Abstract
Problem
Plasminogen activator inhibitor-1 (PAI-1) is elevated in women with polycystic ovary syndrome (PCOS), but the regulation in granulosa cells (GCs) is unclear.
Method of Study
PAI-1 expression in PCOS ovaries were investigated immunohistologically. PAI-1 expressions in HGrC1, a human GC cell line, were investigated at mRNA and activity levels. The expressions of TGF-β and TNF-α in peritoneal fluid mononuclear cells (PFMC) were measured with quantitative PCR.
Results
Little PAI-1 expression is observed in healthy GCs whereas GCs of PCOS and atretic follicle exhibit distinct expression in vivo. In vitro study using HGrC1 shows that TGF-β and TNF-α increase PAI-1 mRNA and its activity, and both together exhibit a synergistic effect. The expression of PAI-1 mRNA is suppressed by simvastatin. Moreover, insulin sensitizing drugs (metformin, pioglitazone and rosiglitazone) suppress LPS-induced TGF-β and TNF-α mRNA expression in PFMC.
Conclusions
Statin and insulin sensitizing drugs may provide a potential therapy for PCOS via down-regulation of PAI-1 expression in GCs and down-regulation of TGF-β and TNF-α expression in PFMC, respectively.
Keywords: PAI-1, Polycystic ovary syndrome, granulosa cell, statin
Introduction
PCOS is a common and complex endocrine disorder with a prevalence of up to 10% in the female population, characterized by anovulation, infertility, hyperandrogenemia and hyperandrogenism1–3. In addition to the hyperandrogenemia and reproductive impacts, metabolic dysfunction is a common feature of PCOS 1, 2. A considerable proportion of women with PCOS display insulin resistance 1, but the actual etiology of PCOS is unknown.
PAI-1 is a molecule which prevents plasminogen activation driven by tissue plasminogen activator (t-PA) and urokinase plasminogen activator (u-PA) 4, 5. In normal ovarian physiology, the plasminogen system regulates the breakdown of the follicular wall during ovulation 5, 6. In the preovulatory follicle, the coordinated expression of the plasminogen system, under the influence of gonadotropins, allows for a window of t-PA excess, which results in the activation of plasminogen to plasmin within the follicular fluid, and ultimately results in controlled proteolytic breakdown of the follicular wall 5, 6. Levels of t-PA peak within the granulosa cells (GCs) of the preovulatory follicle just prior to ovulation, while PAI-1 is at low level, thus leading to selective rupture of the follicle4, 5, 7.
Interestingly, Devin et al. reported that transgenic mice that constitutively express a stable form of human PAI-1 exhibit a hypertrophied theca layer, a characteristic of human PCOS ovaries 8. Moreover, the plasma testosterone concentration was nearly two-fold greater in transgenic female mice than in wild-type mice. The group also reported that ovarian tissues from patients with PCOS exhibit intense staining for PAI-1 in GCs, whereas little PAI-1 is detected in normal ovarian tissues 8.
These data strongly support the idea that over-expressed PAI-1 is a key factor involved in the pathophysiology of PCOS. In accordance with this hypothesis, there are several reports which showed elevated levels of PAI-1 in PCOS 9–13. As PAI-1 is an inhibitor for t-PA which regulates ovulation, higher amounts of PAI-1 may cause the follicle to be refractory to ovulation stimuli, one of the hallmarks of PCOS, although precise mechanisms are still unknown.
Accordingly, a better understanding of PAI-1 regulation in human GC might result in the discovery of new strategies for the treatment of PCOS patients. The purpose of this study was to examine the mechanisms of PAI-1 production using a newly established human non-luteinized GC line, HGrC1 14, and seek to find new strategies to reduce PAI-1 production by GC.
Materials and methods
Reagents and materials
Fetal bovine serum (FBS), DMEM, antibiotics (mixture of penicillin, streptomycin, and amphotericin B) and simvastatin were purchased from Sigma (St. Louis, MO). Recombinant human TNF-α and TGF-β were purchased from R&D Systems (Minneapolis, MN).
Culture of HGrC1cells
HGrC1 cells were cultured with TGF-β (0–300 ng/ml) or TNF-α (0–300 ng/ml) for up to 48 hrs. In some experiments, MAPK inhibitors, PD98059 (ERK inhibitor, 25μM, Calbiochem, San Diego, CA), SB202190 (p38MAPK inhibitor, 10μM, Calbiochem), and SP600125 (JNK inhibitor, 10μM, Calbiochem) and SB431542, (inhibitor of activin receptor-like kinase (ALK)-5, 10μM, Calbiochem) and simvastatin (up to 5 μM) were added 1hr before the treatment with TGF-β and/ or TNF-α 15
Culture of peritoneal fluid mononuclear cells (PFMCs)
To obtain PFMCs, the peritoneal fluid (PF) obtained at surgery were used 16. Briefly, the PFs from benign ovarian tumor patients were layered onto Lymphoprep (Axis-Shield PoC AS. Oslo, Norway) and centrifuged at 1500 rpm for 30 minutes. The PFMCs were recovered from the interface and washed with PBS. Isolated PFMCs were resuspended in Roswell Park Memorial Institute (RPMI)-1640 medium containing 10% fetal bovine serum and plated at a density of 2 × 105 cells/ml in 12-well culture plates. After overnight incubation, media were replaced and the cells were cultured in replenished serum-free media without (control) or with lipopolysaccharides (LPS, 1μg/mL, Sigma, Tokyo) for 8 hrs, followed by quantitative RT-PCR. To examine the effects of insulin sensitizing drugs, metformin (activator of the AMP-activated protein kinase (AMPK), 1mM, Sigma), peroxisome proliferator-activated receptor (PPAR) γ agonist, pioglitazone (10μM, Cayman Chemical) and rosiglitazone (10μM, Cayman Chemical) were added 1hr before the treatment with LPS.
Reverse transcription and quantitative real-time PCR Analysis
Total RNA was extracted from HGrC1, using the ISOGEN II (Nippon Gene, Tokyo, Japan). Reverse transcription was performed using ReverTra Ace qPCR RT Master Mix with gDNA Remover (TOYOBO, Tokyo, Japan). 500 nanogram to one microgram of total RNA was reverse transcribed in a 20μl volume. For the quantification of various mRNA levels, real-time PCR was performed using the Mx3000P Real-Time PCR System (Agilent Technologies, CA, USA), according to the manufacturer’s instructions. PCR primer sets were designed to span introns to discriminate PCR products that might arise from possible chromosomal DNA contaminants. The primer sequences were as follows PAI-1 (NM_00602.4: 546–569 and 737–714), and TGF-β (NM_000660.5: 1305–1326 and 1514–1493), TNF-α (NM_000594.3: 442–461 and 759–740) and GAPDH (NM_002046: 628–648 and 1079–1060). PCR conditions were as follows, PAI-1: 40 cycles of 95º C for 10 sec, 61º C for 10 sec, and 72º C for 9 sec, TGF-β and TNF-α:40 cycles of 95º C for 10 sec, 56º C for 10 sec, and 72º C for 10 sec GAPDH: 40cycles of 95 º C for 10 sec, 64º C for 10 sec, and 72º C for 18 sec. After amplification, melting curve analysis was performed. The relative expression of each mRNA was normalized by GAPDH mRNA.
