bioenergy 33 635–643. 10.1016/j.biombioe.2008.10.005 [DOI] [Google Scholar]
- Banerjee A., Wu Y., Banerjee R., Li Y., Yan H., Sharkey T. D. (2013). Feedback inhibition of deoxy-D-xylulose-5-phosphate synthase regulates the methylerythritol 4-phosphate pathway. J. Biol. Chem. 288 16926–16936. 10.1074/jbc.M113.464636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barta C., Loreto F. (2006). The relationship between the methyl-erythritol phosphate pathway leading to emission of volatile isoprenoids and abscisic acid content in leaves. Plant Physiol. 141 1676–1683. 10.1104/pp.106.083063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckett M., Loreto F., Velikova V., Brunetti C., Di Ferdinando M., Tattini M., et al. (2012). Photosynthetic limitations and volatile and non-volatile isoprenoids in the poikilochlorophyllous resurrection plant Xerophyta humilis during dehydration and rehydration. Plant Cell Environ. 35 2061–2074. 10.1111/j.1365-3040.2012.02536.x [DOI] [PubMed] [Google Scholar]
- Bentley R., Chasteen T. G. (2004). Environmental VOSCs - formation and degradation of dimethyl sulfide, methanethiol and related materials. Chemosphere 55 291–317. 10.1016/j.chemosphere.2003.12.017 [DOI] [PubMed] [Google Scholar]
- Carini P. (2016). Microbial oxidation of DMS to DMSO: a biochemical surprise with geochemical implications. Environ. Microbiol. 18 2302–2304. 10.1111/1462-2920.13317 [DOI] [PubMed] [Google Scholar]
- Catola S., Ganesha S. D. K., Calamai L., Loreto F., Ranieri A., Centritto M. (2016). Headspace-solid phase microextraction approach for dimethylsulfoniopropionate quantification in Solanum lycopersicum plants subjected to water stress. Front. Plant Sci. 7:1257 10.3389/fpls.2016.01257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Centritto M., Brilli F., Fodale R., Loreto F. (2011a). Different sensitivity of isoprene emission, respiration and photosynthesis to high growth temperature coupled with drought stress in black poplar (Populus nigra) saplings. Tree Physiol. 31 275–286. 10.1093/treephys/tpq112 [DOI] [PubMed] [Google Scholar]
- Centritto M., Loreto F., Chartzoulakis K. (2003). The use of low [CO2] to estimate diffusional and non-diffusional limitations of photosynthetic capacity of salt-stressed olive saplings. Plant Cell Environ. 26 585–594. 10.1046/j.1365-3040.2003.00993.x [DOI] [Google Scholar]
- Centritto M., Tognetti R., Leitgeb E., Støelcová K., Cohen S. (2011b). “Above ground processes - anticipating climate change influences,” in Forest Management and the Water Cycle: An Ecosystem-Based Approach, eds Bredemeier M., Cohen S., Godbold D. L., Lode E., Pichler V., Schleppi P. (London: Springer; ), 31–64. [Google Scholar]
- Challenger F., Bywood R., Thomas P., Hayward B. J. (1957). Studies on biological methylation. XVII. The natural occurrence and chemical reactions of some thetins. Archiv. Biochem. Biophys. 69 514–523. 10.1016/0003-9861(57)90516-7 [DOI] [PubMed] [Google Scholar]
- Cochard H. (2002). Xylem embolism and drought-induced stomatal closure in maize. Planta 215 466–471. 10.1007/s00425-002-0766-9 [DOI] [PubMed] [Google Scholar]
- Cochard H., Coll L., Le Roux X., Améglio T. (2002). Unraveling the effects of plant hydraulics on stomatal closure during water stress in walnut. Plant Physiol. 128 282–290. 10.1104/pp.010400 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosentino S. L., Copani V., D’Agosta G. M., Sanzone E., Mantineo M. (2006). First results on evaluation of Arundo donax L. clones collected in Southern Italy. Ind. Crops Prod. 23 212–222. 10.1016/j.indcrop.2005.06.004 [DOI] [Google Scholar]
