Abstract
Background
Dalbavancin is a novel lipoglycopeptide antibiotic that has potent in vitro activity against Gram-positive microorganisms.
Methods
We performed a phase 1, open-label, multi-center study to investigate the pharmacokinetics (PK) and safety of a single-dose of intravenous dalbavancin in hospitalized pediatric subjects 3 months to 11 years of age. We combined these data with previously collected adolescent PK data and performed a population PK analysis.
Results
Model development was performed using 311 dalbavancin plasma concentrations from 43 subjects. The median age was 5.9 years (range 0.3–16.9). A three-compartment, linear PK model was developed. Based on simulations, the following age-dependent dosing regimen was found to achieve similar dalbavancin exposure to that in adults administered a 2-dose regimen: age 6 to < 18 years, 12 mg/kg (1000 mg maximum) on day 1 and 6 mg/kg (500 mg maximum) on day 8; age 3 months to < 6 years, 15 mg/kg (1000 mg maximum) on day 1 and 7.5 mg/kg (500 mg maximum) on day 8. Similarly, the following age-dependent regimen was found to match adult exposure after a single-dose (1500 mg): age 6 to < 18 years, 18 mg/kg (1500 mg maximum) on day 1, and age 3 months to < 6 years, 22.5 mg/kg (1500 mg maximum) on day 1. Nineteen subjects experienced 36 treatment-emergent adverse events. Five out of 36 adverse events were assessed as possibly or probably related to treatment.
Conclusions
Dalbavancin pediatric dosing that matched adult exposure was identified. Overall, dalbavancin was well tolerated in our study population.
Keywords: dalbavancin, pharmacokinetics, adolescents, children, infants, safety, pediatrics
Dalbavancin is a novel lipoglycopeptide antibiotic that exerts its antimicrobial effect by inhibiting synthesis of bacterial cell wall peptidoglycan. It has potent in vitro activity against Gram-positive microorganisms, including drug-resistant strains such as methicillin-resistant Staphylococcus aureus and penicillin-resistant Streptococcus pneumoniae, Gram-positive anaerobes, and most Gram-positive non-acid-fast bacilli, including Bacillus anthracis.
Dalbavancin is approved by the U.S. Food and Drug Administration (FDA) and the European Medicines Agency (EMA) for the treatment of acute bacterial skin and skin structure infections (ABSSSI) in adults. In a phase 3 study, dalbavancin (1000 mg given intravenously on day 1 and 500 mg given intravenously on day 8) was shown to be as effective as linezolid (600 mg given intravenously or intravenously/orally every 12 hours for 14 days) for the treatment of complicated skin and skin structure infections.1 In two separate studies, the same 2-dose dalbavancin regimen was not inferior to twice-daily intravenous vancomycin (1 g or 15 mg/kg of body weight every 12 hours) with subsequent oral linezolid (600 mg every 12 hours) for the treatment of ABSSSI.2 Also, a single dalbavancin infusion of 1500 mg was found to be as effective as the 2-dose regimen.3 Both the single and 2-dose regimen have been approved by the FDA and EMA for the treatment of ABSSSI.
In vitro studies have shown that dalbavancin is not a substrate, inhibitor, or inducer of cytochrome P450 isoenzymes and it is eliminated predominantly (~70%) as the parent compound in feces and urine.4,5 It binds extensively to plasma proteins (93%), and distributes into extracellular fluid with a volume of distribution at steady-state of 15.9 liters.6,7 In healthy volunteers, following a single, 1000 mg dose, the mean (% coefficient of variation) maximal drug concentration (Cmax) and terminal half-life (t1/2) were 287 mg/L (13.9%) and 14.4 days (16.5%), respectively.6 In a population pharmacokinetic (PK) analysis of adult subjects mostly with skin and skin structure infections, a t1/2 of 8.5 days was observed.7 This long half-life and favorable clinical efficacy results led to the approval of a two-dose regimen in adults: 1000 mg followed by 500 mg one week later.
There are limited data available evaluating the use of dalbavancin in the pediatric population. One study evaluated dalbavancin PK following intravenous administration of a single dose in 10 adolescents 12 to 17 years of age, and reported a t1/2 of approximately 9 days, comparable exposures between a 1000 mg and 15 mg/kg single doses, and an acceptable safety profile.8 We performed an open-label, multi-center study to characterize the PK and safety of dalbavancin in hospitalized children 3 months to 11 years of age. We then combined the data from this study with the previously published adolescent data to develop a population PK model that can inform dosing across pediatric age groups.
MATERIALS AND METHODS
Patient population
This was a phase 1, open-label, multi-center (N=10) study to investigate the PK, safety, and tolerability of a single intravenous dose of dalbavancin in hospitalized pediatric patients with a suspected or confirmed bacterial infection (Study DUR001-106; ClinicalTrials.gov #NCT01946568). Subjects were enrolled in three age cohorts: 6 to 11 years of age, inclusive; 2 to <6 years of age; and 3 months to <2 years of age. All study subjects received a single-dose of dalbavancin in addition to standard of care anti-infective treatment chosen at the discretion of the treating physician. The study was reviewed and approved by the each institution’s institutional review board. The first and last subjects were enrolled on August 13, 2013, and March 23, 2015, respectively.
The following study inclusion criteria were used: hospitalized subjects 3 months to 11 years of age (inclusive) who received systemic anti-infective treatment other than glycopeptide antibiotics for suspected or confirmed bacterial infections; legal guardian consent, and if required by the local institutional review board, assent from subjects in the two older age cohorts; and subjects were expected to survive with appropriate antibiotic therapy and supportive care. Exclusion criteria were: treatment with an investigational drug within 30 days, concurrent treatment with intravenous vancomycin, clinically significant laboratory abnormalities not associated with the underlying disease, albumin concentration (ALB) less than half the lower limit of normal, subjects that were less than 1 year post-natal age born with a gestational age less than 32 weeks, positive urine (or serum) pregnancy test, known hypersensitivity to glycopeptides, a calculated creatinine clearance <30 mL/min/1.73 m2 (according to Schwartz method9), pregnant or nursing females, and cystic fibrosis. Because historical data from other antibiotics’ PK suggested that alterations in drug clearance may occur in cystic fibrosis, the DUR001-106 protocol was amended to exclude cystic fibrosis after 3 subjects (2 of whom were dosed with study drug) with cystic fibrosis had already been enrolled.
We also used dalbavancin PK data collected from adolescents in a previously published clinical trial (Study A8841004).8 Briefly, this was an open-label, multi-center study to evaluate the PK and safety of single-dose dalbavancin in adolescents 12–17 years of age. Subjects that were hospitalized and required parenteral antibiotic treatment for known or suspected Gram-positive infections were eligible for enrollment.
