Abstract
Problem
New HIV infections in women are predominantly spread through sexual intercourse. Recent non-human primate studies demonstrated that SIV deposited in the vagina infected immune cells in the ovary. Whether immune cells in the human ovary are susceptible to HIV infection is unknown.
Method of study
Immune cells were isolated from ovaries and characterized by flow cytometry. Cells were exposed to HIV for 2hr. HIV infection was measured by flow cytometry and p24 secretion following 6 days in culture.
Results
CD4+ T cells and CD14+ cells are present in the ovary and susceptible to infection by HIV-BaL. Among the CD45+ cells present, 30% were CD3+ T cells (with similar proportions of CD4+ or CD8+ T cells), and 7–10% were CD14+ cells. Both CD4+ T cells and CD14+ cells were productively infected and supported replication.
Conclusion
Immune cells in the ovary are potential targets for HIV infection.
Keywords: CD4+ T cells, CD14+ cells, female reproductive tract, HIV infection, human ovary, immune cells
Introduction
As of 2015, 36.7 million people were living with HIV, approximately half of which are women.1 The majority of women diagnosed with HIV worldwide contract the virus through genital intercourse with an infected partner.2 Previous studies and trials have focused on the lower female reproductive tract (FRT) as the initial site of infection based on intravaginal simian immunodeficiency virus (SIV) infections in non-human primates.3 These studies presented evidence that CD4+ T cells in the endocervix and ectocervix were the first cells infected.4, 5
More recently, analysis of the FRT in its entirety after vaginal inoculation of non-human primates demonstrated that multiple portals of entry exist.6 In studies designed to identify early events of viral transmission1 Hope and colleagues demonstrated that vaginally deposited SIV rapidly disseminates throughout the lower and upper FRT. Using a dual reporter system, these studies unexpectedly demonstrated that the ovary was the second most frequent site of viral detection, after vagina and ectocervix.6, 7 In addition to the detection of viral RNA in the ovary following vaginal inoculation of macaques, studies by Barouch and colleagues characterized the immune responses to the virus throughout the FRT.8 Their studies demonstrated that vaginal inoculation with SIVmac251 triggers proinflammatory responses including the expression of NLRX1, which inhibits antiviral responses, and activation of the TGFβ pathway.9–12 These specific host mechanisms suppress the generation of antiviral innate and adaptive immune responses at multiple sites, including the FRT, within the first few days of infection.8
While non-human primate studies are extremely informative, what remains to be determined is whether immune cells present in the human ovary are capable of being infected by HIV. Previous studies have shown that the number of potential HIV-target cells in the ovary, such as CD4+ T cells and macrophages, varies with anatomical location within the ovary and stage of ovarian cycle.13–16
However, to the best of our knowledge, nothing is known about the susceptibility of these cells to HIV infection. In previous studies, we observed that immune cells display differential susceptibility to HIV infection between FRT sites and within each tissue.17 For example, we found that CD4+ T cells from the ectocervix were very susceptible to infection by CCR5 strains of HIV, while CD4+ T cells from the endometrium were resistant to infection in vitro. When CD4+ T cell phenotype from each site was analyzed, we found that CD4+ T helper 17 (Th17) cells expressed the highest levels of CCR5 and were the most susceptible to in vitro infection. Importantly, Th17 cells were very low or absent in the endometrium compared with the cervix and ectocervix in premenopausal women.17 Overall, these studies demonstrate that FRT immune cells are differentially susceptible to HIV infection depending upon their anatomical location and menstrual status.
In this study, we evaluated the immune cell populations in the human ovary and determined if these cells were susceptible to HIV infection in vitro. We show that populations of CD4+ T cells and CD14+ cells are present in the human ovary and susceptible to infection by CCR5- tropic HIV. These findings demonstrate that the human ovary, as suggested form non-human primate studies, is a site for potential transmission of HIV into women.
Materials and Methods
Study Subjects
Ovarian tissues were obtained from 8 women undergoing hysterectomy surgery at Dartmouth-Hitchcock Medical Center (Lebanon, NH). Indications for surgery were benign conditions and all tissues used were distal from the sites of pathology and were determined to be unaffected with disease upon inspection by a pathologist. Menopausal status was determined by a pathologist based on the histological evaluation of sections of the endometrial dating. Postmenopausal status was characterized by an atrophic endometrium. Age, menstrual stage and surgical indication of each patient are shown in Table 1. All investigations were conducted according to the principles expressed in the Declaration of Helsinki and carried out with the approval from the Committee for the Protection of Human Subjects (CPHS), Dartmouth Hitchcock Medical Center, and with written informed consent obtained from the patients before surgery.
Table 1.
