Abstract
Malignant mesothelioma (MM) is a rare, aggressive tumor often associated with asbestos exposure and characterized by complex genetic abnormalities, including deletions of chromosome 22. A gene fusion involving EWSR1 and YY1 gene on 14q32 has been reported in 2 patients over the age of 60 with peritoneal MM. However, the incidence of EWSR1 rearrangements in MM and the spectrum of its fusion partners remain unknown. We recently encountered 2 MM cases with EWSR1-ATF1 fusions and sought to investigate the prevalence and clinicopathologic features associated with this abnormality. As both index cases occurred as intra-abdominal tumors in young adults, we searched our files for pleural and peritoneal MM occurring in adults younger than age of 40. All cases were tested by FISH using custom BAC probes for EWSR1, FUS and ATF1 genes. When available, immunohistochemistry for BAP1 was performed. A total of 25 MM from patients aged 40 or less were screened, either from peritoneum (n = 13) or pleura (n = 12), with a median age of 31 (range 7–40). Two additional ATF1-rearranged tumors were identified at pleural and peritoneal sites with EWSR1 and FUS as fusion partners, respectively, for a total of 4 cases (16%, 4/25). The fusion positive cases displayed classic epithelioid morphology, immunoreactivity for cytokeratins and WT1, and negativity for S100. BAP-1 expression was retained in the 3 fusion-positive cases with available material, and in 80% (12/15) of the fusion-negative cases. Our results expand the spectrum of tumor types harboring EWSR1/FUS-ATF1 gene fusions to include a subgroup of conventional epithelioid MM. Other features of this unique MM subset include young age at presentation, lack of asbestos exposure and retained BAP1 expression.
Keywords: EWSR1, FUS, ATF1, gene fusion, mesothelioma, young adults
INTRODUCTION
Malignant mesothelioma (MM) is a rare and aggressive tumor of mesothelial lining, being more prevalent in the pleura than peritoneum (1). A causal relationship with occupational exposure to asbestos is well established (2). As most cases occur after long-term exposure to this carcinogen (3), MM patients are typically diagnosed in their 8-th decade of life (median age of 73 years old in USA; www.cdc.gov). However, a small subset of MM occurs in patients without significant asbestos exposure history and a younger age, lacking well-defined risk factors. Germline mutations in BRCA1 associated protein-1 (BAP1) predisposing to MM may account for a small portion of these cases (4), and have also been implicated in increasing sensitivity to asbestos carcinogenesis (5).
Due to the low incidence of MM, few comprehensive genomic studies have been performed to date, which have identified abnormalities in a number of cancer related genes, such as: CDKN2A, NF2, SETD2, TP53, DDX3 and BAP1 (6, 7). Furthermore, MM were previously shown to exhibit complex chromosomal copy number variations, including frequent losses in chromosomes 1p, 4q, 9p, 13q, 14q and 22q, either by conventional karyotype and comparative genomic hybridization (8). Despite frequent alterations involving chromosome 22, specific gene rearrangements involving EWSR1 (22q12) have been reported only recently in 2 peritoneal MM, harboring an EWSR1-YY1 fusion (9).
We recently encountered 2 similar peritoneal MM cases exhibiting EWSR1 rearrangements, but being fused instead to the ATF1 gene, and sought to investigate the prevalence and clinicopathologic features associated with this abnormality. As both cases occurred as intra-abdominal tumors in young adults and displayed epithelioid morphology, we searched our files for pleural and peritoneal MM occurring in adults younger than age of 40.
METHODS
Index Cases and Extended Cohort Selection
The first index case was a 21 year-old man who presented with a bulky and widely invasive peritoneal tumor at an outside institution. As the initial clinical diagnostic consideration was a desmoplastic round cell tumor, FISH for EWSR1 was performed and revealed a break-apart signal, while the RT-PCR was negative for the EWSR1-WT1 canonical fusion. Available formalin fixed paraffin embedded (FFPE) tissue sections were further investigated for other potential fusion partners of EWSR1. Thus, FISH analysis revealed an ATF1 gene rearrangement.
The second case was that of a 33 year-old female with a peritoneal tumor with histologic features of epithelioid MM, which was investigated by the institutional hybrid-capture based targeted next-generation sequencing assay (MSK-IMPACT) for additional molecular characterization. The results showed an EWSR1-ATF1 fusion candidate.
To further expand the cohort, we searched the files of Department of Pathology at Memorial Sloan Kettering Cancer Center and personal consultations of CDF and CRA for the diagnosis of MM, in patients under the age 40, with available tissue for molecular analysis, from a two-decade period (1995–2016). Review of Hematoxylin and Eosin (H&E) was done for each case. Previously performed and submitted immunohistochemical stains were reviewed and complementary markers were performed if needed in a subset of cases. Clinical follow-up data were obtained from review of medical charts. The relationship between tumor location and age, gender, smoking status or asbestos exposure was assessed using two-sided Fisher/s exact test. The study was approved by the Institutional Review Board.
Fluorescence In Situ Hybridization
Four µm-thick sections from formalin-fixed paraffin-embedded (FFPE) tissue were prepared to perform FISH applying custom probes using bacterial artificial chromosomes (BAC; Supplementary Table 1), as previously described (10). Probes were designed to cover EWSR1, FUS, ATF1, and SMARCB1, chosen according to USSC genome browser and obtained from the Children`s Hospital of Oakland Research Institute (CHORI; Oakland, CA, USA; http://bacpac.chori.org). DNA from individual BACs was isolated according to the manufacturer’s instructions, labeled with different fluorochromes in a nick translation reaction, denatured, and hybridized to pretreated slides. Slides were then incubated, washed, and mounted with DAPI in an antifade solution. The genomic location of each BAC set was verified by hybridizing them to normal metaphase chromosomes. Scoring was performed on two hundred successive nuclei using a Zeiss fluorescence microscope (Zeiss Axioplan, Oberkochen, Germany), controlled by Isis 5 software (Metasystems, Newton, MA). At least 20% of the nuclei showing a break-apart signal were required to interpret as a positive score, and nuclei with an incomplete set of signals were omitted.