Drug dosing and sample collection
For the current study performed in children 3 months to 11 years of age (Study DUR001-106), at study initiation, dalbavancin dosing was 15 mg/kg (not to exceed the 1000 mg adult dose) for subjects ≥5 years of age, and 25 mg/kg for <5 years of age. The dosing regimen was selected using a population PK model that was developed using available adult and adolescent (Study A8841004) dalbavancin concentration versus time data. After performing an interim PK analysis with data from 18 subjects (N=11, 6 to 11 years, inclusive; N=7, 2 to <6 years), a dose of 10 mg/kg (maximum of 1000 mg) was selected for children 3 months to <2 years of age in order to achieve exposure comparable to adults. Dalbavancin was administered as a 30-minute intravenous infusion.
Whole blood samples (0.5 mL) for PK assessment were collected in tubes containing di-potassium ethylenediaminetetraacetic acid (K2-EDTA) at pre-defined time points on days 1, 2, 7, and 28 following dalbavancin administration. On day 1, samples were collected at 0.5 hours after the end of the infusion (±5 minutes), and at 4 hours (±2 hours) and 12 hours (±2 hours) post-start-of-infusion. Additional samples were collected at 24 hours (±4 hours, day 2), 144 hours (±2 days, day 7), and 648 hours (±4 days, day 28). PK samples were collected from the arm contralateral to the infusion, but if not possible, samples (other than the 0.5 hour time point) could be drawn from the same line in which dalbavancin was infused. The whole blood samples were centrifuged at approximately 3000 rpm at 4°C for 10 minutes to harvest the plasma. Plasma samples were then frozen (−20°C freezer) within 5–10 minutes and kept frozen until analysis.
In the adolescent PK study (Study A8841004), a single dose of 1000 mg was given as a 30 minute intravenous infusion to subjects ≥60 kg, or 15 mg/kg intravenously for those less than 60 kg.8 Blood samples were collected at the following time points: pre-dose, 0.5, 1, 2, 4, 12, 24, 48, 144 (day 6), 312 (day 13), 480 (day 20), 648 (day 27), and 1320 hours (day 55) post-dose.8 Of note, a day 55 PK sample was not collected in children enrolled in DUR001-106 because most of the drug was shown to be eliminated by day 27 in the adolescent study.
Analytical methods
Dalbavancin plasma concentrations were measured by a commercial laboratory (Tandem Labs – a LabCorp Company, West Trenton, NJ) using a validated liquid chromatography-tandem mass spectrometry (LC-MS/MS) method. The following instrumentation was used: MDS Sciex API 4000 mass spectrometer (Thornhill, Ontario); Shimadzu SIL-5000 autosampler and LC-10ADVP pump (Columbia, MD); and a Fortis Phenyl, 2.1×50 mm, 5 µm chromatographic column (Cheshire, UK). A gradient mobile phase containing 0.1% formic acid (aq), methanol; acetonitrile (50:50, v:v), and acetone was used. The lower and upper limits of quantification were 0.5 and 500 µg/mL, respectively. Plasma was extracted using protein precipitation. The overall precision and accuracy for quality control samples was 1.8% to 8.9% and −6.7% to −2.0%, respectively. Long term stability studies were performed and showed that dalbavancin plasma samples were stable at 368 days when stored at −20°C. All clinical PK samples were analyzed within the validated period.
Structural model development
Population PK analyses were performed using the software NONMEM (version 7.1.2, Icon Development Solutions, Ellicott City, MD) with the PK dataset of pediatric subjects 3 months to 11 years of age combined with the previously collected adolescent PK data.8 The first-order conditional estimation method with η-ε interaction was used for all model runs. Data assembly was performed using SAS (version 9.4, SAS Institute, Inc., Cary, NC). Dalbavancin concentration values that were below the quantification limit (BQL) were excluded from the analysis.
Dalbavancin plasma concentrations appeared to decline in a poly-phasic manner following completion of 30-minute intravenous infusion. A three-compartment structural model with linear elimination was used to describe the data. The potential effects of clinical covariates on PK parameters were evaluated and simplified models were constructed using only those covariates deemed most significant. To account for the growth effects, total body weight (WT) was included in the base model prior to assessment of other covariates using an allometric scaling approach.10,11 Scaling with total body weight was performed on the following PK parameters: total clearance (CL), distributional clearance to peripheral compartment 1 (CLd1), distributional clearance to peripheral compartment 2 (CLd2), central volume of distribution (Vc), volume of distribution to peripheral compartment 1 (Vp1), and volume of distribution to peripheral compartment 2 (Vp2). The coefficients describing the relationship between WT and population PK parameters were estimated from the data.
Covariate analysis
After accounting for body size differences using WT, the following additional covariates were explored for model inclusion: age, height, body surface area (BSA), sex, estimated creatinine clearance (CrCl), and ALB. Individual values for each of these covariates were available for all study subjects. BSA was calculated using the method of Gehan and George.12 In children 3 months to 11 years of age, CrCl was calculated from baseline serum creatinine and height using the Schwartz equation.13 In adolescents 12 to 17 years of age, CrCl was calculated from serum creatinine, age, and total body weight using the Cockcroft and Gault equation and then normalized to BSA.14
In order to characterize age-dependent changes in renal function, CrCl estimates were fitted as a function of age as shown in Equation 1.
| (1) |
Where Base is the baseline creatinine clearance at birth, Rmax is the maximum increase of CrCl, PNA is post-natal age in months, Hill is a slope parameter, and TMr corresponds to the age that achieves 50% of the maximum increase in CrCl. The fitted CrCl values obtained from this renal function developmental sub-model were then tested as a covariate in the population PK analysis.
The statistical significance of covariate relationships was evaluated using a forward inclusion (p<0.05 and change in objective function value > 3.8) and backward elimination (p<0.001 and change in objective function value >10.8) approach.
Derivation of Dalbavancin Exposure Estimates and Secondary Pharmacokinetic Parameters
Using the dosing history and post-hoc PK parameter estimates for each subject, a simulation was performed to generate a PK profile from 0 to 648 hours after the start of drug administration. Then the following dalbavancin exposure estimates and secondary PK parameters were calculated: Cmax, area under the dalbavancin plasma concentration versus time (AUC) from 0 to 120 hours (AUC0–120), AUC from 0 to 168 hours (AUC0–168), AUC from 0 to 648 hours (AUC0–648), AUC from 0 to infinity (AUC0–inf), steady-state volume of distribution (Vss), and the terminal half-life. AUC0–120, AUC0–168, and AUC0–648 were calculated by integrating the concentration versus time curve over the defined time interval, whereas AUC0–inf was calculated as dose divided by CL. Vss was calculated as the sum of fitted estimates of the three volumes of distribution (Vc, Vp1, and Vp2). The terminal half-life was calculated using the post-hoc slope of gamma phase based upon the three compartment model PK equations.15
Model evaluation
The following criteria were used to guide the model development process: evaluation of individual and population mean PK parameter estimates and their precision, as measured using the percent standard error of the estimate; graphical examination of standard diagnostic plots; graphical examination of the observed and individual post-hoc predicted concentration time data; reduction in both residual and inter-individual variability; and comparison of the objective function value for nested models.