Characteristics of the patients of the study
| Premenopausal | Postmenopausal | |
|---|---|---|
| Number of donors | 1 | 7 |
| Age average (range) | 31 | 54.6 (45–71) |
| Menstrual stage | ||
| Secretory | 100% (1/1) | |
| Atrophic | 100% (7/7) | |
| Surgical indication | ||
| Adenomysis | 100% (1/1) | |
| Fibroids | 28.6% (2/7) | |
| Prolapse | 71.4% (5/7) |
Tissue processing
Ovarian tissues were processed to obtain a mixed cell suspension as described previously,17 using 0.05% collagenase type IV (Sigma-Aldrich, St. Louis, MO) and 0.01% DNAse (Worthington Biochemical, Lakewood, NJ). Average tissue weight obtained was 1.6±1.0g. After tissue digestion, cells were filtered through a 20μm mesh screen (Small Parts) to separate stromal cells from debris. Stromal cells were washed, erythrocytes lysed, and dead cells removed using the Dead cell removal kit (Miltenyi Biotec, San Diego, CA) according to manufacturer instructions. The resulting mixed cell suspension, consisting of immune cells and stromal fibroblasts, was used for all analysis.
Flow cytometry
Prior to HIV infection, mixed cell suspensions were stained for surface markers with combinations of the following mouse anti-human antibodies: CD45-VioletFluor450, CD8-FITC (Tonbo, San Diego, CA), CD3-APC (BioLegend, San Diego, CA), CD4-PE, CD14-e780 (eBiosciences, San Diego, CA). After infection (6 days), cells were stained for surface markers by using same antibody panel above excluded CD8-FITC. Analysis was performed on MACSQuant flow cytometer (Miltenyi Biotec) using MACSQuantify software and data were analyzed with FlowJo software (Tree Star, Inc., Ashland, OR). Expression of surface markers was measured by the percentage of positive cells.
Leukocyte Contamination
Potential leukocyte contamination from peripheral blood of ovarian tissues was assessed as described before.18 Briefly, erythrocytes and non-erythrocytes present in the mixed cell suspension were counted in a hemocytometer chamber prior to lysing red blood cells. After lysis, we then determined via appropriate flow cytometric gating procedure what proportion of the total number of dispersed non-erythrocytes were leukocytes (CD45+). Given that erythrocytes outnumber leukocytes by a factor of 1000 in an average sample of peripheral blood, we were able to estimate what proportion of the total number of leukocytes in our mixed cell suspensions might have been derived from peripheral blood contamination of the tissues being studied. We determined that in n=8 donor tissue preparations, contaminating peripheral blood leukocytes represented only approximately 1.92±0.75% in ovarian cell samples (data not shown). Every tissue was used in HIV infection experiments, levels of blood leukocytes contamination were lower than the infection levels measured.
Viruses
The replication-competent GFP-encoding infectious molecular clone (IMC), pNLENG1i-BaL.ecto,19 was derived from pNLENG1-ires20 to express heterologous BaL env gene sequences in an isogenic backbone following the strategy previously described.21, 22 Such reporter viruses, collectively referred to as Env-IMC-GFP, expresses GFP upon infection of HIV-1 susceptible target cells.19, 23 Throughout the text we refer to this GFP-reporter virus as “HIV-GFP-BaL”.
HIV-infection
Mixed cells suspensions were exposed to HIV-GFP-BaL for 2hr at a MOI=1 in Xvivo 15 without Phenol Red (Invitrogen, Grand Island, NY) supplemented with 10% charcoral dextran-stripped human serum (Valley Biomedical, Winchester, VA), and then washed to remove residual virus. Uninfected controls were incubated with medium without virus for the same amount of time. After incubation, cells were plated in round bottom ultra-low attachment 96-well plates (Corning, Corning, NY) at 200,000cells/well. Cell cultures were maintained for 6 days, with half of the media in each collected and replaced with fresh media on days 2 and 4. At the end of the infection time, cells were washed, stained for surface markers as indicated, and levels of GFP expression measured by flow cytometry. Additionally, p24 released into the culture media was measured by p24 enzyme-linked immunosorbent assay (Advanced Bioscience Laboratories, Rockville, MD) following the manufacturer’s recommendations. Sensitivity of this assay is 3.1pg/ml.
As a control to prove that p24 corresponds to de novo infection and not residual viral inoculum, control cells from each patient were incubated with Zidovudine (AZT; 10uM) (AIDS Research and Reference Reagent program, Division of AIDS, NIAID, NIH) for 15 min prior to HIV challenge, AZT was present throughout the post-infection period. In previous studies of primary immune cells,23 we established that AZT was not cytotoxic at this concentration by Trypan blue exclusion (Trypan Blue Solution, HyClone, Inc; Logan, UT) at the end of the infection period.
Statistics
Data analysis was performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). A two-sided P value <0.05 was considered statistically significant. Comparison of two groups was performed using the non-parametric Wilcoxon matched-pairs signed rank test. Comparison of three groups was performed using Kruskal-Wallis, followed by Dunns post-test to correct for multiple comparisons. Comparison of HIV infections in the absence vs. presence of AZT overtime was analyzed using two-way ANOVA with Bonferroni post-test for multiple comparison correction.
