Abstract
The hypocretin/orexin (HCRT) system is implicated in reward and reinforcement processes through actions on the mesolimbic dopamine (DA) system. Here we provide evidence for the relationship between HCRT and DA in vivo in anesthetized and freely-moving mice. The ability of cocaine to elicit reward-related behaviors in mice lacking the HCRT prepro-peptide (HCRT KO) and wild-type (WT) controls was determined using conditioned place preference. Using a combination of microdialysis and in vivo fast scan cyclic voltammetry in anesthetized and freely-moving mice we investigated the underlying role of HCRT in the regulation of DA release and uptake. We show that, unlike WT mice, HCRT KO mice fail to develop characteristic conditioned place preference for cocaine. These mice also demonstrated reduced DA release and uptake under baseline conditions in both anesthetized and freely-moving experiments. Further, diminished DA signaling in HCRT KO mice persists following administration of cocaine. These findings indicate that HCRT is essential for the expression of behaviors associated with the rewarding effects of cocaine, and suggest that HCRT regulation of reward and reinforcement may be related to disruptions to DA neurotransmission.
Keywords: addiction, conditioned place preference, fast scan cyclic voltammetry, microdialysis, nucleus accumbens, reward
Introduction
The mesolimbic dopamine (DA) system has been repeatedly implicated in reward and reinforcement processing associated with cocaine abuse (Roberts and Koob, 1982; Di Chiara and Imperato, 1988; Pettit and Justice, 1989). While a number of environmental and neurobiological factors have been found to influence this pathway, the hypocretin/orexin (HCRT) system has emerged as a particularly powerful modulator of DA neurotransmission. The HCRT peptides—HCRT-1 and HCRT-2—are synthesized by neurons originating in the lateral hypothalamus and adjacent perifornical areas. HCRT neurons project widely throughout the brain, where they produce effects via actions at the HCRT receptor 1 and HCRT receptor 2 (HCRTr1, HCRTr2; de Lecea et al., 1998; Peyron et al., 1998; Sakurai et al., 1998; Marcus et al., 2001; Fadel and Deutch, 2002). Since their discovery, extensive research has demonstrated that the HCRT system participates in the regulation of sleep/wake processes (Hagan et al., 1999; Piper et al., 2000; España et al., 2001). Accumulating evidence also implicates these neuropeptides in reward and reinforcement (Boutrel et al., 2005; Harris et al., 2005; Smith et al., 2010) and in the mesolimbic dopaminergic processes underlying them, particularly through actions within the ventral tegmental area (VTA; España et al., 2010; España et al., 2011; Prince et al., 2015). HCRT neurons send relatively dense projections to the VTA (Peyron et al., 1998; Fadel and Deutch, 2002), where HCRT both potentiates glutamate-mediated excitatory drive and induces burst firing of DA neurons (Korotkova et al., 2003; Borgland et al., 2006), and promotes cocaine self-administration (España et al., 2011). In contrast, blockade of HCRTr1 via SB-334867 decreases excitation of DA neurons (Moorman and Aston-Jones, 2010) and reduces cocaine-induced uptake inhibition and DA release in the nucleus accumbens (NAc; España et al., 2010). Additionally, SB-334867 blocks locomotor sensitization to the effects of cocaine (Borgland et al., 2006), inhibits cue-induced reinstatement of cocaine seeking (Smith et al., 2009; Mahler et al., 2013) and decreases the motivation to obtain a cocaine reward (Borgland et al., 2009; España et al., 2010; Brodnik et al., 2015; Prince et al., 2015). Considered together, these observations provide compelling evidence for HCRT's involvement in regulating mesolimbic DA signaling and cocaine reward.
Previous studies into HCRT's role in DA neurotransmission have typically used pharmacological interventions such as SB-334867 to block HCRT signaling in the VTA and NAc. Although pharmacological approaches allow for carefully controlled studies comparing DA release and uptake dynamics both prior to and following disruption of HCRT signaling, potential off-target effects (Gotter et al., 2012; Lebold et al., 2013), and hydrolytic instability (McElhinny et al., 2012) introduce some ambiguity into the interpretation of results. The HCRT knock-out (KO) mouse (Chemelli et al., 1999) provides a means to investigate dysfunction in HCRT neurotransmission without these potential confounds, and further supplies complementary information to previous work performed using pharmacological approaches. These mice are engineered to lack prepro-HCRT and display attenuated CPP for (Sharf et al., 2010) and physiological dependence on morphine (Georgescu et al., 2003), alongside a reduced DA response to the drug (Narita et al., 2006). We have previously demonstrated that HCRT KO mice show both altered uptake dynamics and reduced DA release before and in response to cocaine in vitro (España et al., 2010). While this initial evidence is compelling, the effects of cocaine on DA neurotransmission in mice lacking HCRT have not been demonstrated in a whole-animal model. The studies described herein sought to elucidate the role of HCRT in the regulation of mesolimbic DA in drug naïve conditions and in the behavioral and in vivo neurochemical responses to cocaine using HCRT KO mice. We used CPP to determine whether HCRT influences the rewarding effects of cocaine and used both microdialysis and fast scan cyclic voltammetry (voltammetry) to determine HCRT's involvement in regulating DA signaling.
Materials and Methods
Animals
Male HCRT KO and wild-type (WT) mice (12-18 weeks old) were provided ad libitum access to food and water and maintained in a vivarium with controlled humidity and temperature under a 12:12hr light-dark cycle. All experiments and procedures were conducted during the light cycle. HCRT KO and WT mice were created on a C57BL/6J-129/SvEV background and then backcrossed with C57BL/6J mice for more than 10 generations (Chemelli et al., 1999). Mice were bred in house and genotyped using polymerase chain reaction with a neo primer, 5′-CCGCTATCAGGACATAGCGTTGGC-3′, or a genomic primer, 5′-GACGACGGCCTCAGACTTCTTGGG-3′, and a genomic primer, 3′-TCACCCCCTTGGGATAGCCCTTCC-5′, common to KO and WT mice. All protocols and animal procedures were conducted in accordance with the NIH Guide for care and use of laboratory animals under the supervision of the Institutional Animal Care and Use Committee at Drexel University College of Medicine and Wake Forest School of Medicine.
Conditioned Place Preference
Apparatus
The apparatus consisted of a two-chamber insert for Med Associates open field activity monitors (13cm × 13cm × 20.3cm, Med Associates, St. Albans, VT). The chambers were equal in every aspect except that the floor and walls of one chamber were patterned with black and white stripes while the other was patterned with black and white polka dots. Patterns were counterbalanced across the left and right chambers, and drug pairing was further counterbalanced across both side and pattern.