Using the final model, parameter precision was also assessed using a nonparametric bootstrap resampling technique. A total of 1000 resampled new datasets were created using the software SAS (version 9.4, SAS Institute, Inc., Cary, NC), and the median and 90% confidence interval of parameter estimates was computed. Also, a visual predictive check based on 1000 simulations was performed to assess the ability of the final model to describe the observed concentration-time data.
Dosing simulations
A virtual pediatric population was generated to perform dosing simulations. Age was simulated to approximate uniform distributions of 1000 subjects 3 to 23 months of age and 1000 patients 2 to 18 years of age. Approximately 50% of virtual subjects were assigned to each sex. ALB was simulated using a normal distribution with mean of 3.25 g/dL and standard deviation equal to 0.6 g/dL.
Then two z-scores were simulated from a normal distribution for each virtual subject with a mean of zero and standard deviation of 0.75. The first z-score (Z), along with the age- and gender-specific median (M), generalized coefficient of variation (S), and the power in the Box-Cox transformation (L) values from the Centers for Disease Control and Prevention (CDC) growth charts16 were used to obtain a value of height for each patient according to Equation 2 or 3.
| (2) |
| (3) |
WT was also calculated for each virtual subject using a similar approach and age- and gender-specific L, M and S values from the CDC growth charts,16 with one exception: the z-score was obtained by multiplying the first z-score by the probability of the second z-score from a normal distribution. This allowed for some variability in the height and weight-percentiles, as is seen clinically.
Using the final model, Monte Carlo simulations were performed to identify pediatric dosing that visually matches dalbavancin AUC0–120 distributions observed in a phase 3 adult program. The value of AUC0–120 in adults receiving a single dose of 1000 mg IV dalbavancin was obtained from the phase 3 program, where the median [90% confidence interval] was 6900 µg*h/mL [4400, 10600] (sponsor data, Durata Therapeutics, a subsidiary of Actavis plc, Branford, Connecticut). The value of AUC0–120 in adults receiving a single dose of 1500 mg IV dalbavancin was calculated from the 1000 mg IV AUC0–120 estimate given that the PK is linear, where the median [90% confidence interval] was 10350 ug*h/mL [6600, 15900].
Safety evaluation
All subjects that received study drug completed safety assessments, which included adverse event (AE) monitoring, clinical laboratory results, vital signs, weight measurement, physical examination findings, and evaluation of concomitant medications. AE monitoring included documentation of serious and non-serious AEs, timing and severity of AEs, relationship to study drug, actions taken to resolve the AE, and outcome. Both clinical and laboratory AEs were monitored. AEs were reported from the day of consent through 28 calendar days following study drug administration. Among subjects 3 months through 11 years of age, an audiologic assessment was performed by a licensed pediatric audiologist within 7 days before dalbavancin administration and repeated at day 28. Audiologic testing included distortion product otoacoustic emissions (DPOAE) and tympanograms. A few subjects also received pure tone air threshold testing (audiograms).
RESULTS
Patient characteristics
A total of 44 subjects were enrolled; 34 in the current study (DUR001-106) and 10 in the adolescents study (A8841004). A total of 23 (7%) samples were either missing or BQL and were dropped from the analysis, including one subject where all plasma concentrations (N=8) were uninterpretable due to thawing during transit to the bioanalysis laboratory. Dosing and patient demographic information for the remaining 43 subjects are presented in Table 1. These 43 subjects contributed 311 samples for the PK analysis.
Table 1.
Clinical data used for population PK model development.
| Study | DUR001-106 | A88410048 | |||
|---|---|---|---|---|---|
| Variable | 3 months to <2 years (N=11) |
2 years to <6 years (N=11) |
6 to 11 years (N=11) |
12 to 17 years (N=10) |
Total (N=43) |
| Dalbavancin dose (mg/kg) | 10.2 (9.7–10.6) |
25.1 (15.1–25.7) |
15.0 (10.9–15.2) |
15.0 (9.5–16.2) |
15.0 (9.5–25.7) |
| Total dalbavancin dose (mg) | 100.0 (60–130) |
362.5 (275–425) |
465.0 (300–1000) |
943.0 (720 – 1000) |
400.0 (60 – 1000) |
| Age (years) | 0.8 (0.3–1.6) |
3.0 (2.3–5.9) |
9.0 (6.8–11.8) |
15.7 (12.4–16.9) |
5.9 (0.3–16.9) |
| Weight (kg) | 9.6 (5.7–13.0) |
15.7 (10.7–18.9) |
31.4 (19.8–92.0) |
60.4 (47.9–105.2) |
18.9 (5.7–105.2) |
| Albumin (g/dL) | 3.4 (2.9–4.5) |
3.6 (2.2–4.5) |
4.1 (3.1–4.4) |
3.0 (1.9–4.6) |
3.6 (1.9–4.6) |
| Calculated creatinine clearancea (mL/min/1.73 m2) |
70.7 (49.8–174.9) |
154.1 (94.5–206.5) |
127.5 (91.8–191.6) |
101.3 (54.7–132.2) |
115.4 (49.8–206.5) |
| Male (%) | 9 (82) | 6 (55) | 9 (82) | 7 (70) | 31 (72) |
Population Pharmacokinetic analysis
A three-compartment model with zero-order infusion input and first-order, linear elimination described the dalbavancin PK data well. After accounting for body size differences using WT on all PK parameters, the following additional covariate relationships were identified to be statistically significant during the forward covariate selection: ALB on clearance CL and Vc, and CrCl on CL.