Results
CD4+ T cells and CD14+ cells are present in the ovary
Stromal cells in the ovary consist of a heterogeneous population of immune and non-immune cells that contribute to the cyclic changes that control ovulation and prepare the female reproductive tract for fertilization, implantation and pregnancy.24 To characterize tissue resident immune cells in the ovary, as detailed in Methods, mixed cell suspensions from digested ovarian tissues were analyzed by flow cytometry. The gating strategy is shown in Fig. 1 for a representative donor. CD45+ immune cells can be detected (Fig. 1a), with CD3+ T cells (Fig. 1b) and CD14+ cells (Fig. 1c) present in significant numbers. Within CD3+ T cells, both CD4+ T cells (Fig. 1d) and CD8+ T cells (Fig. 1e) were readily measurable.
Fig. 1. Representative flow cytometry of CD3+ T cells and CD14+ cells in the ovary.
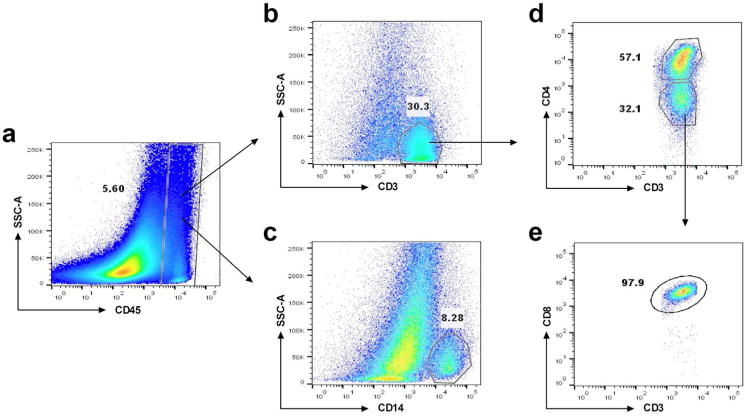
Dot plots showing the gating strategy to select cell populations. (a) CD45+ cells; (b) CD3+ cells; (c) CD14+ T cells; (d) CD3+CD4+ and CD3+CD4− T cells; (e) CD3+CD8+ T cells.
The yield of CD45+ cells in mixed cell suspensions derived from ovaries of 8 women ranged from 2–20% of the total cells present, with a mean value of 12% (Fig. 2a). As seen in Fig. 2b, this proportion corresponded to an average number of 2.9×105 CD45+ cells per gram of tissue.
Fig. 2. Yield of CD45+ cells from the ovary.
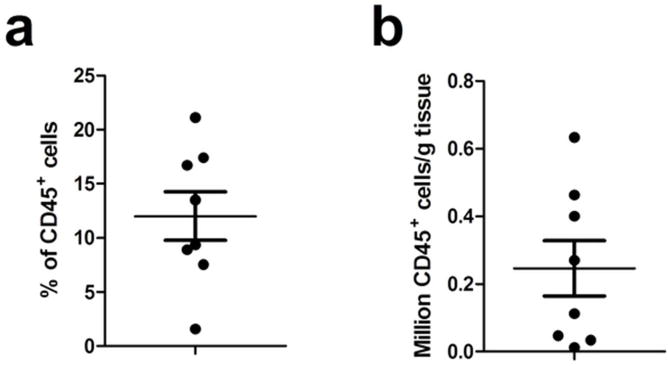
Flow cytometric analysis of CD45+ cells in mixed cells suspension from ovarian tissue (n=8). Data are expressed as (a) % CD45+ cells in the mixed cell population and (b) CD45+ cells per g of tissue. Each dot represents a single patient. Horizontal lines represent the mean ± SEM. Average weight of tissue received was 1.6±1.0g.
We further defined the relative distribution of immune cells in ovarian tissues by flow cytometry and measured the percentage of CD3+ and CD14+ cells after gating on CD45+ cells. As shown in Fig. 3a, the number of CD3+ T cells in ovarian tissues was significantly greater (P<0.05) than the number of CD14+ cells. Among CD3+ cells, CD4+ and CD8+ T cells were equally distributed (Fig. 3b)
Fig. 3. Distribution of CD3+ T cells and CD14+ cells in the ovary.
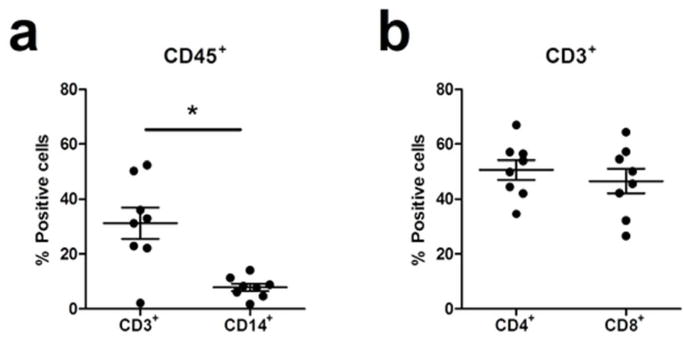
(a) Flow cytometric analysis of the percentage of CD3+ or CD14+ cells after gating on CD45+ cells and (b) CD4+ or CD8+ T cells after gating on CD3+ cells in ovarian tissues (n=8). Each dot represents a single patient. Horizontal lines represent the mean ± SEM. *, p<0.05.