- Cosentino S. L., Patanè C., Sanzone E., Testa G., Scordia D. (2016). Leaf gas exchange, water status and radiation use efficiency of giant reed (Arundo donax L.) in a changing soil nitrogen fertilization and soil water availability in a semi-arid Mediterranean area. Eur. J. Agron. 72 56–69. 10.1016/j.eja.2015.09.011 [DOI] [Google Scholar]
- Cousins A. B., Adam N. R., Wall G. W., Kimball B. A., Pinter P. J., Ottman M. J., et al. (2002). Photosystem II energy use, non-photochemical quenching and the xanthophyll cycle in Sorghum bicolor grown under drought and free-air CO2 enrichment (FACE) conditions. Plant Cell Environ. 25 1551–1559. 10.1046/j.1365-3040.2002.00935.x [DOI] [Google Scholar]
- Curie C., Cassin G., Couch D., Divol F., Higuchi K., Le Jean M., et al. (2009). Metal movement within the plant: contribution of nicotianamine and yellow stripe 1-like transporters. Ann. Bot. 103 1–11. 10.1093/aob/mcn207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dani K. G. S., Loreto F. (2017). Trade-off between dimethyl sulfide and isoprene emissions from marine phytoplankton. Trends Plant Sci. 22 361–372. 10.1016/j.tplants.2017.01.006 [DOI] [PubMed] [Google Scholar]
- Dbara S., Haworth M., Emiliani G., Mimoun M. B., Gómez-Cadenas A., Centritto M. (2016). Partial root-zone drying of olive (Olea europaea var.’Chetoui’) induces reduced yield under field conditions. PLoS ONE 11:e0157089 10.1371/journal.pone.0157089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Pérez J. C., Shackel K. A., Sutter E. G. (1995). Relative water content and water potential of tissue 1. J. Exp. Bot. 46 111–118. 10.1093/jxb/46.1.111 [DOI] [Google Scholar]
- Farooq M., Wahid A., Kobayashi N., Fujita D., Basra S. M. A. (2009). Plant drought stress: effects, mechanisms and management. Agron. Sustain. Dev. 29 185–212. 10.1051/agro:2008021 [DOI] [Google Scholar]
- Genty B., Briantais J.-M., Baker N. R. (1989). The relationship between the quantum yield of photosynthetic electron transport and quenching of chlorophyll fluorescence. Biochim. Biophys. Acta 990 87–92. 10.1016/S0304-4165(89)80016-9 [DOI] [Google Scholar]
- Giovanelli J. (1987). Sulfur amino acids of plants: an overview. Methods Enzymol. 143 419–426. 10.1016/0076-6879(87)43073-5 [DOI] [Google Scholar]
- Groene T. (1995). Biogenic production and consumption of dimethylsulfide (DMS) and dimethylsulfoniopropionate (DMSP) in the marine epipelagic zone: a review. J. Mar. Syst. 6 191–209. 10.1016/0924-7963(94)00023-5 [DOI] [Google Scholar]
- Groene T., Kirst G. (1992). The effect of nitrogen deficiency, methionine and inhibitors of methionine metabolism on the DMSP contents of Tetraselmis subcordiformis (Stein). Mar. Biol. 112 497–503. 10.1007/BF00356296 [DOI] [Google Scholar]
- Habben J. E., Bao X., Bate N. J., DeBruin J. L., Dolan D., Hasegawa D., et al. (2014). Transgenic alteration of ethylene biosynthesis increases grain yield in maize under field drought-stress conditions. Plant Biotechnol. J. 12 685–693. 10.1111/pbi.12172 [DOI] [PubMed] [Google Scholar]
- Haworth M., Centritto M., Giovannelli A., Marino G., Proietti N., Capitani D., et al. (2017a). Xylem morphology determines the drought response of two Arundo donax ecotypes from contrasting habitats. GCB Bioenergy 9 119–131. 10.1111/gcbb.12322 [DOI] [Google Scholar]