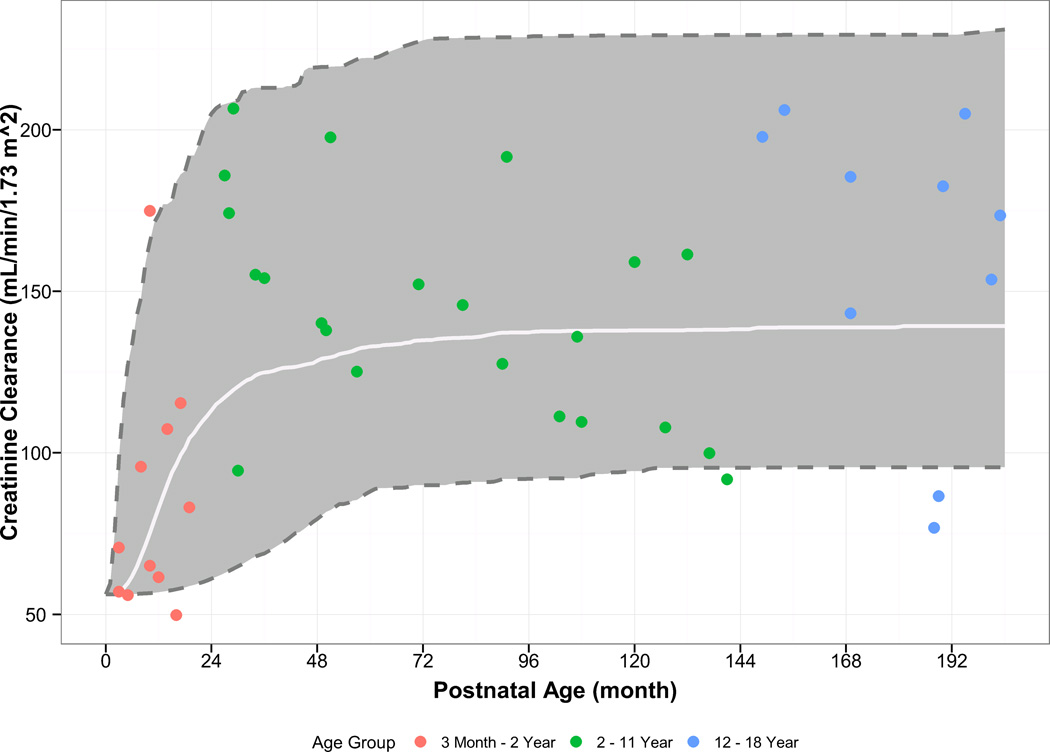
In order to account for reduced renal function in children less than 2 years of age, a hyperbolic sigmoidal function that describes age-varying changes in CrCl was used. The fitted values from this function were then used to account for the relationship between CrCl and CL. The parameter estimates and the fitted relationship are shown in Supplementary Table 1 and Figure 1. The power coefficient of CrCl on CL was estimated at 0.52 with high standard error of the mean (93.1%), suggesting a shallow relationship that is not unexpected given the limited number of subjects with a CrCl below 80 mL/min/1.73 m2. Upon backward elimination the relationship between CrCl and CL was not statistically significant, and was not retained in the final model.
Figure 1.
Visual comparison of observed and simulated creatinine clearance in children and adolescents, stratified by age. The white line/grey area represent the median/90% confidence interval for the model simulations.
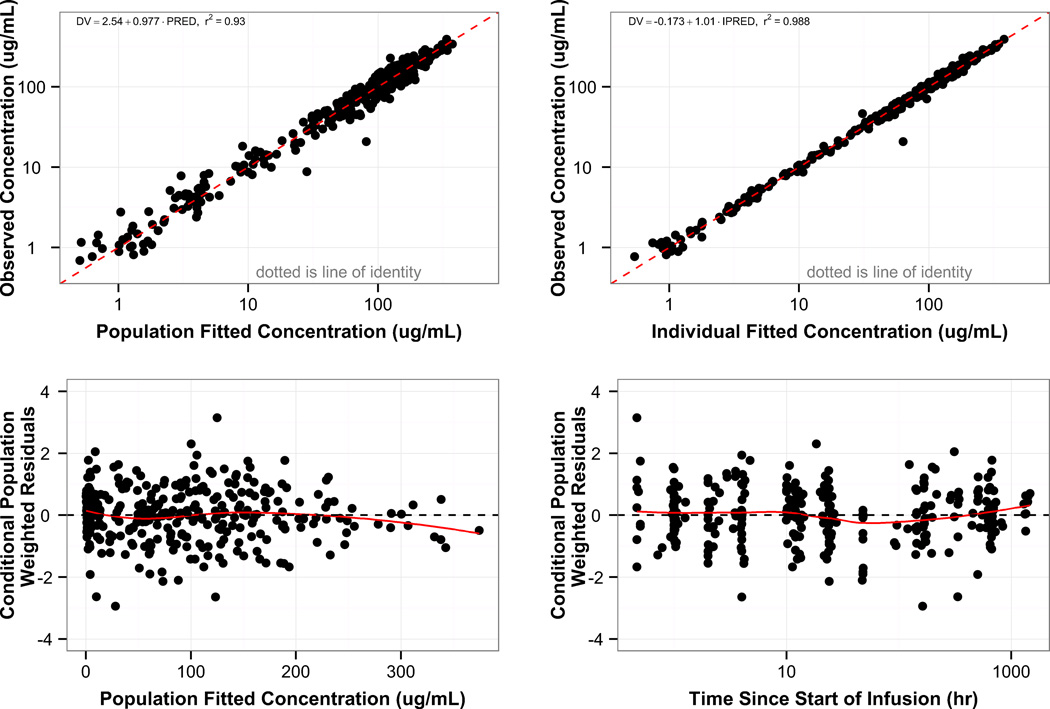
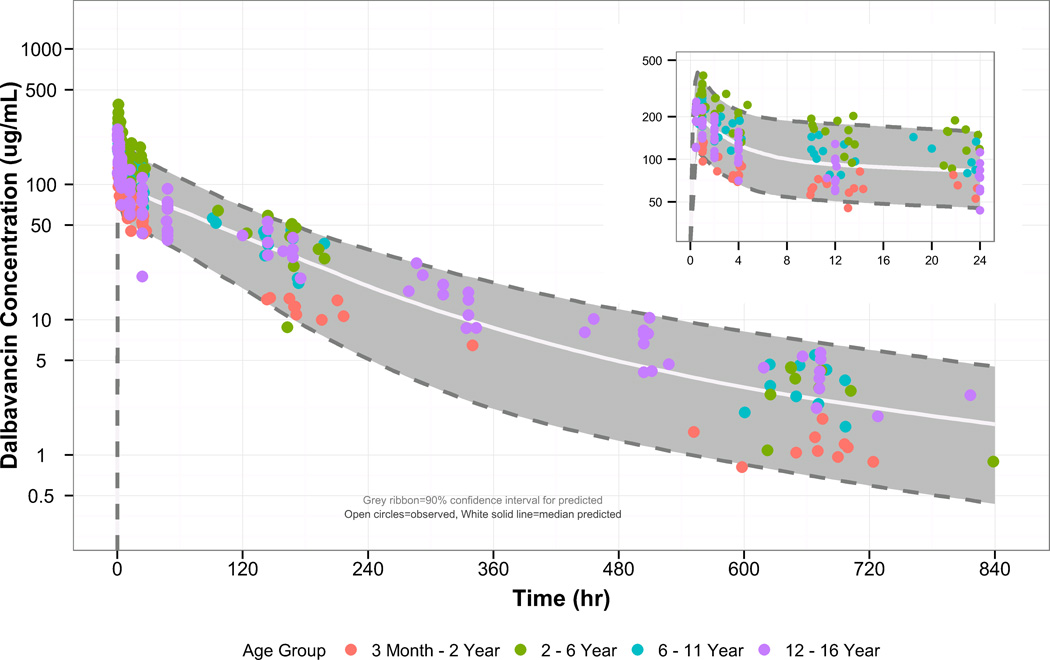
Diagnostic plots and a visual predictive check for the final population PK model are shown in Figures 2 and 3. The final population PK parameter estimates and individual post-hoc estimates are shown in Tables 2 and 3. The final population PK model had the following estimates:
As expected, subjects who were age 2 to 5 years and received a 25 mg/kg dose had the highest exposure estimates (e.g., AUC0–120, Cmax). Also, weight-adjusted CL decreased with age. The individual post-hoc PK parameters and exposure estimates of the two cystic fibrosis subjects were comparable to other subjects within the same age group (6 to 11 years).