HIV infects immune cells from the ovary
To determine whether immune cells from the ovary are susceptible to HIV infection and replication, mixed cell suspensions prepared from ovaries from 4 donors were incubated with HIV-GFP-BaL (MOI=1) for 2hr, after which cells were thoroughly washed to remove free virus and incubated for 6 days. De novo produced, released p24 was measured at days 2, 4 and 6 by ELISA (Fig. 4), and productive infection also assessed at the end of the infection period by GFP expression (Fig. 5). As seen in Fig. 4a, relative to 2 days post infection, the amount of secreted p24 increased significantly both at days 4 and 6. We interpret this to be the result of active viral replication since Zidovudine (AZT; 10μM), a known inhibitor of HIV’s reverse transcriptase, blocked the increase of p24 in the culture medium above the residual input over the course of the culture (Fig. 4a). As seen in Fig. 4b, HIV productively infected and replicated in mixed cell suspensions in 4 out of 4 cell preparations analyzed, and in all cases, AZT addition kept p24 levels at background over the course of the incubation.
Fig. 4. Susceptibility of ovarian immune cells to HIV infection.
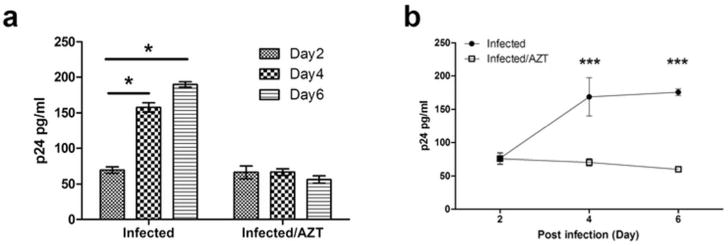
Isolated mixed cell suspensions from ovarian tissues were inoculated with HIV-GFP-BaL in the presence or absence of Zidovudine (AZT; 10μM). Levels of p24 released into the culture media at 2, 4 and 6 days following infection were measured by p24 enzyme-linked immunosorbent assay. “Infected/AZT” indicated background from viral input control. (a) Representative example of released p24 levels in the culture media. The bar represents the mean and Standard Error from triplicate cultures. *, p<0.05. One-way ANOVA (Kruskal-Wallis with Dunns post-test) for infection without AZT. (b) Released p24 levels in the culture media from 4 individual patients. Horizontal lines represent the mean ± SEM. ***, p<0.001. Two-way ANOVA for infection with AZT absence vs. presence.
Fig. 5. Susceptibility of CD4+ T cells and CD14+ cells from ovarian tissue to HIV infection.
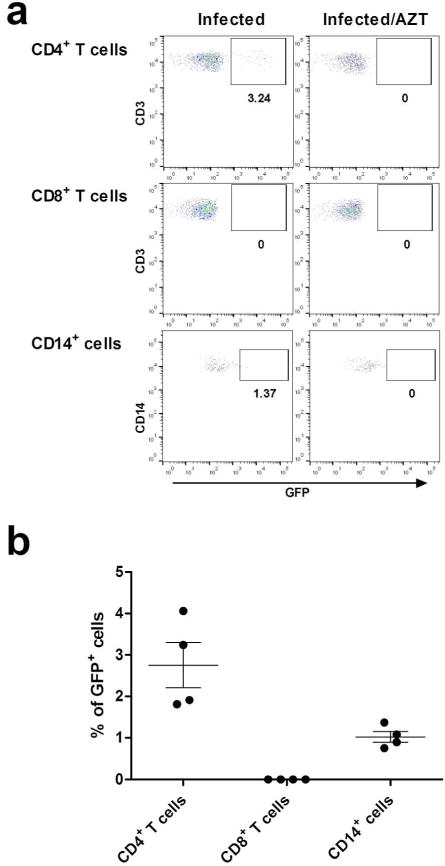
Mixed cells suspensions from ovarian tissues (n=4) were incubated with HIV-GFP-BaL either alone or in the presence of Zidovudine (AZT; 10μM). “Infected/AZT” indicated background from viral input control. (a) Representative experiment showing percent of GFP positive cells after 6 days of infection with HIV-GFP-BaL in CD4+ T cells, CD8+ T cells and CD14+ cells. (b) Percentage of GFP positive cells in CD4+ T cells, CD8+ T cells and CD14+ cells 6 days after in vitro HIV infection (n=4). Each dark circle represents a different patient. The mean and SEM are shown.