Procedure
CPP for cocaine was determined using an unbiased design. In the preconditioning/bias test phase, mice were allowed access to both chambers for 25 min and the time spent in each chamber was recorded. One animal demonstrating ≥15% preference for one side of the apparatus in the preconditioning bias test was removed from the study. During the conditioning phase, WT (n=8) and HCRT KO (n=8) mice received either intraperitoneal (i.p.) saline or 10 mg/kg cocaine (6 mg/ml) and were confined to one chamber for 25 min; drug pairing was counterbalanced across both side and pattern. The mice were then returned to their home cage for 8 h, followed by an injection of the treatment they had not previously received (cocaine or saline) and placement in the opposite chamber. The conditioning phase continued for four consecutive days with two pairings per day, one for each side, alternating saline and cocaine as the first injection each day. CPP was assessed on the fifth day and was determined by the amount of time spent in each chamber over a 25 min period in a drug-free condition.
Microdialysis
HCRT KO (n=6) and WT (n=10) mice used for microdialysis experiments were anesthetized using isoflurane and placed in the stereotaxic apparatus. Guide cannulae for microdialysis probes (CMA/Microdialysis, Stockholm, Sweden) were aimed at the NAc core (+1.5 A, +0.1 L, -2.55 V) and secured into place using acrylic dental cement (Patterson Dental, Saint Paul, MN). Mice received post-surgical antibiotic (Neo-Predef, Pharmacia and Upjohn Co., New York, NY or 5mg/kg Baytril, enrofloxacin; Bayer HealthCare LLC, Shawnee Mission, KS) and analgesic (Ketoprofen, Webster Veterinary, Sterling, MA) and recovered for 48 hr prior to testing. This timing was determined based on previous publications which tested animals 1-5 days after surgery (Narita et al., 2006; Ferris et al., 2014). Microdialysis probes (membrane length 2mm; CMA/Microdialysis) were inserted approximately 16 hr prior to the beginning of sample collections and extended 2 mm beyond the cannula tip to -4.55 V (Mathews et al., 2009).
Microdialysis probes were perfused (0.8-1 μL/min) with sterile artificial cerebrospinal fluid (148 mM NaCl; 2.7 mM KCl; 1.2 mM CaCl2; 0.85 mM MgCl2; pH 7.4). Samples were collected every 15 min and analyzed for DA by high-performance liquid chromatography (HPLC) with electrochemical detection (BAS, West Lafayette, IN). At least three baseline samples were collected prior to administration of a single i.p. injection of 10 mg/kg cocaine. Dialysate samples were subsequently collected every 15 min for 2 hr (España et al., 2010; España et al., 2011).
High Performance Liquid Chromatography
The HPLC (Bioanalytical Systems, Mt. Vernon, IN) consisted of a syringe pump, a glassy carbon working electrode, a reference electrode, and an electrochemical detector. A 2×50 mm (3 μm particle) reverse-phase column (Luna, Phenomenex, Torrance, CA) was used to separate compounds (España et al., 2011). The applied potential was +650 mV as referenced to an Ag/AgCl electrode. The mobile phase (75 mM NaH2PO4, 1.7 mM 1-octanesulfonic acid sodium salt, 100 μL/L triethylamine, 25 μM EDTA, 10% acetonitrile v/v, pH=3.0) was pumped at a rate of 170 μL/min, with a detection limit for DA of 10 pM. DA quantification was achieved by comparing dialysate samples with DA standards of known concentration.
Voltammetry
Mice were anesthetized with isoflurane, placed into a stereotaxic apparatus, and implanted with a carbon fiber microelectrode aimed at the NAc (+1.3A, +1.3L, -4.5V) and a Ag/AgCl reference electrode located in the contralateral cortex. A bipolar stimulating electrode (Plastics One, Roanoke, VA) aimed at the VTA (-3.0P, +1.1L, -4V) was lowered in 100-200 μm increments until a 0.5 s, 60 Hz monophasic (4 ms; 250 μA) stimulation train produced a robust DA response.
Freely Moving
In mice used in freely moving experiments (WT n=7, KO n=6) all electrodes were secured to stainless steel surgical screws (Stoelting, Wood Dale, IL) implanted in the skull using acrylic dental cement as previously described (Clark et al., 2010). Mice received post-surgical antibiotic (5mg/kg; Baytril, enrofloxacin; Bayer HealthCare LLC, Shawnee Mission, KS) and analgesic (5mg/kg; ketoprofen; Webster Veterinary, Sterling, MA) for the first day following surgery, and saline containing vitamin B and dextrose for 2-3 days to aid recovery. After 21-23 days of recovery, animals were briefly anesthetized using isoflurane and connected to a voltammetric potentiometer/amplifier. This recovery time was determined based on preliminary data and previously published work (Clark et al. 2010; Wanat et al., 2013) that performed voltammetry recordings in freely-moving animals 2-16 weeks after electrode implantation. Mice remained in their home cage, which was placed inside an enclosed chamber, for the duration of the experiment. The carbon fiber working electrode was cycled at 60 Hz for 45-60 min, and then at 10 Hz for 20-30 min prior to the beginning of the experiment. Stimulation-evoked (0.5 s, 60 Hz monophasic; 4 ms, ∼90 μA) DA release was recorded in drug naïve animals every 10 min for at least 30 min, followed by an i.p. injection of 10 mg/kg cocaine (6 mg/ml). The effects of cocaine on stimulated DA release were then recorded every 10 min for at least 60 min.
Anesthetized
For mice used in experiments conducted under anesthesia (WT n=8; KO n=7), electrodes were inserted as above but were held in place for the duration of the experiment by stereotactic micromanipulators. Stimulation-evoked (0.5 s, 60 Hz monophasic; 4 ms; ∼270 μA) DA release was recorded every 5 min for at least 30 min until DA peaks in the NAc reached stability (three consecutive collections within 10%). Once stability was achieved, mice were injected i.p. with 10 mg/kg cocaine. Subsequent changes in stimulated DA release were recorded every 5 min for at least 60 min.
Data Acquisition
The electrode potential was linearly scanned (-0.4 to 1.2 V and back to -0.4 V vs Ag/AgCl) and cyclic voltammograms were recorded at the carbon fiber electrode every 100 ms with a scan rate of 400 V/s using a voltammeter/amperometer (freely-moving experiments: Electronics and Materials Engineering, Seattle, WA; anesthetized experiments: Chem-Clamp; Dagan Corporation, Minneapolis, MN). The magnitude of stimulated DA release and transporter-mediated uptake kinetics were monitored. Extracellular concentrations of DA were assessed by comparing the current at the peak oxidation potential for DA in consecutive voltammograms with the average calibration factor from electrodes used in each study (Ehrich et al., 2014) to reduce noise introduced by variance in calibrations. Electrodes were exposed to a known concentration of DA (3 μM) either before (freely-moving) or following (anesthetized) voltammetric experiments. DA overflow curves were first analyzed as previously described (Yorgason et al., 2011) for peak μM concentrations of DA and slope, a first-order measure of uptake. DA overflow curves in anesthetized animals were then fitted to a Michaelis-Menten-based kinetic model using Demon Voltammetry and Analysis software written in LabVIEW language (National Instruments, Austin, TX; Yorgason et al., 2011). DA uptake rates prior to any drug treatment were modeled by setting the affinity of DA for the DA transporter (DAT) to 0.2 μM and then fitting the overflow curve to establish a baseline Vmax (maximal uptake rate) for each subject. Following cocaine injection, Vmax was held constant for the remainder of the experiment and changes in DA uptake rate due to cocaine-induced uptake inhibition were calculated as a change in the apparent affinity for the DAT and defined as appKm. Determination of Michaelis-Menten kinetics relies on sufficient DA release to saturate the DAT (Wightman and Zimmerman, 1990), therefore Michaelis-Menten analysis was not performed on data from freely-moving experiments, as the levels of electrical stimulation necessary to elicit the required concentrations of DA would produce undesirable motor disruptions in awake animals.