PAI-1 activity
The activity of PAI-1 in conditioned media was measured using a PAI-1 activity kit (ASSAYPRO, St.Charles, MO). A fixed amount of t-PA was added in excess to the supernatant of cultured HGrC1 cells, which allowed PAI-1 and t-PA to form an inactive complex. The assay measured plasminogen activation by residual t-PA in coupled assays that contain t-PA, plasminogen, and a plasmin-specific synthetic substrate. The amount of plasmin produced was quantitated using a highly specific plasmin substrate releasing a yellow para-nitroaniline (pNA) chromophore. The absorbance of the pNA at 405 nm was inversely proportional to the PAI-1 enzymatic activity. The intra and inter-assay coefficients of variation were 4.7 % and 7.0% respectively, in the assays.
Immunohistochemistry
A total of 6 ovarian sections was used in the study. Normal ovaries (N=3) were from women with regular menstrual cycles. The surgeries were for non-gynecological reasons. None of the women were receiving exogenous hormones. PCOS ovaries (N=3) were obtained from women with chronic anovulation, hyperandrogenism and elevated serum androgen levels. All had undergone laparotomy with the finding of PCOS ovaries confirmed by histological examination. The protocol was approved by the Institutional Review Board at UCSD.
Antigen retrieval was performed using sodium citrate buffer (10mM, pH 6.0). The sections were stained with anti PAI-1 antibody (Molecular Innovations, Southfield, Michigan, dilution 1:2000) or rabbit IgG as negative control using an Envision+ System/HRP rabbit (DAB+) kit (Dako, Japan).
Western blotting
After pre-treatment of control or simvastatin (5 μM), HGrC1 were stimulated with TGF-β (1ng/ml) and/ or TNF-α (5 ng/ml) for 1 hour. Cultured cells were homogenized in the RIPA Buffer ( Wako, JP) containing 0.1% Protease Inhibitor Cocktail (Sigma) and 1% Phosphatase Inhibitor Cocktail 2 (Sigma) and diluted by 2× Laemmli Sample Buffer (Bio-Rad, USA) containing 5% 2-mercaptoethanol. Samples were resolved by 10% SDS-PAGE. Proteins were blotted onto a nitrocellulose membrane and incubated with rabbit antibody to total human p38 MAPK or rabbit antibody to phospho-specific (Thr180/Tyr182) p38 MAPK (1:1000;Cell Signaling, Inc. Beverly, MA) as a primary antibody, and anti-rabbit IgG HRP-linked antibody (1:2000; Cell Signaling, Inc. Beverly, MA) as a secondary antibody. Immune complexes were visualized by use of WesternSu re ECL Substrate (LI-COR).
Statistical analysis
All experiments were repeated at least three times, and all measurements were done in at least duplicate. Data are presented as means±S.E.M. Non-normally distributed data were analyzed by non-parametric tests (Kruskal–Wallis one-way analysis of variance) using JMP software (SAS Institute Inc., Cary, NC). A p-value of less than 0.05 was considered statistically significant.
Results
1. Localization of human PAI-1 antigen within human ovaries
We confirmed the location of PAI-1 accumulation in human ovary specimens. As reported before 8, in normal ovaries, PAI-1 was scantly detected in the healthy follicles (Fig. 1a, 1d), while PAI-1 accumulation was evident within polycystic ovary specimens and localized lining cystic structures (Fig. 1b). PAI-1 expression was observed in cytoplasm of granulosa cells mainly and some of theca cells (Fig. 1c). In normal ovary, healthy follicles with multilayered GC (Fig. 1d, lower) exhibited very low expression of PAI-1 in GCs, while GCs of unhealthy follicles that were thin-layered and partially detached from membrane exhibited distinct PAI-1 expression (Fig.1d, upper). The above mentioned features of PAI-1 expression pattern were observed in all ovarian sections, 3 normal and 3 PCOS ovaries.
Figure 1. Localization of Plasminogen activator inhibitor-1 (PAI-1) expression in human ovaries.
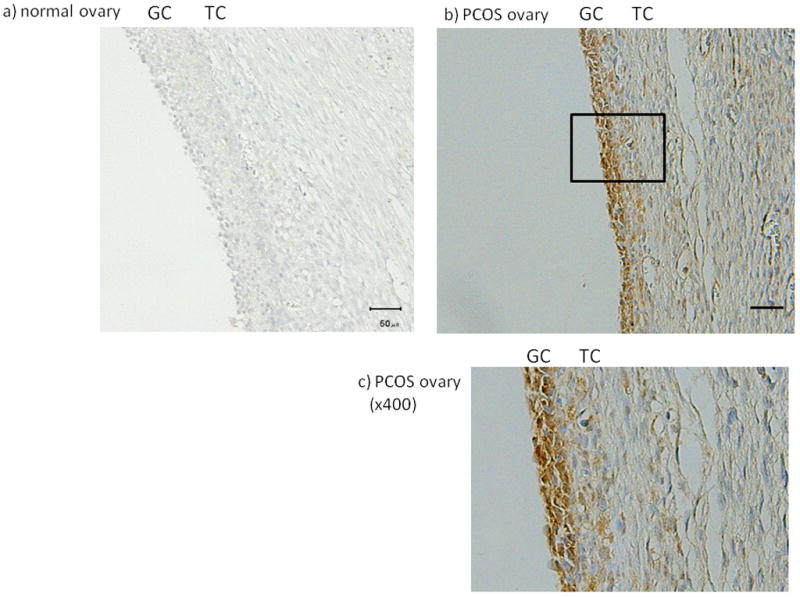
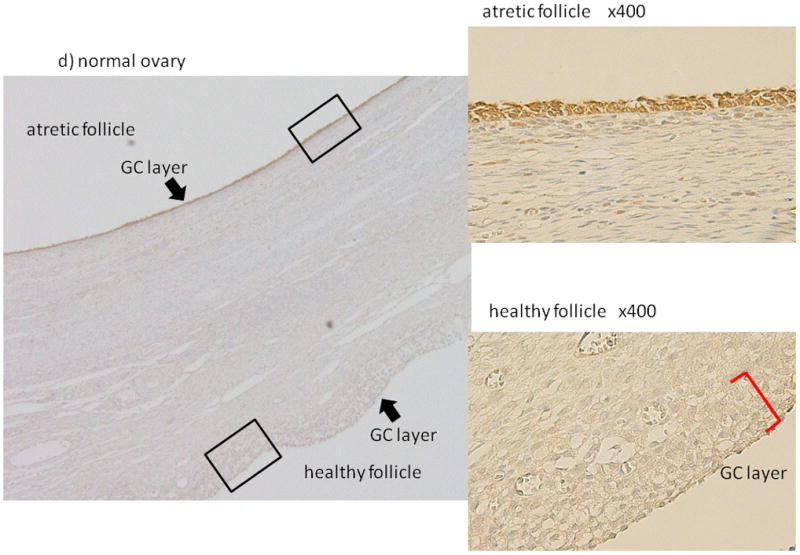
The PAI-1 expression in normal (a, d) and polycystic ovary syndrome (PCOS) human ovaries (b, c) was investigated by immunohistochemistry. (magnification; a, b ×200, c ×400, d ×100). In normal ovary, the PAI-1 expressions in atretic follicle (upper) and healthy follicle (lower) were shown (d). Magnified pictures (×400) of atretic and healthy follicle were also shown. Representative data from 3 normal and 3 PCOS ovaries were shown. GC: granulosa cells, TC: theca cells, bar indicates 50 μm.