Ovarian CD4+ T cells and CD14+ cells are susceptible to HIV-infection
We next tested the hypothesis that both CD4+ T cells and CD14+ cells from the ovary are susceptible to R5-tropic HIV-infection. The same mixed cell suspensions infected in vitro with HIV-GFP-BaL reporter virus in the presence or absence of AZT and described above (Fig. 4), were assessed by flow cytometry for GFP expression, which occurs from integrated provirus after productive infection. As seen in Fig. 5a, HIV infected both CD4+ T cells and CD14+ cells. In contrast, no evidence of infection was observed when cells were pre-treated with AZT. Moreover, analysis of CD8+ T cells, as an internal control, failed to provide any evidence of infection. Interestingly, as seen in Fig. 5b, in mixed cell suspensions from the ovaries of all 4 donors, CD4+ T cells became infected at approximately 2–3 times the rate of CD14+ cells; while we were only able to conduct the experiments with one HIV-1 strain, encoding the Env of R5 reference strain, BaL, this may indicate that ovarian tissue CD4+ T cells are more susceptible to infection than CD14+ cells.
In summary, our studies extend the non-human primate findings6, 7 by demonstrating that human ovarian tissues from pre- and post-menopausal women contain both T cells and CD14+ cells that are susceptible to infection by, and support replication of, prototypic R5 virus.
Discussion
The presence of HIV-1 target cells in the human ovary and their possible roles in sexual acquisition of HIV takes on new importance with the recognition that SIV infects ovarian immune cells in the non-human primate.6, 8 In the present study we examined the presence, abundance, and susceptibility to HIV-infection of CD4+ T cells and CD14+ cells from the ovaries of pre- and postmenopausal women. We found in 8 out of 8 patients that irrespective of menopausal status, CD4+ T cells in general were more prominent than CD14+ cells in individual tissues. Additionally, we found that when mixed cell suspensions were exposed to R5 tropic, GFP-encoding replication competent reporter virus, HIV-GFP-BaL, both CD4+ T cells and CD14+ cells were susceptible to HIV infection in vitro, as measured by flow cytometry and de novo p24 secretion. This study extends the findings in non-human primates by demonstrating that immune cells in the human ovary are potential targets for HIV infection, and that microbicide and vaccine studies designed to prevent the sexual transmission of HIV need to include the entire FRT.
A common concern when working with ovarian tissues is possible contamination with blood leukocytes. In this study, we determined possible blood contamination for each tissue studied. For each sample, the calculated proportion of blood contaminating leukocytes was lower than the percentages of infected cells obtained. In addition, our calculations measured total leukocytes, which includes neutrophils, CD8+ T cells, B cells and NK cells, therefore the actual percentage of contaminating HIV-target cells (CD4+ T cells and monocytes) would be much lower than our calculated numbers. Furthermore, it is well known that resting blood CD4+ T cells are very resistant to infection. Overall, this argues against blood contaminating cells as the main source of infected cells.
Due to the small number of cells available, further characterization of the CD14+ population was not possible. We recently demonstrated that CD14+ cells in the endometrium, endocervix and ectocervix represent a mixed of classical DC (CD1c+CD14+) and monocyte-derived cells25 with the ability to rapidly capture HIV. In addition, CD14 is also a marker for tissue macrophages26, 27 and endometrial macrophages were shown to be the main source of p24 after 11 days in culture.26 Therefore, identification of the populations of DCs and macrophages present in the ovary and whether they each is a major source for viral replication at later stages of the infection remains to be determined.
Early studies in non-human primates reported that the cervix is the first site of detectable SIV-infection.4, 5 More recently, detailed analyses following intravaginal deposition of SIV have extended these findings to demonstrate that in the non-human primate, the entire tract including the ovary are potential sites of viral infection.6 Our results demonstrate that immune cells in the human ovary can be productively infected by HIV. Moreover, we demonstrate that both ovarian CD4+ T cells and CD14+ cells are susceptible to HIV infection.
Just how infection levels detected in human ovarian tissues compare to other sites in the FRT remains to be determined. Due to the difficulty in obtaining ovarian tissues, we were not able to perform side by side comparisons with other FRT tissues to discern possible differences. However, based on our previous studies on HIV infection of immune cells from endometrial, endocervical and ectocervical tissues, our findings with ovarian tissues suggest that they are more susceptible to infection than cells from the endometrium, but less susceptible than the endocervix and ectocervix. However, different between sites cannot exclude the reality that HIV infection conditions used in the present study were different from our previous study.17 Nevertheless, similar differential distribution was also observed in non-human primates.6
Common misconception is that the upper tract (uterus and Fallopian tubes) is sterile and protected from pathogens that enter the lower FRT (ectocervix and vagina). In reality, the upper FRT is continuously exposed to commensals and pathogens, present in the lower FRT.28–30 Others have shown that labeled-albumin microspheres and dyes as well as sperm enter the uterus and Fallopian tubes within minutes of placement in the vagina.31–34 Because HIV in the FRT can be cell-free, cell-associated, and attached to sperm,35, 36 HIV is likely disseminated throughout the entire human FRT within minutes of deposition in the vagina. The recent studies6, 8 demonstrating that SIV placed in the vagina reaches the ovaries within 1–2 days post exposure strongly suggest that the same is true for the human FRT. Our studies extend these important findings to the human by demonstrating that ovaries of pre- and post-menopausal women contain immune cells that can be infected by HIV.