- Haworth M., Cosentino S. L., Marino G., Brunetti C., Scordia D., Testa G., et al. (2017b). Physiological responses of Arundo donax ecotypes to drought: a common garden study. GCB Bioenergy 9 132–143. 10.1111/gcbb.12348 [DOI] [Google Scholar]
- Husband J. D., Kiene R. P. (2007). Occurrence of dimethylsulfoxide in leaves, stems, and roots of Spartina alterniflora. Wetlands 27 224–229. 10.1672/0277-5212(2007)27[224:OODILS]2.0.CO;2 [DOI] [Google Scholar]
- Husband J. D., Kiene R. P., Sherman T. D. (2012). Oxidation of dimethylsulfoniopropionate (DMSP) in response to oxidative stress in Spartina alterniflora and protection of a non-DMSP producing grass by exogenous DMSP plus acrylate. Environ. Exp. Bot. 79 44–48. 10.1016/j.envexpbot.2012.01.006 [DOI] [Google Scholar]
- James F., Paquet L., Sparace S. A., Gage D. A., Hanson A. D. (1995). Evidence implicating dimethylsulfoniopropionaldehyde as an intermediate in dimethylsulfoniopropionate biosynthesis. Plant Physiol. 108 1439–1448. 10.1104/pp.108.4.1439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jardine K. J., Monson R. K., Abrell L., Saleska S. R., Arneth A., Jardine A., et al. (2012). Within-plant isoprene oxidation confirmed by direct emissions of oxidation products methyl vinyl ketone and methacrolein. Global Change Biol. 18 973–984. 10.1111/j.1365-2486.2011.02610.x [DOI] [Google Scholar]
- Kauter D., Lewandowski I., Claupein W. (2003). Quantity and quality of harvestable biomass from Populus short rotation coppice for solid fuel use—a review of the physiological basis and management influences. Biomass Bioenergy 24 411–427. 10.1016/S0961-9534(02)00177-0 [DOI] [Google Scholar]
- Keller M., Kiene R., Kirst G., Visscher P. (2012). Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds. Berlin: Springer Science & Business Media. [Google Scholar]
- Killi D., Anlauf R., Kavdir Y., Haworth M. (2014). Assessing the impact of agro-industrial olive wastes in soil water retention: implications for remediation of degraded soils and water availability for plant growth. Int. Biodeterior. Biodegradation 94 48–56. 10.1016/j.ibiod.2014.06.019 [DOI] [Google Scholar]
- Killi D., Bussotti F., Raschi A., Haworth M. (2017). Adaptation to high temperature mitigates the impact of water deficit during combined heat and drought stress in C3 sunflower and C4 maize varieties with contrasting drought tolerance. Physiol. Plant. 159 130–147. 10.1111/ppl.12490 [DOI] [PubMed] [Google Scholar]
- Kirst G. O. (1996). “Osmotic adjustment in phytoplankton and macroalgae,” in Biological and Environmental Chemistry of DMSP and Related Sulfonium Compounds, eds Kiene R. P., Visscher P. T., Keller M. D., Kirst G. O. (Berlin: Springer; ), 121–129. [Google Scholar]
- Klatte M., Schuler M., Wirtz M., Fink-Straube C., Hell R., Bauer P. (2009). The analysis of Arabidopsis nicotianamine synthase mutants reveals functions for nicotianamine in seed iron loading and iron deficiency responses. Plant Physiol. 150 257–271. 10.1104/pp.109.136374 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klute A. (1986). Methods of Soil Analysis. Part 1. Physical and Mineralogical Methods. Madison, WI: American Society of Agronomy, Inc. [Google Scholar]
- Lauteri M., Haworth M., Serraj R., Monteverdi M. C., Centritto M. (2014). Photosynthetic diffusional constraints affect yield in drought stressed rice cultivars during flowering. PLoS ONE 9:e109054 10.1371/journal.pone.0109054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leustek T., Saito K. (1999). Sulfate transport and assimilation in plants. Plant Physiol. 120 637–644. 10.1104/pp.120.3.637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichtenthaler H. K., Schwender J., Disch A., Rohmer M. (1997). Biosynthesis of isoprenoids in higher plant chloroplasts proceeds via a mevalonate-independent pathway. FEBS Lett. 400 271–274. 10.1016/S0014-5793(96)01404-4 [DOI] [PubMed] [Google Scholar]