Data Analysis and Statistics
Statistical analyses were primarily conducted using SPSS (v22) and all data was assessed for normality using the Shapiro-Wilk test. Homogeneity of variance and sphericity using Levene's and Mauchly's tests, respectively, were determined prior to analysis and corrections applied where noted. Outliers were identified using a two-tailed Grubbs' test (GraphPad QuickCalcs). Reported p-values are one-tailed as considerable previous work has determined the direction of HCRT disruption effects on DA signaling.
Cocaine preference was defined as increased time spent in the chamber paired with cocaine. Scores from CPP experiments were calculated as (seconds spent in the drug-paired chamber during the test session) – (seconds spent in the drug-paired chamber during the preconditioning session) and were analyzed using independent samples t-test with genotype as the grouping factor. For microdialysis experiments, baseline DA dialysate for each animal was determined by taking the average value of three stable collections prior to cocaine injection. One missing value 45 min following cocaine injection was imputed using linear interpolation. DA dialysate was expressed as a percent of baseline before and after cocaine administration and the peak concentration of DA in the first 60 min following cocaine injection was compared between WT and HCRT KO mice using a one-way ANOVA. Additionally, data was analyzed using a mixed design ANOVA with genotype as the between-subjects variable and time as the within-subjects variable with the baseline set at 100%.
To account for the decrease in peak height that can occur across repeated collections in freely moving stimulated voltammetry (Budygin et al., 2001), the first of the three collections acquired prior to cocaine was used to compare drug-naïve DA release and uptake dynamics between WT and HCRT KO mice using a one-way ANOVA. Two mice (n=1 KO, n=1 WT) were identified as statistical outliers and thus were removed from post-drug analysis. The peak concentration of DA in the first 30 min following cocaine injection was compared between HCRT KO and WT mice using a one-way ANOVA. Additionally, a two-way repeated measures ANOVA using genotype as the between-subjects factor and time as the within-subjects factor was performed to evaluate whether there were differences in the time-course of changes in DA release following cocaine compared to the collection immediately prior to cocaine administration.
Baseline values in animals used for anesthetized voltammetry were determined by averaging the last three values prior to cocaine and compared using one-way ANOVA with genotype as the between-subjects factor. One HCRT KO mouse was identified as a statistical outlier and removed from the analysis. Similar to freely-moving experiments, the maximum response in the first 30 min following cocaine administration was compared using one-way ANOVA with genotype as the between-subjects factor and was also examined using a two-way mixed design ANOVA with genotype as the between-subjects factor and time as the within-subjects factor. Where noted, the response to cocaine was also calculated in relation to baseline values, i.e. % of baseline = (response to cocaine) / (baseline value).
Results
Conditioned Place Preference
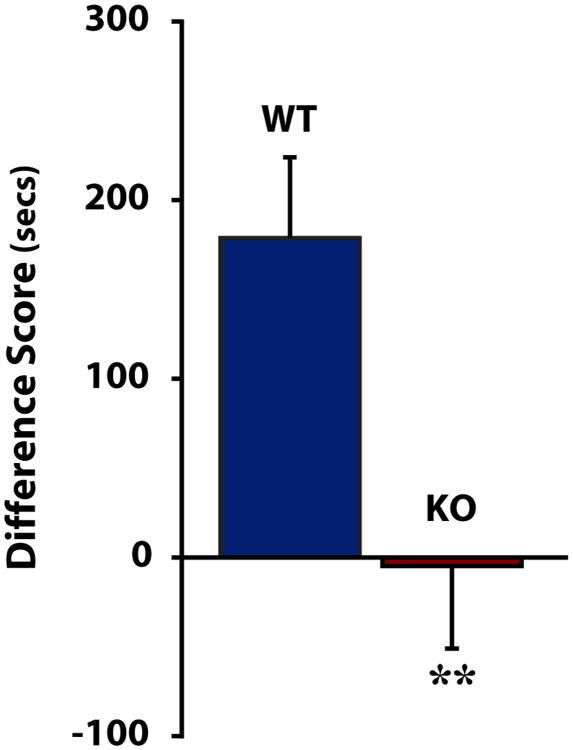
To determine whether HCRT regulated the rewarding properties of cocaine, we tested whether WT and HCRT KO mice displayed different preferences for cocaine using CPP. As demonstrated in numerous reports (for review, see Tzschentke, 1998), WT mice showed a robust preference for the cocaine-paired chamber, spending approximately 3 min longer in that chamber than during the preconditioning phase. HCRT KO mice differed significantly from WT mice [t(14)=2.838, p<0.01], demonstrating no preference for the cocaine-paired chamber (Fig. 1).
Figure 1. HCRT knock-out mice do not display conditioned place preference for cocaine.
Shown are mean ± SEM differences in time spent in the cocaine-paired chamber in wild-type (WT) and HCRT knock-out (KO) mice. **p<0.01
Microdialysis
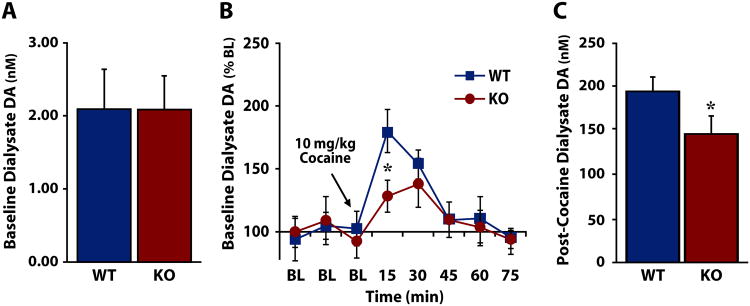
As the mesolimbic DA system has been repeatedly demonstrated to participate in cocaine reward and reinforcement (Roberts and Koob, 1982; Pettit and Justice, 1989; Caine and Koob, 1994; Pierce and Kumaresan, 2006), we next sought to establish the contribution of HCRT signaling to the regulation of DA in the NAc using freely-moving WT and HCRT KO mice. Baseline, non-drug extracellular levels of DA were established over 60 min in HCRT KO and WT mice prior to i.p. injection of 10 mg/kg cocaine. Similar to previous reports using pharmacological agents to block HCRTr1 (Narita et al., 2006; Sharf et al., 2010), there was no significant difference in basal extracellular DA between WT and HCRT KO mice (Fig. 2a) [F(1,14)=0.000163, p=0.495]. Although there was no significant main effect of genotype, a significant effect of time and a significant interaction was present, indicating that WT and HCRT KO mice differed in their response to cocaine (Fig 2b) [Time: F(5,70)=13.688, p<0.0001; Genotype: F(1,14)=0.742, p=0.403; Genotype × Time: F(5,70)=2.186, p<0.05]. Further analysis revealed that HCRT KO mice had significantly reduced DA response to cocaine relative to WT mice at the 15 min time point following cocaine administration [t(14)=-2.141, p<0.05]. Additionally, HCRT KO mice showed a significantly lower maximal response to cocaine than WT mice (Fig 2c) [F(1,14)=3.517, p<0.05].