Less clear is the pathway through which ovarian cells become infected. One likely pathway is through the reproductive tract, similar to the pathway through which sperm reach the Fallopian tubes. However, given that the ovary is encapsulated, then direct exposure of immune cells to virus would be restricted to a short time interval when ovulation occurs and underlying cells are exposed. Whether alternative pathways for infection of ovarian immune cells exist (e.g. through blood or lymphatic vessels) remains to be determined.
Our findings have important implications for the development of anti-HIV strategies to prevent infection. The recognition that immune cells in the human ovary, distant from the site of viral deposition in the vagina, are vulnerable to HIV-infection, suggests that a detailed analysis of antiretroviral (ARV) dissemination throughout the FRT, including the ovary, is needed for the development of intravaginal microbicides as Pre-exposure Prophylaxis (PrEP), to ensure that protective levels are reached and sustained. Beyond the protective effects of ARVs, the recognition that the human ovary is a target site for HIV infection also has implications for studies designed to test the effectiveness of vaccines as well as antibodies, which need to ensure protection of the entire FRT to prevent HIV acquisition in women.
Acknowledgments
Study supported by NIH grants AI102838 and AI117739 (CRW). We thank all study participants, Pathologists, Obstetrics and Gynecology surgeons, operating room nurses and support personnel at Dartmouth-Hitchcock Medical Center. Flow Cytometry studies were carried out in DartLab, the Immune Monitoring and Flow Cytometry Shared Resource. The Shared Resource is supported in part by a Norris Cotton Cancer Center Support Grant (P30CA023108-36) and an Immunology COBRE Grant (P30GM103415-14) from the National Institute of General Medical Sciences. Contributions by CO were supported through access to services from the Birmingham Center for AIDS Research (CFAR) Virology and Sequencing Cores at the University of Alabama at Birmingham (NIH/NIAID P30 AI27767).
Footnotes
Conceived and designed the experiments: ZS, MRG, CRW. Performed the experiments and analyzed the data: ZS, MRG. Contributed materials, reviewed the paper and provided comments: CO. Wrote the paper: ZS, MRG, CRW.
References
- 1.JUNPo HIV/AIDS, editor. UNAIDS. Global AIDS Update. Geneva: UNAIDS; 2016. [Google Scholar]
- 2.UNAIDS. UNAIDS report on the global AIDS. 2013. [Google Scholar]
- 3.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annual review of medicine. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 4.Li Q, Estes JD, Schlievert PM, Duan L, Brosnahan AJ, Southern PJ, Reilly CS, Peterson ML, Schultz-Darken N, Brunner KG, Nephew KR, Pambuccian S, Lifson JD, Carlis JV, Haase AT. Glycerol monolaurate prevents mucosal SIV transmission. Nature. 2009;458:1034–1038. doi: 10.1038/nature07831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang Z, Schuler T, Zupancic M, Wietgrefe S, Staskus KA, Reimann KA, Reinhart TA, Rogan M, Cavert W, Miller CJ, Veazey RS, Notermans D, Little S, Danner SA, Richman DD, Havlir D, Wong J, Jordan HL, Schacker TW, Racz P, Tenner-Racz K, Letvin NL, Wolinsky S, Haase AT. Sexual transmission and propagation of SIV and HIV in resting and activated CD4+ T cells. Science. 1999;286:1353–1357. doi: 10.1126/science.286.5443.1353. [DOI] [PubMed] [Google Scholar]
- 6.Stieh DJ, Maric D, Kelley ZL, Anderson MR, Hattaway HZ, Beilfuss BA, Rothwangl KB, Veazey RS, Hope TJ. Vaginal challenge with an SIV-based dual reporter system reveals that infection can occur throughout the upper and lower female reproductive tract. PLoS pathogens. 2014;10:e1004440. doi: 10.1371/journal.ppat.1004440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stieh DJ, Matias E, Xu H, Fought AJ, Blanchard JL, Marx PA, Veazey RS, Hope TJ. Th17 Cells Are Preferentially Infected Very Early after Vaginal Transmission of SIV in Macaques. Cell host & microbe. 2016;19:529–540. doi: 10.1016/j.chom.2016.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barouch DH, Ghneim K, Bosche WJ, Li Y, Berkemeier B, Hull M, Bhattacharyya S, Cameron M, Liu J, Smith K, Borducchi E, Cabral C, Peter L, Brinkman A, Shetty M, Li H, Gittens C, Baker C, Wagner W, Lewis MG, Colantonio A, Kang HJ, Li W, Lifson JD, Piatak M, Jr, Sekaly RP. Rapid Inflammasome Activation following Mucosal SIV Infection of Rhesus Monkeys. Cell. 2016;165:656–667. doi: 10.1016/j.cell.2016.