- Loreto F., Schnitzler J.-P. (2010). Abiotic stresses and induced BVOCs. Trends Plant Sci. 15 154–166. 10.1016/j.tplants.2009.12.006 [DOI] [PubMed] [Google Scholar]
- Malin G., Turner S. M., Liss P. S. (1992). Sulfur: the plankton-climate connection. J. Phycol. 28 590–597. 10.1111/j.0022-3646.1992.00590.x [DOI] [Google Scholar]
- Mantineo M., D’Agosta G. M., Copani V., Patanè C., Cosentino S. L. (2009). Biomass yield and energy balance of three perennial crops for energy use in the semi-arid Mediterranean environment. Field Crops Res. 114 204–213. 10.1016/j.fcr.2009.07.020 [DOI] [Google Scholar]
- Pinheiro C., Chaves M. M. (2011). Photosynthesis and drought: can we make metabolic connections from available data? J. Exp. Bot. 62 869–882. 10.1093/jxb/erq340 [DOI] [PubMed] [Google Scholar]
- Plettner I., Steinke M., Malin G. (2005). Ethene (ethylene) production in the marine macroalga Ulva (Enteromorpha) intestinalis L. (Chlorophyta, Ulvophyceae): effect of light-stress and co-production with dimethyl sulphide. Plant Cell Environ. 28 1136–1145. 10.1111/j.1365-3040.2005.01351.x [DOI] [Google Scholar]
- Pollastri S., Tsonev T., Loreto F. (2014). Isoprene improves photochemical efficiency and enhances heat dissipation in plants at physiological temperatures. J. Exp. Bot. 65 1565–1570. 10.1093/jxb/eru033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reisch C. R., Moran M. A., Whitman W. B. (2011). Bacterial catabolism of dimethylsulfoniopropionate (DMSP). Front. Microbiol. 2:172 10.3389/fmicb.2011.00172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sánchez E., Scordia D., Lino G., Arias C., Cosentino S., Nogués S. (2015). Salinity and water stress effects on biomass production in different Arundo donax L. clones. Bioenergy Res. 8 1461–1479. 10.1007/s12155-015-9652-8 [DOI] [Google Scholar]
- Sato T., Theologis A. (1989). Cloning the mRNA encoding 1-aminocyclopropane-1-carboxylate synthase, the key enzyme for ethylene biosynthesis in plants. Proc. Natl. Acad. Sci. U.S.A. 86 6621–6625. 10.1073/pnas.86.17.6621 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Z., Haworth M., Feng Q., Cheng R., Centritto M. (2015). Growth habit and leaf economics determine gas exchange responses to high elevation in an evergreen tree, a deciduous shrub and a herbaceous annual. AoB Plants 7 1–14. 10.1093/aobpla/plv115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singsaas E. L., Lerdau M., Winter K., Sharkey T. D. (1997). Isoprene increases thermotolerance of isoprene-emitting species. Plant Physiol. 115 1413–1420. 10.1104/pp.115.4.1413 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stefels J. (2000). Physiological aspects of the production and conversion of DMSP in marine algae and higher plants. J. Sea Res. 43 183–197. 10.1016/S1385-1101(00)00030-7 [DOI] [Google Scholar]
- Stefels J., Dijkhuizen L., Gieskes W. (1995). DMSP-lyase activity in a spring phytoplankton bloom off the Dutch coast, related to Phaeocystis sp. abundance. Mar. Ecol. Prog. Ser. 123 235–243. 10.3354/meps123235 [DOI] [Google Scholar]
- Stefels J., Steinke M., Turner S., Malin G., Belviso S. (2007). Environmental constraints on the production and removal of the climatically active gas dimethylsulphide (DMS) and implications for ecosystem modelling. Biogeochemistry 83 245–275. 10.1007/s10533-007-9091-5 [DOI] [Google Scholar]