2. TGF-β and TNF-α induced PAI-1 mRNA expression and PAI-1 activity in HGrC1 cells
In a pilot study, various stimuli including anti-mullerian hormone (AMH, 100 ng/ml), androstendione (5 nM), luteinizing hormone / human chorionic gonadotropin (LH/hCG, 10 IU/ml), TGF-β and TNF-α, which could be involved in the pathophysiology of PCO, were added to HGrC1 cells for 24 hrs followed by quantitative PCR for PAI-1 mRNA expression. HGrC1 cells are known to respond to androstendione and LH/hCG 14. TGF-β and TNF-α were found to significantly induce PAI-1 mRNA relative expression (Fig. 2-a). In a dose response study, the maximum induction of PAI-1 mRNA was obtained with 1 ng/ml of TGF-β and 5 ng/ml of TNF-α. A time course study demonstrated that TNF-β or TNF-α stimulation from the 3 hr through 24 hr period induced similar levels of PAI-1 mRNA expression (data not shown). Therefore, in subsequent experiments, HGrC1 cells were stimulated with 1 ng/ml of TGF-β and/or 5 ng/ml of TNF-α for 24 hrs. As shown in Fig. 2-a, with TGF-β or TNF-α stimulation, the PAI-1 mRNA expression levels were over 10- fold or 5-fold higher than control, respectively. Moreover, simultaneous addition of TGF-β and TNF-α induced PAI-1 mRNA expression synergistically.
Figure 2. TGF-β and TNF-α induced PAI-1 mRNA expression and PAI-1 activity in a human granulosa cell line (HGrC1).
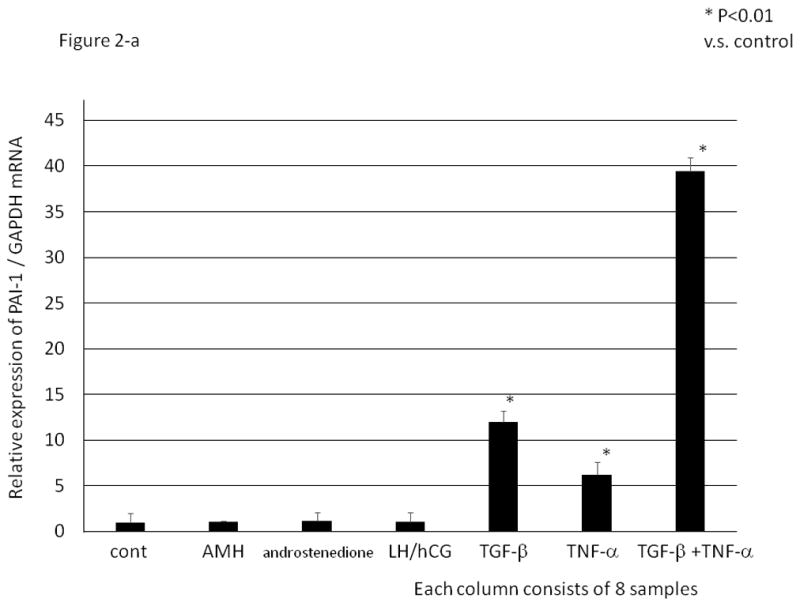
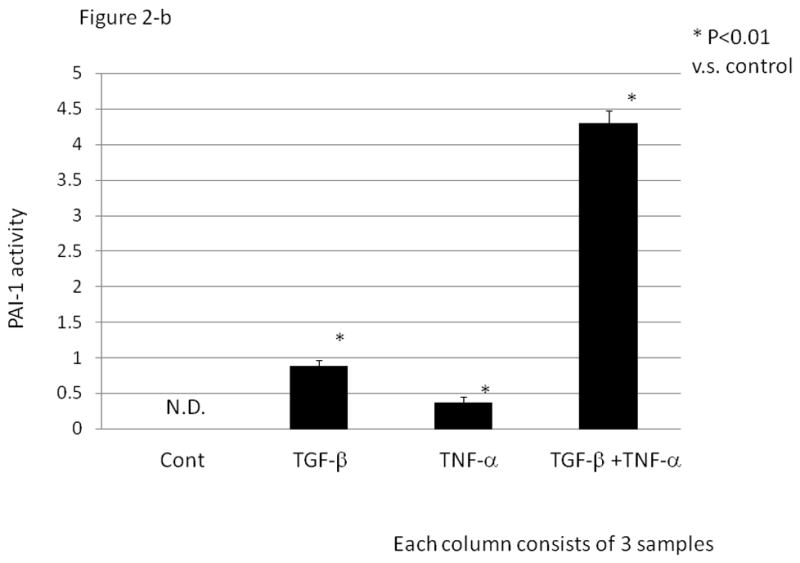
HGrC1 cells were stimulated with anti-mullerian hormone (AMH, 100 ng/ml), androstenedione (5 nM), luteinizing hormone / human chorionic gonadotropin (LH/hCG, 10 IU/ml), TGF-β (1 ng/ml) and/ or TNF-α (5 ng/ml) for 24 hrs. (Fig. 2-a) Total RNA was extracted from the cells and subjected to real-time PCR to determine the PAI-1 mRNA levels. Data were normalized by GAPDH mRNA levels to show the relative abundance. Representative data from three different experiments were shown as the mean ± SD relative to an adjusted value of 1.0 for the mean value of the control. *: P<0.01 (vs. control). (Fig. 2-b) PAI-1 activity (IU/ml) in the supernatant of cultured HGrC1 cells was measured. Representative data from three different experiments were shown as the mean ±S.E.M. N.D.: not detected.