Figure 2.
Goodness of fit for the final population PK model. Individual and population predicted concentrations are plotted versus observed dalbavancin concentrations (top row). Conditional weighted residuals are plotted versus population predicted concentrations and time since start of the infusion (bottom row). The red solid line in the bottom row represents the loess curve.
Figure 3.
Visual comparison of the observed and simulated dalbavancin concentrations in pediatric subjects, stratified by post-natal age. The grey region denotes the 90% confidence interval for the predictions, the circles denote the observed concentrations, and the white solid line is the median for the predictions. The inset graph is a visual comparison of the data using a smaller time scale (0 – 24 hours after first dose).
Table 2.
Final population pharmacokinetic model parameter estimates.
| Final Model | Bootstrap (N=1000) | ||||
|---|---|---|---|---|---|
| Parameter | Estimate | % SEM | 5th Percentile |
Median | 95th Percentile |
| CL (L/hr) | 0.033 | 6.90 | 0.031 | 0.033 | 0.035 |
| Vc (L) | 2.46 | 7.80 | 2.28 | 2.45 | 2.67 |
| CLd1 (L/hr) | 0.51 | 15.0 | 0.44 | 0.50 | 0.58 |
| Vp1 (L) | 2.90 | 9.40 | 2.52 | 2.86 | 3.23 |
| CLd2 (L/hr) | 0.0065 | 12.3 | 0.006 | 0.007 | 0.008 |
| Vp2 (L) | 2.93 | 62.1 | 2.24 | 2.87 | 3.99 |
| Power coefficient of weight on CL parametersa |
0.71 | 8.70 | 0.66 | 0.71 | 0.77 |
| Power coefficient of weight on volume parametersb |
0.95 | 5.80 | 0.88 | 0.95 | 1.03 |
| Power coefficient of albumin on CLc | −0.78 | 34.1 | −1.02 | −0.76 | −0.41 |
| Power coefficient of albumin on Vcd | −0.73 | 47.1 | −1.02 | −0.72 | −0.46 |
| ω2_CL (IIV as %) | 0.034 (18.4) | 44.4 | 0.015 (12.4) | 0.030 (17.4) | 0.051 (22.5) |
| ω2_Vc (IIV as %) | 0.035 (18.8) | 35.7 | 0.019 (13.8) | 0.032 (17.8) | 0.050 (22.3) |
| ω2_Vp1 (IIV as %) | 0.090 (29.9) | 36.9 | 0.032 (17.9) | 0.081 (28.5) | 0.139 (37.3) |
| σ2_Plasma proportional error | 0.01 | 5.30 | 0.005 | 0.01 | 0.02 |
| σ2_Plasma additive error | 0.25 | Fixed | NE | NE | NE |
CL: clearance; Vc central volume of distribution; CLd1 distributional clearance to peripheral compartment 1; CLd2 distributional clearance to peripheral compartment 2; Vp1 volume of distribution for peripheral compartment 1; Vp2 volume of distribution for peripheral compartment 2. IIV: inter-individual variability. NE: not evaluable. ω2 variance of random effect parameter for inter-individual variability in a specific PK parameter. σ2 variance of random effect parameter for intra-individual (i.e., residual) variability.
The same power coefficient used for all clearance parameters (CL, CLd1, CLd2). The following relationship was used to account for the effect of body weight on clearance parameters: ((Weight in kg)/40.9)0.71.
The same power coefficient used for all volume of distribution parameters (Vc, Vp1, Vp2). The following relationship was used to account for the effect of body weight on volume of distribution parameters: ((Weight in kg)/40.9)0.95.
Effect of albumin on CL expressed using the following relationship: (Albumin/3.7)−0.78.
Effect of albumin on Vc expressed using the following relationship: (Albumin/3.7)−0.73.
Table 3.
Empirical Bayesian estimates and derived PK parameters stratified by age group.
| Study | DUR001-106 | A8841004 | |||
|---|---|---|---|---|---|
| Variable | 3 months to <2 years (N=11) |
2 years to <6 years (N=11) |
6 to 11 years (N=11) |
12-17 years (N=10) |
Total (N=43) |
| AUC0–120 (µg*h/mL) | 5120 (4090–6460) |
12400 (7060–16300) |
9000 (6660–12100) |
7830 (5530–11100) |
8210 (4090–16300) |
| AUC0–168 (µg*h/mL) | 5900 (4780–7530) |
14600 (7690–19100) |
11000 (7870–14500) |
9670 (6940–13700) |
10000 (4780–19100) |
| AUC0–648 (µg*h/mL) | 7500 (6330–10200) |
20500 (8520–26500) |
16300 (10800–21400) |
15900 (11300–22500) |
15100 (6330–26500) |
| AUC0–inf (µg*h/mL) | 7890 (6630–11000) |
22100 (8670–28800) |
18200 (11500–24000) |
18000 (12400–25800) |
16700 (6630–28800) |
| Cmax(µg/mL) | 141 (114–192) |
328 (221–443) |
247 (183–289) |
198 (129–252) |
219 (114–443) |
| CL (mL/h) | 12.9 (8.79–16.6) |
15.8 (9.56–47.5) |
27.7 (17.0–54.8) |
51.6 (38.1–80.5) |
19.5 (8.79–80.5) |
| CL_WT (mL/h/kg) | 1.23 (0.957–1.55) |
1.05 (0.893–2.90) |
0.795 (0.596–1.32) |
0.798 (0.494–1.09) |
1.01 (0.494–2.90) |
| Vc (L) | 0.604 (0.421–0.887) |
1.01 (0.546–1.54) |
2.08 (1.35–4.12) |
4.37 (2.68–6.60) |
1.35 (0.421–6.60) |
| Vss (L)a | 2.21 (1.39–2.69) |
3.30 (1.99–4.19) |
6.93 (4.55–16.2) |
14.4 (11.8–22.1) |
4.19 (1.39–22.1) |
| Terminal half-life (h) | 279 (244–298) |
315 (271–332) |
390 (317–490) |
435 (395–519) |
328 (244–519) |
Estimates are reported as median (range) for all parameters.