Figure 2. HCRT knock-out mice display a reduced DA response to cocaine.
A) Mean ± SEM basal levels of extracellular dopamine (DA) in wild-type (WT) and HCRT knock-out (KO) mice prior to cocaine treatment. B) Mean ± SEM extracellular DA expressed as a percent of baseline (BL) in WT and KO mice before and after cocaine delivery. The arrow indicates administration of 10 mg/kg cocaine. C) Mean ± SEM extracellular DA levels following 10 mg/kg i.p. cocaine. *p<0.05
Freely Moving Voltammetry
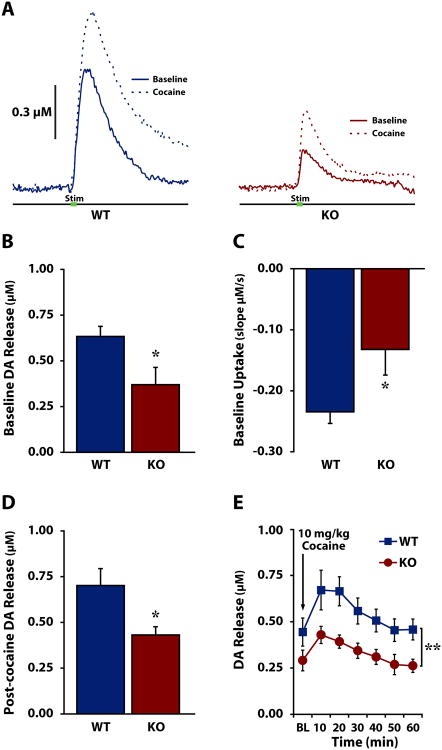
We then used voltammetry in freely-moving WT and HCRT KO mice to determine the contribution of HCRT neurotransmission to phasic DA release under baseline, non-drug conditions and in response to cocaine in an awake, freely-moving animal model. As shown in Fig. 3A and Fig. 3B, baseline DA release was significantly lower in HCRT KO mice (n=6) compared to WT mice (n=7) [F(1,11)=6.140, p<0.05]. Additionally, HCRT KO mice showed slower uptake compared to WT mice as measured by the slope of DA clearance (Fig. 3c) [F(1,11)=5.411, p<0.05]. HCRT KO mice (n=5) also showed significantly reduced DA release compared to WT mice (n=6) following i.p. 10 mg/kg cocaine (Figs. 3d, 3e) [F(1,9)=6.056, p<0.05], although there was no interaction effect [Time: F(6,54)=10.694, p<0.001; Genotype: F(1,9)=8.538, p<0.01; Genotype × Time: F(6,54)=1.199, p=0.161]. Despite this finding, neither cocaine-induced increases in DA release expressed as a percent of baseline [F(1,9)=0.142, p=0.358], nor DA uptake inhibition as measured by slope [F(1,9)=3.043, p=0.058] differed significantly between WT and HCRT KO mice (Fig. S1).
Figure 3. Freely-moving HCRT knock-out mice display disrupted DA signaling at baseline and in response to cocaine.
A) Examples of dopamine (DA) overflow curves collected in freely-moving wild-type (WT, left) and HCRT knock-out (KO, right) mice before (solid) and after (dashed) 10 mg/kg i.p. cocaine. Green bar indicates stimulation (0.5 s). B) Mean ± SEM stimulated DA release and C) DA uptake in freely-moving WT and KO mice under baseline conditions. D) Mean ± SEM stimulated DA release following 10 mg/kg i.p. cocaine. E) Mean ± SEM stimulated DA release in WT (square) and KO (circle) mice across experimental sessions, at baseline (BL) and following cocaine. The arrow indicates administration of 10 mg/kg cocaine. *p<0.05, **p<0.01
Anesthetized Voltammetry
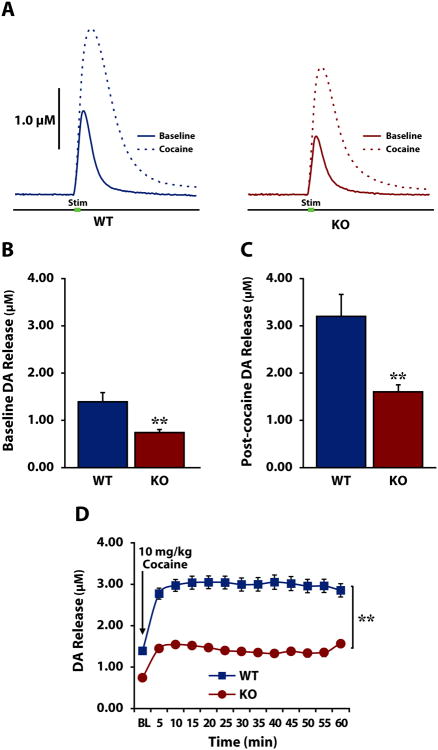
To further evaluate the role of HCRT neurotransmission in DA release in the NAc in vivo, we used voltammetry in isoflurane-anesthetized WT and HCRT KO mice. This approach allows for higher levels of stimulation than is obtainable in studies using freely-moving animals, producing greater DA release and therefore more complete saturation of the DAT (Wightman and Zimmerman, 1990). This in turn allows for more accurate determination of rates of uptake and cocaine-induced uptake inhibition without confounding locomotor effects. Under baseline, non-drug conditions, HCRT KO mice had lower levels of stimulated DA release (Fig. 4a and Fig. 4b) compared to WT mice [F(1,12)=7.728, p<0.01]. Similar to results in freely-moving mice, this relationship persisted after experimenter delivered cocaine; DA release following cocaine (Fig. 4c) was significantly lower in HCRT KO compared to WT mice [Welch's ANOVA for unequal variances: F(1,8.375)=10.651, p<0.01]. Further, repeated measures ANOVA found that DA release differed significantly between the groups across time (Fig. 4d) [Time: F(2.086,25.034 Greenhouse-Geisser corrected)=19.427, p<0.0001); Genotype: F(1,12)=8.896, p<0.01; Genotype × Time: F(2.086,25.034 Greenhouse-Geisser corrected)=3.704, p<0.05]. Similar to observations under freely-moving conditions, there was no significant effect of genotype on cocaine-induced increases in DA release when expressed as a percent of baseline [F(1,12)=0.598, p=0.227](Fig.S2).