In PAI-1 activity experiments using supernatant of cultured HGrC1 cells (Fig. 2-b), no PAI-1 activity was observed in the absence of TGF-β or TNF-α. In the presence of TGF-β and/or TNF-α, PAI-1 activity was observed in the same tendency as mRNA levels; as with mRNA expression, simultaneous addition of TGF-β and TNF-α resulted in a synergistic effect on PAI-1 activity.
3. p38MAPK inhibitor and ALK-5 inhibitor suppressed TGF-β and/or TNF-α induced PAI-1 mRNA expression
Various inhibitors were used to investigate the signaling pathway of TGF-β or TNF-α-induced PAI-1 expression. As shown in Fig. 3-a, SB431542 (ALK-5 inhibitor) completely abrogated the TGF-β-induced PAI-1 expression. SB202190 (p38MAPK inhibitor), and SP600125 (JNK inhibitor) partially inhibited the TGF-β-induced PAI-1 mRNA expression. On the other hand, SB202190 totally abolished the stimulatory effect of TNF-α on PAI-1 mRNA expression, whereas SP600125 and SB431542 partially suppressed the effect of TNF-α on PAI-1 mRNA expression.
Figure 3. The effect of various inhibitors on TGF-β and/ or TNF –α induced PAI-1 mRNA expression in a human granulosa cell line (HGrC1).
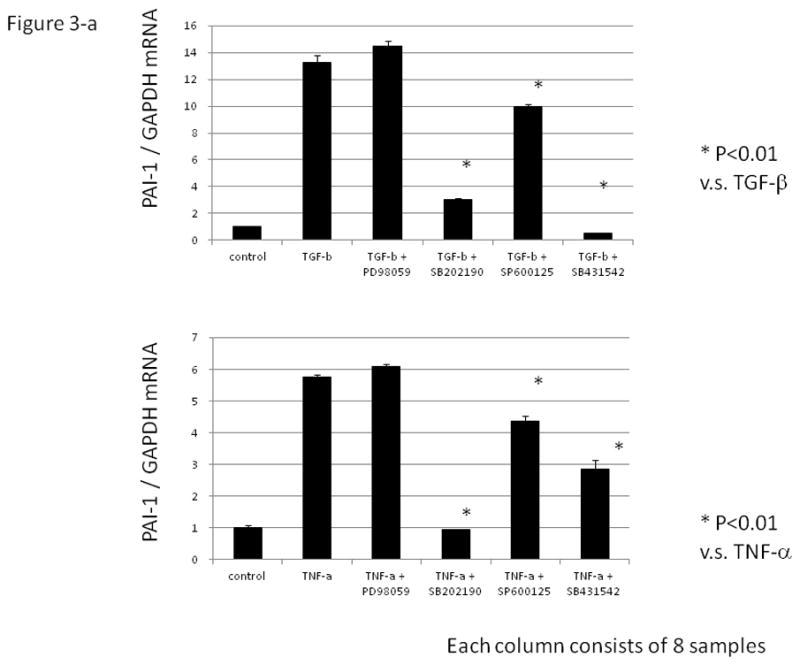
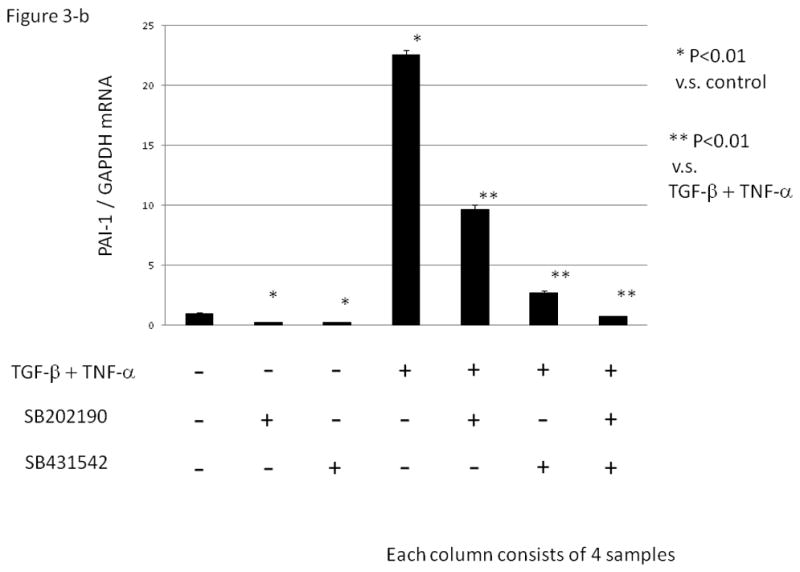
(Fig. 3-a) HGrC1 cells were stimulated with TGF-β (1 ng/ml) or TNF-α (5 ng/ml) for 24 hrs. MAPK inhibitors, PD98059 (ERK inhibitor 25μM), SB202190 (p38MAPK inhibitor, 10μM), and SP600125 (JNK inhibitor, 10 μM) and SB431542, (inhibitor of activin receptor-like kinase (ALK)-5, 10 μM) were added 1hr before the treatment with TGF-β or TNF-α. (Fig. 3-b) HGrC1 cells were stimulated with TGF-β (1 ng/ml) and TNF-α (5 ng/ml) for 24 hrs. SB202190 (p38MAPK inhibitor, 10 μM) and/ or SB431542 (ALK-5 inhibitor, 10μM) were added 1hr before the treatment with TGF-β and TNF-α. Total RNA was extracted from the cells and subjected to real-time PCR to determine the PAI-1 mRNA levels. Data were normalized by GAPDH mRNA levels to show the relative abundance. Representative data from three different experiments were shown as the mean ± S.E.M relative to an adjusted value of 1.0 for the mean value of the control. *: P<0.01 (vs. control), **: P<0.01 (vs. TGF-β and TNF-α).
PAI-1 mRNA expression induced by simultaneous addition of TGF-β and TNF-α was partially suppressed with the addition of either SB202190 or SB431542. A combination of both inhibitors completely abolished the synergistic effect by TGF-β and TNF-α on PAI-1 mRNA expression (Fig. 3-b, P<0.01).