All cases were first tested for EWSR1 gene abnormalities. The EWSR1-rearranged tumors were then evaluated for break-apart signal using the ATF1 BACs. The EWSR1 negative tumors were then tested for FUS break-apart, as FUS gene has previously been shown to substitute for EWSR1 in gene fusions involving members of the CREB transcription factors, including ATF1 (11, 12). As deletions of 22q have been reported as a common finding in MM, we have also examined abnormalities of this region by FISH using EWSR1, SMARCB1 and a 22q11 reference (RP11–960P211 and RP11–81B3), as previously described (13). A monosomy pattern (or large deletion) was defined if one allele copy of both EWSR1 and SMARCB1 genes were lost, with a ratio of 1:1. In MM2 index case, FISH fusion assay was also performed to confirm the association of the EWSR1 and ATF1 genes. For the FISH fusion assay the BAC probes were labeled as follows: centromeric EWSR1 (red) and telomeric ATF1 (green). A positive result was interpreted when the red and green signals came-together as one signal (yellow).
Next-Generation Sequencing
One patient was consented for molecular testing with the Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT). This hybridization capture-based next-generation sequencing (NGS) assay detects somatic single nucleotide mutations, small indels, copy number alterations and selected structural variants in several cancer-related genes (410 for the version used) (14). Briefly, DNA extracted from FFPE tumor sample was prepared in libraries and sequenced on an Illumina HiSeq2500, using patient`s blood DNA as a reference to ensure the somatic nature of the variant calls.
Immunohistochemistry
When FFPE material was available, IHC was performed on FFPE tissue sections using a fully automated system (Benchmark ULTRA; Ventana Medical Systems, Tucson, AZ). The following antibodies were used: BAP1 (Santa Cruz, 1:50), Calretinin (Ventana, SP65), Cytokeratin-Pan (DAKO, M3515, 1:1600), CK5/6 (Ventana, 790–4554), Desmin (Ventana, 760–2513), EMA (Ventana, E29), ER (Leica, 6F11), Myogenin (Cell Marque, 760–2832), OCT4 (Cell Marque, 760–4392), PAX8 (Proteintech, 10336-I-AP, 1:100), S100 (Z0311, 1:8000, Dako), Vimentin (Ventana, 790–2917), and WT-1 (Leica, PA0562).
RESULTS
Clinico-pathologic and Molecular Findings of Index Cases
The first index case (MM1) was a 21 year-old man, who presented with symptoms of acute appendicitis, and upon resection showed an extensive peritoneal tumor, infiltrating omentum and involving loco-regional lymph nodes. Morphologically, the tumor showed sheets of epithelioid cells with focally papillary architecture, abundant eosinophilic cytoplasm, small nuclei with well-defined borders, open chromatin and inconspicuous nucleoli (Fig. 1A–C). Psammoma bodies were present in moderate number (Fig. 1B). On immunostaining, the tumor revealed diffuse positivity for AE1/AE3, EMA and WT-1, focal positivity for calretinin, but negativity for PAX8, Vimentin, OCT3/4 and S100. FISH studies performed revealed EWSR1 break-apart signal and a concurrent gene rearrangement of ATF1 (Fig. 2).
Figure 1. Histologic features of EWSR1-ATF1 fusion positive mesotheliomas.
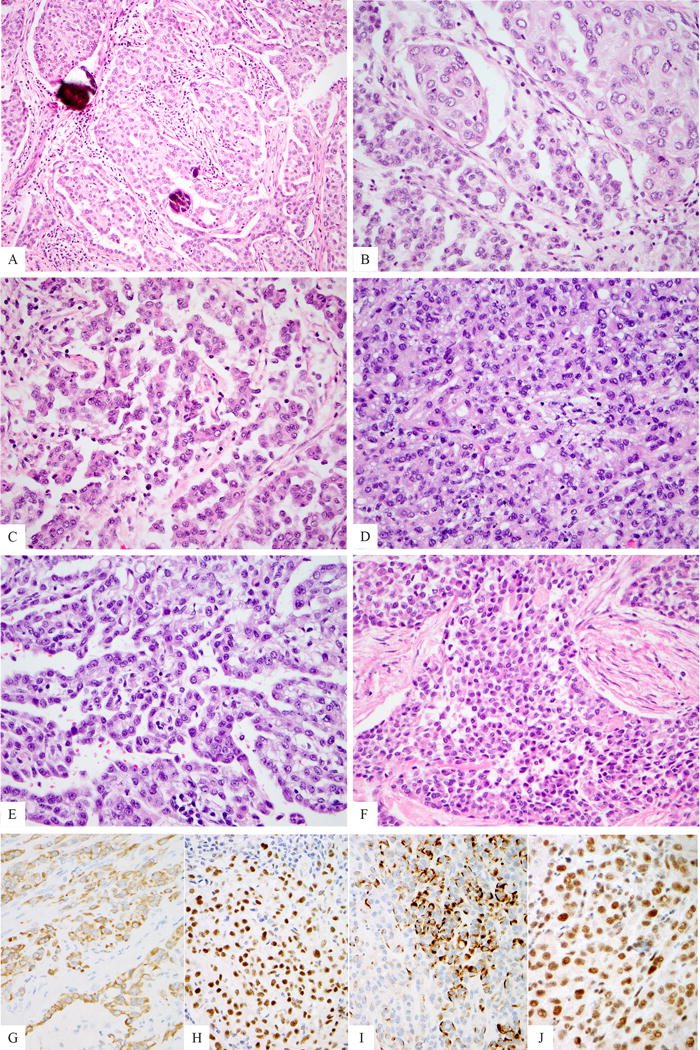
Medium power view of peritoneal index case (MM1) exhibiting focal psammoma bodies (A). The tumor displayed a conventional epithelioid morphology with focal papillary architecture, as well as abundant eosinophilic cytoplasm and open chromatin (B, C). MM2 showed a predominantly solid growth (D), with only focal papillary architecture (E). MM3 exhibited a predominant round cell phenotype with scant cytoplasm (F). All cases were immunohistochemically positive for AE1/AE3 (G, MM2), WT1 (H, MM2) and focal desmin expression was observed in a single case (I, MM2). Immunohistochemical expression of BAP1 was retained in the 3 fusion positive cases tested (J, MM2).
Figure 2. Fluorescence in situ hybridization showing EWSR1.