AUC0–120 area under the dalbavancin plasma concentration versus time curve from time zero to 120 hours; AUC0–168 area under the dalbavancin plasma concentration versus time curve from time zero to 168 hours; AUC0–648 area under the dalbavancin plasma concentration versus time curve from time zero to 648 hours; AUC0–inf area under the dalbavancin plasma concentration versus time curve from time zero to infinity; Cmax maximal plasma concentration; CL: clearance; CL_WT: body-weight adjusted clearance; Vc central volume of distribution; Vss steady-state volume of distribution. Vp1 volume of distribution for peripheral compartment 1; Vp2 volume of distribution for peripheral compartment 2.
Vss was calculated as the sum of the individual fitted estimates for the three volumes of distribution (Vc, Vp1, and Vp2).
Simulations
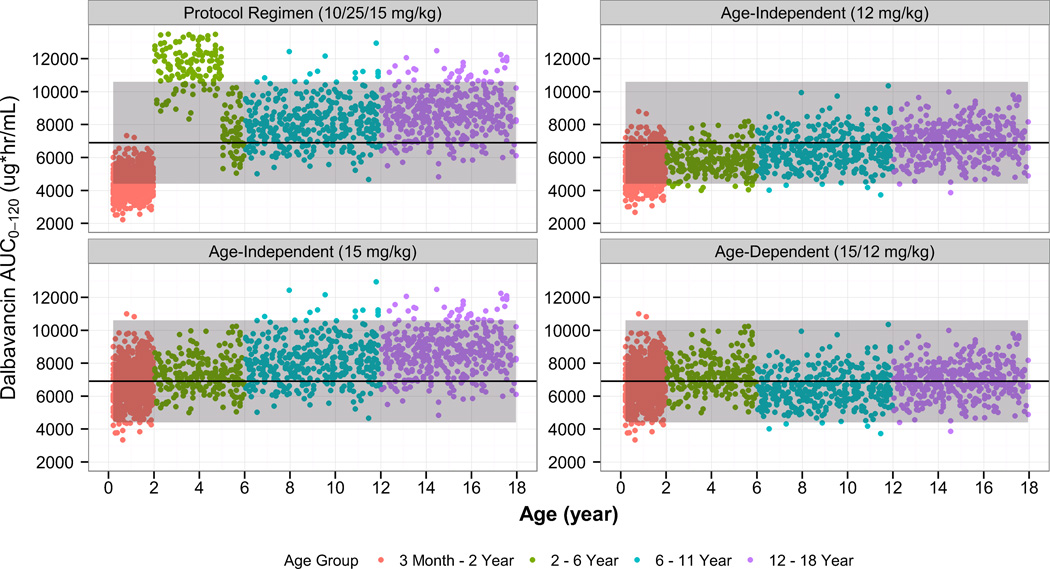
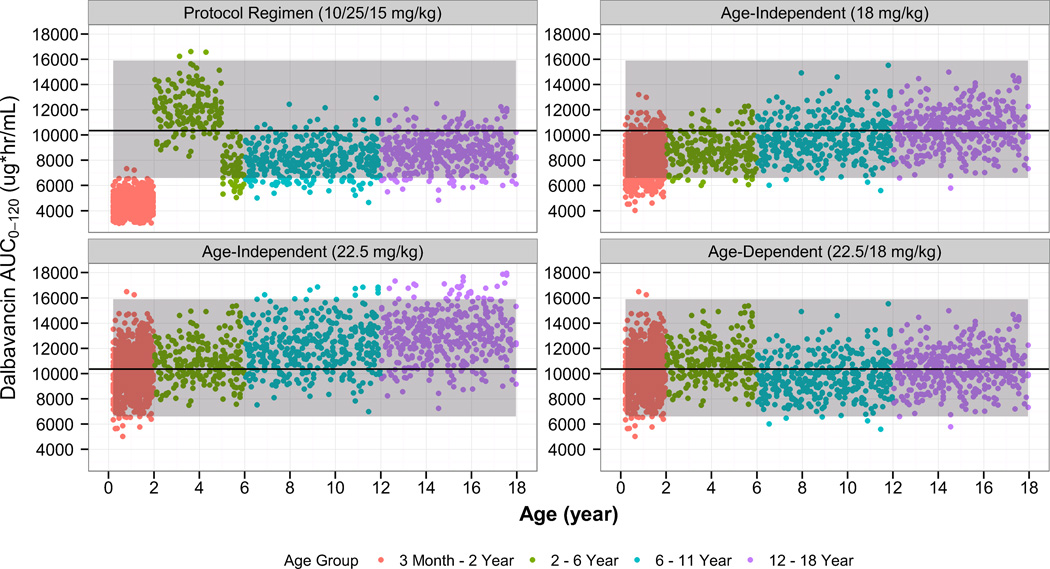
Monte Carlo simulations were performed using the final population PK model in order to identify optimal pediatric dosing. Simulated AUC0–120 estimates following dalbavancin dosing regimens designed to match a 1000 mg and 1500 mg adult dose are shown in Figures 4 and 5, respectively.
Figure 4.
Comparison of the simulated dalbavancin AUC0–120 estimates in pediatric subjects of age 3 months to 18 years using various age-dependent dosing regimens to the distribution of AUC0–120 estimates from the adult, phase 3 program (dalbavancin day 1 dose of 1000 mg). The solid line/shaded area represent median/90% confidence interval of AUC0–120 estimates from the adult, phase 3 program (dalbavancin day 1 dose of 1000 mg).
Figure 5.
Comparison of the simulated dalbavancin AUC0–120 estimates in pediatric subjects of age 3 months to 18 years using various age-dependent dosing regimens to the distribution of AUC0–120 estimates from the adult, phase 3 program (dalbavancin day 1 dose of 1500 mg). The solid line/shaded area represent median/90% confidence interval of AUC0–120 estimates from the adult, phase 3 program (dalbavancin day 1 dose of 1500 mg).
Based on these results, the following age-dependent dosing regimen was found to achieve similar dalbavancin exposure to that in adults administered a 2-dose regimen (1000 mg on day 1 plus 500 mg on day 8): children age 6 to < 18 years, 12 mg/kg on day 1 (1000 mg maximum) and 6 mg/kg on day 8 (500 mg maximum); children age 3 months to < 6 years, 15 mg/kg (1000 mg maximum) on day 1, and 7.5 mg/kg (500 mg maximum) on day 8.
Similarly, the following age-dependent regimen was found to match adult single-dose exposure (1500 mg): children age 6 to <18 years, 18 mg/kg (1500 mg maximum) on day 1; children age 3 months up to < 6 years, 22.5 mg/kg (1500 mg maximum) on day 1.