4. Therapeutic agents which suppress PAI-1 expression
We next examined the ability of various agents to suppress PAI-1 expression in HGrC1 cells. As shown in Fig. 4-a, simvastatin (1 or 5 μM) did not suppress basal levels of PAI-1, but significantly suppressed TGF-β/TNF-α-induced PAI-1 mRNA expression. Western blotting analysis showed that TGF-β and TNF-α induced phospho-p38 in a synergistic fashion, which was abolished with pre-treatment of simvastatin (Fig. 4-b).
Figure 4. The effect of simvastatin on TGF-β and/ or TNF –α induced PAI-1 expression in a human granulosa cell line (HGrC1).
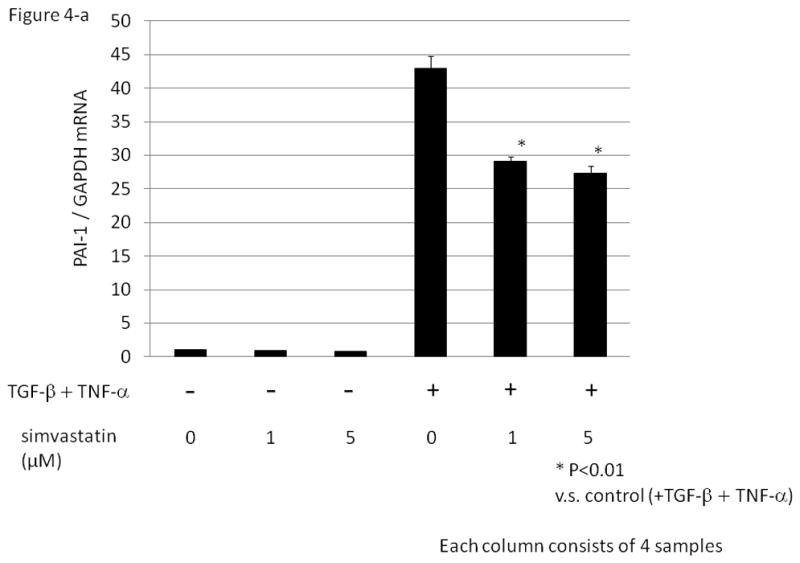
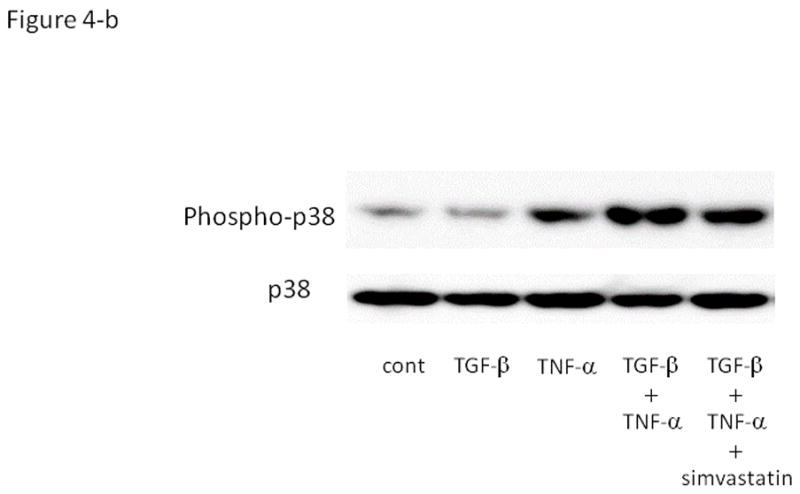
(Fig. 4-a) HGrC1 cells were stimulated with or without TGF-β (1 ng/ml) and TNF-α (5 ng/ml) for 3 hrs. Simvastatin (1 or 5 μM, dissolved in DMSO) or control (DMSO) were added 1hr before the treatment with TGF-β and TNF-α. Total RNA was extracted from the cells and subjected to real-time PCR to determine the PAI-1 mRNA levels. Data were normalized by GAPDH mRNA levels to show the relative abundance. Representative data from three different experiments were shown as the mean ± S.E.M relative to an adjusted value of 1.0 for the mean value of the control. *: P<0.01 (vs. TGF-β and TNF-α). (Fig. 4-b) After pre-treatment of control or simvastatin (5 μM), HGrC1 were stimulated with TGF-β (1 ng/ml) and/ or TNF-α (5 ng/ml) for 1 hr, followed by Western blotting for p38 and phospho-p38 MAPK.
We also examined the effects of insulin sensitizing agents, such as metformin (an activator of AMPK), and the PPAR γ agonists, pioglitazone and rosiglitazone. In HGrC1 cells, all of insulin sensitizing agents failed to suppress basal PAI-1 mRNA expression levels and also had no effect on the mRNA expression induced by simultaneous addition of TGF-β and TNF-α (data not shown).
As TNF-α and TGF-β are reported to be produced in macrophages of PCOS patients 17, 18, we investigated the expression of TGF-β and TNF-α in PFMCs. Insulin sensitizing drugs failed to suppress basal levels of TGF-β and TNF-α, but significantly suppressed LPS-induced TGF-β and TNF-α mRNA expression in PFMCs (Fig.5-a and b).
Figure 5. The effect of insulin sensitizing drugs on TGF-β and TNF-α expression in human peritoneal fluid mononuclear cell (PFMCs).