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Allen IC, Moore CB, Schneider M, Lei Y, Davis BK, Scull MA, Gris D, Roney KE, Zimmermann AG, Bowzard JB, Ranjan P, Monroe KM, Pickles RJ, Sambhara S, Ting JP. NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-kappaB signaling pathways. Immunity. 2011;34:854–865. doi: 10.1016/j.immuni.2011.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Moore CB, Bergstralh DT, Duncan JA, Lei Y, Morrison TE, Zimmermann AG, Accavitti-Loper MA, Madden VJ, Sun L, Ye Z, Lich JD, Heise MT, Chen Z, Ting JP. NLRX1 is a regulator of mitochondrial antiviral immunity. Nature. 2008;451:573–577. doi: 10.1038/nature06501. [DOI] [PubMed] [Google Scholar]
- 11.Guo H, Konig R, Deng M, Riess M, Mo J, Zhang L, Petrucelli A, Yoh SM, Barefoot B, Samo M, Sempowski GD, Zhang A, Colberg-Poley AM, Feng H, Lemon SM, Liu Y, Zhang Y, Wen H, Zhang Z, Damania B, Tsao LC, Wang Q, Su L, Duncan JA, Chanda SK, Ting JP. NLRX1 Sequesters STING to Negatively Regulate the Interferon Response, Thereby Facilitating the Replication of HIV-1 and DNA Viruses. Cell host & microbe. 2016;19:515–528. doi: 10.1016/j.chom.2016.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen ML, Pittet MJ, Gorelik L, Flavell RA, Weissleder R, von Boehmer H, Khazaie K. Regulatory T cells suppress tumor-specific CD8 T cell cytotoxicity through TGF-beta signals in vivo. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:419–424. doi: 10.1073/pnas.0408197102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Best CL, Pudney J, Welch WR, Burger N, Hill JA. Localization and characterization of white blood cell populations within the human ovary throughout the menstrual cycle and menopause. Human reproduction. 1996;11:790–797. doi: 10.1093/oxfordjournals.humrep.a019256. [DOI] [PubMed] [Google Scholar]
- 14.Castro A, Castro O, Troncoso JL, Kohen P, Simon C, Vega M, Devoto L. Luteal leukocytes are modulators of the steroidogenic process of human mid-luteal cells. Human reproduction. 1998;13:1584–1589. doi: 10.1093/humrep/13.6.1584. [DOI] [PubMed] [Google Scholar]
- 15.Brannstrom M, Pascoe V, Norman RJ, McClure N. Localization of leukocyte subsets in the follicle wall and in the corpus luteum throughout the human menstrual cycle. Fertility and sterility. 1994;61:488–495. [PubMed] [Google Scholar]
- 16.Gaytan F, Morales C, Garcia-Pardo L, Reymundo C, Bellido C, Sanchez-Criado JE. Macrophages, cell proliferation, and cell death in the human menstrual corpus luteum. Biology of reproduction. 1998;59:417–425. doi: 10.1095/biolreprod59.2.417. [DOI] [PubMed] [Google Scholar]
- 17.Rodriguez-Garcia M, Barr FD, Crist SG, Fahey JV, Wira CR. Phenotype and susceptibility to HIV infection of CD4+ Th17 cells in the human female reproductive tract. Mucosal immunology. 2014;7:1375–1385. doi: 10.1038/mi.2014.26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Givan AL, White HD, Stern JE, Colby E, Gosselin EJ, Guyre PM, Wira CR. Flow cytometric analysis of leukocytes in the human female reproductive tract: comparison of fallopian tube, uterus, cervix, and vagina. Am J Reprod Immunol. 1997;38:350–359. doi: 10.1111/j.1600-0897.1997.tb00311.x. [DOI] [PubMed] [Google Scholar]
- 19.Ochiel DO, Ochsenbauer C, Kappes JC, Ghosh M, Fahey JV, Wira CR. Uterine epithelial cell regulation of DC-SIGN expression inhibits transmitted/founder HIV-1 trans infection by immature dendritic cells. PloS one. 2010;5:e14306. doi: 10.1371/journal.pone.0014306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gelderblom HC, Vatakis DN, Burke SA, Lawrie SD, Bristol GC, Levy DN. Viral complementation allows HIV-1 replication without integration. Retrovirology. 2008;5:60. doi: 10.1186/1742-4690-5-60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ochsenbauer C, Edmonds TG, Ding H, Keele BF, Decker J, Salazar MG, Salazar-Gonzalez JF, Shattock R, Haynes BF, Shaw GM, Hahn BH, Kappes JC. Generation of transmitted/founder HIV-1 infectious molecular clones and characterization of their replication capacity in CD4 T lymphocytes and monocyte-derived macrophages. Journal of virology. 