Figure 4. Anesthetized HCRT knock-out mice display disrupted DA signaling at baseline and in response to cocaine.
A) Examples of dopamine (DA) overflow curves collected in anesthetized wild-type (WT, left) and HCRT knock-out (KO, right) mice before (solid) and after (dashed) 10 mg/kg i.p. cocaine. Green bar indicates stimulation (0.5 s). B) Mean ± SEM stimulated DA release under baseline conditions and C) after cocaine delivery in WT and HCRT KO mice. D) Mean ± SEM stimulated DA release in WT (square) and KO (circle) mice across the experimental session. The arrow indicates administration of 10 mg/kg cocaine. *p<0.05; **p<0.01
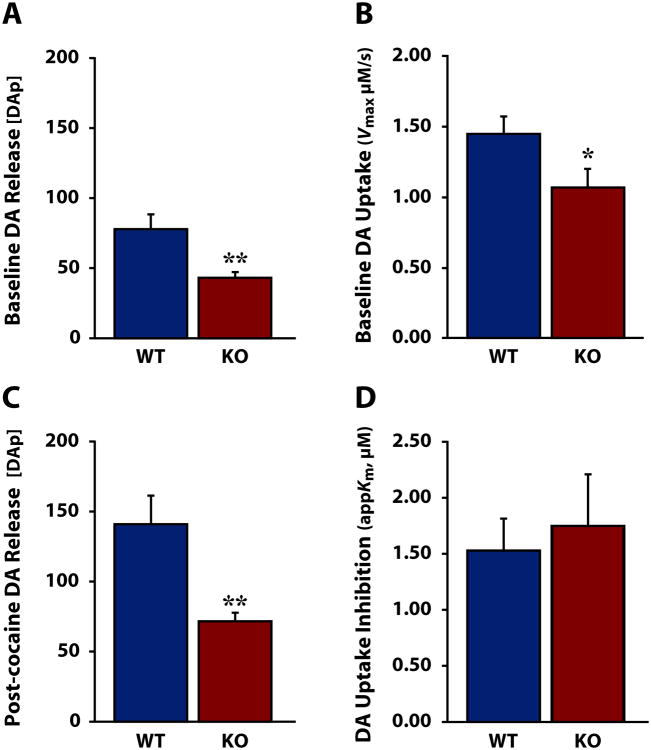
To more fully elucidate the role HCRT plays in the DA response to cocaine and to facilitate comparison to previous work, we next conducted Michaelis-Menten kinetic analysis to determine HCRT's effects on DA release and uptake. As shown in Fig. 5, HCRT KO mice demonstrated significantly lower DA release per stimulation pulse ([DAp]) relative to WT mice under both baseline conditions (Fig. 5a) [Welch's ANOVA for unequal variances: F(1,9.040)=9.635, p<0.01] and in response to cocaine (Fig. 5c) [Welch's ANOVA for unequal variances: F(1,8.125)=10.566, p<0.01]. Although HCRT KO mice displayed a significantly slower rate of uptake (Vmax) prior to cocaine (Fig. 5b) [one-way ANOVA: F(1,12)=4.269, p<0.05] there was no significant difference between WT and HCRT KO mice in cocaine-induced uptake inhibition (appKm) (Fig. 5d) [one-way ANOVA: F(1,12)=0.73, p=0.396].
Figure 5. Anesthetized HCRT knock-out mice display disrupted DA release and uptake dynamics.
A) Mean ± SEM dopamine (DA) release per stimulation pulse ([DAp]) and B) maximal uptake rate (Vmax) in wild-type (WT) and HCRT knock-out (KO) mice under baseline conditions. C) Mean ± SEM [DAp] and D) uptake inhibition expressed as apparent affinity for the DA transporter (appKm) in WT and KO mice following 10 mg/kg cocaine. *p<0.05 **p<0.01
Discussion
Here we sought to determine the nature of HCRT modulation of reward and underlying dopaminergic processes using mice lacking the HCRT prepro-peptide. The current observations demonstrate that, in contrast to WT mice, HCRT KO mice do not exhibit cocaine-induced CPP. Additionally, mice lacking HCRT show reduced baseline levels of DA signaling, reflecting HCRT's role in the maintenance of DA neurotransmission. This was recapitulated by the attenuated DA response to cocaine in anesthetized and freely-moving mice. The current report suggests that HCRT is an essential modulator of DA processes underlying reward and reinforcement, and confirms the HCRT system as a potential target for pharmacotherapies treating cocaine addiction.
HCRT modulates the rewarding efficacy of cocaine
We first used CPP to determine the role of HCRT signaling in cocaine reward, finding that a lack of HCRT blocked cocaine-elicited place preference. This result is contrary to a previous report indicating that HCRTr1 blockade using SB-334867 in mice attenuated morphine but not cocaine CPP (Sharf et al., 2010). A possible explanation for this discrepancy is that SB-334867 preferentially blocks only the HCRTr1, whereas HCRT KO mice necessarily lack activity at both HCRT receptors by virtue of complete loss of the HCRT peptide. It is also worth noting that HCRT neurons project to multiple areas of the brain and produce actions on many neurotransmitters that may be involved in the development and expression of CPP. For example, serotonin neurons receive HCRT projections and serotonergic function has been implicated in cocaine-induced CPP (Hnasko 2007). Further, dynorphin has been demonstrated to co-transmit with HCRT in the VTA (Muschamp 2014), suggesting that in the absence of HCRT there may be disproportionate release of dynorphin. It is therefore possible that the CPP results reflect a loss of excitatory input to multiple converging pathways, including the DA systems investigated here.