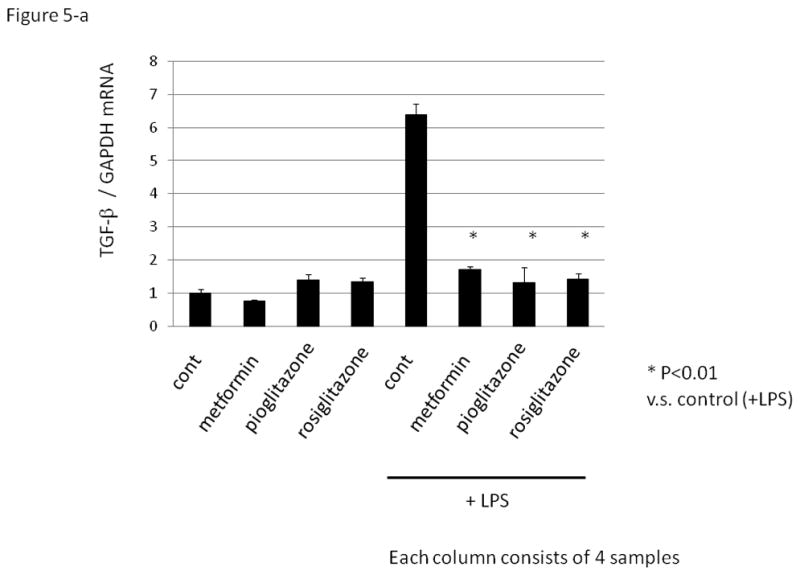
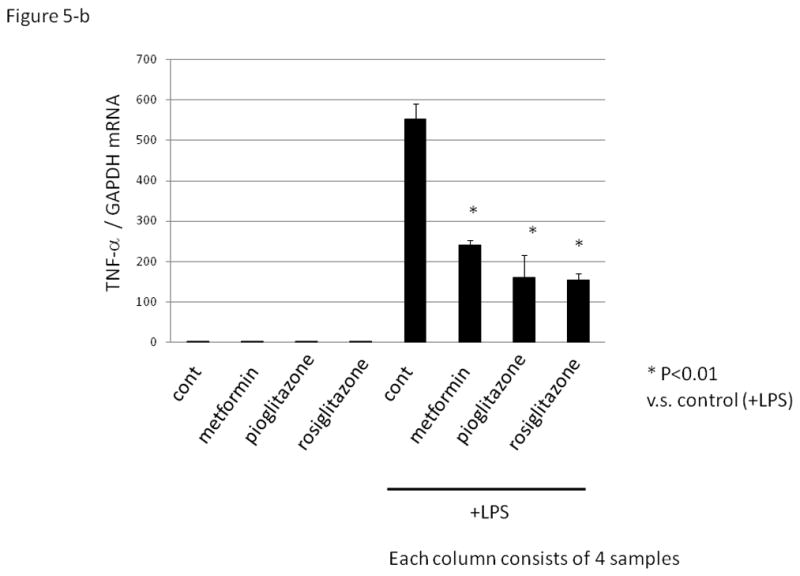
PFMCs were cultured without (control) or with lipopolysaccharides (LPS, 1μg/mL) for 8 hrs followed by quantitative RT-PCR. Insulin sensitizing drugs, metformin (activator of the AMP-activated protein kinase, 1mM), peroxisome proliferator-activated receptor γ agonist; pioglitazone (10μM), and rosiglitazone (10μM) were added 1hr before the treatment with LPS. Total RNA was extracted from the cells and subjected to real-time PCR to determine the expression of TGF-β (Fig. 5a) and TNF-α (Fig. 5b). Data were normalized by GAPDH mRNA levels to show the relative abundance. Representative data from five different experiments were shown as the mean ± S.E.M relative to an adjusted value of 1.0 for the mean value of the each control. *: P<0.01 (vs. LPS-treated control).
Discussion
In the present study, we found that little PAI-1 expression was observed in healthy GCs whereas GCs of PCOS and atretic follicle exhibited distinct expression in vivo (Fig 1). As PAI-1 is a potential factor in the etiology of PCOS, we firstly investigated the regulation of PAI-1 expression in GC using a newly established human GC line, HGrC1, derived from a small antral follicle 14. We firstly clarified that TGF-β and TNF-α induce PAI-1 mRNA expression and activity. PCOS is described as a low-grade chronic inflammatory state mainly triggered by an increase in activated macrophage infiltration in visceral fat 19. It has also been reported that aberrant expression of TGF-β and TNF-α is related to PCOS, and macrophages are known to primarily secrete these cytokines 17, 18, 20, 21.
In the present study, simultaneous addition of TGF-β and TNF-α had a synergistic effect on PAI-1 mRNA expression and activity, which was completely abrogated by a combination of ALK-5 and p38 MAPK inhibitors. Western blotting analysis also revealed that p38 MAPK was involved in this synergistic effect. In rat astrocytes in vitro, p38 and JNK are involved in PAI-1 up-regulation 22. In our experiments, JNK inhibitor (SP600125) also partially suppressed PAI-1 mRNA in HGrC1 cells.
Statins are known to interfere with the rate-limiting step of cholesterol biosynthesis; hence, they are first-line therapies for treatment of hypercholesterolemia. However, statins appear to have pleiotropic effects, including immunomodulation 23 and anti-inflammatory actions 24, which occur independently of their cholesterol-lowering abilities. In the present study, simvastatin significantly suppressed TGF-β/TNF-α-induced PAI-1 expression in HGrC1 cells. Therefore, statins could be used as a potential therapy for PCOS that works by suppressing PAI-1 expression. Although statins are not commonly used for the treatment of PCOS currently, there are other studies demonstrating the efficacy of statins on PCOS in patients, where statins reduced hyperandrogenism significantly 25, 26.
We also examined the effect of metformin, an activator of the AMPK, and the PPAR γ agonists, pioglitazone and rosiglitazone, on PAI-1 mRNA expression, because these medicines are used for the treatment of PCOS, but the actual mechanism of insulin-sensitizing agents has not been fully understood. In our current study, these agents did not regulate basal as well as TGF-β– and TNF-α–stimulated levels of PAI-1 mRNA expression in HGrC1 cells (data not shown). However, insulin sensitizing agents markedly suppressed LPS-induced TGF-β and TNF-α mRNA expression in PFMCs (Fig. 5).
As shown in Fig. 6, we hypothesize that activated macrophages might induce inflammation, leading to increased TNF-α and TGF-β synthesis, which results in the stimulation of PAI-1 expression in GCs via p38- and Smad2/3-mediated signaling, respectively. In mice, increased PAI-1 expression results in polycystic ovaries and a hypertrophied theca layer with elevated testosterone 8. Simvastatin directly suppressed PAI-1 expression in GCs, while insulin sensitizing agents suppressed the production of TNF-α and TGF-β in macrophages, which might further suppress PAI-1 expression in GCs. As statin and insulin sensitizing agents can suppress PAI-1 expression via different mechanisms, a combination of these drugs might be useful for the treatment of PCOS. In fact, a pilot study showed that the concomitant use of metfromin and statin led to a better reduction of hyperandrogenism and atherosclerosis-related factors in PCOS patients 26. Interestingly, Koiou et al. reported that metformin reduces serum PAI-1 levels in PCOS 27. Further study is warranted to determine if elevated PAI-1 levels in GCs would be remedied with statin and insulin sensitizing agents in PCOS patients, effects that were observed in serum of patients with diabetes, metabolic syndrome or PCOS. 27–30.
Figure 6. Schema of the hypothesis.