2012;86:2715–2728. doi: 10.1128/JVI.06157-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Edmonds TG, Ding H, Yuan X, Wei Q, Smith KS, Conway JA, Wieczorek L, Brown B, Polonis V, West JT, Montefiori DC, Kappes JC, Ochsenbauer C. Replication competent molecular clones of HIV-1 expressing Renilla luciferase facilitate the analysis of antibody inhibition in PBMC. Virology. 2010;408:1–13. doi: 10.1016/j.virol.2010.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodriguez-Garcia M, Biswas N, Patel MV, Barr FD, Crist SG, Ochsenbauer C, Fahey JV, Wira CR. Estradiol reduces susceptibility of CD4+ T cells and macrophages to HIV-infection. PloS one. 2013;8:e62069. doi: 10.1371/journal.pone.0062069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wira CR, Rodriguez-Garcia M, Patel MV. The role of sex hormones in immune protection of the female reproductive tract. Nat Rev Immunol. 2015;15:217–230. doi: 10.1038/nri3819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rodriguez-Garcia M, Shen Z, Barr FD, Boesch AW, Ackerman ME, Kappes JC, Ochsenbauer C, Wira CR. Dendritic cells from the human female reproductive tract rapidly capture and respond to HIV. Mucosal immunology. 2017;10:531–544. doi: 10.1038/mi.2016.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Quillay H, El Costa H, Marlin R, Duriez M, Cannou C, Chretien F, Fernandez H, Lebreton A, Ighil J, Schwartz O, Barre-Sinoussi F, Nugeyre MT, Menu E. Distinct characteristics of endometrial and decidual macrophages and regulation of their permissivity to HIV-1 infection by SAMHD1. Journal of virology. 2015;89:1329–1339. doi: 10.1128/JVI.01730-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen AL, Collins J, Shipman EP, Wira CR, Guyre PM, Pioli PA. A subset of human uterine endometrial macrophages is alternatively activated. Am J Reprod Immunol. 2012;68:374–386. doi: 10.1111/j.1600-0897.2012.01181.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wira CR, Fahey JV. A new strategy to understand how HIV infects women: identification of a window of vulnerability during the menstrual cycle. Aids. 2008;22:1909–1917. doi: 10.1097/QAD.0b013e3283060ea4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wira CR, Patel MV, Ghosh M, Mukura L, Fahey JV. Innate immunity in the human female reproductive tract: endocrine regulation of endogenous antimicrobial protection against HIV and other sexually transmitted infections. Am J Reprod Immunol. 2011;65:196–211. doi: 10.1111/j.1600-0897.2011.00970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Verstraelen H, Vilchez-Vargas R, Desimpel F, Jauregui R, Vankeirsbilck N, Weyers S, Verhelst R, De Sutter P, Pieper DH, Van De Wiele T. Characterisation of the human uterine microbiome in non-pregnant women through deep sequencing of the V1-2 region of the 16S rRNA gene. PeerJ. 2016;4:e1602. doi: 10.7717/peerj.1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kunz G, Beil D, Deininger H, Wildt L, Leyendecker G. The dynamics of rapid sperm transport through the female genital tract: evidence from vaginal sonography of uterine peristalsis and hysterosalpingoscintigraphy. Human Reprod. 1996;11:627–632. doi: 10.1093/humrep/11.3.627. [DOI] [PubMed] [Google Scholar]
- 32.Kunz GD, Beil H, Deiniger A, Einspanier G, Mall G, Leyendecke G. The uterine peristaltic pump. Normal and impeded sperm transport within the female genital tract. Adv Exp Med Biol. 1997;424:267–277. [PubMed] [Google Scholar]
- 33.Parsons AK, Cone RA, Moench TR. Uterine uptake of vaginal fluids: implications for microbicides; Presented at Microbicides 2002; Antwerp, Belgium. 2002. p. 136. pp Abstract B-175. [Google Scholar]
- 34.Settlage D, Motoshima M, Tredway D. Sperm transport from the external cervical os to the fallopian tubes in women: a time and quantitation study. Fertil Steril. 1973;24:655–661. doi: 10.1016/s0015-0282(16)39908-3. [DOI] [PubMed] [Google Scholar]
- 35.Bagasra O, Freund M, Weidmann J, Harley G. Interaction of human immunodeficiency virus with human sperm in vitro. J Acquir Immune Defic Syndr. 1988;1:431–435. [PubMed] [Google Scholar]
- 36.Brogi A, Presentini R, Solazzo D, Piomboni P, Costantino-Ceccarini E. Interaction of human immunodeficiency virus type 1 envelope glycoprotein gp120 with a galactoglycerolipid associated with human sperm. AIDS Res Hum Retroviruses. 1996;12:483–489. doi: 10.1089/aid.1996.12.483. [DOI] [PubMed] [Google Scholar]