- Sun P., Wahbi S., Tsonev T., Haworth M., Liu S., Centritto M. (2014). On the use of leaf spectral indices to assess water status and photosynthetic limitations in Olea europaea L. during water-stress and recovery. PLoS ONE 9:e105165 10.1371/journal.pone.0105165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunda W., Kieber D., Kiene R., Huntsman S. (2002). An antioxidant function for DMSP and DMS in marine algae. Nature 418 317–320. 10.1038/nature00851 [DOI] [PubMed] [Google Scholar]
- Triana F., Nassi o Di Nasso N., Ragaglini G., Roncucci N., Bonari E. (2015). Evapotranspiration, crop coefficient and water use efficiency of giant reed (Arundo donax L.) and miscanthus (Miscanthus × giganteus Greef et Deu.) in a Mediterranean environment. GCB Bioenergy 7 811–819. 10.1111/gcbb.12172 [DOI] [Google Scholar]
- Trossat C., Nolte K. D., Hanson A. D. (1996). Evidence that the pathway of dimethylsulfoniopropionate biosynthesis begins in the cytosol and ends in the chloroplast. Plant Physiol. 111 965–973. 10.1104/pp.111.4.965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyree M. T., Sperry J. S. (1989). Vulnerability of ylem to cavitation and embolism. Annu. Rev. Plant Biol. 40 19–36. 10.1146/annurev.pp.40.060189.000315 [DOI] [Google Scholar]
- Van Alstyne K. L., Houser L. T. (2003). Dimethylsulfide release during macroinvertebrate grazing and its role as an activated chemical defense. Mar. Ecol. Prog. Ser. 250 175–181. 10.3354/meps250175 [DOI] [Google Scholar]
- Van Bergeijk S. A., Van der Zee C., Stal L. J. (2003). Uptake and excretion of dimethylsulphoniopropionate is driven by salinity changes in the marine benthic diatom Cylindrotheca closterium. Eur. J. Phycol. 38 341–349. 10.1080/09670260310001612600 [DOI] [Google Scholar]
- Velikova V., Loreto F. (2005). On the relationship between isoprene emission and thermotolerance in Phragmites australis leaves exposed to high temperatures and during the recovery from a heat stress. Plant Cell Environ. 28 318–327. 10.1111/j.1365-3040.2004.01314.x [DOI] [Google Scholar]
- Velikova V., Varkonyi Z., Szabo M., Maslenkova L., Nogues I., Kovacs L., et al. (2011). Increased thermostability of thylakoid membranes in isoprene-emitting leaves probed with three biophysical techniques. Plant Physiol. 157 905–916. 10.1104/pp.111.182519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vickers C. E., Gershenzon J., Lerdau M. T., Loreto F. (2009a). A unified mechanism of action for volatile isoprenoids in plant abiotic stress. Nat. Chem. Biol. 5 283–291. 10.1038/nchembio.158 [DOI] [PubMed] [Google Scholar]
- Vickers C. E., Possell M., Cojocariu C. I., Velikova V. B., Laothawornkitkul J., Ryan A., et al. (2009b). Isoprene synthesis protects transgenic tobacco plants from oxidative stress. Plant Cell Environ. 32 520–531. 10.1111/j.1365-3040.2009.01946.x [DOI] [PubMed] [Google Scholar]
- Yan W., Zhong Y., Shangguan Z. (2016). A meta-analysis of leaf gas exchange and water status responses to drought. Sci. Rep. 6:20917 10.1038/srep20917 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zivcak M., Brestic M., Balatova Z., Drevenakova P., Olsovska K., Kalaji H. M., et al. (2013). Photosynthetic electron transport and specific photoprotective responses in wheat leaves under drought stress. Photosynth. Res. 117 529–546. 10.1007/s11120-013-9885-3 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.