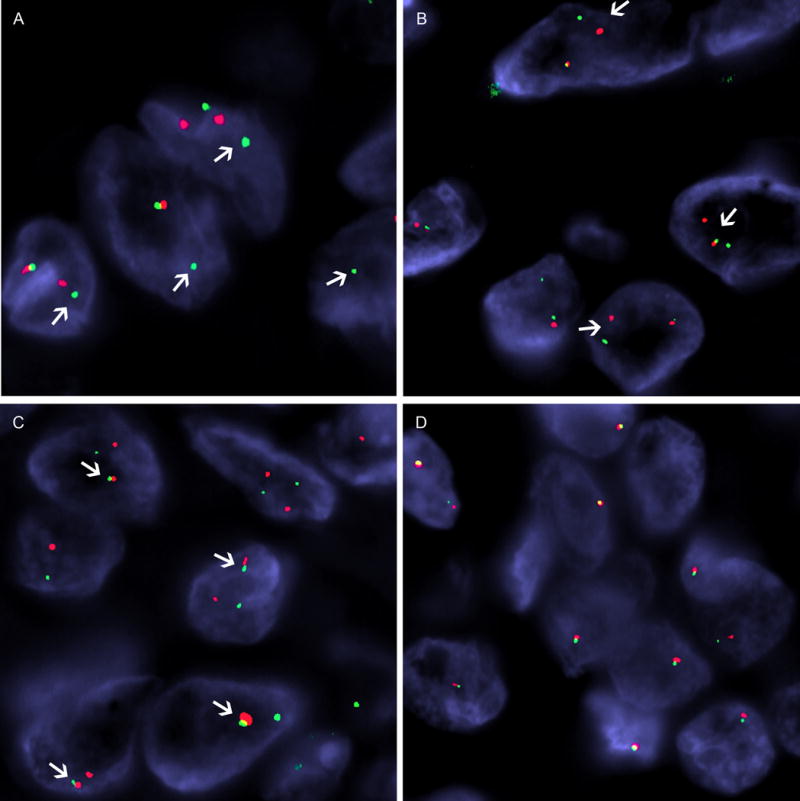
(A, red, centromeric; green, telomeric) and ATF1 (B, red, centromeric; green, telomeric) break-apart signals (arrows) in MM3. C) FISH fusion assay in MM2 illustrates the come-together signals (arrows) between centromeric EWSR1 (red) and telomeric ATF1 (green), confirming the fusion result detected by next-generation sequencing assay (MSK-IMPACT). D) Large deletion or monosomy of 22q12 showing only one copy of EWSR1 (red, centromeric, green telomeric) in a fusion-negative pleural mesothelioma from a 37 years-old patient.
The second case (MM2) occurred in a 33 year-old female with a mesenteric tumor and retroperitoneal lymphadenopathy. Microscopically, the tumor showed typical features of an epithelioid MM, with predominantly solid sheets of eosinophilic cells with focal gland-like and papillary structures (Fig. 1 D–E). The tumor cells exhibited immunostaining for AE1/AE3, CK5/6, WT-1 (diffuse) and calretinin (focal). A focal area of desmin reactivity was observed in the absence of myogenin staining in the solid epithelioid component (Fig. 1I). MOC-31, PAX8, S100 and SOX10 were negative in the tumor cells. The tumor was subjected to further molecular testing by a hybrid-capture based targeted next-generation sequencing assay (MSK-IMPACT), with a median sequencing coverage of 897X. This revealed fusion of EWSR1 exons 1–14 (NM_013986) to ATF1 exons 5–7 (NM_005171)(Fig. 3). No somatic mutations, copy number alterations or other structural variants were identified. The rearrangement of both genes was also confirmed by FISH (Fig. 2C).
Figure 3.
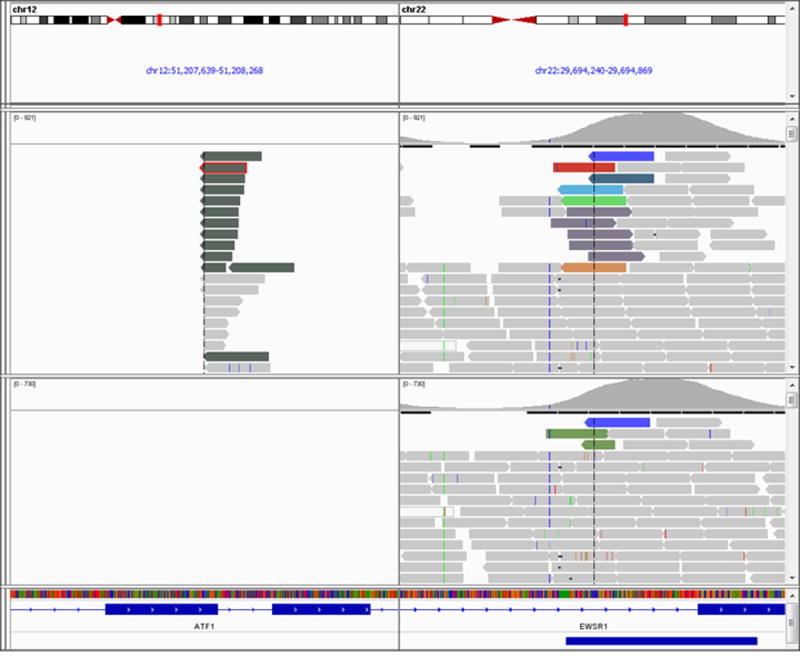
Representation of the EWSR1-ATF1 fusion in Integrated Genome Viewer for MM2, where each bar represents a single sequenced read. The sets of reads (on top) map to EWSR1 on chromosome 22, corresponding paired end reads map to ATF1 on chromosome 12, supporting a somatic fusion involving both genes. Upper panel: tumor; lower panel: normal blood control.
Further FISH Screening in Young Patients Identifies an Additional Pleural MM with EWSR1-ATF1 fusion
As both index cases were identified in young patients, we conducted our screen for additional MM cases in this age population. From 1995–2016, 23 additional cases were identified in patients of age 40 or less, 11 from the peritoneum and 12 from the pleura. The clinical and pathologic findings of the entire cohort (n = 25) are listed in Table 2. Briefly, the cohort had a median age of 31 years (range 7–40) at diagnosis, with a gender ratio varying based on the anatomic site involved (p = 0.047). Most (77%) peritoneal tumors occurred in males, while only 33% of the thoracic tumors presented in male patients. Among the 16 patients with information available, none had a history of direct asbestos exposure, although 3 patients with fusion-negative tumors had a possible secondary (indirect) exposure through relatives. Smoking history was available for 14 (56%) of patients, with only 5 patients being identified as heavy-smokers.