Alternatively, the failure of SB-334867 treatment to disrupt cocaine CPP in mice may be explained by the method of testing employed. In Sharf et al., WT mice were trained to make associations with cocaine without interference and SB-334867 was administered only on the test day, after CPP for cocaine had already been established. HCRT KO mice have a congenital absence of HCRT signaling, thus DA release is disrupted throughout all experimental phases, suggesting that HCRT neurotransmission may be necessary for the acquisition—but not expression—of cocaine CPP. This is supported by previous research which found that reducing the ability of DA to act on the D1 receptor attenuated the acquisition of cocaine CPP but did not affect expression of previously acquired associations for cocaine (Cervo and Samanin, 1995). Thus, congenitally reduced DA release in HCRT KO mice may have prevented acquisition of cocaine CPP. The role of HCRT itself in learning remains uncertain, with HCRT-1 peptide both facilitating passive-avoidance learning (Telegdy and Adamik, 2002) and impairing spatial learning in the Morris Water Maze (Aou et al., 2003). Nonetheless, as disruption of HCRT neurotransmission does not prevent conditioned place aversion (Di Sebastiano et al., 2011) or conditioned taste aversion (Mediavilla et al., 2011) to lithium chloride, the failure of HCRT KO mice to form CPP for cocaine is not likely the result of an inability to learn contextual associations or, similarly, the result of a narcoleptic phenotype interfering with normal motor and learning behaviors. Nevertheless, the results of our cocaine-induced CPP experiments are consistent with a wealth of data demonstrating that disruption to HCRTr1 signaling results in diminished motivation to obtain a cocaine reward in rats and mice (Hollander et al., 2012; Muschamp et al., 2014; Prince et al., 2015).
HCRT maintains DA signaling and modulates DA responses to cocaine
Consistent with a failure to develop cocaine-induced CPP, HCRT KO mice show disrupted DA signaling both under baseline non-drug conditions and in response to cocaine. Using microdialysis, we found that basal levels of extracellular DA do not differ between HCRT KO and WT mice, but that the DA response to cocaine was significantly blunted in mice lacking HCRT. We then used voltammetry in freely-moving mice and found significantly reduced stimulated DA release in HCRT KO mice under baseline, non-drug conditions compared to WT mice that persisted following the administration of cocaine. These observations indicate that HCRT KO mice have disrupted DA signaling under baseline conditions and in response to cocaine. Nevertheless, whereas previous studies found that disruption of HCRT signaling altered the ability of cocaine to inhibit uptake via the DAT (España et al., 2010), we found no significant differences in DA clearance following cocaine administration between HCRT KO and WT mice using a first-order measure of uptake (slope), although the rate of uptake differed at baseline.
To more fully understand the relationship between HCRT transmission and DA release and uptake dynamics, we then used in vivo voltammetry in anesthetized mice. This technique permits the higher levels of stimulation necessary to evoke sufficient DA release to saturate the DA transporters (DAT), thus allowing for analysis of Vmax (maximal uptake) and the inhibitory effect of cocaine on Km (affinity of DA for the DAT) using Michaelis-Menten kinetics (Wightman and Zimmerman, 1990). Results from these studies confirmed that DA signaling under baseline conditions and in response to cocaine differ between WT and HCRT KO mice and suggested that this relationship does not rely on differences in uptake inhibition. Although the absolute concentration of DA release differed significantly between WT and KO mice before and following administration of cocaine, there was no difference in DA release when expressed as a percent of baseline (i.e., the increase in DA following cocaine was proportional across groups). As deficits in DA release were observed both before and after cocaine, it is possible that this is the neural substrate underlying the failure of HCRT KO mice to develop cocaine-induced CPP. In this capacity, in HCRT KO mice the effects of cocaine may not have been sufficient to elevate DA concentrations to levels needed to produce rewarding effects.
Although Vmax was slower in HCRT KO mice compared to WT mice we found no significant difference in the ability of cocaine to inhibit DA uptake between genotypes, thus the affinity of cocaine for the DAT appears unaffected. This lack of an effect of HCRT on cocaine-induced uptake inhibition is surprising as we have previously demonstrated that acute SB-334867 attenuates the ability of cocaine to inhibit DA uptake (España et al., 2011; Prince et al., 2015), and that HCRT KO mice show similarly attenuated cocaine-induced uptake inhibition in an in vivo preparation (España et al., 2010). It is possible, however, that compensation of the mesolimbic DA system in animals congenitally lacking HCRT may account for the disparity of results between this and previous, largely pharmacological studies, as intervention with SB-334867 disrupts dopaminergic processes already in place. Further, experiments in an in vitro preparation were performed in a NAc isolated from midbrain dopaminergic input with bath application of cocaine at higher concentrations than may occur in vivo, suggesting that the two models may not be fully comparable.
The similar levels of basal extracellular DA between WT and HCRT KO mice may be explained by results obtained from our freely-moving and anesthetized voltammetry experiments. Although DA release is lower in HCRT KO mice when measured using voltammetry, this decrease may be countered by the significantly slower rate of uptake also seen in these animals. Given that microdialysis reflects a balance between DA release and uptake, the reduced DA release in HCRT KO would be cleared more slowly, resulting in normalized extracellular DA concentrations.
Conclusion
We sought to assess the role of HCRT in reward and its underlying dopaminergic processes. Here we found that the HCRT system is essential for the development of CPP for cocaine, and for the regulation of DA neurotransmission in both drug-naïve and post-cocaine conditions. When considered alongside an amassing literature using HCRT pharmacological manipulations, the current observations provide further support for the hypothesis that HCRT influences reward and reinforcement processes via actions on the mesolimbic DA system.
Supplementary Material
Acknowledgments
We would like to thank Dr. Thomas E. Scammell for generously providing the Hypocretin Knock-out line of mice. We would also like to thank Rochelle España, Courtney Prince, Douglas Fox, and Preeti Badve for their expert technical assistance. This work was supported by grants NARSAD-17738, DA031900 (RE) and DA021325, P50 DA006634 (SJ).
Footnotes
Author Contributions: RE, MF, SJ and JS were responsible for study concept and design. MF, JL, JS, and ZB assisted in acquisition and analysis of animal data. JS drafted the manuscript and RE, MF and SJ provided critical revision for content and style. All authors critically reviewed content and approve the final version for publication.