Safety
For children 3 months to 11 years of age (N=34; including the child that was excluded from the PK analysis), a total of 39 AEs occurred in 19 subjects---9 in the age 6 to 11 years cohort (N=6, 54.5%), 23 in the age 2 to <6 years cohort (N=9, 75.0%), and 4 in the age 3 months to <2 years cohort (N=4, 36.4%). Among the 36 treatment-emergent AEs, 4 were considered severe, 6 were moderate, and 26 were mild. Five subjects experienced a serious AE. None of the serious or severe treatment-emergent AEs were considered related to dalbavancin treatment. The following AEs were deemed possibly related to dalbavancin treatment (N=1 each): rash, diaper dermatitis, urticaria, and asymptomatic hepatic enzyme elevation. One subject had infusion site discomfort that was probably related to study treatment.
One cystic fibrosis patient, receiving concomitant medications including aztreonam and azithromycin, developed urticaria on day 11. On day 12, piperacillin/tazobactam and tobramycin were added. The subject was diagnosed with drug eruption and the rash had completely resolved by day 78. The other cystic fibrosis patient that developed an asymptomatic hepatic enzyme elevation (day 7) had a history of aspartate aminotransferase (AST) and alanine aminotransferase (ALT) elevation that had resolved spontaneously. Mildly elevated hepatic enzymes were observed on the day prior to study drug administration. The subject had received concomitant medications that are known to cause elevation of AST and ALT, including ceftazidime, tobramycin and ciprofloxacin. Hepatic enzymes returned to normal by day 27.
Audiology testing was difficult to perform and interpret in this subject population. Difficulties included lack of cooperation due to age and underlying illness. Considering these limitations, there was no observed evidence of ototoxicity due to dalbavancin administration in a majority of subjects. Of the 34 treated subjects, 31 had both tympanogram and DPOAE results for both ears at baseline, and 11 subjects completed baseline audiograms. End of study results were available for 32 subjects (2 were lost to follow-up), 29 of whom had tympanogram and DPOAE results, and 12 subjects had audiogram results available. Of 34 treated subjects, 21 (62%) had no evidence of ototoxicity due to dalbavancin administration. For the remainder of subjects, no determination of ototoxicity could be made.
DISCUSSION
Dalbavancin PK, safety, and tolerability have been previously studied in adolescents, age 12 to 17 years, receiving a single-dose of dalbavancin 1000 mg (60 kg or greater) or 15 mg/kg (< 60 kg).8 Both groups were found to have similar exposures and apparent half-life but exposure was slightly lower (~30%) than that observed in randomized comparative studies of adults with skin and skin structure infections.8 In the current study, a population PK model was developed using these previously published adolescent data and PK samples collected from children age 3 months to 11 years. We focused on analyzing the data using a population PK approach because we wanted to leverage the data from the previous adolescent PK trial to develop a robust pediatric population PK model that incorporates all available data. Combining data from both studies allowed us to fully categorize disposition characteristics of dalbavancin in pediatric subjects’ age greater than 3 months. Also, a population PK analysis was performed because we sought to identify optimal dalbavancin dosing in children, and development of this would help fulfill this objective. Last, a population PK analysis allowed for estimation of inter-individual variability and identification of covariates that explain some of this variability, which is important when you then apply the model to perform dosing simulations.
The dalbavancin population PK model accounted for the effect of WT using allometric coefficients that were estimated to be 0.71 and 0.95 for CL and Vc, respectively, which are similar to physiologically relevant values of 0.75 and 1.10 This is an interesting finding because the primary elimination mechanism for dalbavancin is not well understood. In spite of this unknown, estimates of allometric coefficients closely matched physiologically relevant coefficients characterized for drugs with renal and hepatic elimination.10,17 In addition to WT, albumin was found to result in a significant reduction in the objective function value, which is consistent with the high degree of protein binding (93% in human plasma) of dalbavancin. A hyperbolic sigmoidal function was used to account for age-varying renal function in study participants. Using this function, CrCl was found to be a significant covariate in the forward selection step, but was not retained in the model during backward elimination. Although the average CrCl was lower in children age < 2 years, the relationship did not rise to the level of clinical and statistical significance, likely due to the limited number of participants with a calculated CrCl < 80 mL/min/1.73 m2.
In adults, a 2-dose dalbavancin regimen (1000 mg on day 1 and 500 mg on day 8) has been shown to be as effective as linezolid for complicated skin and skin structure infections.1 In addition, the 2-dose regimen in adults has been shown to be noninferior to intravenous vancomycin followed by oral linezolid treatment.2 Subsequently, the one dose dalbavancin regimen (1500 mg on day 1, the same total dose as the previous 2-dose regimen) has been shown to be noninferior to the 2-dose regimen in adults.3 We performed dosing simulations using the final population PK model to identify pediatric doses that match exposure observed in the adult phase 3 program following a 1- or 2-dose regimen. Based on these simulations, an increased mg/kg dosing regimen in younger subjects is suggested, which is consistent with the increased and expected weight-normalized clearance values observed in children age 3 months to < 6 years. Additionally, a shallow relationship between dalbavancin exposure (i.e., AUC) and albumin was identified, which has no clinical relevance (i.e., relatively small changes in exposure with varying albumin concentrations), and therefore dalbavancin dose modification on the basis of albumin in children 3 months to less than 18 years is not warranted.
Dalbavancin was well tolerated in the current study and there were no serious or severe AEs related to treatment. Also, no deaths were reported. There was no evidence of ototoxicity due to dalbavancin administration in the majority of subjects; for the remainder, no determination could be made. The safety profile we observed in children is consistent with that observed in adults.
Supplementary Material
Acknowledgments
Durata Therapeutics International B.V., Branford, CT: Xilla T. Ussery, MD, Sailaja Puttagunta, MD, Michael W. Dunne, MD
Allergan, Jersey City, NJ: T.J. Carrothers, ScD
Institute for Clinical Pharmacodynamics, Latham, NY: Harish Ganesan, MS, Patricia A. Queeno, BA, Christopher M. Rubino, PharmD, Michael Trang, PharmD, Scott Van Wart, MS, PhD, Li Zhang, MD, PhD
Study Team, Principal Investigators, and Study Coordinators:
Duke Clinical Research Institute: Michael Cohen-Wolkowiez, MD, PhD (Study PI) and Jennifer Murphy, MS, CCRA, PMP (Project Leader)
Clinical Trial Sites: John Bradley, MD (PI) and Sara Hingtgen (SC), Rady Children’s Hospital, San Diego, CA; Jeffrey Blumer, PhD, MD (PI) and Bonnie Rosolowski, RRT, CCRC (SC), University of Toledo Medical Center, Toledo, OH; Ram Yogev, MD (PI) and Laura Fearn (SC), Ann & Robert H. Lurie Children’s Hospital of Chicago, Chicago, IL; Kevin M. Watt, MD (PI) and Samantha Wrenn (SC), Duke University Medical Center, Durham, NC; Laura James, MD (PI), and Lee Howard, RN, CCRC (SC), Arkansas Children’s Hospital Research Institute, Little Rock, AR; Debra Palazzi, MD (PI), and Farida Khetani, MBBS, MPH (SC), Baylor College of Medicine, Houston, TX; Varsha Bhatt-Mehta, PharmD (PI) and Nicholas Harris (SC), University of Michigan Medical Center, Ann Arbor, MI; Janice E. Sullivan, MD (PI) and Susan Poff (SC), Kosair Charities Pediatric Clinical Research Unit, Department of Pediatrics, University of Louisville, and Kosair Children’s Hospital, Louisville, KY.