Table 2.
Clinicopathological Features of Malignant Mesotheliomas in the Screening Cohort
| Characteristics | Localization
|
||
|---|---|---|---|
| Peritoneal n = 13 (52%) |
Thoracic n = 12 (48%) |
Total n = 25 |
|
| Age, years; median (range) | 30 (7–40) | 33 (8–38) | 31 (7–40) |
| Sex, n (%) | |||
| Male | 10* (77) | 4 (33) | 14 (56) |
| Female | 3 (23) | 8 (67) | 11 (44) |
| Asbestos exposure, n (%) | |||
| None | 6 (46) | 7 (58) | 13 (52) |
| Potential Indirect | 0 (0) | 3 (25) | 3 (12) |
| N/A | 7 (54) | 2 (17) | 9 (36) |
| Smoking | |||
| Never smoker | 3 (31) | 2 (17) | 6 (24) |
| Light smoker | 0 | 3 (25) | 3 (12) |
| Heavy smoker | 1(8) | 4 (33) | 5 (20) |
| N/A | 8 (61) | 3 (25) | 11 (44) |
| Morphology | |||
| Epithelioid | 13 (100) | 11 (92) | 24 (96) |
| Sarcomatoid | 0 | 0 | 0 |
| Biphasic | 0 | 1 (8) | 1 (4) |
| BAP1 IHC, n (%) | |||
| Retained | 6 (46) | 9 (75) | 15 (60) |
| Loss | 0 | 3 (25) | 3 (12) |
| Failure/N/A | 1/6 (54) | 0/0 | 7 (28) |
| EWSR1/FUS-ATF1 fusion, n (%) | |||
| Positive | 3 (23) | 1 (8) | 4 (16) |
| Negative | 10 (77) | 11 (92) | 21 (84) |
male: female ratio statistically significant between thoracic and peritoneal localization (p = 0.047); N/A: not available; IHC, immunohistochemistry; FISH, fluorescence in situ hybridization
FISH screening identified an additional positive case (MM3; Fig. 2 A–B) for the EWSR1-ATF1 fusion. The tumor occurred in a 34-year-old female and originated from the pleura (Table 1). Like the prior 2 index cases, it displayed epithelioid cells with eosinophilic cytoplasm and round nuclei (Fig. 1F). The tumor cells were positive for cytokeratins and WT1, while negative for S100.
Table 1.
Clinicopathological Features of EWSR1/FUS-ATF1 Fusion Positive Malignant Mesotheliomas
| Case | Age/Sex | Location | Asbestos exposure | Smoking | Morphology | Stage | Follow up | BAP-1 IHC | Fusion | Method |
|---|---|---|---|---|---|---|---|---|---|---|
| MM1 | 21/M | Peritoneal | N/A | N/A | Epithelioid | Advanced, pN1 | N/A | Failure | EWSR1-ATF1 | FISH |
| MM2 | 33/F | Peritoneal | None | Never smoker | Epithelioid | Advanced, cM1 | POD | Retained | EWSR1-ATF1 | FISH + targeted NGS |
| MM3 | 34/F | Thoracic | None | 10 pack-year | Epithelioid | pT2N0/II | POD | Retained | EWSR1-ATF1 | FISH |
| MM4 | 25/F | Peritoneal | None | Never smoker | Epithelioid | Advanced | POD | Retained | FUS-ATF1 | FISH |
IHC, immunohistochemistry; FISH, fluorescence in situ hybridization; POD progression of disease; NGS: Next-Generation Sequencing; stage: c: clinical, p: pathologic
FISH Screening Allows Detection of a FUS-ATF1 Rearranged Peritoneal MM
Fusion-negative tumors were also further tested for FUS gene abnormalities by FISH. Among the 22 tumors tested, one revealed a FUS-ATF1 rearrangement (1/22) (Supplementary Fig. 1), for a total of 4/25 (16%) EWSR1 or FUS-ATF1 fusion positive MM. The FUS-ATF1 fusion positive tumor occurred in a 25-years-old woman with widely extensive peritoneal tumor. Tumor exhibited epithelioid morphology with clear cell changes, and was immunohistochemically positive for AE1-AE3, WT1, D2/40 (focal) and CK5/6, while negative for calretinin, Ber-EP4, MOC31, B72.3, PAX8, ER, inhibin, desmin, OCT3/4, GATA3 and S100. Electronic microscopy performed at an outside institution reported the presence of long and thin cellular projections, consistent with the non-intestinal microvilli of mesothelioma.
Additionally, among the 21 fusion-negative MM, 5 (24%) cases showed large deletions by FISH of EWSR1 and SMARCB1, with a 1:1 ratio (Fig. 2D).
All patients with fusion positive MM had advanced stage at presentation and progression of disease after debulking surgery and radio-chemotherapy occurred in three patients who had clinical follow up data available (Table 2).
MM with FUS/EWSR1-ATF1 fusion show retained BAP1 Immunoexpression
BAP-1 immunostaining was assessed in 18 tumors with available FFPE material. Expression was retained in 15/18 (83%) cases and only 3 pleural MM showed loss of BAP1 staining (Table 2). The FUS-ATF1 fusion-positive and two of the EWSR1-ATF1 rearranged tumors showed retained expression of BAP1 (Table 1 and Fig. 1I). The third EWSR1-ATF1 case had no remaining material for immunotesting. In the fusion negative tumors, nuclear expression of BAP1 was lost in only 3/15 (20%).
DISCUSSION
We report 4 cases of conventional epithelioid MM exhibiting recurrent EWSR1 or FUS-ATF1 gene fusions among a comparatively large cohort of 25 children and young adults, under the age of 40. These findings further expand understanding of the pathogenesis of MM, as well as enrich the growing list of pathologic entities harboring fusions of EWSR1 with members of the CREB family of transcription factors. All 4 cases presented in young adults, lacking history of asbestos exposure, and microscopically displayed a typical epithelioid phenotype, with retained expression of BAP1, suggesting a novel MM subset.