References
- Aou S, Li XL, Li AJ, Oomura Y, Shiraishi T, Sasaki K, Imamura T, Wayner MJ. Orexin-A (hypocretin-1) impairs Morris water maze performance and CA1-Schaffer collateral long-term potentiation in rats. Neuroscience. 2003;119:1221–1228. doi: 10.1016/s0306-4522(02)00745-5. [DOI] [PubMed] [Google Scholar]
- Borgland SL, Chang SJ, Bowers MS, Thompson JL, Vittoz N, Floresco SB, Chou J, Chen BT, Bonci A. Orexin A/hypocretin-1 selectively promotes motivation for positive reinforcers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2009;29:11215–11225. doi: 10.1523/JNEUROSCI.6096-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgland SL, Taha SA, Sarti F, Fields HL, Bonci A. Orexin A in the VTA is critical for the induction of synaptic plasticity and behavioral sensitization to cocaine. Neuron. 2006;49:589–601. doi: 10.1016/j.neuron.2006.01.016. [DOI] [PubMed] [Google Scholar]
- Boutrel B, Kenny PJ, Specio SE, Martin-Fardon R, Markou A, Koob GF, de Lecea L. Role for hypocretin in mediating stress-induced reinstatement of cocaine-seeking behavior. Proc Natl Acad Sci USA. 2005;102:19168–19173. doi: 10.1073/pnas.0507480102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodnik ZD, Bernstein DL, Prince CD, España RA. Hypocretin receptor 1 blockade preferentially reduces high effort responding for cocaine without promoting sleep. Behavioural brain research. 2015;291:377–384. doi: 10.1016/j.bbr.2015.05.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Budygin EA, Phillips PE, Robinson DL, Kennedy AP, Gainetdinov RR, Wightman RM. Effect of acute ethanol on striatal dopamine neurotransmission in ambulatory rats. J Pharmacol Exp Ther. 2001;297:27–34. [PubMed] [Google Scholar]
- Caine SB, Koob GF. Effects of mesolimbic dopamine depletion on responding maintained by cocaine and food. J Exp Anal Behav. 1994;61:213–221. doi: 10.1901/jeab.1994.61-213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cervo L, Samanin R. Effects of dopaminergic and glutamatergic receptor antagonists on the acquisition and expression of cocaine conditioning place preference. Brain research. 1995;673:242–250. doi: 10.1016/0006-8993(94)01420-m. [DOI] [PubMed] [Google Scholar]
- Chemelli RM, Willie JT, Sinton CM, Elmquist JK, Scammell T, Lee C, Richardson JA, Williams SC, Xiong Y, Kisanuki Y, Fitch TE, Nakazato M, Hammer RE, Saper CB, Yanagisawa M. Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell. 1999;98:437–451. doi: 10.1016/s0092-8674(00)81973-x. [DOI] [PubMed] [Google Scholar]
- Clark JJ, Sandberg SG, Wanat MJ, Gan JO, Horne EA, Hart AS, Akers CA, Parker JG, Willuhn I, Martinez V, Evans SB, Stella N, Phillips PE. Chronic microsensors for longitudinal, subsecond dopamine detection in behaving animals. Nat Methods. 2010;7:126–129. doi: 10.1038/nmeth.1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Danielson PE, Fukuhara C, Battenberg EL, Gautvik VT, Bartlett FS, 2nd, Frankel WN, van den Pol AN, Bloom FE, Gautvik KM, Sutcliffe JG. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Chiara G, Imperato A. Drugs abused by humans preferentially increase synaptic dopamine concentrations in the mesolimbic system of freely moving rats. Proc Natl Acad Sci USA. 1988;85:5274–5278. doi: 10.1073/pnas.85.14.5274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sebastiano AR, Wilson-Perez HE, Lehman MN, Coolen LM. Lesions of orexin neurons block conditioned place preference for sexual behavior in male rats. Horm Behav. 2011;59:1–8. doi: 10.1016/j.yhbeh.2010.09.006. [DOI] [PubMed] [Google Scholar]
- Ehrich JM, Phillips PE, Chavkin C. Kappa opioid receptor activation potentiates the cocaine-induced increase in evoked dopamine release recorded in vivo in the mouse nucleus accumbens. Neuropsychopharmacology. 2014;39:3036–3048. doi: 10.1038/npp.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Baldo BA, Kelley AE, Berridge CW. Wake-promoting and sleep-suppressing actions of hypocretin (orexin): basal forebrain sites of action. Neuroscience. 2001;106:699–715. doi: 10.1016/s0306-4522(01)00319-0. [DOI] [PubMed] [Google Scholar]
- España RA, Melchior JR, Roberts DC, Jones SR. Hypocretin 1/orexin A in the ventral tegmental area enhances dopamine responses to cocaine and promotes cocaine self-administration. Psychopharmacology. 2011;214:415–426. doi: 10.1007/s00213-010-2048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- España RA, Oleson EB, Locke JL, Brookshire BR, Roberts DC, Jones SR. The hypocretin-orexin system regulates cocaine self-administration via actions on the mesolimbic dopamine system. The European journal of neuroscience. 2010;31:336–348. doi: 10.1111/j.1460-9568.2009.07065.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fadel J, Deutch AY. Anatomical substrates of orexin-dopamine interactions: lateral hypothalamic projections to the ventral tegmental area. Neuroscience. 2002;111:379–387. doi: 10.1016/s0306-4522(02)00017-9. [DOI] [PubMed] [Google Scholar]
- Ferris MJ, España RA, Locke JL, Konstantopoulos JK, Rose JH, Chen R, Jones SR. Dopamine transporters govern diurnal variation in extracellular dopamine tone. Proc Natl Acad Sci USA. 2014;111:E2751–2759. doi: 10.1073/pnas.1407935111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Georgescu D, Zachariou V, Barrot M, Mieda M, Willie JT, Eisch AJ, Yanagisawa M, Nestler EJ, DiLeone RJ. Involvement of the lateral hypothalamic peptide orexin in morphine dependence and withdrawal. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:3106–3111. doi: 10.1523/JNEUROSCI.23-08-03106.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotter AL, Webber AL, Coleman PJ, Renger JJ, Winrow CJ. International Union of Basic and Clinical Pharmacology. LXXXVI. Orexin receptor function, nomenclature and pharmacology. Pharmacol Rev. 2012;64:389–420. doi: 10.1124/pr.111.005546. [DOI] [PubMed] [Google Scholar]
- Hagan JJ, Leslie RA, Patel S, Evans ML, Wattam TA, Holmes S, Benham CD, Taylor SG, Routledge C, Hemmati P, Munton RP, Ashmeade TE, Shah AS, Hatcher JP, Hatcher PD, Jones DN, Smith MI, Piper DC, Hunter AJ, Porter RA, Upton N. Orexin A activates locus coeruleus cell firing and increases arousal in the rat. Proc Natl Acad Sci USA. 1999;96:10911–10916. doi: 10.1073/pnas.96.19.10911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris GC, Wimmer M, Aston-Jones G. A role for lateral hypothalamic orexin neurons in reward seeking. Nature. 2005;437:556–559. doi: 10.1038/nature04071. [DOI] [PubMed] [Google Scholar]