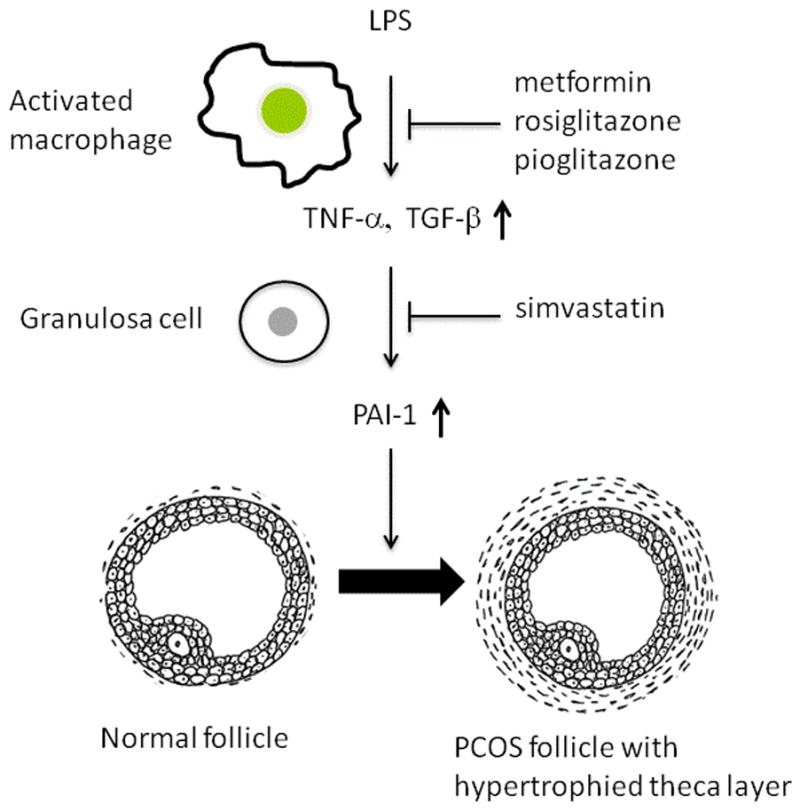
In PCOS, activated macrophages produce TNF-α and TGF-β 17, 18, 20, 21, which up-regulates the expression of PAI-1 in GCs. PAI-1 is a cause of polycystic ovary appearance and hypertrophied theca layer with elevated testosterone 8. Insulin sensitizing agents (metformin, pioglitazone, and rosiglitazone) suppresses TNF-α and TGF-β expression in activated macrophages, which leads to the reduction of PAI-1 expression in GCs. Statin suppresses PAI-1 expression directly in GCs.
Ultimately, the investigation into pharmacological agents that are capable of suppressing p38MAPK or Smad2/3 signaling, or directly antagonizing PAI-1, might result in discovery of potential treatments for PCOS 8. Further study is needed to test this hypothesis.
Acknowledgments
We thank Dr. Heather M. Martinez for her helpful discussion and critical reading of the manuscript. The authors thank Ms. Maiko Mizuguchi for her excellent technical assistance.
Grants
This work was supported by Health and Labor Sciences Research Grants from the Ministry of Health, Labor and Welfare of Japan, Grant-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology. This research was also funded by the National Institute of Child Health and Human Development/NIH through a cooperative agreement (U54 HD012303) as part of the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
Footnotes
Disclosure statement; the authors have nothing to disclose.
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Goodarzi MO, Dumesic DA, Chazenbalk G, Azziz R. Polycystic ovary syndrome: etiology, pathogenesis and diagnosis. Nat Rev Endocrinol. 2011;7:219–231. doi: 10.1038/nrendo.2010.217. [DOI] [PubMed] [Google Scholar]
- 2.Ehrmann DA, Sturis J, Byrne MM, Karrison T, Rosenfield RL, Polonsky KS. Insulin secretory defects in polycystic ovary syndrome. Relationship to insulin sensitivity and family history of non-insulin-dependent diabetes mellitus. J Clin Invest. 1995;96:520–527. doi: 10.1172/JCI118064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El Hayek S, Bitar L, Hamdar LH, Mirza FG, Daoud G. Poly Cystic Ovarian Syndrome: An Updated Overview. Front Physiol. 2016;7:124. doi: 10.3389/fphys.2016.00124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu K, Liu YX, Hu ZY, Zou RY, Chen YJ, Mu XM, Ny T. Temporal expression of urokinase type plasminogen activator, tissue type plasminogen activator, plasminogen activator inhibitor type 1 in rhesus monkey corpus luteum during the luteal maintenance and regression. Mol Cell Endocrinol. 1997;133:109–116. doi: 10.1016/s0303-7207(97)00152-4. [DOI] [PubMed] [Google Scholar]
- 5.Liu YX, Liu XM, Nin LF, Shi L, Chen SR. Serine protease and ovarian paracrine factors in regulation of ovulation. Front Biosci (Landmark Ed) 2013;18:650–664. doi: 10.2741/4128. [DOI] [PubMed] [Google Scholar]
- 6.Yoshimura Y, Maruyama K, Shiraki M, Kawakami S, Fukushima M, Nakamura Y. Prolactin inhibits plasminogen activator activity in the preovulatory follicles. Endocrinology. 1990;126:631–636. doi: 10.1210/endo-126-1-631. [DOI] [PubMed] [Google Scholar]
- 7.Liu K, Brandstrom A, Liu YX, Ny T, Selstam G. Coordinated expression of tissue-type plasminogen activator and plasminogen activator inhibitor type 1 during corpus luteum formation and luteolysis in the adult pseudopregnant rat. Endocrinology. 1996;137:2126–2132. doi: 10.1210/endo.137.5.8612557. [DOI] [PubMed] [Google Scholar]
- 8.Devin JK, Johnson JE, Eren M, Gleaves LA, Bradham WS, Bloodworth JR, Jr, Vaughan DE. Transgenic overexpression of plasminogen activator inhibitor-1 promotes the development of polycystic ovarian changes in female mice. J Mol Endocrinol. 2007;39:9–16. doi: 10.1677/JME-06-0057. [DOI] [PubMed] [Google Scholar]
- 9.Orio F, Jr, Palomba S, Cascella T, Tauchmanova L, Nardo LG, Di Biase S, Labella D, Russo T, Savastano S, Tolino A, Zullo F, Colao A, Lombardi G. Is plasminogen activator inhibitor-1 a cardiovascular risk factor in young women with polycystic ovary syndrome? Reprod Biomed Online. 2004;9:505–510. doi: 10.1016/s1472-6483(10)61634-3. [DOI] [PubMed] [Google Scholar]
- 10.Atiomo WU, Bates SA, Condon JE, Shaw S, West JH, Prentice AG. The plasminogen activator system in women with polycystic ovary syndrome. Fertil Steril. 1998;69:236–241. doi: 10.1016/s0015-0282(97)00486-x. [DOI] [PubMed] [Google Scholar]
- 11.Gonzalez F, Rote NS, Minium J, Kirwan JP. Evidence of proatherogenic inflammation in polycystic ovary syndrome. Metabolism. 2009;58:954–962. doi: 10.1016/j.metabol.2009.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lindholm A, Bixo M, Eliasson M, Hudecova M, Arnadottir R, Holte J, Poromaa IS. Tissue plasminogen activator and plasminogen activator inhibitor 1 in obese and lean patients with polycystic ovary syndrome. Gynecol Endocrinol. 2010;26:743–748. doi: 10.3109/09513590.2010.487592. [DOI] [PubMed] [Google Scholar]