EWSR1 (22q12) encodes a protein with a carboxy-terminus RNA binding domain, which has roles in mitosis, microtubule processing, DNA repair and cellular ageing (15). EWSR1 has gained the infamous reputation of providing a promiscuous 5` gene partner in many different fusions involved in mesenchymal neoplasia and beyond (16, 17). Particularly complex and heterogeneous is the spectrum of pathologic entities recently described as sharing fusions between EWSR1 and one of the c-AMP dependent transcription factor member of the CREB-ATF1 family (18). An EWSR1-ATF1 fusion transcript has been described in several tumor types without an unifying cell lineage, morphology or behavior, spanning both benign and malignant tumors of either mesenchymal or epithelial differentiation (19). These include clear cell sarcoma (20), angiomatoid fibrous histiocytoma (21, 22), GI clear cell sarcoma (23), hyalinizing clear cell carcinoma (24), myoepithelial carcinoma (25), primary pulmonary myxoid sarcoma (19) and more recently in a myxoid mesenchymal tumor with intracranial predilection (26). To our knowledge, EWSR1/FUS-ATF1 fusions have not been previously described in a MM case. Two studies involving whole genome sequencing did not reveal rearrangements of EWSR1 or ATF1 in 99 and 22 MM cases, respectively (6, 27). Data available from the Cancer Genomic BioPortal (www.cbioportal.com; 01/2017) (28) showed no evidence of further rearrangement of FUS or CREB1 in these cohorts. Our index case 2 represents the single MM case with alterations in either EWSR1, FUS or ATF1 genes from the MSK-IMPACT cohort, which contains genomic data for 131 patients with MM (data not shown). In the series reporting 2 MM cases with EWSR1-YY1 fusion (9), a control cohort consisting of 14 MM patients, ranging from 43–78 years (median 66), was studied but no additional cases with EWSR1 rearrangement were found. Recently, a biphasic pleural tumor with features of mesothelioma and undifferentiated round cell harboring EWSR1 rearrangement was reported, but the gene partner was not identified (29).
Taken together, our data suggest that EWSR1/FUS rearrangements are rare events in the general population of MM. The 4 EWSR1/FUS-ATF1 fusion positive cases were remarkably identified among the cohort of 25 young patients, suggesting that this genetic alteration has a higher prevalence in this rare clinical subset of MM. As most of the previous genomic studies included either none or only a minute fraction of patients under the age of 40, this likely explains why this abnormality was not previously identified in MM. Indeed, MM occurring in adults younger than age of 40 is estimated to be quite rare (2% of cases) (30). A recent review suggests that some clinical features might be unique to this subset, including a more even ratio of pleural to peritoneal tumors and more importantly a better prognosis compared to MM in older patients (30). Recently, an ALK-related fusion was reported in a pediatric peritoneal MM case (31), further support that a subset of MM in children and young adults is driven by alternative pathogenetic mechanisms. As expected, none of the young patients in our cohort, including the fusion-positive cases, had a history of direct exposure to asbestos. Risk factors for MM in patients without significant exposure to asbestos, accounting for 20% of all MM, are not well defined, but include radiation exposure, non-asbestos mineral fibers, as well as genetic predisposition such as BAP1 germline loss (30). While the germline status of BAP1 remains unknown in our cohort, retained immunohistochemical expression of BAP1 in most cases was observed. This argues against a germline BAP1 predisposition in these patients, given that germline cases have been found to exhibit the loss of BAP1 expression (4).
Indeed, loss of BAP1 expression was found in only 3/18 tested cases (17%) in our overall cohort of mesotheliomas in patients under 40. This is a lower frequency than reported in different series (27–70%) using negative BAP1 expression by IHC as a surrogate for BAP1 mutation status (32–35). Noteworthy, the median age of patients in these studies is either unknown or > 60 years old, sharply contrasting with ours. Although limited by the size of our cohort, our findings suggest that BAP1 loss might be less common in younger patients with MM, including the EWSR1-rearranged cases. Both young age and loss of BAP1 expression were independently associated with better prognosis in one of these studies (35). However, a more definitive conclusion regarding the clinical behavior of EWSR1/FUS-rearranged MM is precluded due to the low number of cases, but the information available in our cases suggests an advanced stage at diagnosis (3/3 peritoneal; 0/1 pleural) and subsequent recurrence (3/3 with follow up).
Concordantly with the frequent deletions of chromosome 22 observed in MM, including band 22q12 harboring EWSR1 (8, 36), 5/21 of fusion-negative MM from our screening cohort showed loss of one EWSR1 or SMARCB1 allele by FISH. In contrast, copy number alterations in chromosome 12, where ATF1 is located, appear to be less frequent in MM. However, one case showing a translocation involving chromosomes 12 and X has been described in a patient with MM lacking a history of asbestos exposure (37).
Several entities with EWSR1-ATF1 fusions are considered in the differential diagnosis of epithelioid malignancies that could occur in abdomen or thorax of young patients, though given classic morphology and immunoprofile of fusion-positive MM these entities are discussed for general reference. Amongst them is the GI clear cell sarcoma, a tumor of children and young adults, with epithelioid morphology arranged in solid nests and occasionally pseudo-papillary growth, and with rare multinucleated, osteoclast-like giant cells (38). In contrast to MM, the GI clear cell sarcoma show diffuse immunostaining for S100 and SOX10, but lacks cytokeratin reactivity as well as reactivity for mesothelial markers (39). A single case report of an EWSR1-ATF1 positive myoepithelial tumor was described in the pelvis, exhibiting myxoid background and focal EMA reactivity, as well as S100 positivity (25). Although rare in the thoracic or abdominal cavity, angiomatoid fibrous histiocytomas have been reported at these sites, harboring EWSR1-ATF1 fusions (40). Additionally, rare cases with endobronchial or pulmonary presentation have been described (41, 42). However, angiomatoid fibrous histiocytomas typically stain for desmin, while being consistently negative for cytokeratin. Although one of our EWSR1-ATF1 positive MM showed patchy desmin reactivity in the solid epithelioid component it also co-expressed diffuse cytokeratin and WT1 in keeping with the diagnosis of MM. Hyalinizing clear cell carcinoma, described initially as a distinct salivary gland tumor with EWSR1-ATF1 fusion (24), was subsequently reported in the bronchus (43), but so far not in abdominal locations. The tumor is characterized by bland epithelioid cells exhibiting at least focally clear cytoplasm, arranged in cords, sheets, small nests and trabeculae. They are often surrounded in a background of hyalinized fibrous tissue and show reactivity for CK7, CK5/6, but also for squamous cell markers (p63/p40). Primary pulmonary myxoid sarcoma is a rare pulmonary/endobronchial tumor with lobulated architecture, consisting of spindle to epithelioid cells arranged in solid and reticular patterns, within a diffusely myxoid stroma (42). The tumor is typically negative for cytokeratins and harbors the related EWSR1-CREB1 fusion (42); while no example with EWSR1-ATF1 fusion has been yet reported. Finally, a group of unclassified myxoid mesenchymal neoplasms with predilection for the intracranial compartment and characterized by fusions of EWSR1 with members of the CREB gene family, including ATF1, was recently described (26). From this series of 5 tumors occurring in young patients (range: 12–23 years), one presented in the pelvic soft tissues. These tumors were characterized by lobulated growth, with variably myxoid to solid areas of round to ovoid cells, in addition to distinctive amianthoid collagen fibers forming rosette-type structures. None of the cases showed cytokeratin expression.