CONFLICT OF INTEREST
The study was funded by Durata Therapeutics, Inc. D.G. receives support for research from the National Institute for Child Health and Human Development (K23HD083465), the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org), and from industry (Cempra, Inc. and Jacobus Pharmaceutical Company, Inc.) for drug development in adults and children. J.S.B. received no personal compensation for participation in the study or generation of the manuscript, but his employer, the University of California, received funds for conduct of the study and consultation with Durata Therapeutics, Inc. J.B., R.Y., K.M.W., L.P.J., D.L.P., V.B-M, and J.E.S. received research support from Durata Therapeutics, Inc. to serve as an investigator in this study. X.U. was a consultant to Allergan, Inc. and received a consultant fee for her contributions to this study. S.P. was an employee of Allergan, Inc. and received salary and stock options for her contributions to this study. M.W.D. was an employee of Allergan, Inc. and received salary and speaker honorarium for his contributions to this study. M.C-W. receives support for research from the NIH (1R01-HD076676-01A1), the National Center for Advancing Translational Sciences of the NIH (UL1TR001117), the National Institute of Allergy and Infectious Disease (HHSN272201500006I and HHSN272201300017I), the National Institute for Child Health and Human Development of the NIH (HHSN275201000003I), the Food and Drug Administration (1U01FD004858-01), the Biomedical Advanced Research and Development Authority (BARDA) (HHSO100201300009C), the nonprofit organization Thrasher Research Fund (www.thrasherresearch.org), and from industry for drug development in adults and children (www.dcri.duke.edu/research/coi.jsp). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Footnotes
AUTHOR CONTRIBUTIONS
D.G. and M.C-W. wrote the manuscript. L.Z. analyzed the data. M.C-W. designed the research. D.G., J.S.B., J.B., R.Y., K.M.W., L.P.J., D.L.P., V.B-M, J.E.S., J.M., X.T.U, S. P., M.W.D., and M.C-W. performed the research.
REFERENCES
- 1.Jauregui LE, Babazadeh S, Seltzer E, et al. Randomized, double-blind comparison of once-weekly dalbavancin versus twice-daily linezolid therapy for the treatment of complicated skin and skin structure infections. Clin Infect Dis. 2005;41(10):1407–1415. doi: 10.1086/497271. [DOI] [PubMed] [Google Scholar]
- 2.Boucher HW, Wilcox M, Talbot GH, Puttagunta S, Das AF, Dunne MW. Once-weekly dalbavancin versus daily conventional therapy for skin infection. N Engl J Med. 2014;370(23):2169–2179. doi: 10.1056/NEJMoa1310480. [DOI] [PubMed] [Google Scholar]
- 3.Dunne MW, Puttagunta S, Giordano P, Krievins D, Zelasky M, Baldassarre J. A Randomized Clinical Trial of Single Dose vs Weekly Dalbavancin for Treatment of Acute Bacterial Skin and Skin Structure Infection. Clin Infect Dis. 2015;62(5):545–551. doi: 10.1093/cid/civ982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marbury T, Dowell JA, Seltzer E, Buckwalter M. Pharmacokinetics of dalbavancin in patients with renal or hepatic impairment. J Clin Pharmacol. 2009;49(4):465–476. doi: 10.1177/0091270008330162. [DOI] [PubMed] [Google Scholar]
- 5.Leighton A, Gottlieb AB, Dorr MB, et al. Tolerability, Pharmacokinetics, and Serum Bactericidal Activity of Intravenous Dalbavancin in Healthy Volunteers. Antimicrob Agents Chemother. 2004;48(3):940–945. doi: 10.1128/AAC.48.3.940-945.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Allergan, Plc. Dalvance® (dalbavancin) prescribing infromation. [Accessed May 22, 2016]; Available at: http://www.allergan.com/assets/pdf/dalvance_pi. [Google Scholar]
- 7.Buckwalter M, Dowell Ja. Population pharmacokinetic analysis of dalbavancin, a novel lipoglycopeptide. J Clin Pharmacol. 2005;45(11):1279–1287. doi: 10.1177/0091270005280378. [DOI] [PubMed] [Google Scholar]
- 8.Bradley J, Puttagunta S, Rubino C, Blumer J, Dunne M, Sullivan J. Pharmacokinetics, safety and tolerability of single dose dalbavancin in children 12 through 17 years of age. Pediatr Infect Dis J. 2015;34(7):748–752. doi: 10.1097/INF.0000000000000646. [DOI] [PubMed] [Google Scholar]
- 9.Schwartz GJ, Work DF. Measurement and estimation of GFR in children and adolescents. Clin J Am Soc Nephrol. 2009;4(11):1832–1843. doi: 10.2215/CJN.01640309. [DOI] [PubMed] [Google Scholar]
- 10.Holford N, Heo Y, Anderson B. A pharmacokinetic standard for babies and adults. J Pharm Sci. 2013;102(9):2941–2952. doi: 10.1002/jps.23574. [DOI] [PubMed] [Google Scholar]
- 11.Anderson BJ, Holford NHG. Mechanistic basis of using body size and maturation to predict clearance in humans. Drug Metab Pharmacokinet. 2009;24(1):25–36. doi: 10.2133/dmpk.24.25. [DOI] [PubMed] [Google Scholar]
- 12.Gehan EA, George SL. Estimation of human body surface area from height and weight. Cancer Chemother Rep. 1970;54(4):225–235. [PubMed] [Google Scholar]
- 13.Schwartz GJ, Muñoz A, Schneider MF, Mak RH, Kaskel F, Warady BAFS. New Equations to Estimate GFR in Children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16(1):31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- 15.Gibaldi M, Perrier D. Pharmacokinetics. Chapter 2. New York, NY: Marcel Dekker; 1982. Multicompartment Models; pp. 84–111. [Google Scholar]
- 16.Centers for Disease Control and Prevention. [Accessed January 19, 2011];Growth charts. 2000 Available at: http://www.cdc.gov/Growthcharts/Percentile_Data_Files.htm.
- 17.Rhodin MM, Anderson BJ, Peters aM, et al. Human renal function maturation: a quantitative description using weight and postmenstrual age. Pediatr Nephrol. 2009;24(1):67–76. doi: 10.1007/s00467-008-0997-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.