- 13.Polak K, Czyzyk A, Simoncini T, Meczekalski B. New markers of insulin resistance in polycystic ovary syndrome. J Endocrinol Invest. 2016 doi: 10.1007/s40618-016-0523-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bayasula, Iwase A, Kiyono T, Takikawa S, Goto M, Nakamura T, Nagatomo Y, Nakahara T, Kotani T, Kobayashi H, Kondo M, Manabe S, Kikkawa F. Establishment of a human nonluteinized granulosa cell line that transitions from the gonadotropin-independent to the gonadotropin-dependent status. Endocrinology. 2012;153:2851–2860. doi: 10.1210/en.2011-1810. [DOI] [PubMed] [Google Scholar]
- 15.Yoshino O, Osuga Y, Hirota Y, Koga K, Hirata T, Harada M, Morimoto C, Yano T, Nishii O, Tsutsumi O, Taketani Y. Possible pathophysiological roles of mitogen-activated protein kinases (MAPKs) in endometriosis. Am J Reprod Immunol. 2004;52:306–311. doi: 10.1111/j.1600-0897.2004.00231.x. [DOI] [PubMed] [Google Scholar]
- 16.Koga K, Osuga Y, Yoshino O, Hirota Y, Yano T, Tsutsumi O, Taketani Y. Elevated interleukin-16 levels in the peritoneal fluid of women with endometriosis may be a mechanism for inflammatory reactions associated with endometriosis. Fertil Steril. 2005;83:878–882. doi: 10.1016/j.fertnstert.2004.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Raja-Khan N, Urbanek M, Rodgers RJ, Legro RS. The Role of TGF-beta in Polycystic Ovary Syndrome. Reprod Sci. 2013;21:20–31. doi: 10.1177/1933719113485294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Figueroa F, Davicino R, Micalizzi B, Oliveros L, Forneris M. Macrophage secretions modulate the steroidogenesis of polycystic ovary in rats: effect of testosterone on macrophage pro-inflammatory cytokines. Life Sci. 2012;90:733–739. doi: 10.1016/j.lfs.2012.03.019. [DOI] [PubMed] [Google Scholar]
- 19.Alanbay I, Ercan CM, Sakinci M, Coksuer H, Ozturk M, Tapan S. A macrophage activation marker chitotriosidase in women with PCOS: does low-grade chronic inflammation in PCOS relate to PCOS itself or obesity? Arch Gynecol Obstet. 2012;286:1065–1071. doi: 10.1007/s00404-012-2425-0. [DOI] [PubMed] [Google Scholar]
- 20.Raja-Khan N, Kunselman AR, Demers LM, Ewens KG, Spielman RS, Legro RS. A variant in the fibrillin-3 gene is associated with TGF-beta and inhibin B levels in women with polycystic ovary syndrome. Fertil Steril. 2010;94:2916–2919. doi: 10.1016/j.fertnstert.2010.05.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiao J, Feng HL. Extra- and intra-ovarian factors in polycystic ovary syndrome: impact on oocyte maturation and embryo developmental competence. Hum Reprod Update. 2010;17:17–33. doi: 10.1093/humupd/dmq032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nakajima K, Yamamoto S, Tohyama Y, Kohsaka S. Close association of p38 and JNK with plasminogen-dependent upregulation of PAI-1 in rat astrocytes in vitro. Neurosci Lett. 2010;471:66–69. doi: 10.1016/j.neulet.2010.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Danesh FR, Anel RL, Zeng L, Lomasney J, Sahai A, Kanwar YS. Immunomodulatory effects of HMG-CoA reductase inhibitors. Arch Immunol Ther Exp (Warsz) 2003;51:139–148. [PubMed] [Google Scholar]
- 24.Kwak BR, Mulhaupt F, Mach F. Atherosclerosis: anti-inflammatory and immunomodulatory activities of statins. Autoimmun Rev. 2003;2:332–338. doi: 10.1016/s1568-9972(03)00049-1. [DOI] [PubMed] [Google Scholar]
- 25.Gao L, Zhao FL, Li SC. Statin is a reasonable treatment option for patients with Polycystic Ovary Syndrome: a meta-analysis of randomized controlled trials. Exp Clin Endocrinol Diabetes. 2012;120:367–375. doi: 10.1055/s-0032-1304619. [DOI] [PubMed] [Google Scholar]
- 26.Celik O, Acbay O. Effects of metformin plus rosuvastatin on hyperandrogenism in polycystic ovary syndrome patients with hyperlipidemia and impaired glucose tolerance. J Endocrinol Invest. 2012;35:905–910. doi: 10.3275/8371. [DOI] [PubMed] [Google Scholar]
- 27.Koiou E, Tziomalos K, Katsikis I, Delkos D, Tsourdi EA, Panidis D. Disparate effects of pharmacotherapy on plasma plasminogen activator inhibitor-1 levels in women with the polycystic ovary syndrome. Hormones (Athens) 2014;12:559–566. doi: 10.14310/horm.2002.1444. [DOI] [PubMed] [Google Scholar]
- 28.Cefalu WT, Schneider DJ, Carlson HE, Migdal P, Gan Lim L, Izon MP, Kapoor A, Bell-Farrow A, Terry JG, Sobel BE. Effect of combination glipizide GITS/metformin on fibrinolytic and metabolic parameters in poorly controlled type 2 diabetic subjects. Diabetes Care. 2002;25:2123–2128. doi: 10.2337/diacare.25.12.2123. [DOI] [PubMed] [Google Scholar]
- 29.Kruszynska YT, Yu JG, Olefsky JM, Sobel BE. Effects of troglitazone on blood concentrations of plasminogen activator inhibitor 1 in patients with type 2 diabetes and in lean and obese normal subjects. Diabetes. 2000;49:633–639. doi: 10.2337/diabetes.49.4.633. [DOI] [PubMed] [Google Scholar]
- 30.Wang L, Rockwood J, Zak D, Devaraj S, Jialal I. Simvastatin reduces circulating plasminogen activator inhibitor 1 activity in volunteers with the metabolic syndrome. Metab Syndr Relat Disord. 2008;6:149–152. doi: 10.1089/met.2008.0012. [DOI] [PMC free article] [PubMed] [Google Scholar]