From a morphologic perspective, the main differential diagnosis of epitheloid peritoneal MM in young patients is with serous carcinoma, although they tend to occur in females of older age (44). In contrast to high grade serous carcinoma, MM typically reveal a non-hierarchical papillary growth, with mild to moderate atypia and a relatively low mitotic activity. Immunohistochemically, serous carcinomas express MOC31, Ber-EP4 as well as PAX8, which is only rarely expressed in MM (45). Although our cohort showed a male predominance in the peritoneal location, PAX8 was tested in all peritoneal tumors occurring in female patients and was negative, arguing against a serous carcinoma diagnosis. A less common diagnostic consideration was mesonephric adenocarcinoma occurring in the peritoneum of young patients. These tumors show mixed growth patterns, variable degree of atypia and brisk mitotic activity. While exhibiting immunolabelling for cytokeratin and PAX8, they are typically negative for WT1 (46). To exclude this rare diagnostic consideration, we have tested 4 cases from our files but no EWSR1/FUS gene rearrangements were found by FISH (data not shown).
In summary, we identified 4 MM cases harboring EWSR1-ATF1 (3) and FUS-ATF1 (1) fusions from a large cohort of 25 MM patients under the age of 40. Although the number of positive cases is relatively small, this is the largest MM series to date focusing on this age group. This finding expands the spectrum of heterogeneous entities harboring EWSR1/FUS-ATF1 fusions, to include MM, among other mesenchymal and epithelial malignancies. Taken together, our findings suggest that fusions involving EWSR1 or FUS and ATF1 are rare events in mesothelioma, which appear to be restricted to younger patients without significant exposure to asbestos or BAP1 germline or somatic loss, and indistinguishable from conventional MM. In keeping with the simple genomic profile of other translocation-associated neoplasms, one of the index cases tested by targeted NGS showed that the EWSR1-ATF1 fusion was the sole genetic abnormality identified, without other mutations or copy number changes. Whether the genetic profile of MM occurring in children and young adults is similarly heterogeneous to the older age group will require further study, but our findings suggest that at least a small subset harbors abnormalities such as losses of 22q and BAP1 at chromosomal and immunohistochemical levels, respectively.
Supplementary Material
Supplementary Figure 1. Peritoneal malignant mesothelioma with FUS-ATF1 fusion (MM4). Histologic findings include typical epithelioid features arranged in papillary architecture (A), as well as solid growth with epithelioid cells harboring cytoplasmic clearing (B). Fluorescence in situ hybridization showing FUS (A, red, centromeric; green, telomeric) and ATF1 (B, red, centromeric; green, telomeric) break-apart signals (arrows).
Acknowledgments
The authors thank Milagros Soto for her excellent assistance and Allyne Manzo for help in photo editing.
Supported in part by: P50 CA140146-01 (CRA); Kristen Ann Carr Foundation (CRA); Cycle for Survival (CRA); P30 CA008748
Footnotes
Conflicts of interest: none
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With County, Total U.S. (1990–2010) - Linked To County Attributes - Total U.S., 1969–2011 Countries.
References
- 1.Moolgavkar SH, Meza R, Turim J. Pleural and peritoneal mesotheliomas in SEER: age effects and temporal trends, 1973–2005. Cancer Causes Control. 2009;20:935–944. doi: 10.1007/s10552-009-9328-9. [DOI] [PubMed] [Google Scholar]
- 2.Wagner JC, Sleggs CA, Marchand P. Diffuse pleural mesothelioma and asbestos exposure in the North Western Cape Province. Br J Ind Med. 1960;17:260–271. doi: 10.1136/oem.17.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Reid A, de Klerk NH, Magnani C, et al. Mesothelioma risk after 40 years since first exposure to asbestos: a pooled analysis. Thorax. 2014;69:843–850. doi: 10.1136/thoraxjnl-2013-204161. [DOI] [PubMed] [Google Scholar]
- 4.Testa JR, Cheung M, Pei J, et al. Germline BAP1 mutations predispose to malignant mesothelioma. Nat Genet. 2011;43:1022–1025. doi: 10.1038/ng.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Xu J, Kadariya Y, Cheung M, et al. Germline mutation of Bap1 accelerates development of asbestos-induced malignant mesothelioma. Cancer Res. 2014;74:4388–4397. doi: 10.1158/0008-5472.CAN-14-1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bueno R, Stawiski EW, Goldstein LD, et al. Comprehensive genomic analysis of malignant pleural mesothelioma identifies recurrent mutations, gene fusions and splicing alterations. Nat Genet. 2016;48:407–416. doi: 10.1038/ng.3520. [DOI] [PubMed] [Google Scholar]
- 7.Hylebos M, Van Camp G, van Meerbeeck JP, et al. The Genetic Landscape of Malignant Pleural Mesothelioma: Results from Massively Parallel Sequencing. J Thorac Oncol. 2016;11:1615–1626. doi: 10.1016/j.jtho.2016.05.020. [DOI] [PubMed] [Google Scholar]