- Hnasko TS, Sotak BN, Palmiter RD. Cocaine-conditioned place preference by dopamine-deficient mice is mediated by serotonin. J Neurosci. 2007;27:12484–12488. doi: 10.1523/JNEUROSCI.3133-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollander JA, Pham D, Fowler CD, Kenny PJ. Hypocretin-1 receptors regulate the reinforcing and reward-enhancing effects of cocaine: pharmacological and behavioral genetics evidence. Frontiers in behavioral neuroscience. 2012;6:47. doi: 10.3389/fnbeh.2012.00047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korotkova TM, Sergeeva OA, Eriksson KS, Haas HL, Brown RE. Excitation of ventral tegmental area dopaminergic and nondopaminergic neurons by orexins/hypocretins. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2003;23:7–11. doi: 10.1523/JNEUROSCI.23-01-00007.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebold TP, Bonaventure P, Shireman BT. Selective orexin receptor antagonists. Bioorganic & medicinal chemistry letters. 2013;23:4761–4769. doi: 10.1016/j.bmcl.2013.06.057. [DOI] [PubMed] [Google Scholar]
- Mahler SV, Smith RJ, Aston-Jones G. Interactions between VTA orexin and glutamate in cue-induced reinstatement of cocaine seeking in rats. Psychopharmacology. 2013;226:687–698. doi: 10.1007/s00213-012-2681-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marcus JN, Aschkenasi CJ, Lee CE, Chemelli RM, Saper CB, Yanagisawa M, Elmquist JK. Differential expression of orexin receptors 1 and 2 in the rat brain. J Comp Neurol. 2001;435:6–25. doi: 10.1002/cne.1190. [DOI] [PubMed] [Google Scholar]
- Mathews TA, Brookshire BR, Budygin EA, Hamre K, Goldowitz D, Jones SR. Ethanol-induced hyperactivity is associated with hypodopaminergia in the 22-TNJ ENU-mutated mouse. Alcohol. 2009;43:421–431. doi: 10.1016/j.alcohol.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhinny CJ, Jr, Lewin AH, Mascarella SW, Runyon S, Brieaddy L, Carroll FI. Hydrolytic instability of the important orexin 1 receptor antagonist SB-334867: possible confounding effects on in vivo and in vitro studies. Bioorganic & medicinal chemistry letters. 2012;22:6661–6664. doi: 10.1016/j.bmcl.2012.08.109. [DOI] [PubMed] [Google Scholar]
- Mediavilla C, Cabello V, Risco S. SB-334867-A, a selective orexin-1 receptor antagonist, enhances taste aversion learning and blocks taste preference learning in rats. Pharmacol Biochem Behav. 2011;98:385–391. doi: 10.1016/j.pbb.2011.01.021. [DOI] [PubMed] [Google Scholar]
- Moorman DE, Aston-Jones G. Orexin/hypocretin modulates response of ventral tegmental dopamine neurons to prefrontal activation: diurnal influences. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30:15585–15599. doi: 10.1523/JNEUROSCI.2871-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muschamp JW, Hollander JA, Thompson JL, Voren G, Hassinger LC, Onvani S, Kamenecka TM, Borgland SL, Kenny PJ, Carlezon WA., Jr Hypocretin (orexin) facilitates reward by attenuating the antireward effects of its cotransmitter dynorphin in ventral tegmental area. Proc Natl Acad Sci USA. 2014;111:E1648–1655. doi: 10.1073/pnas.1315542111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita M, Nagumo Y, Hashimoto S, Narita M, Khotib J, Miyatake M, Sakurai T, Yanagisawa M, Nakamachi T, Shioda S, Suzuki T. Direct involvement of orexinergic systems in the activation of the mesolimbic dopamine pathway and related behaviors induced by morphine. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:398–405. doi: 10.1523/JNEUROSCI.2761-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettit HO, Justice JB., Jr Dopamine in the nucleus accumbens during cocaine self-administration as studied by in vivo microdialysis. Pharmacol Biochem Behav. 1989;34:899–904. doi: 10.1016/0091-3057(89)90291-8. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, Kilduff TS. Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Kumaresan V. The mesolimbic dopamine system: the final common pathway for the reinforcing effect of drugs of abuse? Neurosci Biobehav Rev. 2006;30:215–238. doi: 10.1016/j.neubiorev.2005.04.016. [DOI] [PubMed] [Google Scholar]
- Piper DC, Upton N, Smith MI, Hunter AJ. The novel brain neuropeptide, orexin-A, modulates the sleep-wake cycle of rats. The European journal of neuroscience. 2000;12:726–730. doi: 10.1046/j.1460-9568.2000.00919.x. [DOI] [PubMed] [Google Scholar]
- Prince CD, Rau AR, Yorgason JT, España RA. Hypocretin/Orexin regulation of dopamine signaling and cocaine self-administration is mediated predominantly by hypocretin receptor 1. ACS chemical neuroscience. 2015;6:138–146. doi: 10.1021/cn500246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DC, Koob GF. Disruption of cocaine self-administration following 6-hydroxydopamine lesions of the ventral tegmental area in rats. Pharmacol Biochem Behav. 1982;17:901–904. doi: 10.1016/0091-3057(82)90469-5. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, Williams SC, Richarson JA, Kozlowski GP, Wilson S, Arch JR, Buckingham RE, Haynes AC, Carr SA, Annan RS, McNulty DE, Liu WS, Terrett JA, Elshourbagy NA, Bergsma DJ, Yanagisawa M. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:1. doi: 10.1016/s0092-8674(02)09256-5. page following 696. [DOI] [PubMed] [Google Scholar]
- Sharf R, Guarnieri DJ, Taylor JR, DiLeone RJ. Orexin mediates morphine place preference, but not morphine-induced hyperactivity or sensitization. Brain research. 2010;1317:24–32. doi: 10.1016/j.brainres.2009.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, See RE, Aston-Jones G. Orexin/hypocretin signaling at the orexin 1 receptor regulates cue-elicited cocaine-seeking. The European journal of neuroscience. 2009;30:493–503. doi: 10.1111/j.1460-9568.2009.06844.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith RJ, Tahsili-Fahadan P, Aston-Jones G. Orexin/hypocretin is necessary for context-driven cocaine-seeking. Neuropharmacology. 2010;58:179–184. doi: 10.1016/j.neuropharm.2009.06.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Telegdy G, Adamik A. The action of orexin A on passive avoidance learning. Involvement of transmitters. Regulatory peptides. 2002;104:105–110. doi: 10.1016/s0167-0115(01)00341-x. [DOI] [PubMed] [Google Scholar]
- Tzschentke TM. Measuring reward with the conditioned place preference paradigm: a comprehensive review of drug effects, recent progress and new issues. Prog Neurobiol. 1998;56:613–672. doi: 10.1016/s0301-0082(98)00060-4. [DOI] [PubMed] [Google Scholar]
- Wanat MJ, Bonci A, Phillips PE. CRF acts in the midbrain to attenuate accumbens dopamine release to rewards but not their predictors. Nat Neurosci. 2013;16:383–385. doi: 10.1038/nn.3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman RM, Zimmerman JB. Control of dopamine extracellular concentration in rat striatum by impulse flow and uptake. Brain Res Brain Res Rev. 1990;15:135–144. doi: 10.1016/0165-0173(90)90015-g. [DOI] [PubMed] [Google Scholar]
- Yorgason JT, España RA, Jones SR. Demon voltammetry and analysis software: analysis of cocaine-induced alterations in dopamine signaling using multiple kinetic measures. Journal of neuroscience methods. 2011;202:158–164. doi: 10.1016/j.jneumeth.2011.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.