- 8.Taniguchi T, Karnan S, Fukui T, et al. Genomic profiling of malignant pleural mesothelioma with array-based comparative genomic hybridization shows frequent non-random chromosomal alteration regions including JUN amplification on 1p32. Cancer Sci. 2007;98:438–446. doi: 10.1111/j.1349-7006.2006.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Panagopoulos I, Thorsen J, Gorunova L, et al. RNA sequencing identifies fusion of the EWSR1 and YY1 genes in mesothelioma with t(14;22)(q32;q12) Genes Chromosomes Cancer. 2013;52:733–740. doi: 10.1002/gcc.22068. [DOI] [PubMed] [Google Scholar]
- 10.Antonescu CR, Zhang L, Chang NE, et al. EWSR1-POU5F1 fusion in soft tissue myoepithelial tumors. A molecular analysis of sixty-six cases, including soft tissue, bone, and visceral lesions, showing common involvement of the EWSR1 gene. Genes Chromosomes Cancer. 2010;49:1114–1124. doi: 10.1002/gcc.20819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Waters BL, Panagopoulos I, Allen EF. Genetic characterization of angiomatoid fibrous histiocytoma identifies fusion of the FUS and ATF-1 genes induced by a chromosomal translocation involving bands 12q13 and 16p11. Cancer Genet Cytogenet. 2000;121:109–116. doi: 10.1016/s0165-4608(00)00237-5. [DOI] [PubMed] [Google Scholar]
- 12.Raddaoui E, Donner LR, Panagopoulos I. Fusion of the FUS and ATF1 genes in a large, deep-seated angiomatoid fibrous histiocytoma. Diagn Mol Pathol. 2002;11:157–162. doi: 10.1097/00019606-200209000-00006. [DOI] [PubMed] [Google Scholar]
- 13.Le Loarer F, Zhang L, Fletcher CD, et al. Consistent SMARCB1 homozygous deletions in epithelioid sarcoma and in a subset of myoepithelial carcinomas can be reliably detected by FISH in archival material. Genes Chromosomes Cancer. 2014;53:475–486. doi: 10.1002/gcc.22159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng DT, Mitchell TN, Zehir A, et al. Memorial Sloan Kettering-Integrated Mutation Profiling of Actionable Cancer Targets (MSK-IMPACT): A Hybridization Capture-Based Next-Generation Sequencing Clinical Assay for Solid Tumor Molecular Oncology. J Mol Diagn. 2015;17:251–264. doi: 10.1016/j.jmoldx.2014.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cantile M, Marra L, Franco R, et al. Molecular detection and targeting of EWSR1 fusion transcripts in soft tissue tumors. Med Oncol. 2013;30:412. doi: 10.1007/s12032-012-0412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mertens F, Antonescu CR, Mitelman F. Gene fusions in soft tissue tumors: Recurrent and overlapping pathogenetic themes. Genes Chromosomes Cancer. 2016;55:291–310. doi: 10.1002/gcc.22335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Antonescu CR, Dal Cin P. Promiscuous genes involved in recurrent chromosomal translocations in soft tissue tumours. Pathology. 2014;46:105–112. doi: 10.1097/PAT.0000000000000049. [DOI] [PubMed] [Google Scholar]
- 18.Hai TW, Liu F, Coukos WJ, et al. Transcription factor ATF cDNA clones: an extensive family of leucine zipper proteins able to selectively form DNA-binding heterodimers. Genes Dev. 1989;3:2083–2090. doi: 10.1101/gad.3.12b.2083. [DOI] [PubMed] [Google Scholar]
- 19.Thway K, Fisher C. Tumors with EWSR1-CREB1 and EWSR1-ATF1 fusions: the current status. Am J Surg Pathol. 2012;36:e1–e11. doi: 10.1097/PAS.0b013e31825485c5. [DOI] [PubMed] [Google Scholar]
- 20.Antonescu CR, Tschernyavsky SJ, Woodruff JM, et al. Molecular diagnosis of clear cell sarcoma: detection of EWS-ATF1 and MITF-M transcripts and histopathological and ultrastructural analysis of 12 cases. J Mol Diagn. 2002;4:44–52. doi: 10.1016/S1525-1578(10)60679-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hallor KH, Mertens F, Jin Y, et al. Fusion of the EWSR1 and ATF1 genes without expression of the MITF-M transcript in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer. 2005;44:97–102. doi: 10.1002/gcc.20201. [DOI] [PubMed] [Google Scholar]
- 22.Antonescu CR, Dal Cin P, Nafa K, et al. EWSR1-CREB1 is the predominant gene fusion in angiomatoid fibrous histiocytoma. Genes Chromosomes Cancer. 2007;46:1051–1060. doi: 10.1002/gcc.20491. [DOI] [PubMed] [Google Scholar]
- 23.Stockman DL, Miettinen M, Suster S, et al. Malignant gastrointestinal neuroectodermal tumor: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of 16 cases with a reappraisal of clear cell sarcoma-like tumors of the gastrointestinal tract. Am J Surg Pathol. 2012;36:857–868. doi: 10.1097/PAS.0b013e31824644ac. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Antonescu CR, Katabi N, Zhang L, et al. EWSR1-ATF1 fusion is a novel and consistent finding in hyalinizing clear-cell carcinoma of salivary gland. Genes Chromosomes Cancer. 2011;50:559–570. doi: 10.1002/gcc.20881. [DOI] [PubMed] [Google Scholar]
- 25.Flucke U, Mentzel T, Verdijk MA, et al. EWSR1-ATF1 chimeric transcript in a myoepithelial tumor of soft tissue: a case report. Hum Pathol. 2012;43:764–768. doi: 10.1016/j.humpath.2011.08.004. [DOI] [PubMed] [Google Scholar]
- 26.Kao YC, Sung YS, Zhang L, et al. EWSR1 Fusions With CREB Family Transcription Factors Define a Novel Myxoid Mesenchymal Tumor With Predilection for Intracranial Location. Am J Surg Pathol. 2016 doi: 10.1097/PAS.0000000000000788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guo G, Chmielecki J, Goparaju C, et al. Whole-exome sequencing reveals frequent genetic alterations in BAP1, NF2, CDKN2A, and CUL1 in malignant pleural mesothelioma. Cancer Res. 2015;75:264–269. doi: 10.1158/0008-5472.CAN-14-1008. [DOI] [PubMed] [Google Scholar]
- 28.Gao J, Aksoy BA, Dogrusoz U, et al. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal. 2013;6:l1. doi: 10.1126/scisignal.2004088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hackman S, Hammer RD, Layfield L. A Biphasic Pleural Tumor with Features of an Epithelioid and Small Cell Mesothelioma: Morphologic and Molecular Findings. Case Rep Pathol. 2016;2016:1532424. doi: 10.1155/2016/1532424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thomas A, Chen Y, Yu T, et al. Distinctive clinical characteristics of malignant mesothelioma in young patients. Oncotarget. 2015;6:16766–16773. doi: 10.18632/oncotarget.4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Loharamtaweethong K, Puripat N, Aoonjai N, et al. Anaplastic lymphoma kinase (ALK) translocation in paediatric malignant peritoneal mesothelioma: a case report of novel ALK-related tumour spectrum. Histopathology. 2016;68:603–607. doi: 10.1111/his.12779. [DOI] [PubMed] [Google Scholar]
- 32.Shinozaki-Ushiku A, Ushiku T, Morita S, et al. Diagnostic utility of BAP1 and EZH2 expression in malignant mesothelioma. Histopathology. 2016 doi: 10.1111/his.13123. [DOI] [PubMed] [Google Scholar]
- 33.Cigognetti M, Lonardi S, Fisogni S, et al. BAP1 (BRCA1-associated protein 1) is a highly specific marker for differentiating mesothelioma from reactive mesothelial proliferations. Mod Pathol. 2015;28:1043–1057. doi: 10.1038/modpathol.2015.65. [DOI] [PubMed] [Google Scholar]
- 34.Sheffield BS, Hwang HC, Lee AF, et al. BAP1 immunohistochemistry and p16 FISH to separate benign from malignant mesothelial proliferations. Am J Surg Pathol. 2015;39:977–982. doi: 10.1097/PAS.0000000000000394. [DOI] [PubMed] [Google Scholar]
- 35.Farzin M, Toon CW, Clarkson A, et al. Loss of expression of BAP1 predicts longer survival in mesothelioma. Pathology. 2015;47:302–307. doi: 10.1097/PAT.0000000000000250. [DOI] [PubMed] [Google Scholar]
- 36.Bott M, Brevet M, Taylor BS, et al. The nuclear deubiquitinase BAP1 is commonly inactivated by somatic mutations and 3p21.1 losses in malignant pleural mesothelioma. Nat Genet. 2011;43:668–672. doi: 10.1038/ng.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dal Cin P, De Wever I, Moerman P, et al. Translocation X;12 in mesothelioma. Cancer Genet Cytogenet. 1991;55:115–118. doi: 10.1016/0165-4608(91)90245-p. [DOI] [PubMed] [Google Scholar]
- 38.Zambrano E, Reyes-Mugica M, Franchi A, et al. An osteoclast-rich tumor of the gastrointestinal tract with features resembling clear cell sarcoma of soft parts: reports of 6 cases of a GIST simulator. Int J Surg Pathol. 2003;11:75–81. doi: 10.1177/106689690301100202. [DOI] [PubMed] [Google Scholar]
- 39.Antonescu CR, Nafa K, Segal NH, et al. EWS-CREB1: a recurrent variant fusion in clear cell sarcoma–association with gastrointestinal location and absence of melanocytic differentiation. Clin Cancer Res. 2006;12:5356–5362. doi: 10.1158/1078-0432.CCR-05-2811. [DOI] [PubMed] [Google Scholar]
- 40.Chen G, Folpe AL, Colby TV, et al. Angiomatoid fibrous histiocytoma: unusual sites and unusual morphology. Mod Pathol. 2011;24:1560–1570. doi: 10.1038/modpathol.2011.126. [DOI] [PubMed] [Google Scholar]
- 41.Tay CK, Koh MS, Takano A, et al. Primary Angiomatoid Fibrous Histiocytoma of the Lung with Mediastinal Lymph Node Metastasis. Hum Pathol. 2016 doi: 10.1016/j.humpath.2016.07.022. [DOI] [PubMed] [Google Scholar]
- 42.Thway K, Nicholson AG, Wallace WA, et al. Endobronchial pulmonary angiomatoid fibrous histiocytoma: two cases with EWSR1-CREB1 and EWSR1-ATF1 fusions. Am J Surg Pathol. 2012;36:883–888. doi: 10.1097/PAS.0b013e31824b1ee0. [DOI] [PubMed] [Google Scholar]
- 43.Garcia JJ, Jin L, Jackson SB, et al. Primary pulmonary hyalinizing clear cell carcinoma of bronchial submucosal gland origin. Hum Pathol. 2015;46:471–475. doi: 10.1016/j.humpath.2014.11.010. [DOI] [PubMed] [Google Scholar]
- 44.Grimley PM, Matsuno RK, Rosenberg PS, et al. Qualitative age interactions between low-grade and high-grade serous ovarian carcinomas. Cancer Epidemiol Biomarkers Prev. 2009;18:2256–2261. doi: 10.1158/1055-9965.EPI-09-0240. [DOI] [PubMed] [Google Scholar]
- 45.Husain AN, Colby T, Ordonez N, et al. Guidelines for pathologic diagnosis of malignant mesothelioma: 2012 update of the consensus statement from the International Mesothelioma Interest Group. Arch Pathol Lab Med. 2013;137:647–667. doi: 10.5858/arpa.2012-0214-OA. [DOI] [PubMed] [Google Scholar]
- 46.Kenny SL, McBride HA, Jamison J, et al. Mesonephric adenocarcinomas of the uterine cervix and corpus: HPV-negative neoplasms that are commonly PAX8, CA125, and HMGA2 positive and that may be immunoreactive with TTF1 and hepatocyte nuclear factor 1-beta. Am J Surg Pathol. 2012;36:799–807. doi: 10.1097/PAS.0b013e31824a72c6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Peritoneal malignant mesothelioma with FUS-ATF1 fusion (MM4). Histologic findings include typical epithelioid features arranged in papillary architecture (A), as well as solid growth with epithelioid cells harboring cytoplasmic clearing (B). Fluorescence in situ hybridization showing FUS (A, red, centromeric; green, telomeric) and ATF1 (B, red, centromeric; green, telomeric) break-apart signals (arrows).


